Revealing the Corrosion Resistance Mechanism of Plain Carbon Steel Micro-Alloyed by La in Simulated Industrial Atmosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Tensile Property Testes and Microstructure Characterizations
2.3. Alternate Immersion Tests
2.4. Electrochemical Tests
2.5. Corrosion Inhibition Tests
2.6. Rust Analysis
3. Results
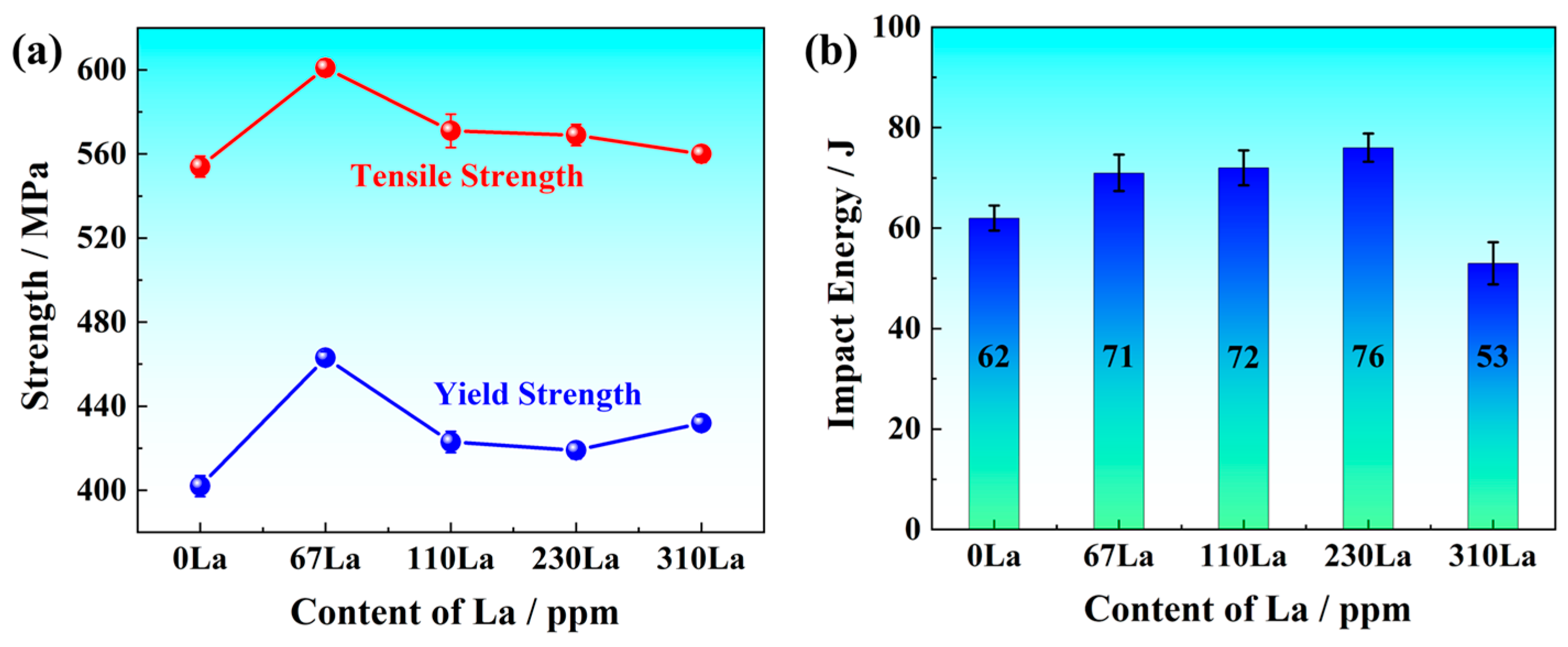
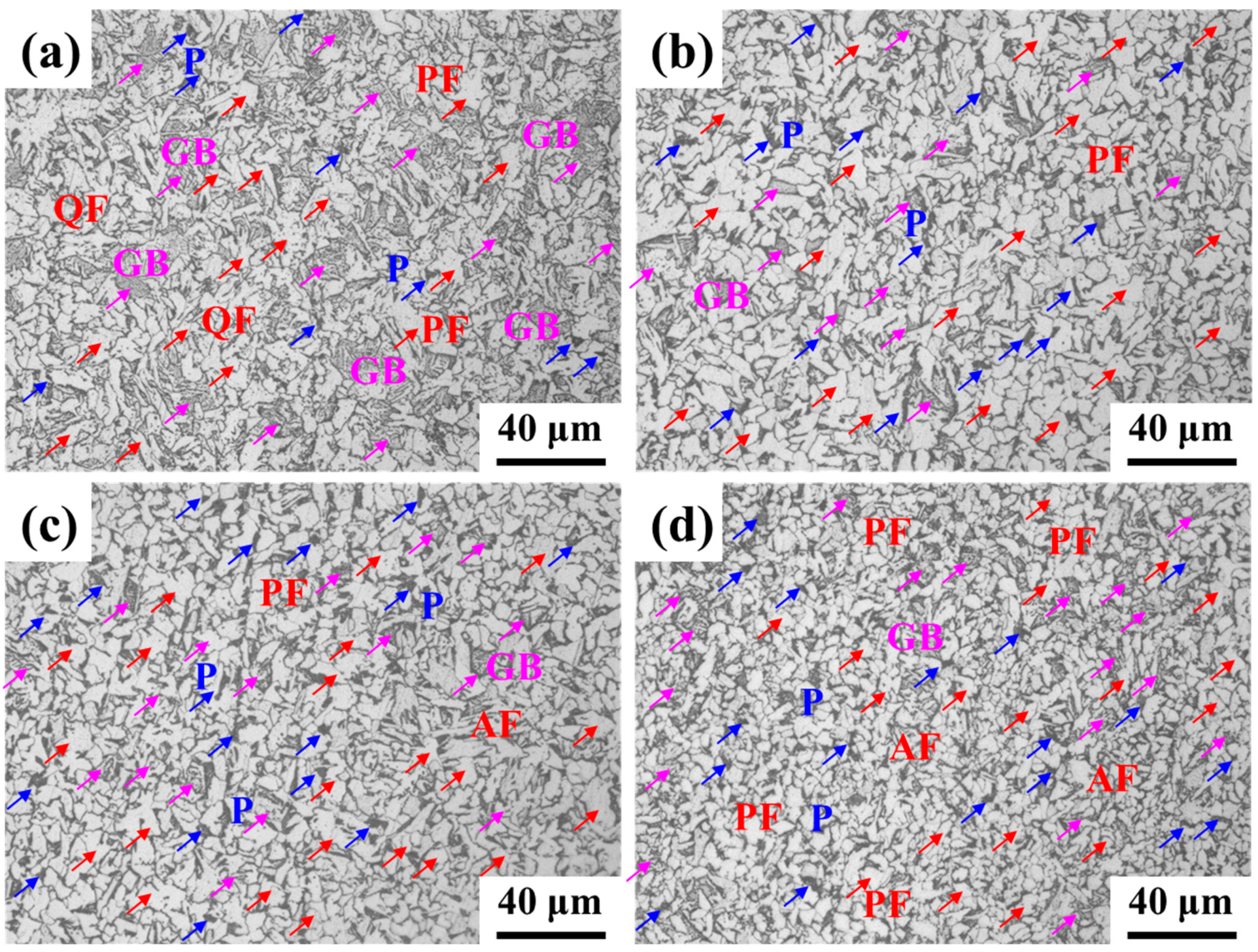
3.1. Mechanical Properties and Microstructure Characterization
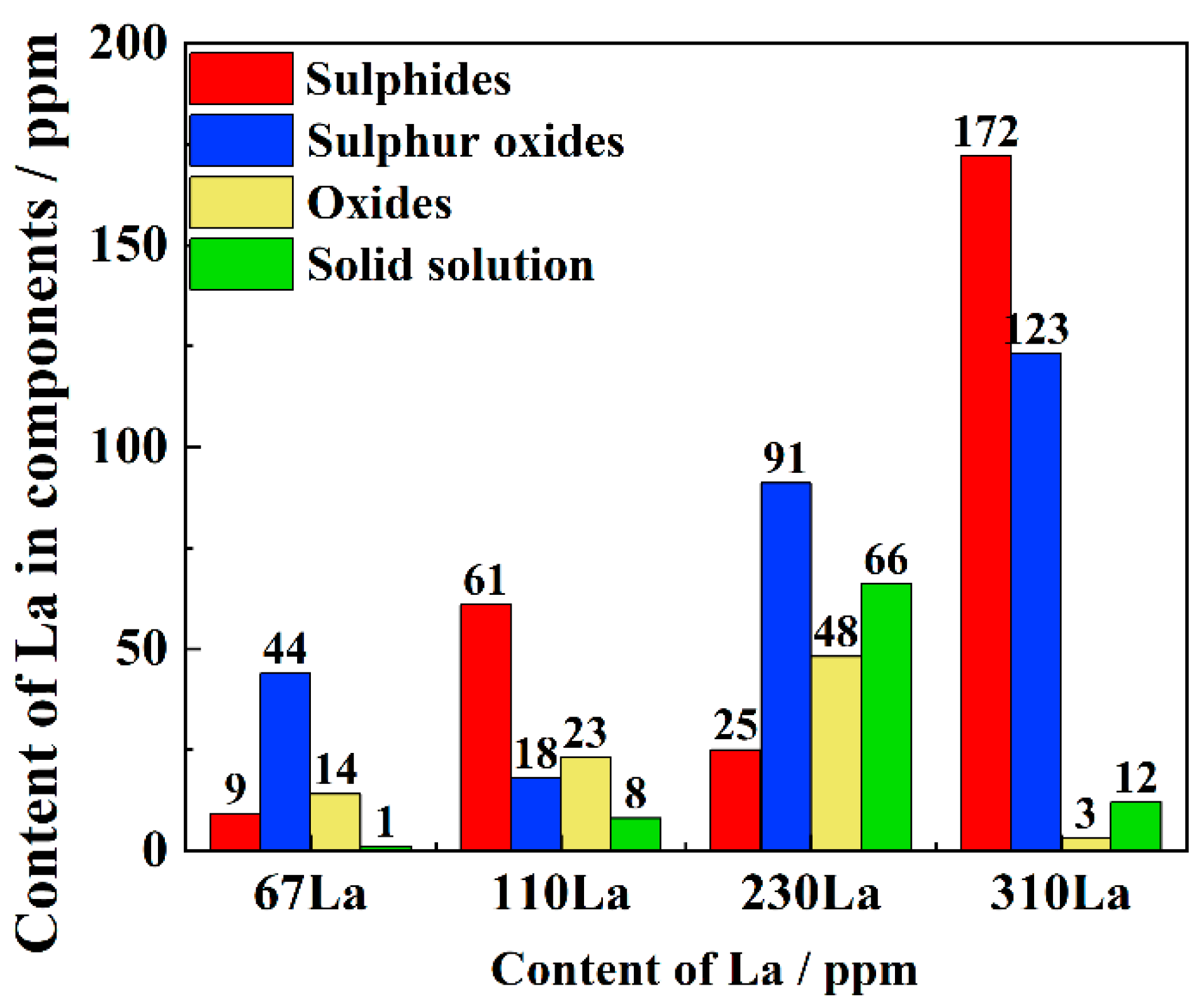
3.2. Inclusion Analysis
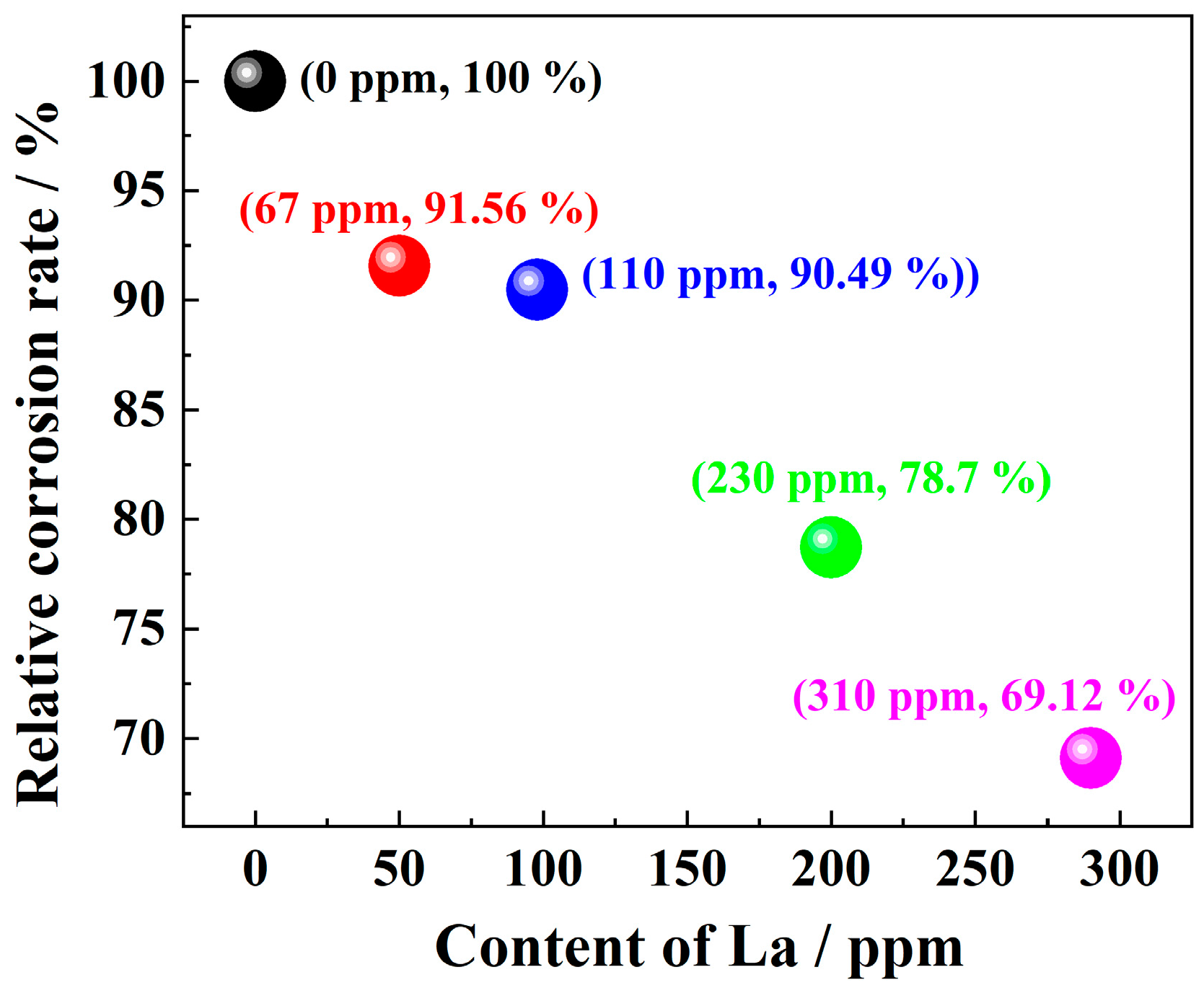
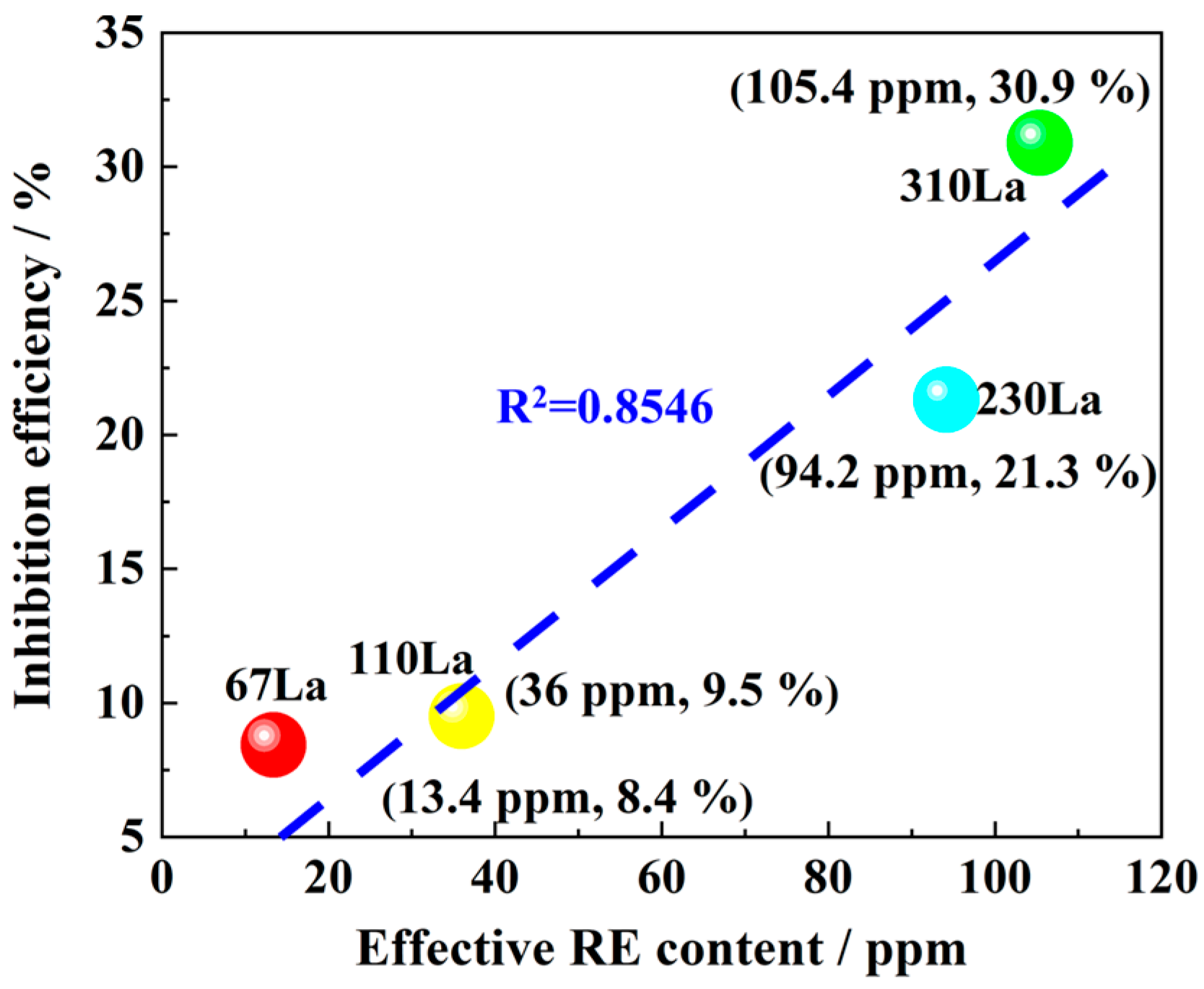
3.3. Corrosion Inhibition Rate
3.4. Alternate Immersion Test
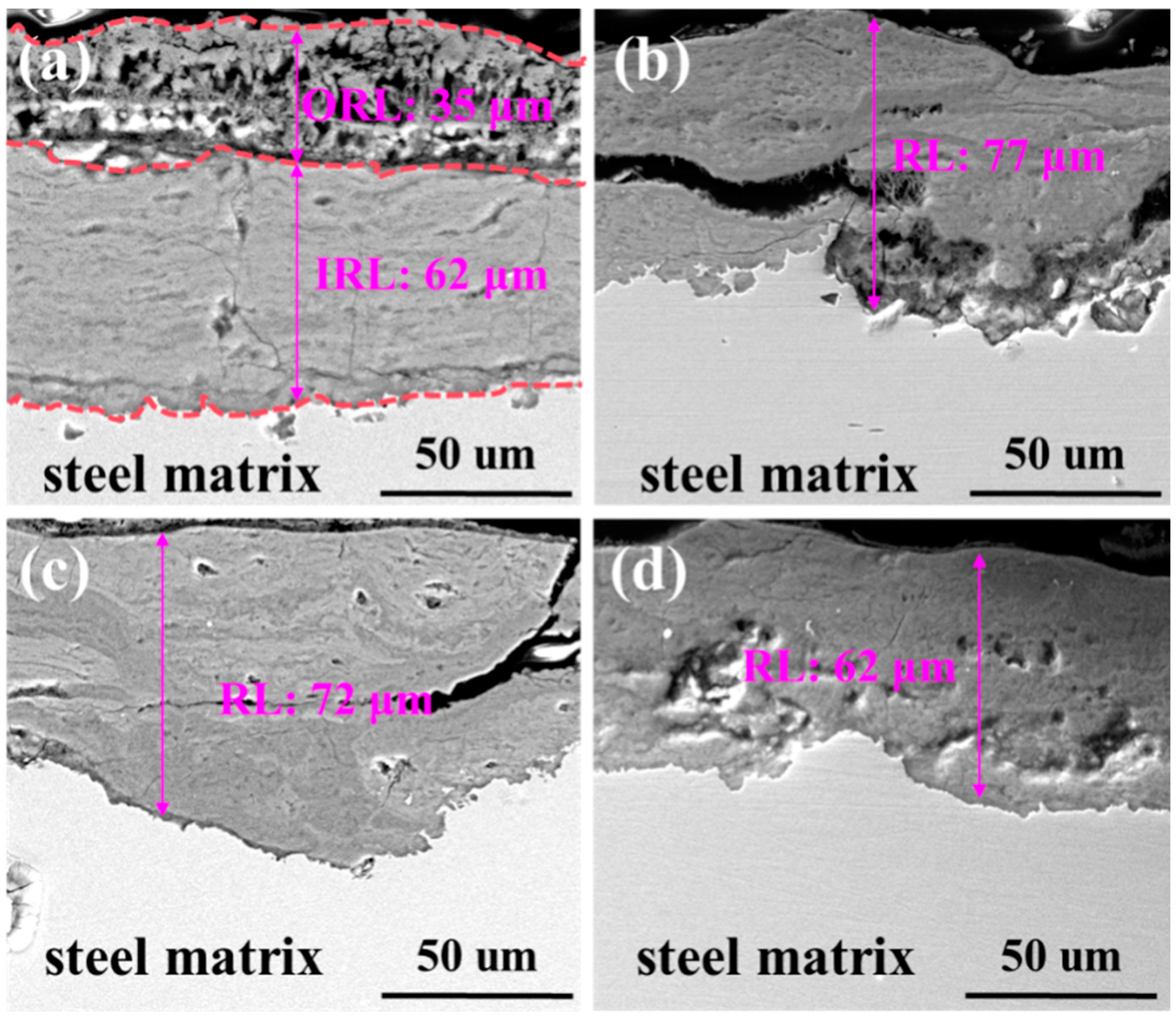
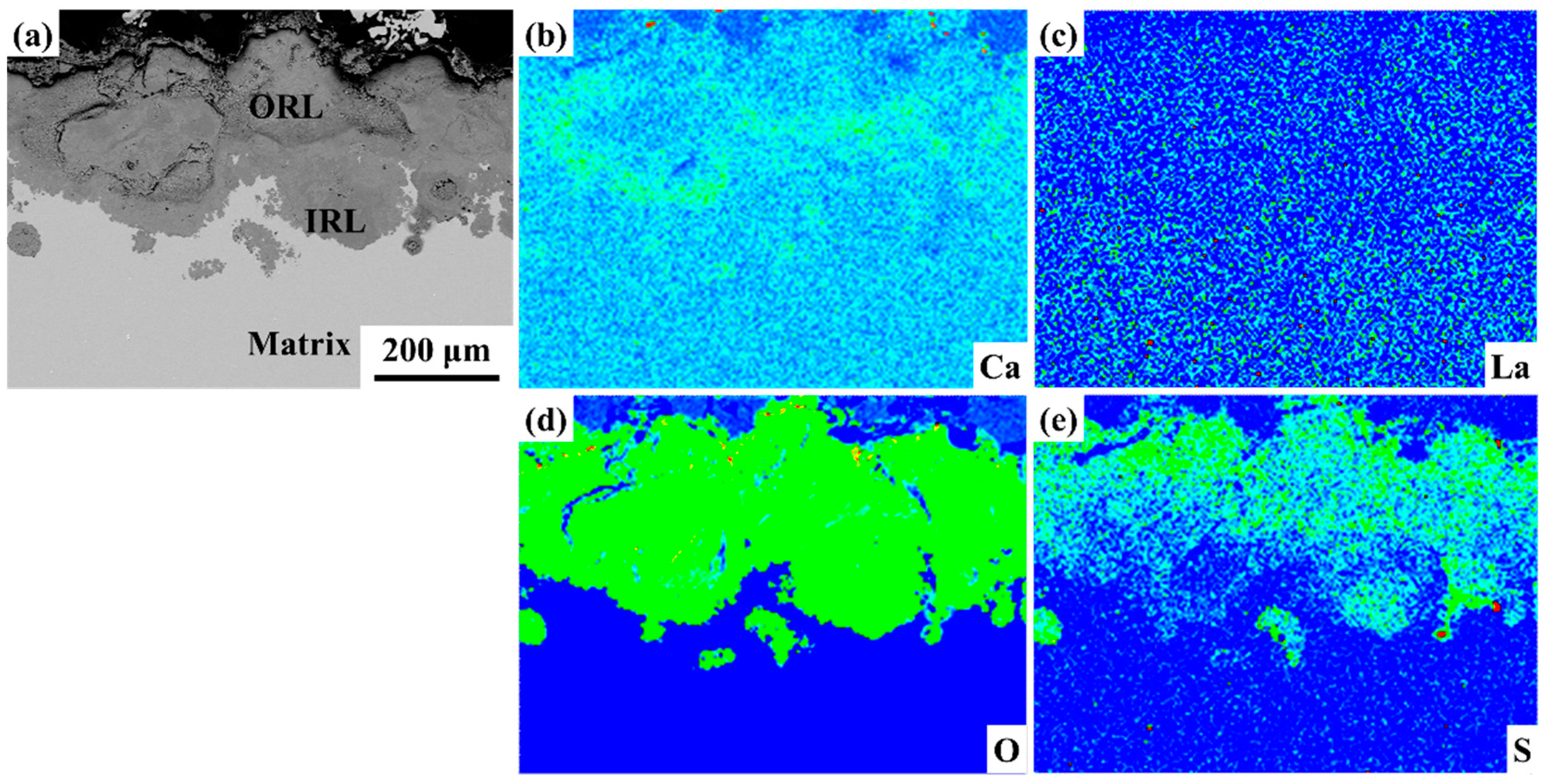
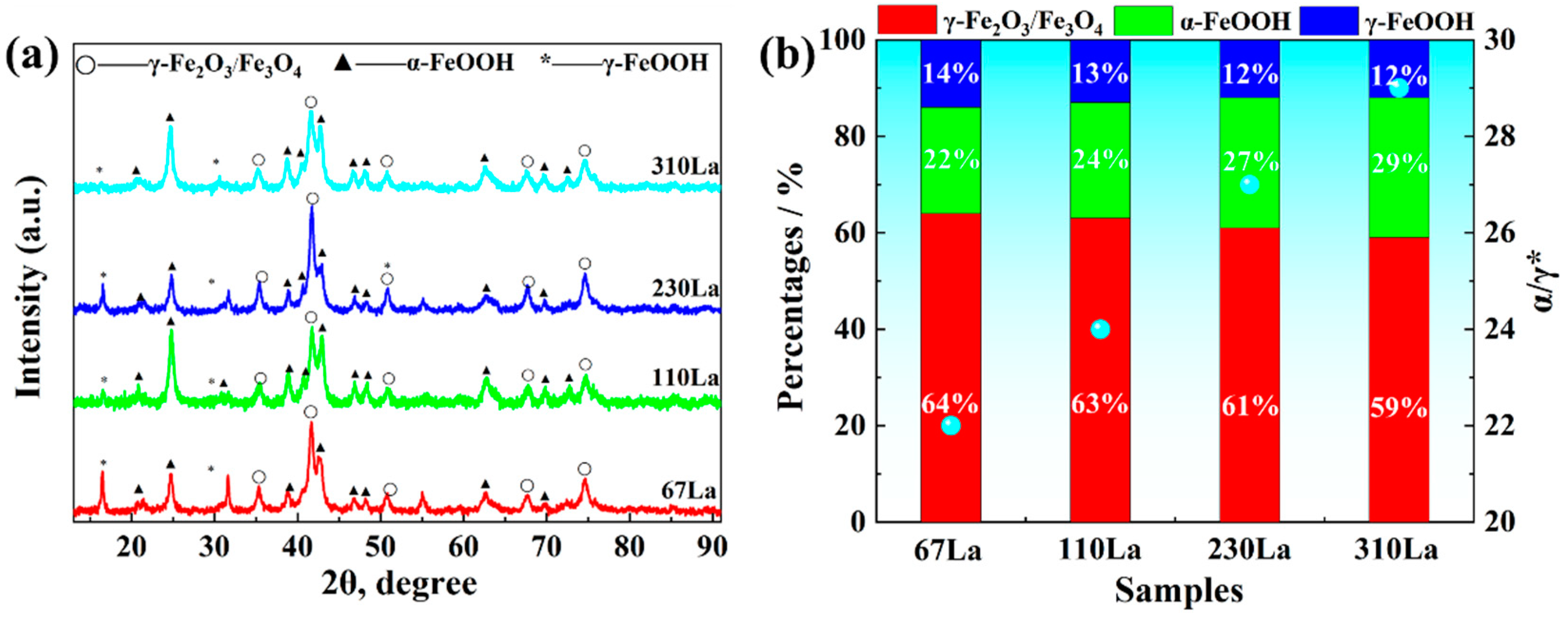
3.5. Rust Analysis
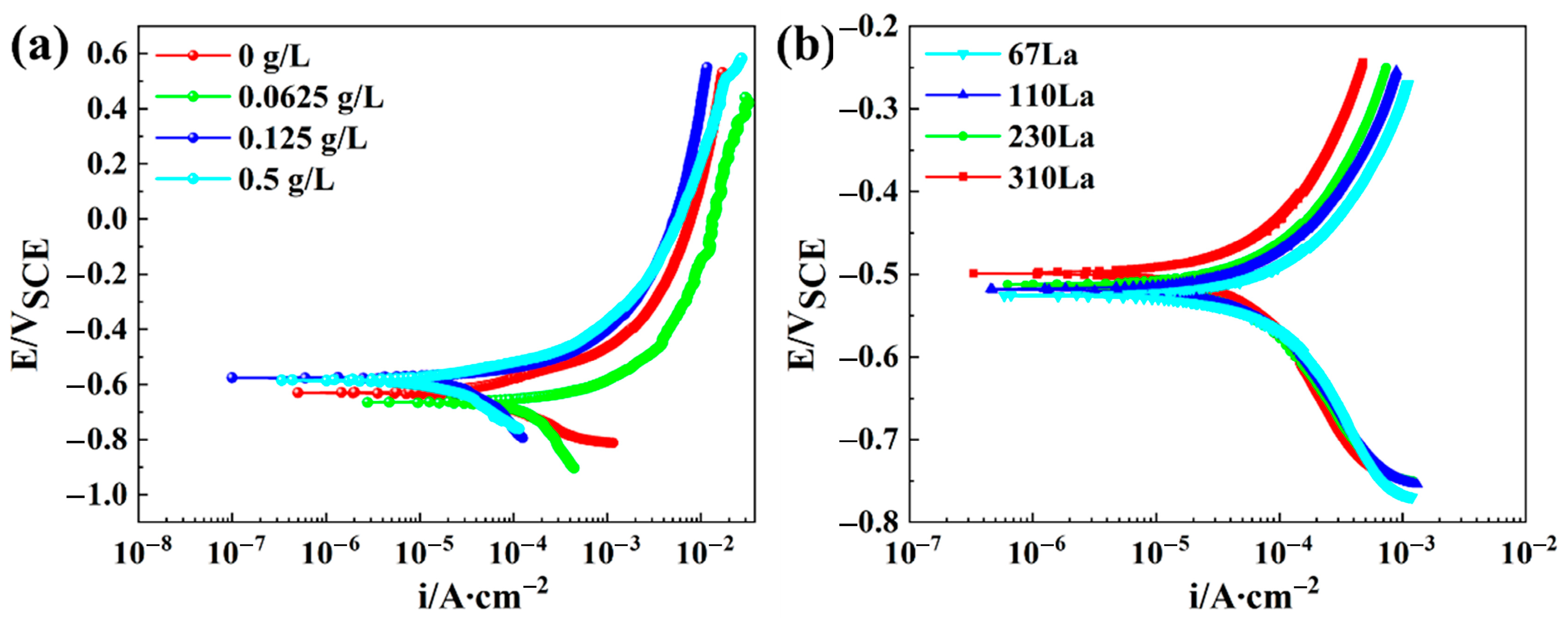
3.6. Potentiodynamic Polarization Tests
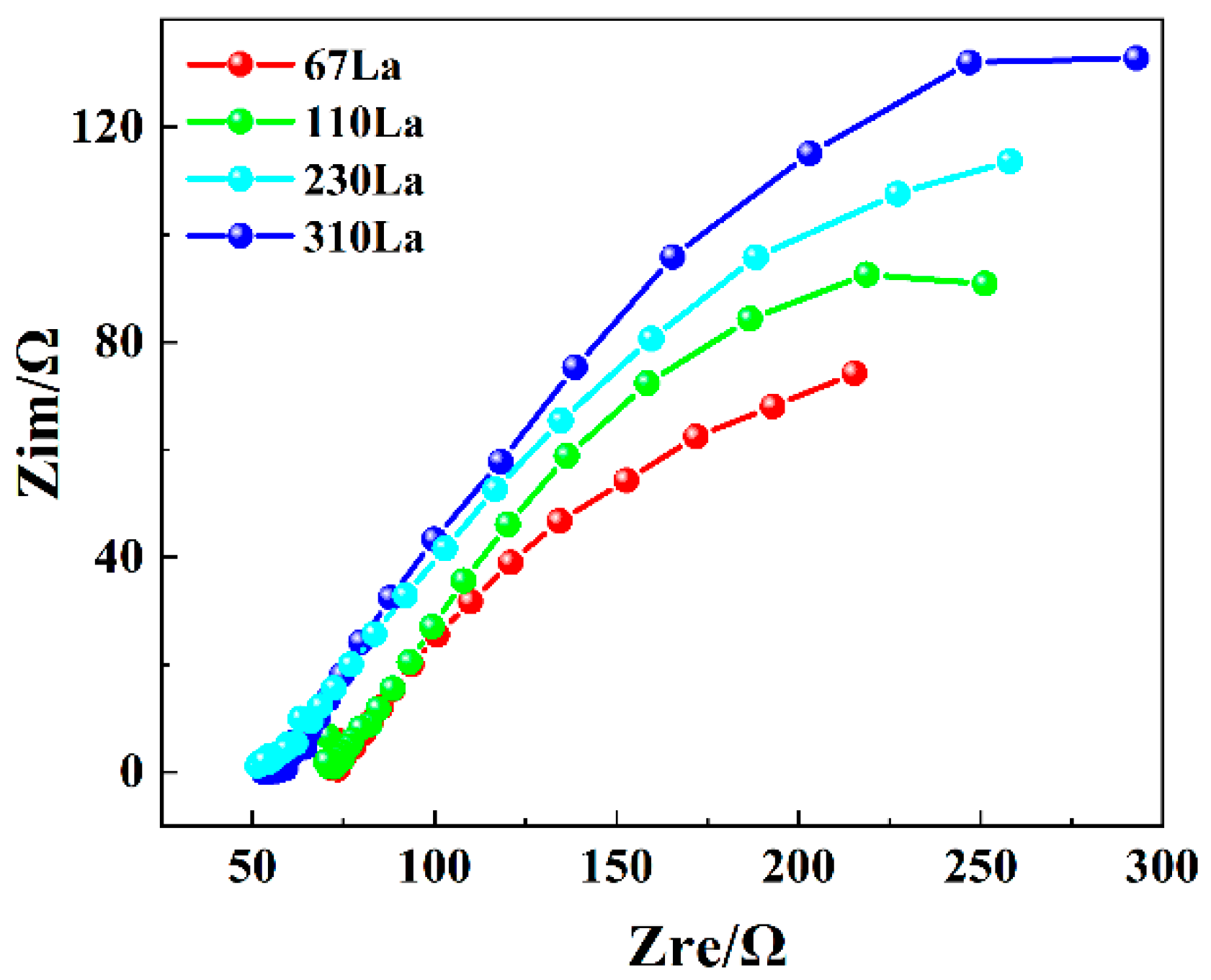
3.7. Electrochemical Impedance Spectroscopy (EIS)
4. Discussions
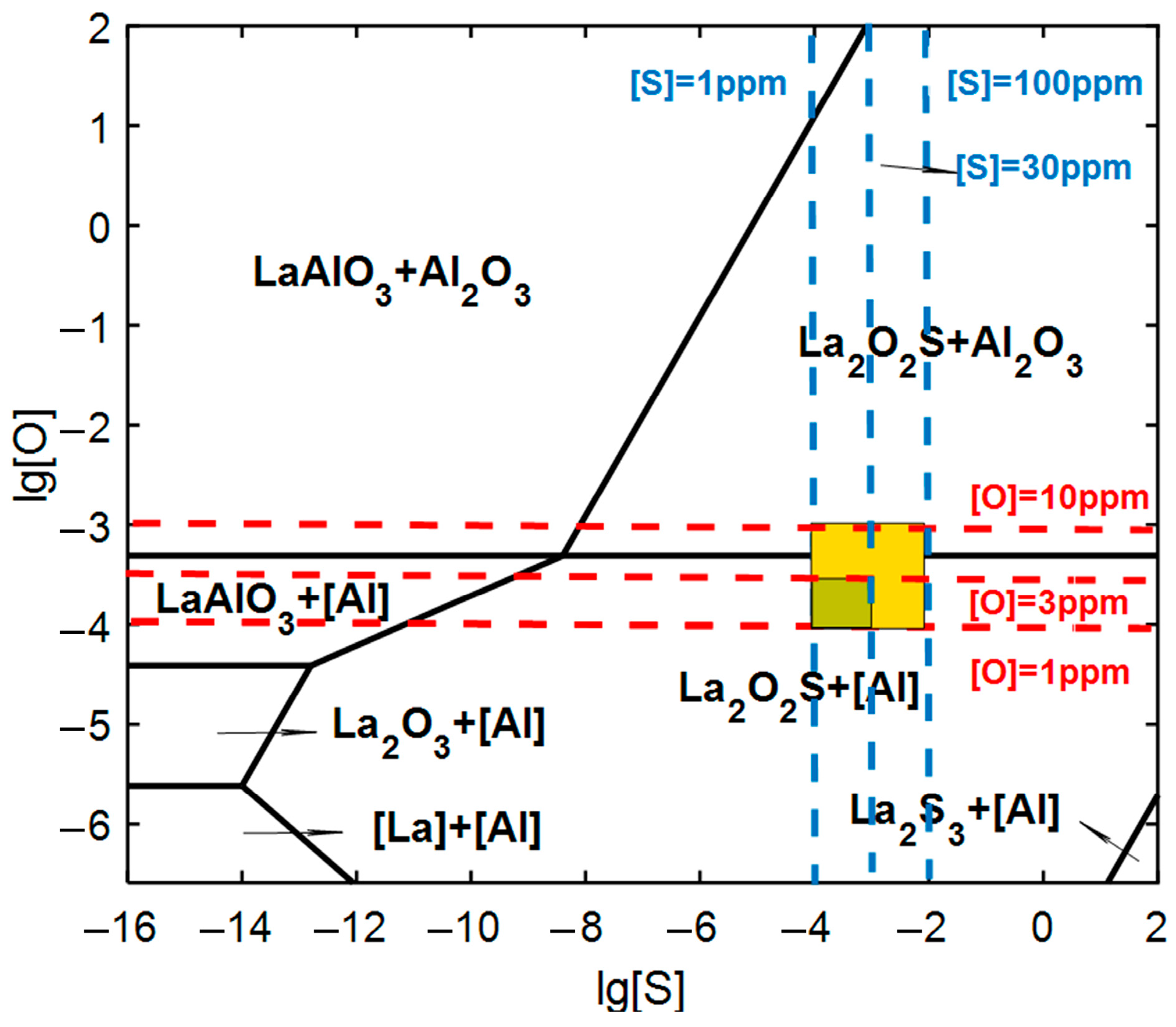
4.1. Microalloying Mechanism of La
4.2. Effect of La on Microstructure, Strength, and Toughness
4.3. Effect of La Element on Corrosion Resistance
5. Conclusions
- (1)
- The microalloying of La element modified the inclusion from mixed Al2O3·CaO·CaS inclusion to LaS, LaO and La3(SO)2 inclusion. Although the total content of inclusion increased with the content of La, the particle size decreased from 4.69 μm of 67La steel to 1.68 μm of 310La steel. The maximum La content in the solid solution state reached 66 ppm in 230La steel, while the content of La in the solid solution state decreased to only 12 ppm.
- (2)
- As the content of La increased, the microstructure was refined. The fraction of pearlite decreased, and the content of acicular ferrite increased. The modified inclusions and microstructure jointly improved the strength and toughness within the La addition of 230 ppm for Q355 steel. The yield strength of 419 MPa, tensile strength of 569 MPa, and impact energy of 76 J were obtained.
- (3)
- La element plays a significant role in corrosion inhibition. The addition of La significantly reduces the cracks and holes in the rust layer and reduces the thickness of the rust layer. The release of La3+ ions promoted the formation of a dense and continuous protective rust layer, which effectively inhibited the corrosion process. The effective proposed RE formula accurately quantified the corrosion inhibition efficiency of La elements, and revealed the positive correlation between the effective rare earth content and the corrosion inhibition rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, S. Interrelationships between yield strength, low-temperature impact toughness, and microstructure in low-carbon, copper-precipitation-strengthened, high-strength low-alloy plate steels. Mater. Sci. Eng. A 2018, 711, 424–433. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, H.; Zhu, L. Effect of interaction between corrosion and high temperature on mechanical properties of Q355 structural steel. Constr. Build. Mater. 2021, 271, 121605. [Google Scholar] [CrossRef]
- Kong, Z.; Jin, Y.; Hossen, G.M.S.; Hong, S.; Wang, Y.; Vu, Q.-V.; Truong, V.-H.; Tao, Q.; Kim, S.-E. Experimental and theoretical study on mechanical properties of mild steel after corrosion. Ocean Eng. 2022, 246, 110652. [Google Scholar] [CrossRef]
- Butt, A.M.; Wang, Y.; Ma, H.; Li, H. The preparation of cerium nitrate and attapulgite based superhydrophobic epoxy coatings for the corrosion protection of Q355 mild steel surface. Surf. Coat. Technol. 2023, 473, 129977. [Google Scholar] [CrossRef]
- Si, Q.; Ding, Y.; Zong, L.; Meng, X. Effect of pre-fatigue damage on static and hysteretic behavior of Q355 steel. Int. J. Fatigue 2022, 160, 106874. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, X.; Chen, Z.; Lin, Z.; Dong, J.; Daniel, E.F.; Qi, J.; Ke, W. Understanding the role of alloyed Cu and P in the initial rust composition of weathering steel formed in a simulated coastal-industrial atmosphere. Corros. Sci. 2021, 193, 109912. [Google Scholar] [CrossRef]
- Morcillo, M.; Díaz, I.; Chico, B.; Cano, H.; De la Fuente, D. Weathering steels: From empirical development to scientific design. A Rev. Corros. Sci. 2014, 83, 6–31. [Google Scholar] [CrossRef]
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; De la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part I Basic Concepts. Constr. Build. Mater. 2019, 213, 723–737. [Google Scholar] [CrossRef]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Yin, Z.; Dong, B.; Zhao, Y.; Fan, Y.; Wu, J.; Zhang, Z.; Li, X. Effects of the addition of Cu and Ni on the corrosion behavior of weathering steels in corrosive industrial environments. J. Mater. Eng. Perform. 2020, 29, 2531–2541. [Google Scholar] [CrossRef]
- Hoche, H.; Pusch, C.; Oechsner, M. Corrosion and wear protection of mild steel substrates by innovative PVD coatings. Surf. Coat. Technol. 2020, 391, 125659. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Tang, M.; Zhang, X.; Wu, K. Role of rare earth elements on the improvement of corrosion resistance of micro-alloyed steels in 3.5 wt.% NaCl solution. J. Mater. Res. Technol. 2021, 11, 519–534. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Liu, Z.; Zhang, D.; Li, X.; Terryn, H. Effect of inclusions modified by rare earth elements (Ce, La) on localized marine corrosion in Q460NH weathering steel. Corros. Sci. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Lijie, Y.; Longmei, W.; Jinsheng, H. Effects of rare earth on inclusions and corrosion resistance of 10PCuRE weathering steel. J. Rare Earths 2010, 28, 952–956. [Google Scholar]
- Liu, C.; Revilla, R.I.; Zhang, D.; Liu, Z.; Lutz, A.; Zhang, F.; Zhao, T.; Ma, H.; Li, X.; Terryn, H. Role of Al2O3 inclusions on the localized corrosion of Q460NH weathering steel in marine environment. Corros. Sci. 2018, 138, 96–104. [Google Scholar] [CrossRef]
- Lian, X.; Zhu, J.; Wang, R.; Liu, T.; Xu, J.; Xu, D.; Dong, H. Effects of rare earth (Ce and La) on steel corrosion behaviors under Wet-Dry cycle immersion conditions. Metals 2020, 10, 1174. [Google Scholar] [CrossRef]
- Liu, X.; Yang, C.; Ren, H.; Li, Y.; Jin, Z.; Zhang, F.; Yang, J. Origin mechanism of pitting corrosion induced by cerium inclusions. J. Rare Earths 2023, 41, 1448–1458. [Google Scholar] [CrossRef]
- Xue, W.; Li, Z.; Xiao, K.; Yu, W.; Song, J.; Chen, J.; Dong, C.; Li, X. Initial microzonal corrosion mechanism of inclusions associated with the precipitated (Ti, Nb) N phase of Sb-containing weathering steel. Corros. Sci. 2020, 163, 108232. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Q.; Ji, J.; Lan, D. New study concerning development of application of rare earth metals in steels. J. Alloys Compd. 2006, 408–412, 384–386. [Google Scholar] [CrossRef]
- Wang, L.-M.; Lin, Q.; Yue, L.-J.; Liu, L.; Guo, F.; Wang, F.-M. Study of application of rare earth elements in advanced low alloy steels. J. Alloys Compd. 2008, 451, 534–537. [Google Scholar] [CrossRef]
- TB/T 2375-1993; Test Method for Cycle Infiltration and Corrosion of Railway Weather-resistance Steel. Ministry of Railways Institute of Measurement Standards: Beijing, China, 1993.
- Torkamani, H.; Raygan, S.; Garcia-Mateo, C.; Rassizadehghani, J.; Palizdar, Y.; San-Martin, D. Evolution of pearlite microstructure in low-carbon cast microalloyed steel due to the addition of La and Ce. Metall. Mater. Trans. A 2018, 49, 4495–4508. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.; Zang, M.; Yin, S.; Wang, Y.; Qi, X.; Gao, X.; Misra, R. On the determining role of acicular ferrite in VN microalloyed steel in increasing strength-toughness combination. Mater. Charact. 2016, 118, 446–453. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; You, Y.; Ruolan, W.; Min, C.; Jie, Z.; Rui, J.; Yulei, L. Analysis of the GB/T 10561-2023: Determination of Non-metallic Inclusion Content in Steel—Standard Rating Chart Microscopic Examination Method. J. Iron Steel Vanadium Titan. 2024, 45, 197–204. [Google Scholar]
- Feng, G.; Qin, L. Existing Forms of Lanthanum in Purity Steels. J. Rare Earths 2006, 24 (Suppl. 1), 405–408. [Google Scholar] [CrossRef]
- Ning, Z.; Li, C.; Wang, J.; Zhai, Y.; Xiong, X.; Chen, L. Refinement and Modification of Al2O3 Inclusions in High-Carbon Hard Wire Steel via Rare Earth Lanthanum. Materials 2023, 16, 5070. [Google Scholar] [CrossRef] [PubMed]
- Torkamani, H.; Raygan, S.; Mateo, C.G.; Rassizadehghani, J.; Vivas, J.; Palizdar, Y.; San-Martin, D. The influence of La and Ce addition on inclusion modification in cast niobium microalloyed steels. Metals 2017, 7, 377. [Google Scholar] [CrossRef]
- Lin, Q.; Guo, F.; Zhu, X. Behaviors of lanthanum and cerium on grain boundaries in carbon manganese clean steel. J. Rare Earths 2007, 25, 485–489. [Google Scholar] [CrossRef]
- Mao, N.; Yang, W.; Chen, D.; Lu, W.; Zhang, X.; Chen, S.; Xu, M.; Pan, B.; Han, L.; Zhang, X. Effect of Lanthanum Addition on Formation Behaviors of Inclusions in Q355B Weathering Steel. Materials 2022, 15, 7952. [Google Scholar] [CrossRef] [PubMed]
- Du, T. Thermodynamics of rare earth elements in iron-base solutions. J. Iron Steel Res 1994, 6, 6–12. [Google Scholar]
- Vahed, A.; Kay, D. Thermodynamics of rare earths in steelmaking. Metall. Trans. B 1976, 7, 375–383. [Google Scholar] [CrossRef]
- Xie, Y.; Song, M.; Zhu, H.; Li, J.; Ma, G.; Xue, Z. Effect of lanthanum content on the formation of acicular ferrite. Metall. Mater. Trans. B 2022, 53, 1484–1494. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, M.-X.; Ren, H. Roles of lanthanum and cerium in grain refinement of steels during solidification. Metals 2018, 8, 884. [Google Scholar] [CrossRef]
- Liu, H.-L.; Liu, C.-J.; Jiang, M.-F. Effect of rare earths on impact toughness of a low-carbon steel. Mater. Des. 2012, 33, 306–312. [Google Scholar] [CrossRef]
- Li, H.; McLean, A.; Rutter, J.; Sommerville, I. Influence of rare earth metals on the nucleation and solidification behavior of iron and 1045 steel. Metall. Trans. B 1988, 19, 383–395. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.; Kim, N.J. Transformation behavior and microstructural characteristics of acicular ferrite in linepipe steels. Mater. Sci. Eng. A 2008, 478, 361–370. [Google Scholar] [CrossRef]
- Garrison, W.M., Jr.; Maloney, J.L. Lanthanum additions and the toughness of ultra-high strength steels and the determination of appropriate lanthanum additions. Mater. Sci. Eng. A 2005, 403, 299–310. [Google Scholar] [CrossRef]



| Samples | C | Si | Mn | P | S | O | N | La |
|---|---|---|---|---|---|---|---|---|
| 0La | 0.16 | 0.29 | 1.24 | 0.012 | 0.0120 | 0.0007 | 0.020 | 0 |
| 67La | 0.16 | 0.28 | 1.36 | 0.0068 | 0.002 | 0.0028 | / | 0.0067 |
| 110La | 0.16 | 0.28 | 1.33 | 0.007 | 0.0081 | 0.0017 | 0.0035 | 0.011 |
| 230La | 0.16 | 0.28 | 1.33 | 0.0066 | 0.0032 | 0.0026 | / | 0.023 |
| 310La | 0.16 | 0.28 | 1.34 | 0.0069 | 0.0076 | 0.0010 | 0.0058 | 0.031 |
| Sample | Ecorr/mV | icorr/A·cm−2 |
|---|---|---|
| 0La–0 g/L | −785.0 | 4.04 × 10−4 |
| 0La–0.0625 g/L | −840.0 | 3.22 × 10−4 |
| 0La–0.125 g/L | −732.0 | 8.76 × 10−5 |
| 0La–0.5 g/L | −740.0 | 9.01 × 10−5 |
| 67La–0 g/L | −525.72 | 2.52 × 10−5 |
| 110La–0 g/L | −520.61 | 1.91 × 10−5 |
| 230La–0 g/L | −513.03 | 1.15 × 10−5 |
| 310La–0 g/L | −498.07 | 6.65 × 10−6 |
| Reaction Equation | ΔGθ = A + B × T (J/mol) | |
|---|---|---|
| A | B | |
| [Mn] + [S] = MnS(s) | −158,365 | 93.966 |
| 2[Al] + 3[O] = Al2O3(s) | −122,500 | 393.8 |
| 2[La] + [O] = La2O3(s) | −1,443,880 | 337 |
| 2[La] + 2[O] + [S] = La2O2S(s) | −1,341,200 | 301 |
| [La] + [S] = LaS(s) | −445,180 | 141.5 |
| [La] + [Al] + 3[O] = LaAlO3(s) | −801,616 | 28.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, S.; Yang, F.; He, J.; Liu, Z.; Fu, T.; Wang, B.; Chen, X.; Jia, S.; Liu, Q. Revealing the Corrosion Resistance Mechanism of Plain Carbon Steel Micro-Alloyed by La in Simulated Industrial Atmosphere. Materials 2024, 17, 4467. https://doi.org/10.3390/ma17184467
Sha S, Yang F, He J, Liu Z, Fu T, Wang B, Chen X, Jia S, Liu Q. Revealing the Corrosion Resistance Mechanism of Plain Carbon Steel Micro-Alloyed by La in Simulated Industrial Atmosphere. Materials. 2024; 17(18):4467. https://doi.org/10.3390/ma17184467
Chicago/Turabian StyleSha, Sha, Feng Yang, Jianzhong He, Zhi Liu, Tianle Fu, Bing Wang, Xiaoping Chen, Shujun Jia, and Qingyou Liu. 2024. "Revealing the Corrosion Resistance Mechanism of Plain Carbon Steel Micro-Alloyed by La in Simulated Industrial Atmosphere" Materials 17, no. 18: 4467. https://doi.org/10.3390/ma17184467
APA StyleSha, S., Yang, F., He, J., Liu, Z., Fu, T., Wang, B., Chen, X., Jia, S., & Liu, Q. (2024). Revealing the Corrosion Resistance Mechanism of Plain Carbon Steel Micro-Alloyed by La in Simulated Industrial Atmosphere. Materials, 17(18), 4467. https://doi.org/10.3390/ma17184467





