A New Mechanism for the Inhibition of SA106 Gr.B Carbon Steel Corrosion by Nitrite in Alkaline Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen and Solution Preparation
2.2. Potentiodynamic Polarization Test
2.3. Immersion Corrosion Test
2.4. Surface Characterization
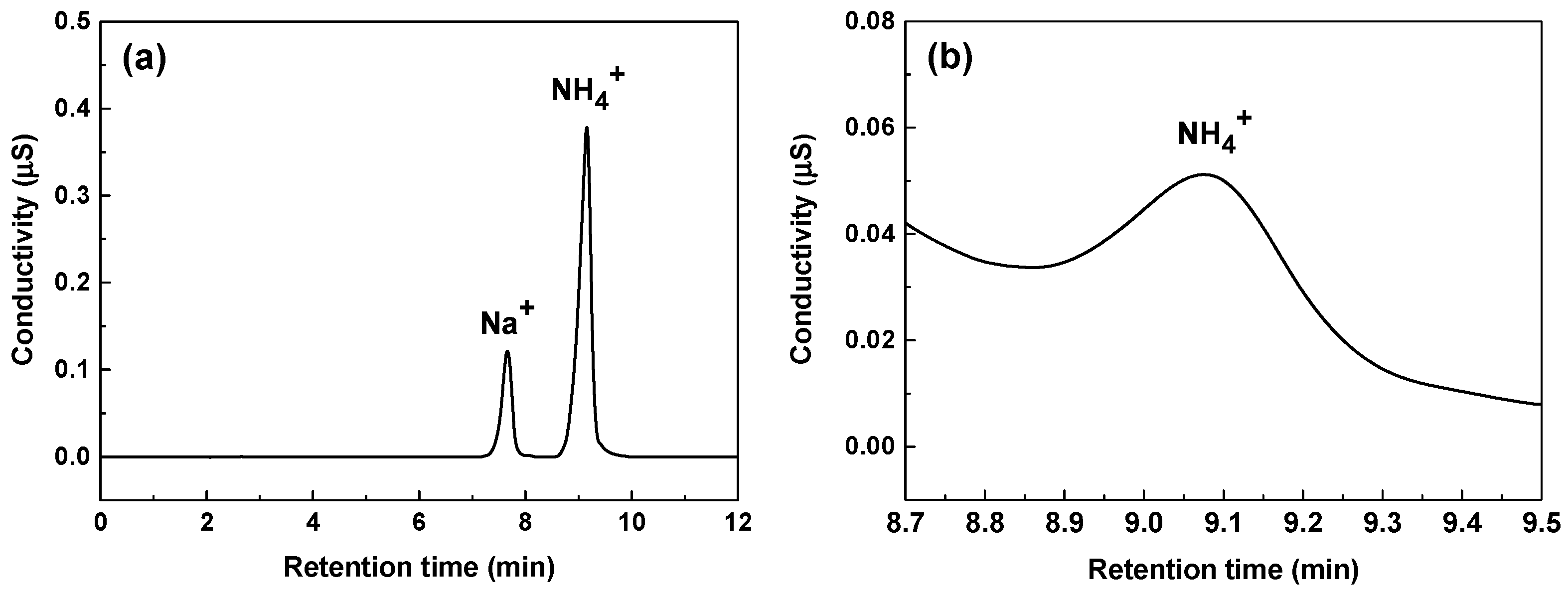
2.5. Chromatographic Conditions
3. Results and Discussion
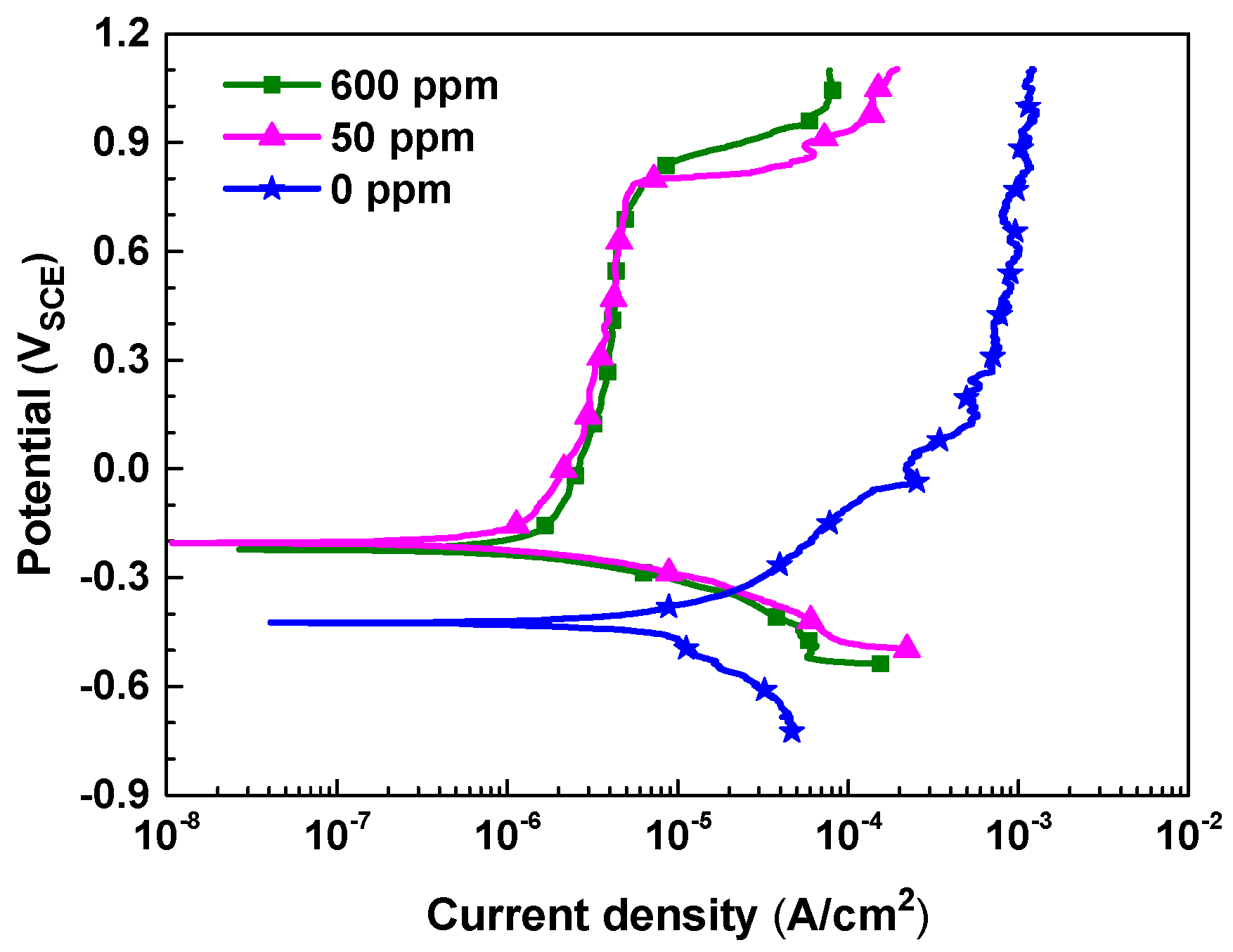
3.1. Polarization Behavior
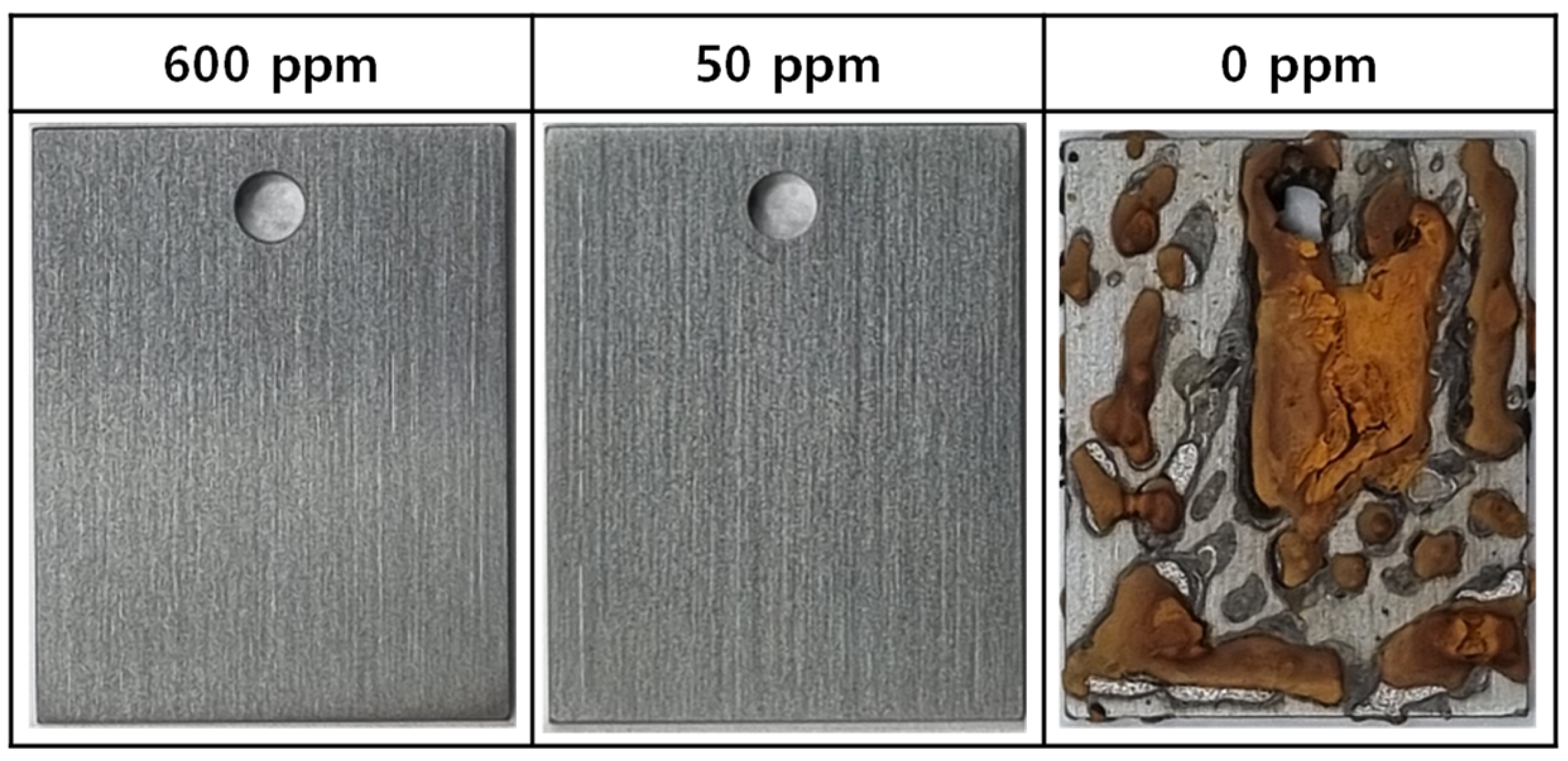
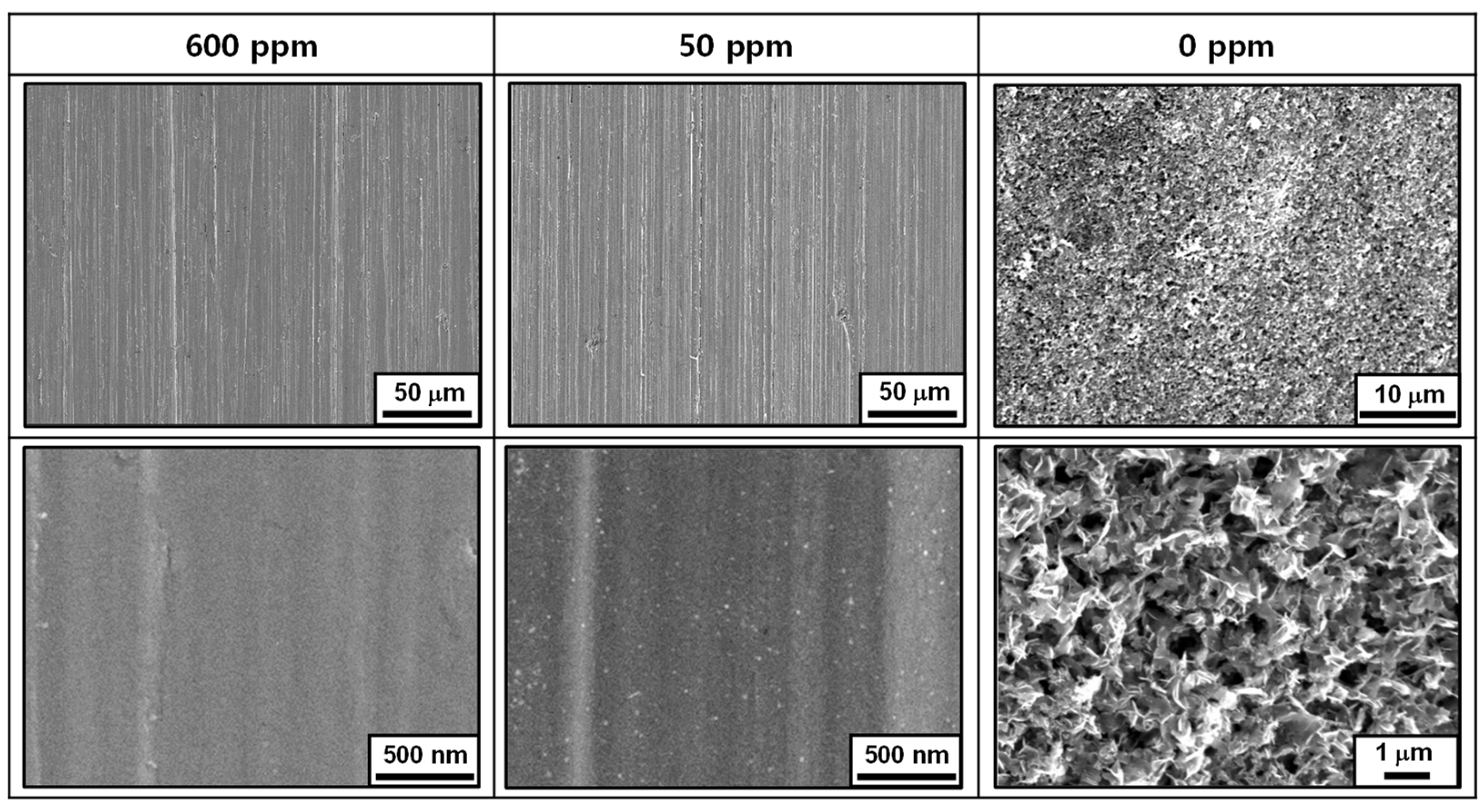
3.2. Immersion Corrosion Behavior
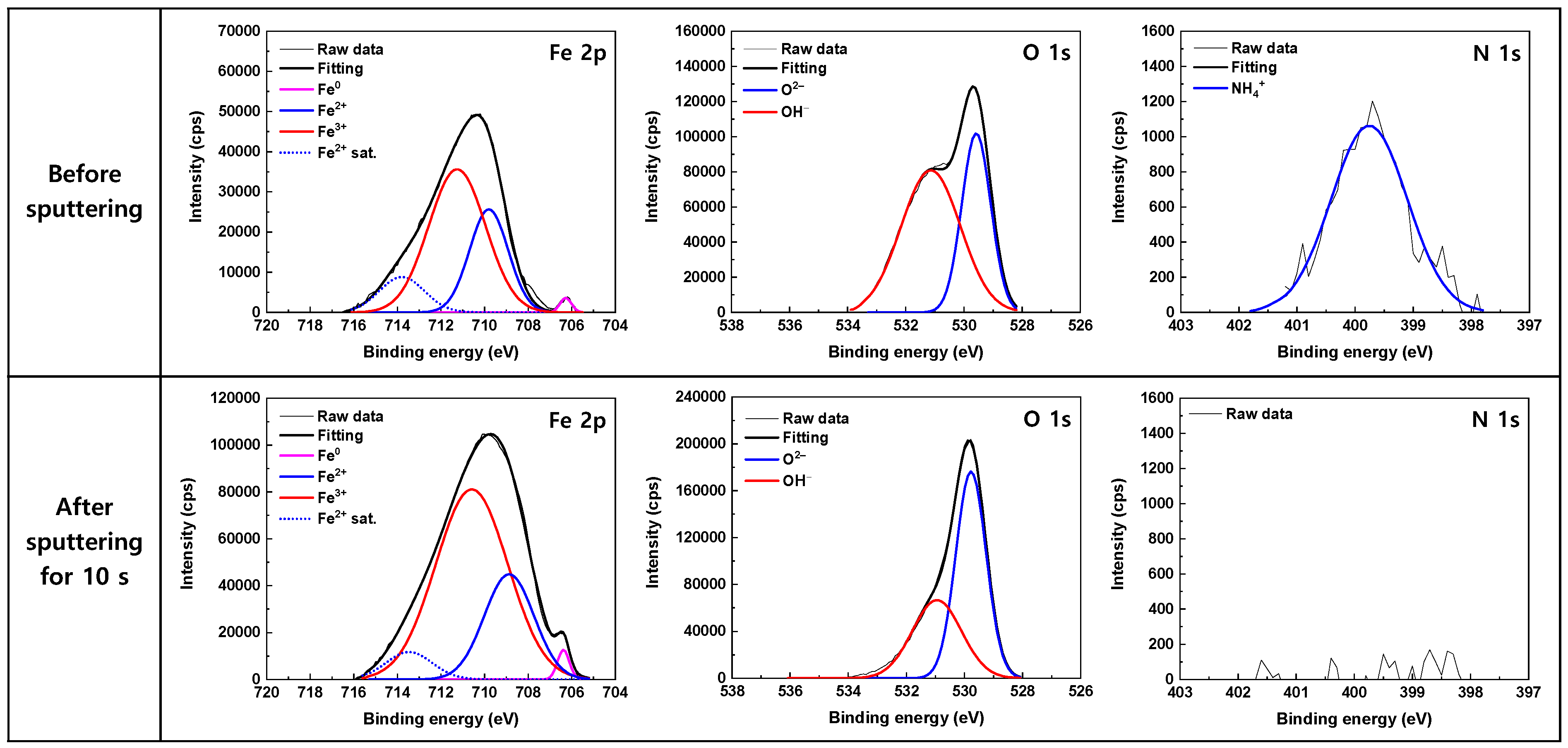
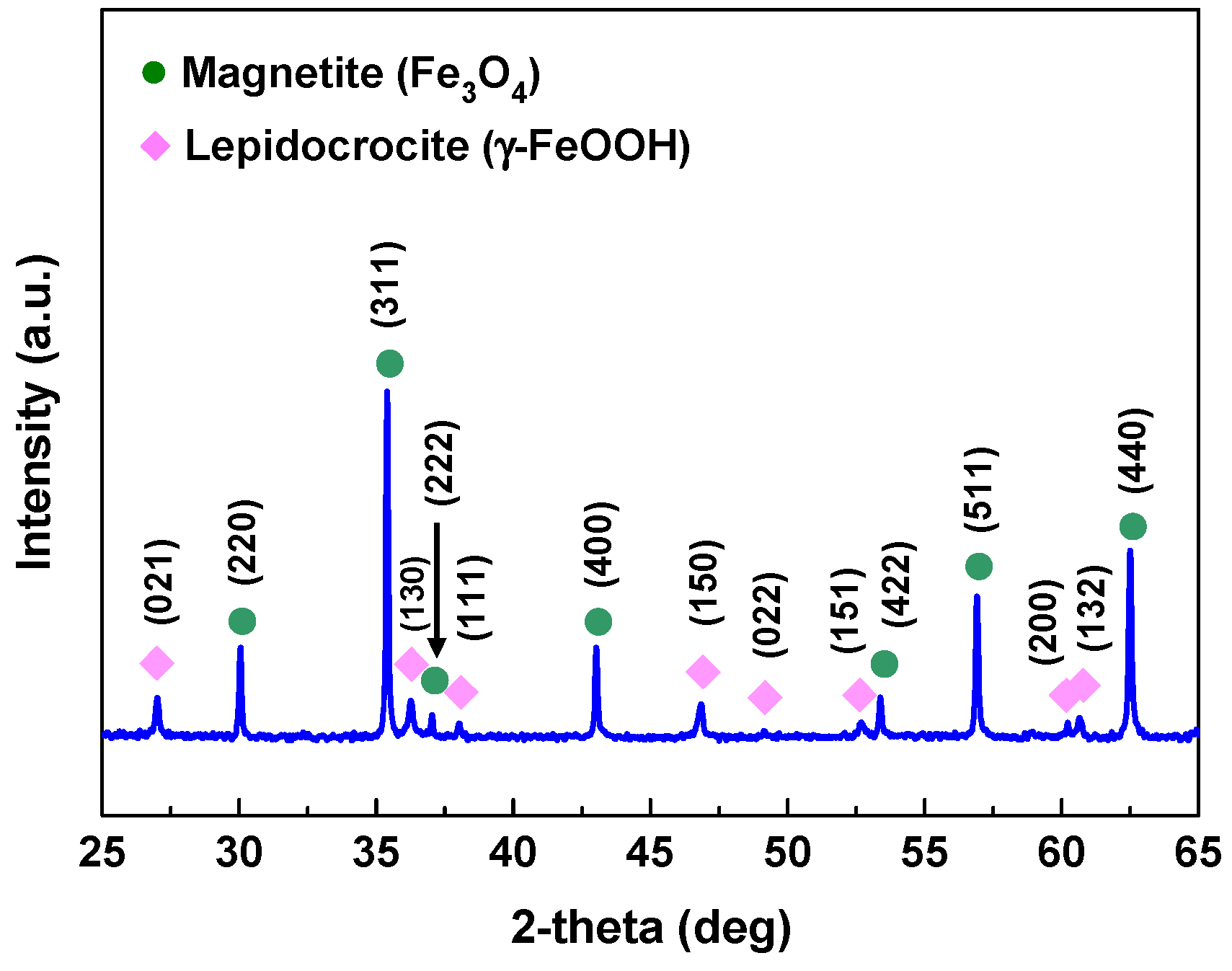
3.3. Oxide Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sani, F.M.; Brown, B.; Nesic, S. An electrochemical study of the effect of high salt concentration on uniform corrosion of carbon steel in aqueous CO2 solutions. J. Electrochem. Soc. 2021, 168, 051501. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Guo, X.P. Initiation and repassivation of pitting corrosion of carbon steel in carbonated concrete pore solution. Corros. Sci. 2011, 53, 1322–1330. [Google Scholar] [CrossRef]
- Han, J.; Hur, D.H.; Lee, Y.K. Effect of turbulent flow on the oxide properties of carbon steel in alkaline water. J. Mater. Res. Technol. 2024, 28, 4694–4702. [Google Scholar] [CrossRef]
- Khani, H.; Arefinia, R. Inhibition mechanism of nitrite on the corrosion of carbon steel in simulated cooling water systems. Mater. Corros. 2018, 69, 337–347. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, W.C.; Kim, J.G. Effect of nitrite concentration on the corrosion behaviour of carbon steel pipelines in synthetic tap water. Corros. Sci. 2012, 64, 105–114. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Luo, J.L. A comparison of the pitting susceptibility and semiconducting properties of the passive films on carbon steel in chromate and bicarbonate solutions. Appl. Surf. Sci. 2000, 167, 113–121. [Google Scholar] [CrossRef]
- Samiento-Bustos, E.; Rodriguez, J.G.G.; Uruchurtu, J.; Dominguez-Patino, G.; Salinas-Bravo, V.M. Effect of inorganic inhibitors on the corrosion behavior of 1018 carbon steel in the LiBr + ethylene glycol + H2O mixture. Corros. Sci. 2008, 50, 2296–2303. [Google Scholar] [CrossRef]
- Rashid, K.H.; Khadom, A.A. Optimization of inhibitive action of sodium molybdate (VI) for corrosion of carbon steel in saline water using response surface methodology. Korean J. Chem. Eng. 2019, 36, 1350–1359. [Google Scholar] [CrossRef]
- Lin, B.; Zuo, Y. Inhibition of Q235 carbon steel by calcium lignosulfonate and sodium molybdate in carbonated concrete pore solution. Molecules 2019, 24, 518. [Google Scholar] [CrossRef]
- Sanni, S.E.; Ewetade, A.P.; Emetere, M.E.; Agboola, O.; Okoro, E.; Olorunshola, S.J.; Olugbenga, T.S. Enhancing the inhibition potential of sodium tungstate towards mitigating the corrosive effect of Acidithiobaccillus thiooxidan on X-52 carbon steel. Mater. Today Commun. 2019, 19, 238–251. [Google Scholar] [CrossRef]
- Deyab, M.A.; Abd El-Rehim, S.S. Inhibitory effect of tungstate, molybdate and nitrite ions on the carbon steel pitting corrosion in alkaline formation water containing Cl− ion. Electrochim. Acta 2007, 53, 1754–1760. [Google Scholar] [CrossRef]
- Mohamed, A.; Martin, U.; Bastidas, D.M. Adsorption and surface analysis of sodium phosphate corrosion inhibitor on carbon steel in simulated concrete pore solution. Materials 2022, 15, 7429. [Google Scholar] [CrossRef] [PubMed]
- Yohai, L.; Schreiner, W.; Vazquez, M.; Valcarce, M.B. Phosphate ions as effective inhibitors for carbon steel in carbonated solutions contaminated with chloride ions. Electrochim. Acta 2016, 202, 231–242. [Google Scholar] [CrossRef]
- Bouoidina, A.; El-Hajjaji, F.; Drissi, M.; Taleb, M.; Hammouti, B.; Chung, I.M.; Jodeh, S.; Lgaz, H. Towards a deeper understanding of the anticorrosive properties of hydrazine derivatives in acid medium: Experimental, DFT and MD simulation assessment. Metall. Mater. Trans. A 2018, 49, 5180–5191. [Google Scholar] [CrossRef]
- El Azzouzi, M.; Aouniti, A.; Tighadouin, S.; Elmsellem, H.; Radi, S.; Hammouti, B.; El Assyry, A.; Bentiss, F.; Zarrouk, A. Some hydrazine derivatives as corrosion inhibitors for mild steel in 1.0 M HCl: Weight loss, electrochemical, SEM and theoretical studies. J. Mol. Liq. 2016, 221, 633–641. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Wang, H.; Shu, H. Research progress of nitrite corrosion inhibitor in concrete. Int. J. Corros. 2019, 2019, 3060869. [Google Scholar] [CrossRef]
- Eyu, G.D.; Will, G.; Dekkers, W.; MacLeod, J. The synergistic effect of iodide and sodium nitrite on the corrosion inhibition of mild steel in bicarbonate–chloride solution. Materials 2016, 9, 868. [Google Scholar] [CrossRef]
- Valcarce, M.B.; Lopez, C.; Vazquez, M. The role of chloride, nitrite and carbonate ions on carbon steel passivity studied in simulating concrete pore solutions. J. Electrochem. Soc. 2012, 159, C244–C251. [Google Scholar] [CrossRef]
- Li, W.S.; Luo, J.L. Uniformity of passive films formed on ferrite and martensite by different inorganic inhibitors. Corros. Sci. 2002, 44, 1695–1712. [Google Scholar] [CrossRef]
- Frontini, M.A.; Schreiner, W.; Vazquez, M.; Valcarce, M.B. Nitrite corrosion inhibition in chloride-rich electrolytes correlated to the electrical properties of surface films on carbon steel. Constr. Build. Mater. 2019, 227, 116650. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Zhang, G.A.; Guo, X.P. The role of inhibitors on the repassivation of pitting corrosion of carbon steel in synthetic carbonated concrete pore solution. Electrochim. Acta 2011, 56, 5890–5897. [Google Scholar] [CrossRef]
- Zhou, Y.; Zuo, Y. The inhibitive mechanisms of nitrite and molybdate anions on initiation and propagation of pitting corrosion for mild steel in chloride solution. Appl. Surf. Sci. 2015, 353, 924–932. [Google Scholar] [CrossRef]
- Valcarce, M.B.; Vazquez, M. Carbon steel passivity examined in solutions with a low degree of carbonation: The effect of chloride and nitrite ions. Mater. Chem. Phys. 2009, 115, 313–321. [Google Scholar] [CrossRef]
- Sideris, K.K.; Savva, A.E. Durability of mixtures containing calcium nitrite based corrosion inhibitor. Cem. Concr. Compos. 2005, 27, 277–287. [Google Scholar] [CrossRef]
- Rizvi, M.; Gerengi, H.; Kaya, S.; Uygur, I.; Yildiz, M.; Sarioglu, I.; Cingiz, Z.; Mielniczek, M.; Ibrahimi, B.E. Sodium nitrite as a corrosion inhibitor of copper in simulated cooling water. Sci. Rep. 2021, 11, 8353. [Google Scholar] [CrossRef]
- Reffass, M.; Sabot, R.; Jeannin, M.; Berziou, C.; Refait, P. Effects of NO2− ions on localised corrosion of steel in NaHCO3 + NaCl electrolytes. Electrochim. Acta 2007, 52, 7599–7606. [Google Scholar] [CrossRef]
- Zhou, Y.; Zuo, Y. The passivation behavior of mild steel in CO2 saturated solution containing nitrite anions. J. Electrochem. Soc. 2015, 162, C47–C54. [Google Scholar] [CrossRef]
- Joseph, G.; Perret, R.; Pourrat, G.; Arce, M.T. Contribution to the study of NaNO2 iron corrosion inhibition. In Proceedings of the 3rd European Symposium, Ferrara, Italy, 14−17 September 1970; pp. 791–804. [Google Scholar]
- Balasubramaniyan, S.; Mohan, S.; Vishnudevan, M. Novel corrosion inhibitor for mild steel in simulated concrete pore solution with chloride contamination. Chem. Sci. Trans. 2015, 4, 27–36. [Google Scholar]
- Al-Refaie, A.A.; Walton, J.; Cottis, R.A.; Lindsay, R. Photoelectron spectroscopy study of the inhibition of mild steel corrosion by molybdate and nitrite anions. Corros. Sci. 2010, 52, 422–428. [Google Scholar] [CrossRef]
- Tang, Z. A review of corrosion inhibitors for rust preventative fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759. [Google Scholar] [CrossRef]
- Rosenberg, A.M.; Gaidis, J.M. The mechanism of nitrite inhibition of chloride attack on reinforcing steel in alkaline aqueous environments. Mater. Perform. 1979, 18, 45–48. [Google Scholar]
- Mohana, K.N.; Badiea, A.M. Effect of sodium nitrite–borax blend on the corrosion rate of low carbon steel in industrial water medium. Corros. Sci. 2008, 50, 2939–2947. [Google Scholar] [CrossRef]
- Wachter, A.; Smith, S.S. Preventing internal corrosion of pipe lines. Ind. Eng. Chem. 1943, 35, 358–367. [Google Scholar] [CrossRef]
- Cohen, M. The breakdown and repair of inhibitive films in neutral solutions. Corrosion 1976, 32, 461–465. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, J.; Li, G.; Wang, P.; Wang, P.; Li, F.; Zhang, B.; Chi, H. Corrosion inhibition efficiency of compound nitrite with D-sodium gluconate on carbon steel in simulated concrete pore solution. Constr. Build. Mater. 2021, 288, 123101. [Google Scholar] [CrossRef]
- Tian, Y.; Wen, C.; Wang, G.; Deng, P.; Mo, W. Inhibiting property of nitrite intercalated layered double hydroxide for steel reinforcement in contaminated concrete condition. J. Appl. Electrochem. 2020, 50, 835–849. [Google Scholar] [CrossRef]
- Gireiene, O.; Samulevieiene, M.; Sudavieius, A.; Ramanauskas, R. Efficiency of steel corrosion inhibitor calcium nitrite in alkaline solutions and concrete structures. Chemija 2005, 16, 1–6. [Google Scholar]
- Girciene, O.; Ramanauskas, R.; Gudaviciute, L.; Martusiene, A. Inhibition effect of sodium nitrite and silicate on carbon steel corrosion in chloride-contaminated alkaline solutions. Corrosion 2011, 67, 125001-1–125001-12. [Google Scholar] [CrossRef]
- Bolzoni, F.; Brenna, A.; Ormellese, M. Recent advances in the use of inhibitors to prevent chloride-induced corrosion in reinforced concrete. Cem. Concr. Res. 2022, 154, 106719. [Google Scholar] [CrossRef]
- Gaidis, J.M. Chemistry of corrosion inhibitors. Cem. Concr. Compos. 2004, 26, 181–189. [Google Scholar] [CrossRef]
- Moraghan, J.T.; Buresh, R.J. Chemical reduction of nitrite and nitrous oxide by ferrous iron. Soil Sci. Soc. Am. J. 1977, 41, 47–50. [Google Scholar] [CrossRef]
- Fujioka, E.; Nishihara, H.; Aramaki, K. The inhibition of pit nucleation and growth on the passive surface of iron in a borate buffer solution containing Cl− by oxidizing inhibitors. Corros. Sci. 1996, 38, 1915–1933. [Google Scholar] [CrossRef]
- Selby, K.; Licina, G. Closed Cooling Water Chemistry Guideline: Revision 2; 3002000590; EPRI: Palo Alto, CA, USA, 2013. [Google Scholar]
- Ciji, A.; Akhtar, M.S. Nitrite implications and its management strategies in aquaculture: A review. Rev. Aquac. 2020, 12, 878–908. [Google Scholar] [CrossRef]
- NACE TM0169/G31–21; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. NACE International/ASTM International: Houston, TX, USA; West Conshohocken, PA, USA, 2021.
- NIST X-ray Photoelectron Spectroscopy Database (SRD 20). Available online: http://srdata.nist.gov/xps/ (accessed on 5 June 2024).
- Crist, B.V. Handbooks of Monochromatic XPS Spectra; XPS International LLC.: Salem, OR, USA, 2019. [Google Scholar]
- Tougaard, S. Practical guide to the use of backgrounds in quantitative XPS. J. Vac. Sci. Technol. A 2021, 39, 011201. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Jayaweera, P.M.; Grassian, V.H. XPS study of nitrogen dioxide adsorption on metal oxide particle surfaces under different environmental conditions. Phys. Chem. Chem. Phys. 2009, 11, 8295–8305. [Google Scholar] [CrossRef]
- Bischoff, J.L.; Lutz, F.; Bolmont, D.; Kubler, L. Use of multilayer techniques for XPS identification of various nitrogen environments in the Si/NH3 system. Surf. Sci. 1991, 251–252, 170–174. [Google Scholar] [CrossRef]
- Jirsak, T.; Kuhn, M.; Rodriguez, J.A. Chemistry of NO2 on Mo(110): Decomposition reactions and formation of MoO2. Surf. Sci. 2000, 457, 254–266. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Jirsak, T.; Liu, G.; Hrbek, J.; Dvorak, J.; Maiti, A. Chemistry of NO2 on oxide surfaces: Formation of NO3 on TiO2(110) and NO2↔O vacancy interactions. J. Am. Chem. Soc. 2001, 123, 9597–9605. [Google Scholar] [CrossRef]
- Liu, G.; Tao, C.; Zhang, M.; Gu, X.; Meng, F.; Zhang, X.; Chen, Y.; Ruan, S. Effects of surface self-assembled NH4+ on the performance of TiO2-based ultraviolet photodetectors. J. Alloys Compd. 2014, 601, 104–107. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, L.; Zhao, K.; Sun, X.; Wu, Q. Layered ferric vanadate nanosheets as a high-rate NH4+ storage electrode. Electrochim. Acta 2020, 360, 137008. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lee, C.T. Surface analysis of (NH4)2Sx-treated InGaN using x-ray photoelectron spectroscopy. J. Vac. Sci. Technol. B 2001, 19, 1734. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, F.; Chen, D.; Wang, P.; Liu, Y.; Meng, C.; Zhang, Y. Modulating NH4+ in vanadium oxide framework for high-efficient aqueous NH4+ storage. Chem. Eng. J. 2024, 489, 151119. [Google Scholar] [CrossRef]
- Vinod, C.P.; Hans, J.W.N.; Nieuwenhuys, B.E. Interaction of small molecules with Au(3 1 0): Decomposition of NO. Appl. Catal. A Gen. 2005, 291, 93–97. [Google Scholar] [CrossRef]
- Rienks, E.D.L.; Bakker, J.W.; Baraldi, A.; Carabineiro, S.A.C.; Lizzit, S.; Weststrate, C.J.; Nieuwenhuys, B.E. Interaction of nitric oxide with Pt(1 0 0). A fast X-ray photoelectron spectroscopy study. Surf. Sci. 2002, 516, 109–117. [Google Scholar] [CrossRef]
- Au, C.T.; Carley, A.F.; Roberts, M.W. Photoelectron spectroscopy: A strategy for the study of reactions at solid surfaces. Int. Rev. Phys. Chem. 1986, 5, 57–87. [Google Scholar] [CrossRef]
- Smirnov, M.Y.; Kalinkin, A.V.; Nazimov, D.A.; Bukhtiyarov, V.I.; Vovk, E.I.; Ozensoy, E. An XPS study of the interaction of model Ba/TiO2 and Ba/ZrO2 NSR catalysts with NO2. J. Struct. Chem. 2014, 55, 757–763. [Google Scholar] [CrossRef]
- Mudiyanselage, K.; Yi, C.W.; Szanyi, J. Reactivity of a thick BaO film supported on Pt(111): Adsorption and reaction of NO2, H2O, and CO2. Langmuir 2009, 25, 10820–10828. [Google Scholar] [CrossRef] [PubMed]
- Staudt, T.; Desikusumastuti, A.; Happel, M.; Vesselli, E.; Baraldi, A.; Gardonio, S.; Lizzit, S.; Rohr, F.; Libuda, J. Modeling NOx storage materials: A high-resolution photoelectron spectroscopy study on the interaction of NO2 with Al2O3/NiAl(110) and BaO/Al2O3/NiAl(110). J. Phys. Chem. C 2008, 112, 9835–9846. [Google Scholar] [CrossRef]
- Yi, C.W.; Kwak, J.H.; Szanyi, J. Interaction of NO2 with BaO: From cooperative adsorption to Ba(NO3)2 formation. J. Phys. Chem. C 2007, 111, 15299–15305. [Google Scholar] [CrossRef]
- Rosseler, O.; Sleiman, M.; Montesinos, V.N.; Shavorskiy, A.; Keller, V.; Keller, N.; Litter, M.I.; Bluhm, H.; Salmeron, M.; Destaillats, H. Chemistry of NOx on TiO2 surfaces studied by ambient pressure XPS: Products, effect of UV irradiation, water, and coadsorbed K+. J. Phys. Chem. Lett. 2013, 4, 536–541. [Google Scholar] [CrossRef]
- Aduru, S.; Contarini, S.; Rabalais, J.W. Electron-, X-ray-, and ion-stimulated decomposition of nitrate salts. J. Phys. Chem. 1986, 90, 1683–1688. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Roobottom, H.K. Lattice Energies. In CRC Handbook of Chemistry and Physics, 95th ed.; Haynes, W.M., Lide, D.R., Bruno, T.J., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 12-21–12-33. [Google Scholar]
- Baer, D.R.; Engelhard, M.H.; Lea, A.S.; Nachimuthu, P.; Droubay, T.C.; Kim, J.; Lee, B.; Mathews, C.; Opila, R.L.; Saraf, L.V.; et al. Comparison of the sputter rates of oxide films relative to the sputter rate of SiO2. J. Vac. Sci. Technol. A 2010, 28, 1060–1072. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, H.; Zhang, P.; Liu, D.; Yan, F. The effects of nitrite anions on surface passive film properties for Q235 carbon steels. Surf. Rev. Lett. 2019, 26, 1850218. [Google Scholar] [CrossRef]
- HSC Chemistry 6, version 6.12; Outotec Research Oy: Pori, Finland, 2006.
- Mandal, S.; Arts, K.; Knoops, H.C.M.; Cuenca, J.A.; Klemencic, G.M.; Williams, O.A. Surface zeta potential and diamond growth on gallium oxide single crystal. Carbon 2021, 181, 79–86. [Google Scholar] [CrossRef]
- Lee, J.M.; Lim, D.S.; Jeon, S.H.; Hur, D.H. Zeta potentials of magnetite particles and alloy 690 surfaces in alkaline solutions. Materials 2020, 13, 3999. [Google Scholar] [CrossRef]
- Xia, C.; Ma, X.; Zhang, X.; Li, K.; Tan, J.; Qiao, Y.; Liu, X. Enhanced physicochemical and biological properties of C/Cu dual ions implanted medical titanium. Bioact. Mater. 2020, 5, 377–386. [Google Scholar] [CrossRef]
- Hedberg, Y.; Wang, X.; Hedberg, J.; Lundin, M.; Blomberg, E.; Wallinder, I.O. Surface-protein interactions on different stainless steel grades: Effects of protein adsorption, surface changes and metal release. J. Mater. Sci. Mater. Med. 2013, 24, 1015–1033. [Google Scholar] [CrossRef]
- Boulange-Petermann, L.; Doren, A.; Baroux, B.; Bellon-Fontaine, M.N. Zeta potential measurements on passive metals. J. Colloid Interface Sci. 1995, 171, 179–186. [Google Scholar] [CrossRef]
- Thomas, D.H.; Rey, M.; Jackson, P.E. Determination of inorganic cations and ammonium in environmental waters by ion chromatography with a high-capacity cation-exchange column. J. Chromatogr. A 2002, 956, 181–186. [Google Scholar] [CrossRef]
- Huang, Y.; Mou, S.; Riviello, J.M. Determination of ammonium in seawater by column-switching ion chromatography. J. Chromatogr. A 2000, 868, 209–216. [Google Scholar] [CrossRef]
- Moed, D.H.; Verliefde, A.R.D.; Rietveld, L.C. Role of metal surface catalysis in the thermolysis of morpholine and ethanolamine under superheater conditions. Ind. Eng. Chem. Res. 2014, 53, 19392–19397. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE: Houston, TX, USA, 1974; p. 495. [Google Scholar]

| Ni | Cr | Mo | Cu | C | Fe |
|---|---|---|---|---|---|
| 0.02 | 0.04 | 0.01 | 0.01 | 0.19 | Balance |
| Nitrite Concentration (ppm) | Ecorr (VSCE) | icorr (10−6 A/cm2) | η (%) |
|---|---|---|---|
| 0 | −0.424 | 7.78 | - |
| 50 | −0.204 | 1.39 | 82 |
| 600 | −0.223 | 1.41 | 82 |
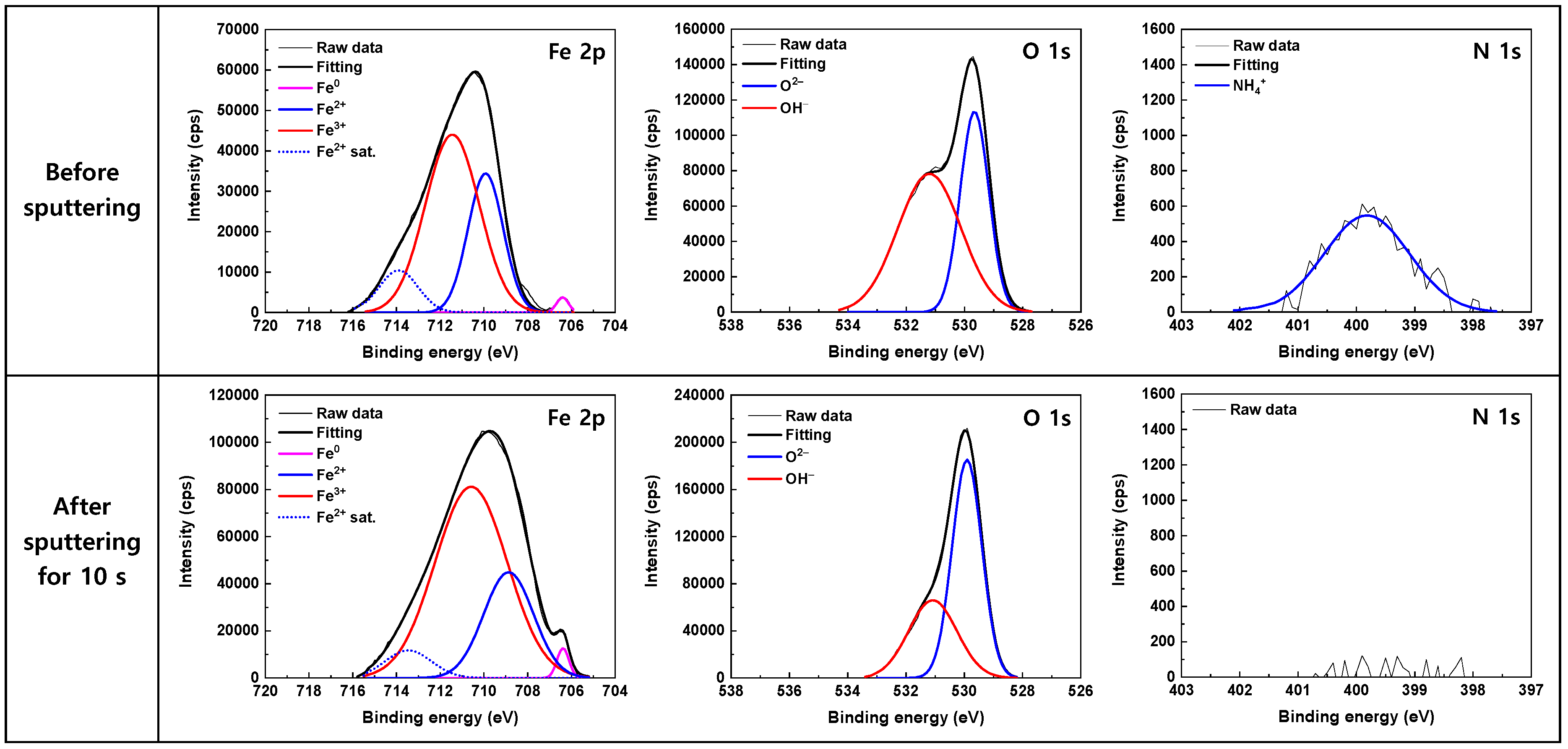
| Component | 600 ppm NO2− | 50 ppm NO2− | |||||
|---|---|---|---|---|---|---|---|
| BE (eV) | FWHM * (eV) | Relative Area (%) | BE (eV) | FWHM (eV) | Relative Area (%) | ||
| Before sputtering | Fe0 2p3/2 | 706.27 | 0.61 | 1.2 | 706.40 | 0.65 | 1.1 |
| Fe2+ 2p3/2 | 709.84 | 2.10 | 29.4 | 709.93 | 1.89 | 30.0 | |
| Fe3+ 2p3/2 | 711.33 | 3.22 | 60.5 | 711.45 | 2.91 | 58.9 | |
| Fe2+ sat. 2p3/2 | 713.97 | 2.30 | 8.9 | 713.90 | 2.09 | 10.0 | |
| O2– 1s | 529.57 | 1.17 | 38.1 | 529.57 | 1.17 | 38.1 | |
| OH− 1s | 531.14 | 2.40 | 61.9 | 531.14 | 2.40 | 61.9 | |
| NH4+ 1s | 399.76 | 1.54 | 100 | 399.76 | 1.54 | 100 | |
| After a 10 s sputtering | Fe0 2p3/2 | 706.23 | 0.63 | 1.6 | 706.38 | 0.64 | 1.7 |
| Fe2+ 2p3/2 | 708.88 | 2.91 | 27.7 | 708.87 | 2.68 | 25.4 | |
| Fe3+ 2p3/2 | 710.69 | 4.24 | 64.2 | 710.59 | 3.89 | 66.7 | |
| Fe2+ sat. 2p3/2 | 714.16 | 2.64 | 6.5 | 713.46 | 2.50 | 6.2 | |
| O2– 1s | 529.78 | 1.26 | 62.0 | 529.91 | 1.20 | 63.3 | |
| OH− 1s | 531.00 | 2.07 | 38.0 | 531.08 | 1.95 | 36.7 | |
| NH4+ 1s | N.D. * | - | - | N.D. | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hur, D.-H.; Han, J.; Lee, J.-H.; Jeon, S.-H.; Shim, H.-S. A New Mechanism for the Inhibition of SA106 Gr.B Carbon Steel Corrosion by Nitrite in Alkaline Water. Materials 2024, 17, 4470. https://doi.org/10.3390/ma17184470
Hur D-H, Han J, Lee J-H, Jeon S-H, Shim H-S. A New Mechanism for the Inhibition of SA106 Gr.B Carbon Steel Corrosion by Nitrite in Alkaline Water. Materials. 2024; 17(18):4470. https://doi.org/10.3390/ma17184470
Chicago/Turabian StyleHur, Do-Haeng, Jeoh Han, Joung-Hae Lee, Soon-Hyeok Jeon, and Hee-Sang Shim. 2024. "A New Mechanism for the Inhibition of SA106 Gr.B Carbon Steel Corrosion by Nitrite in Alkaline Water" Materials 17, no. 18: 4470. https://doi.org/10.3390/ma17184470






