Mixed-Ligand Engineering to Enhance Luminescence of Mn2+-Based Metal Halides for Wide Color Gamut Display
Abstract
:1. Introduction
2. Experiment and Characterization
2.1. Materials
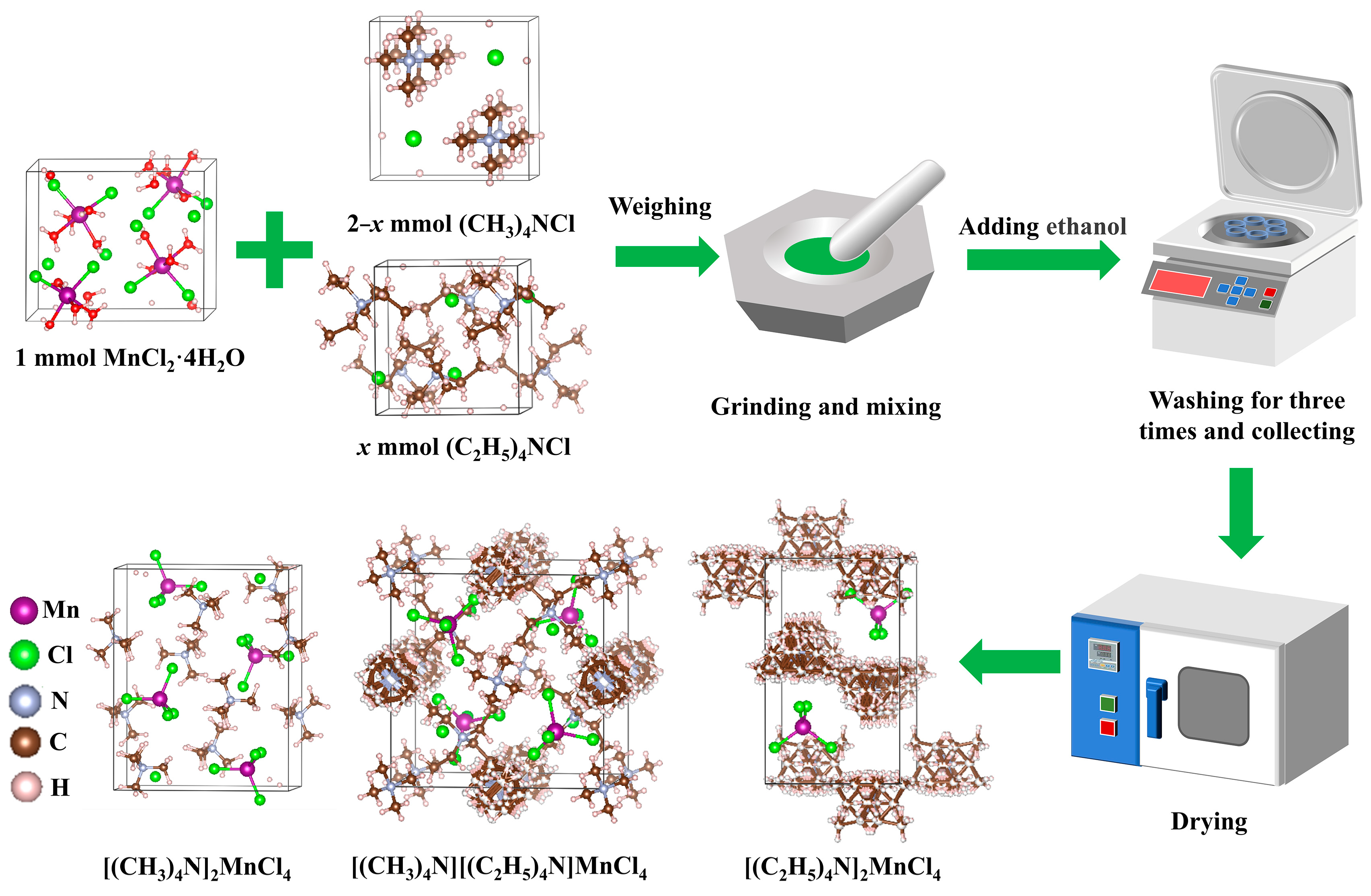
2.2. Synthesis of [(CH3)4N]2−x[(C2H5)4N]xMnCl4 Phosphors
2.3. Preparation of WLED Device
2.4. Characterization
2.5. First Principles Calculations
3. Results and Discussion
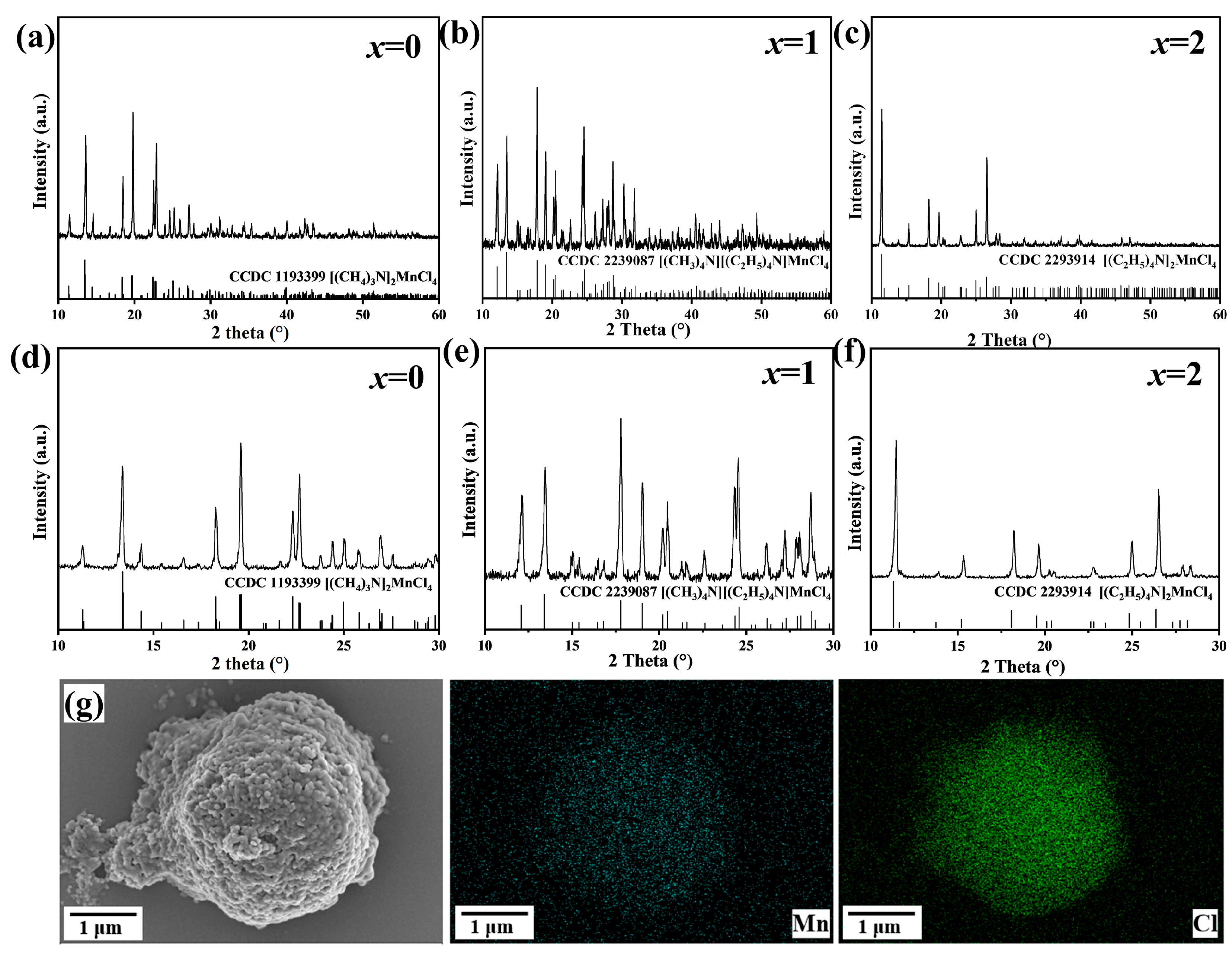
3.1. The Structure and Morphology of [(CH3)4N]2−x[(C2H5)4N]xMnCl4 Phosphors
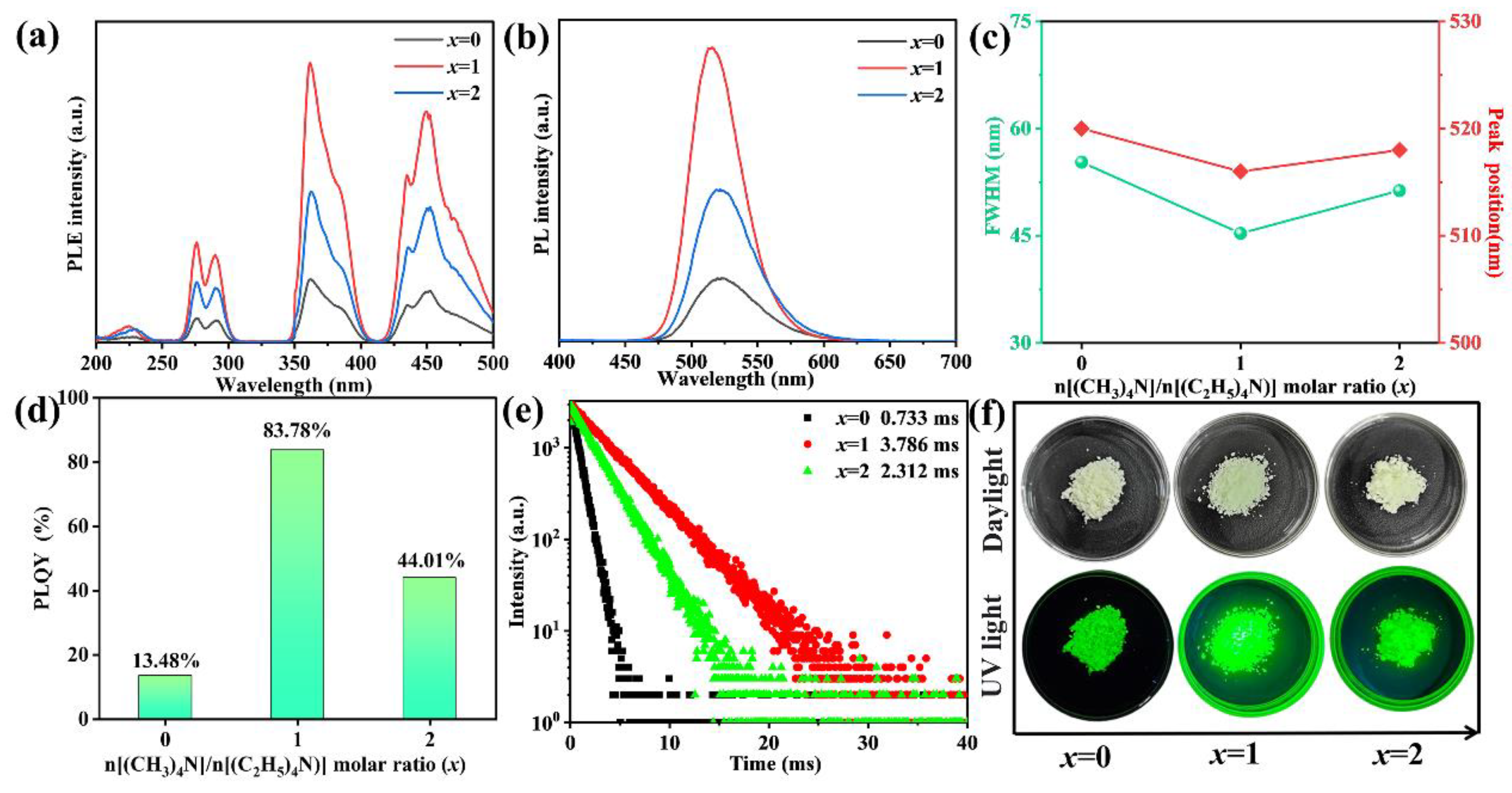
3.2. PL Properties of [(CH3)4N]2−x[(C2H5)4N]xMnCl4 Phosphors
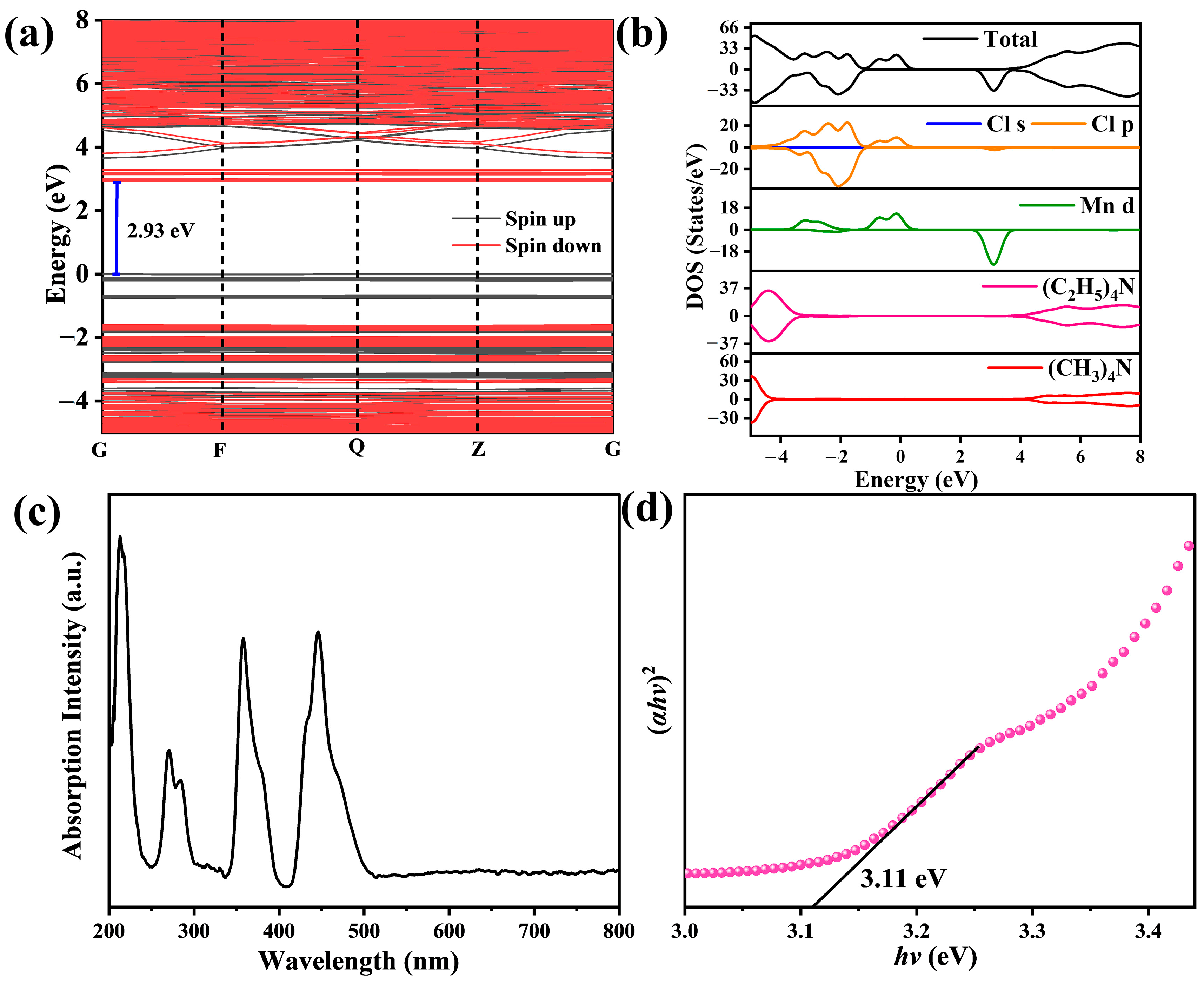
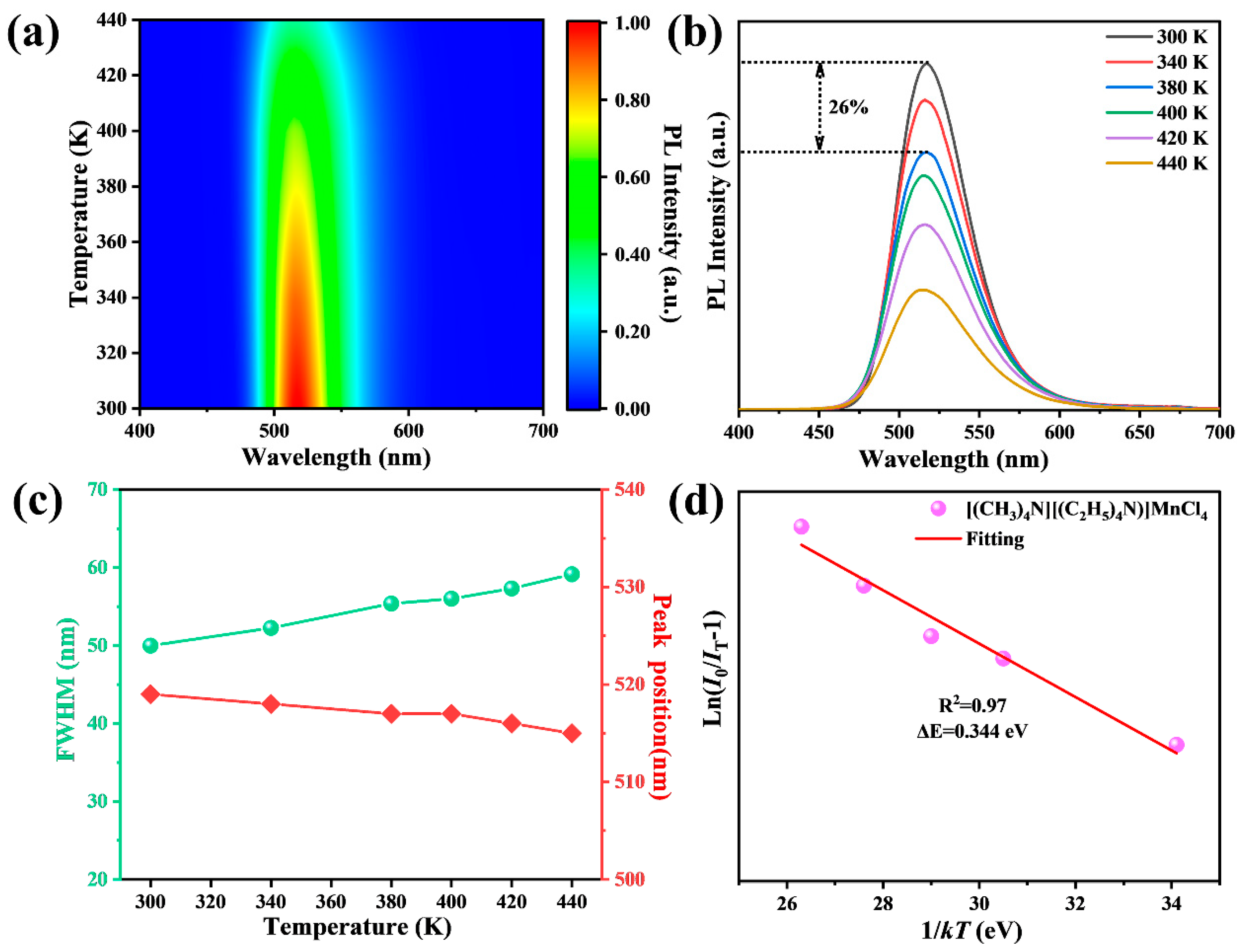
3.3. Emission Mechanism and Stability of [(CH3)4N][(C2H5)4N]MnCl4 Phosphors
3.4. Application in WLED for Wide Color Gamut Display
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Zhang, Q.; Xia, Z. Narrow-band emitters in LED backlights for liquid-crystal displays. Mater. Today 2020, 40, 246–265. [Google Scholar] [CrossRef]
- Zhan, C.Y.; Zhu, H.M.; Liang, S.S.; Huang, Y.P.; Wang, Z.H.; Hong, M.C. A highly Mn2+-doped narrowband green phosphor toward wide color-gamut display applications. Inorg. Chem. Front. 2024, 11, 826–836. [Google Scholar] [CrossRef]
- Liao, H.X.; Zhao, M.; Zhou, Y.Y.; Molokeev, M.S.; Liu, Q.L.; Zhang, Q.Y.; Xia, Z.G. Polyhedron transformation toward stable narrow–band green phosphors for wide–color–gamut liquid crystal display. Adv. Funct. Mater. 2019, 29, 1901988. [Google Scholar] [CrossRef]
- Zhang, B.H.; Lu, Z.T.; Zhang, H.R.; Li, W.; Zhuang, J.L.; Hu, C.F.; Liu, Y.L.; Lei, B.F.; Zhang, X.J. Enhanced narrow green emission via efficient energy transfer from Eu2+ to Mn2+ in KAl4Ga7O17 for backlit LCD displays. J. Mater. Chem. C 2024, 12, 8852–8860. [Google Scholar] [CrossRef]
- Kong, X.; Qiu, Z.; Wu, L.; Lei, Y.; Chi, L. Luminescence Properties of green phosphor Ca2Ga2(Ge1-xSix)O7:y%Eu2+ and application. Materials 2023, 16, 3671. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Fukunaga, H.; Izumi, M.; Masuda, M.; Uemura, T.; Takahashi, K.; Xie, R.; Hirosaki, N. White LEDs using the sharp β-sialon: Eu phosphor and Mn-doped red phosphor for wide-color gamut display applications. J. Soc. Inf. Disp. 2016, 24, 449–453. [Google Scholar] [CrossRef]
- Zhao, M.; Liao, H.X.; Ning, L.X.; Zhang, Q.Y.; Liu, Q.L.; Xia, Z.G. Next-generation narrow-band green-emitting RbLi(Li3SiO4)2: Eu2+ phosphor for backlight display application. Adv. Mater. 2018, 30, 1802489. [Google Scholar] [CrossRef]
- Wan, Y.J.; Dang, P.P.; Liu, D.J.; Zhang, Q.Q.; Wei, Y.; Lian, H.Z.; Li, G.G.; Lin, J. Highly efficient narrow-band green-emitting Na3K5(Li3SiO4)8: Eu2+ phosphor with low thermal quenching. Chem. Mater. 2023, 35, 10702–10712. [Google Scholar] [CrossRef]
- Tian, H.D.; Seto, T.; Zhou, Y.H.; Liu, Z.Q.; Wang, Y.H. Nano-coprecipitation route synthesis of highly-efficient submicron (Sr, Ba)2SiO4: Eu2+ phosphors with enhanced thermal stability for microLED color conversion. ACS Appl. Mater. Interfaces 2023, 15, 28215–28227. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Liang, Y.J.; Liu, S.Q.; Li, H.R.; Chen, J.H. Narrow-band green-emitting Sr2MgAl22O36: Mn2+ phosphors with superior thermal stability and wide color gamut for backlighting display applications. Adv. Opt. Mater. 2019, 7, 1801419. [Google Scholar] [CrossRef]
- Lu, G.G.; Wang, Y.C.; Ma, K.L.; Chen, X.Z.; Geng, W.Y.; Liu, T.S.; Xu, S.; Zhang, J.S.; Chen, B.J. A high-efficiency near-ultraviolet excited Mn2+ doped narrow-band emission phosphor via a strong charge transfer for wide color gamut WLED. Ceram. Int. 2024, 50, 16190–16200. [Google Scholar] [CrossRef]
- Wu, X.D.; Liang, Y.J.; Xue, Y.L.; Li, H.R.; Dou, Y.; Zhang, W.L.; Wang, Q.K.; Han, C. Designing ultra-highly efficient Mn2+-activated Zn2GeO4 green-emitting persistent phosphors toward versatile applications. Mater. Today Chem. 2022, 23, 100693. [Google Scholar]
- Chen, S.X.; Lin, J.D.; Huang, J.; Pang, T.; Ye, Q.Y.; Zheng, Y.H.; Li, X.Y.; Yu, Y.L.; Zhuang, B.; Chen, D.Q. CsPbBr3@glass nanocomposite with green-emitting external quantum efficiency of 75% for backlit display. Adv. Funct. Mater. 2024, 34, 2309293. [Google Scholar] [CrossRef]
- He, K.; Chen, D.J.; Yuan, L.F.; Xu, J.M.; Xu, K.Y.; Hu, J.; Liang, S.S.; Zhu, H.M. Crystallization engineering in PVDF enables ultrastable and highly efficient CsPbBr3 quantum dots film for wide color gamut Mini-LED backlight. Chem. Eng. J. 2024, 480, 148066. [Google Scholar]
- Ren, C.; Wu, Y.; Zou, J.; Cai, B. Employing the interpretable ensemble learning approach to predict the bandgaps of the halide perovskites. Materials 2024, 17, 2686. [Google Scholar] [CrossRef]
- Khan, S.-A.; Khan, N.-Z.; Sohail, M.; Runowski, M.; Xu, X.; Agathopoulos, S. Recent developments of lead-free halide-perovskite nanocrystals: Synthesis strategies, stability, challenges, and potential in optoelectronic applications. Mater. Today Phys. 2023, 34, 101079. [Google Scholar]
- Tao, P.; Liu, S.J.; Wong, W.Y. Phosphorescent manganese (II) complexes and their emerging applications. Adv. Opt. Mater. 2020, 8, 2000985. [Google Scholar] [CrossRef]
- Gao, P.; Cheng, S.; Liu, J.; Li, J.; Guo, Y.; Deng, Z.; Qin, T.; Wang, A. Facile synthesis of highly emissive all-inorganic manganese bromide compounds with perovskite-related structures for white LEDs. Molecules 2022, 27, 8259. [Google Scholar] [CrossRef]
- Fu, P.F.; Sun, Y.L.; Xia, Z.G.; Xiao, Z.W. Photoluminescence behavior of zero-dimensional manganese halide tetrahedra embedded in conjugated organic matrices. J. Phys. Chem. Lett. 2021, 12, 7394–7399. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.F.; Zhou, J.D.; Ming, H.; Wang, C.H.; Jing, X.P.; Ye, S.; Zhang, Q.Y. Structural design enables highly–efficient green emission with preferable blue light excitation from zero–dimensional manganese (II) hybrids. Chem. Eng. J. 2021, 421, 129886. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, Y.; Liu, K.J.; He, S.H.; Cao, J.D.; Zhang, X.S.; Zhao, J.; Liu, Q.L. Efficient narrow–band green light–emitting hybrid halides for wide color gamut display. ACS Appl. Electron. Mater. 2022, 4, 4068–4076. [Google Scholar] [CrossRef]
- Liu, X.T.; Yang, J.P.; Chen, W.Y.; Yang, F.; Chen, Y.H.; Liang, X.J.; Pan, S.; Xiang, W.D. [(CH3)4N]2Mn0.6Zn0.4Br4: Lead-free MnII-based hybrid halide with high photoluminescence quantum yield for backlight displays. Nano Res. 2023, 16, 5894–5899. [Google Scholar] [CrossRef]
- Zhou, G.J.; Ren, Q.Q.; Molokeev, M.S.; Zhou, Y.Y.; Zhang, J.; Zhang, X.-M. Unraveling the ultrafast self-assembly and photoluminescence in zero-dimensional Mn2+-based halides with narrow-band green emissions. ACS Appl. Electron. Mater. 2021, 3, 4144–4150. [Google Scholar] [CrossRef]
- Tan, G.-H.; Chen, Y.-N.; Chuang, Y.-T.; Lin, H.-C.; Hsieh, C.-A.; Chen, Y.-S.; Lee, T.-Y.; Miao, W.-C.; Kuo, H.-C.; Chen, L.-Y.; et al. Highly luminescent earth-benign organometallic manganese halide crystals with ultrahigh thermal stability of emission from 4 to 623 K. Small 2023, 19, 2205981. [Google Scholar] [CrossRef]
- Yu, F.; Li, S.Y.; Yang, H.R.; Shen, J.; Yin, M.X.; Tian, Y.R.; Zhang, Y.T.; Kong, X.W.; Lei, X.W. Crystal-rigidifying strategy in hybrid manganese halide to achieve narrow green emission and high structural stability. Inorg. Chem. 2024, 63, 14116–14125. [Google Scholar] [CrossRef]
- Tang, H.D.; Xu, Y.Q.; Hu, X.B.; Chen, H.J.; Wang, S.H.; Jiang, W.H.; Hu, Q.; Wang, L.J.; Jiang, W. Scalable synthesis of lead-free tetramethylammonium manganese halides for highly efficient backlight displays. Laser Photonics Rev. 2024, 18, 2300672. [Google Scholar] [CrossRef]
- Hu, G.C.; Xu, B.; Wang, A.F.; Guo, Y.; Wu, J.J.; Muhammad, F.; Meng, W.; Wang, C.Y.; Sui, S.Q.; Liu, Y.; et al. Stable and bright pyridine manganese halides for efficient white light-emitting diodes. Adv. Funct. Mater. 2021, 31, 2011191. [Google Scholar] [CrossRef]
- Peng, H.; Zou, B.S.; Guo, Y.C.; Xiao, Y.H.; Zhi, R.N.; Fan, X.Y.; Zou, M.; Wang, J.P. Evolution of the structure and properties of mechanochemically synthesized pyrrolidine incorporated manganese bromide powders. J. Mater. Chem. C 2020, 8, 6488–6495. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Song, Y.-R.; Xu, W.-J.; Zhong, Q.-Q.; Fu, H.-Q.; Liu, X.-L.; Yue, C.-Y.; Lei, X.W. Solvent-free mechanochemical syntheses of microscale lead-free hybrid manganese halides as efficient green light phosphors. J. Mater. Chem. C 2021, 9, 9952–9961. [Google Scholar] [CrossRef]
- Mao, L.L.; Guo, P.J.; Wang, S.X.; Cheetham, A.K.; Seshadri, R. Design principles for enhancing photoluminescence quantum yield in hybrid manganese bromides. J. Am. Chem. Soc. 2020, 142, 13582–13589. [Google Scholar] [CrossRef]
- Pan, H.-M.; Yang, Q.-L.; Xing, X.-X.; Li, J.-P.; Meng, F.-L.; Zhang, X.; Xiao, P.-C.; Yue, C.-Y.; Lei, X.-W. Enhancement of the photoluminescence efficiency of hybrid manganese halides through rational structural design. Chem. Commun. 2021, 57, 6907–6910. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Setyawan, W.; Curtarolo, S. High-throughput electronic band structure calculations: Challenges and tools. Comput. Mater. Sci. 2010, 49, 299–312. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, B.K.; Xie, L.L.; Li, X.T.; Lu, B.H.; Wang, M.; Wu, Y.F.; Jiang, T.; Zhang, F.; Li, X.; et al. Vacancy–ordered double perovskite Rb2ZrCl6−xBrx: Facile synthesis and insight into efficient intrinsic self-trapped emission. Adv. Opt. Mater. 2022, 10, 2101661. [Google Scholar] [CrossRef]
- Palazon, F.; Ajjouri, Y.E.; Bolink, H.J. Making by grinding: Mechanochemistry boosts the development of halide perovskites and other multinary metal halides. Adv. Energy Mater. 2020, 10, 1902499. [Google Scholar] [CrossRef]
- Fang, S.F.; Wang, Y.; Li, H.X.; Fang, F.; Jiang, K.; Liu, Z.X.; Li, H.N.; Shi, Y.M. Rapid synthesis and mechanochemical reactions of cesium copper halides for convenient chromaticity tuning and efficient white light emission. J. Mater. Chem. C 2020, 8, 4895–4901. [Google Scholar] [CrossRef]
- Wu, H.D.; Chen, X.; Song, Z.H.; Zhang, A.; Du, X.Y.; He, X.; Wang, H.Q.; Xu, L.; Zheng, Z.P.; Niu, G.D.; et al. Mechanochemical synthesis of high–entropy perovskite toward highly sensitive and stable X–ray flat-panel detectors. Adv. Mater. 2023, 35, 2301406. [Google Scholar] [CrossRef]
- Hasan, M.; Pawar, T.B.; Sawant, A.B. Density and viscosity studies of symmetrical tetra-n-alkylammonium bromides in water-ethanol mixtures at 303.15 K1. Russ. J. Phys. Chem. A 2010, 84, 429–433. [Google Scholar] [CrossRef]
- Masterton, W.L.; Bolocofsky, D.; Lee, T.P. Ionic radii from scaled particle theory of the salt effect. J. Phys. Chem. 1971, 75, 2809–2815. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, M.-Q.; Su, Z.-A.; Pei, Z.; Peng, D.F.; Peng, G.; Ren, X.-M. Two Organic–inorganic manganese (II) halide hybrids showing compelling photo-and mechanoluminescence as well as rewritable anticounterfeiting printing. Inorg. Chem. 2023, 62, 5791–5798. [Google Scholar] [CrossRef]
- Hao, X.R.; Liu, H.L.; Ding, W.G.; Zhang, F.; Li, X.G.; Wang, S.R. Zn2+–doped lead-free CsMnCl3 nanocrystals enable efficient red emission with a high photoluminescence quantum yield. J. Phys. Chem. Lett. 2022, 13, 4688–4694. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.J.; Ding, J.L.; Jiang, X.X.; Zhang, J.; Molokeev, M.S.; Ren, Q.Q.; Zhou, J.; Li, S.L.; Zhang, X.M. Coordination units of Mn2+ modulation toward tunable emission in zero–dimensional bromides for white light–emitting diodes. J. Mater. Chem. C 2022, 10, 2095–2102. [Google Scholar] [CrossRef]
- Ma, W.; Liang, D.H.; Qian, Q.K.; Mo, Q.H.; Zhao, S.Y.; Cai, W.S.; Chen, J.Z.; Zang, Z.G. Near-unity quantum yield in zero-dimensional lead–free manganese–based halides for flexible X–ray imaging with high spatial resolution. eScience 2023, 3, 100089. [Google Scholar] [CrossRef]
- Wang, S.X.; Wang, Y.C.; Yoon, D.H.; Li, T.R.; Wang, Y.H. Mn2+-doped organic–inorganic hybrids (C8H20N)2Zn1−xMnxBr4 as sub-micrometer green phosphors for Mini-LEDs/Micro-LEDs. J. Mater. Chem. C 2023, 11, 9281–9290. [Google Scholar] [CrossRef]
- Zhou, G.J.; Liu, Z.Y.; Molokeev, M.S.; Xiao, Z.W.; Xia, Z.G.; Zhang, X.M. Manipulation of Cl/Br transmutation in zero-dimensional Mn2+-based metal halides toward tunable photoluminescence and thermal quenching behaviors. J. Mater. Chem. C 2021, 9, 2047–2053. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Tang, H.; Dai, T.; Long, Y.; Luo, D.; Jiang, P.; Xiong, X.; Xu, Y.; Zhang, X.; Hu, Q. Mixed-Ligand Engineering to Enhance Luminescence of Mn2+-Based Metal Halides for Wide Color Gamut Display. Materials 2024, 17, 4459. https://doi.org/10.3390/ma17184459
Wu Z, Tang H, Dai T, Long Y, Luo D, Jiang P, Xiong X, Xu Y, Zhang X, Hu Q. Mixed-Ligand Engineering to Enhance Luminescence of Mn2+-Based Metal Halides for Wide Color Gamut Display. Materials. 2024; 17(18):4459. https://doi.org/10.3390/ma17184459
Chicago/Turabian StyleWu, Zhi, Huidong Tang, Tianhao Dai, Yuxi Long, Dan Luo, Pengcheng Jiang, Xin Xiong, Yanqiao Xu, Xiaojun Zhang, and Qing Hu. 2024. "Mixed-Ligand Engineering to Enhance Luminescence of Mn2+-Based Metal Halides for Wide Color Gamut Display" Materials 17, no. 18: 4459. https://doi.org/10.3390/ma17184459




