Effect of Alloying on Microstructure and Mechanical Properties of AlCoCrFeNi2.1 Eutectic High-Entropy Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructures and Phase Compositions
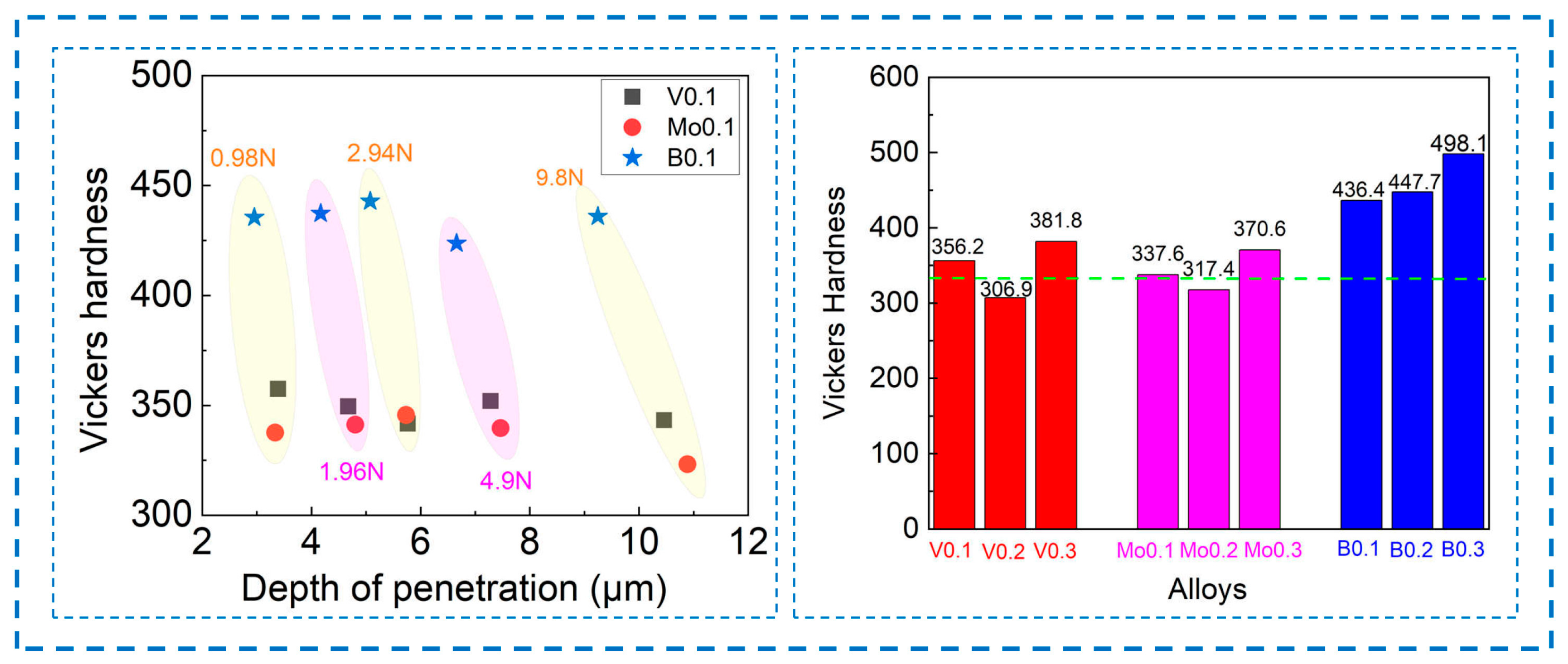
3.2. Mechanical Properties
4. Conclusions
- (1)
- The AlCoCrFeNi2.1 alloy is still composed of two solid solutions with FCC and BCC structures after V, Mo, and B are added. The content of the FCC phase decreases and then increases when high-melting-point elements V and Mo are added, while the content increases and then decreases when non-metallic element B is added.
- (2)
- Typical microstructures composed of dendrites and interdendrites were observed in the AlCoCrFeNi2.1Xy (X = V, Mo, B; y = 0.1, 0.2, 0.3) alloys. Co, Cr, and Fe elements are enriched in the FCC phase, Al and Ni elements are enriched in the BCC phase. The high-melting-point elements V and Mo are distributed in both the FCC phase and the BCC phase.
- (3)
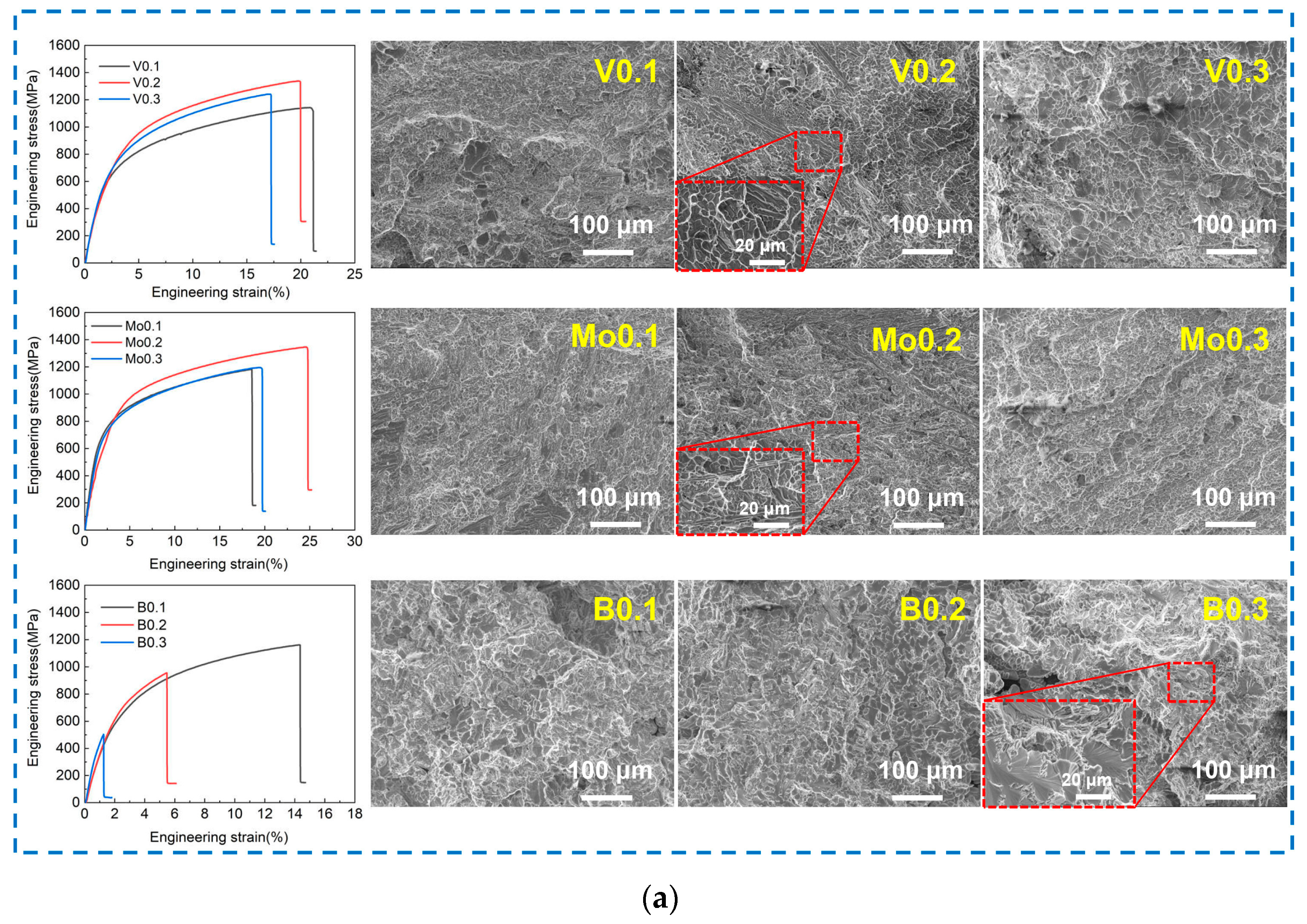
- The tensile strength of the alloy increases and then decreases with the increase in the content of V and Mo elements, while the strength of the alloy decreases significantly with B added. The Mo0.2 alloy achieves a balance of strength and plasticity, and the tensile strength and elongation are 1346.3 MPa and 24.6%, respectively.
- (4)
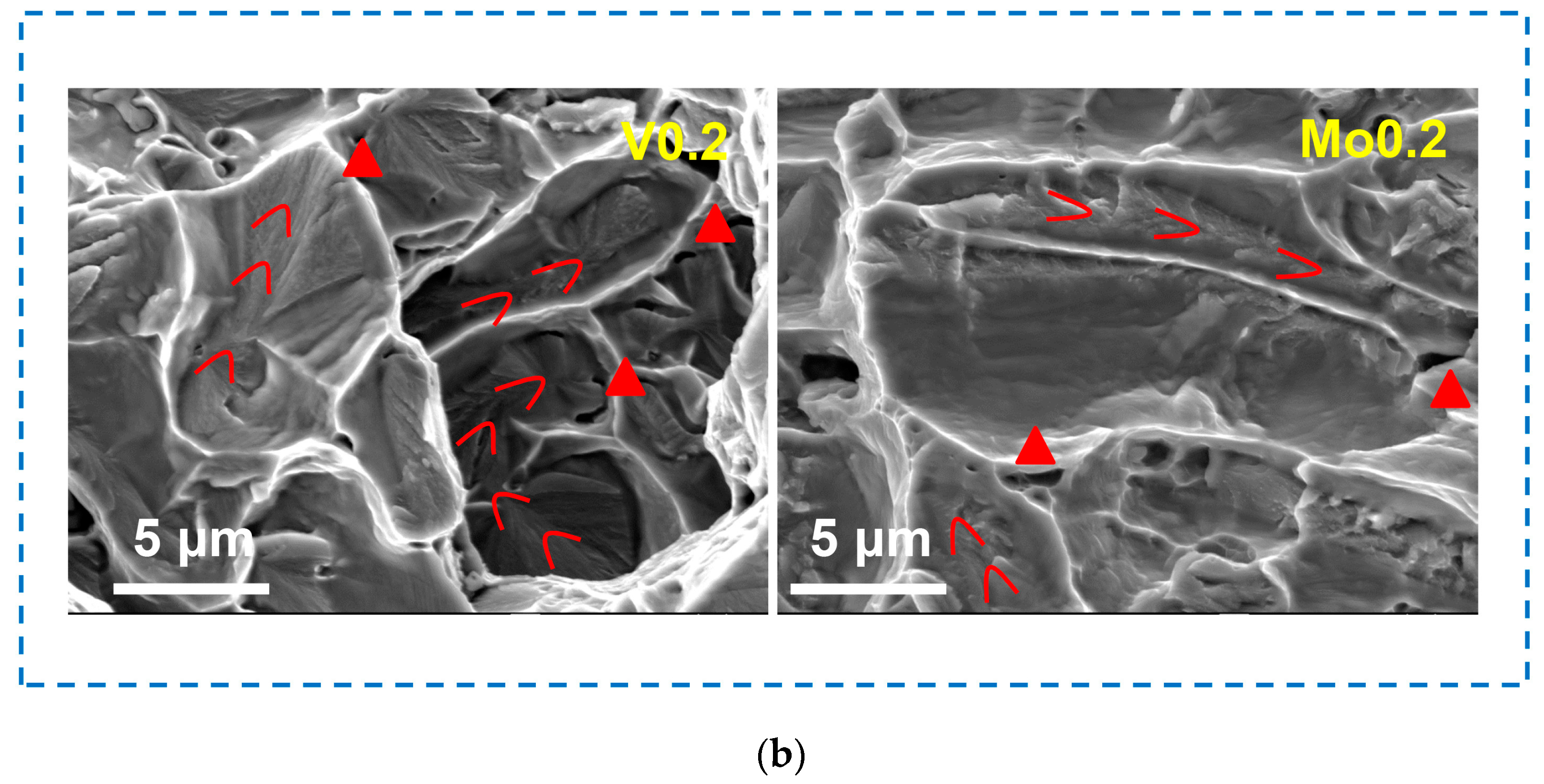
- The fracture mechanism of the alloys with the addition of V and Mo is a tough–brittle mixed fracture, while the alloy with the addition of B is a cleavage fracture. The propagation of cracks in BCC lamellae was observed in the V0.2 and Mo0.2 alloys, and the FCC dendritic lamellae exhibited perfect plastic deformation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A novel low-density, high-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2015, 3, 95–99. [Google Scholar] [CrossRef]
- Yu, P.F.; Zhang, L.J.; Cheng, H.; Zhang, H.; Ma, M.Z.; Li, Y.C.; Li, G.; Liaw, P.K.; Liu, R.P. The high-entropy alloys with high hardness and soft magnetic property prepared by mechanical alloying and high-pressure sintering. Intermetallics 2016, 70, 82–87. [Google Scholar] [CrossRef]
- Tian, F.; Varga, L.K.; Chen, N.; Shen, J.; Vitos, L. Empirical design of single phase high-entropy alloys with high hardness. Intermetallics 2015, 58, 1–6. [Google Scholar] [CrossRef]
- Maresca, F.; Curtin, W.A. Mechanistic origin of high strength in refractory BCC high entropy alloys up to 1900 K. Acta Mater. 2020, 182, 235–249. [Google Scholar] [CrossRef]
- Poletti, M.G.; Fiore, G.; Gili, F.; Mangherini, D.; Battezzati, L. Development of a new high entropy alloy for wear resistance: FeCoCrNiW0.3 and FeCoCrNiW0.3 + 5 at.% of C. Mater. Design. 2017, 115, 247–254. [Google Scholar] [CrossRef]
- Feng, R.; Feng, B.; Gao, M.C.; Zhang, C.; Neuefeind, J.C.; Poplawsky, J.D.; Ren, Y.; An, K.; Widom, M.; Liaw, P.K. Superior High-Temperature Strength in a Supersaturated Refractory High-Entropy Alloy. Adv. Mater. 2021, 33, 2102401. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Su, Y.; Liu, S.Y.; Stahl, K.; Wu, Y.D.; Hui, X.D.; Ruett, U.; Gutowski, O.; Glazyrin, K.; Liermann, H.P.; et al. Structural stability of high entropy alloys under pressure and temperature. J. Appl. Phys. 2017, 121, 235901. [Google Scholar] [CrossRef]
- Raphel, A.; Kumaran, S.; Kumar, K.V.; Varghese, L. Oxidation and Corrosion resistance of AlCoCrFeTiHigh Entropy Alloy. Mater. Today 2017, 4, 195–202. [Google Scholar] [CrossRef]
- Qiu, X.W.; Zhang, Y.P.; He, L.; Liu, C. Microstructure and corrosion resistance of AlCrFeCuCo high entropy alloy. J. Alloy Compd. 2013, 549, 195–199. [Google Scholar] [CrossRef]
- Chang, F.; Cai, B.; Zhang, C.; Huang, B.; Li, S.; Dai, P. Thermal stability and oxidation resistance of FeCrxCoNiB high-entropy alloys coatings by laser cladding. Surf. Coat. Technol. 2019, 359, 132–140. [Google Scholar] [CrossRef]
- Sun, S.J.; Tian, Y.Z.; Lin, H.R.; Dong, X.G.; Wang, Y.H.; Zhang, Z.J.; Zhang, Z.F. Enhanced strength and ductility of bulk CoCrFeMnNi high entropy alloy having fully recrystallized ultrafine-grained structure. Mater. Design. 2017, 133, 122–127. [Google Scholar] [CrossRef]
- Wei, R.; Sun, H.; Han, Z.H.; Chen, C.; Wang, T.; Guan, S.K.; Li, F.S. Strengthening of Fe40Mn40Co10Cr10 high entropy alloy via Mo/C alloying. Mater. Lett. 2018, 219, 85–88. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A Promising New Class of High-Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Zheng, S.; Pang, J.; Ma, X. High-strength and high-ductility AlCoCrFeNi2.1 eutectic high-entropy alloy achieved via precipitation strengthening in a heterogeneous structure. Scr. Mater. 2020, 186, 336–340. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; Zhang, B.; Liang, N.; Wu, G.; Sha, G.; Liu, J.; Zhao, Y. Microstructural origins of high strength and high ductility in an AlCoCrFeNi2.1 eutectic high-entropy alloy. Acta Mater. 2017, 141, 59–66. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Wani, I.S.; Sheikh, S.; Clark, I.T.; Okawa, T.; Guo, S.; Bhattacharjee, P.P.; Tsuji, N. Simultaneous Strength-Ductility Enhancement of a Nano-Lamellar AlCoCrFeNi2.1 Eutectic High Entropy Alloy by Cryo-Rolling and Annealing. Sci. Rep. 2018, 8, 3276. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yao, Z.; Huang, X.; Du, F.; Li, C.; Chen, A.; Wu, F.; Cheng, Y.; Zhang, Z. Microstructure and mechanical properties of AlCoxCrFeNi3-x eutectic high-entropy-alloy system. J. Alloy Compd. 2020, 823, 153886. [Google Scholar] [CrossRef]
- Jiang, H.; Qiao, D.; Jiao, W.; Han, K.; Lu, Y.; Liaw, P.K. Tensile deformation behavior and mechanical properties of a bulk cast Al0.9CoFeNi2 eutectic high-entropy alloy. J. Mater. Sci. Technol. 2021, 61, 119–124. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Niu, H.; Yang, G.; Yang, L.; Xu, M.; Yi, J. BCC + L21 dual-phase Cu40Al20Ti20V20 near-eutectic high-entropy alloy with a combination of strength and plasticity. J. Alloy Compd. 2022, 908, 164683. [Google Scholar] [CrossRef]
- Chen, X.; Xie, W.; Zhu, J.; Wang, Z.; Wang, Y.; Ma, Y.; Yang, M.; Jiang, W.; Yu, H.; Wu, Y.; et al. Influences of Ti additions on the microstructure and tensile properties of AlCoCrFeNi2.1 eutectic high entropy alloy. Intermetallics 2021, 128, 107024. [Google Scholar] [CrossRef]

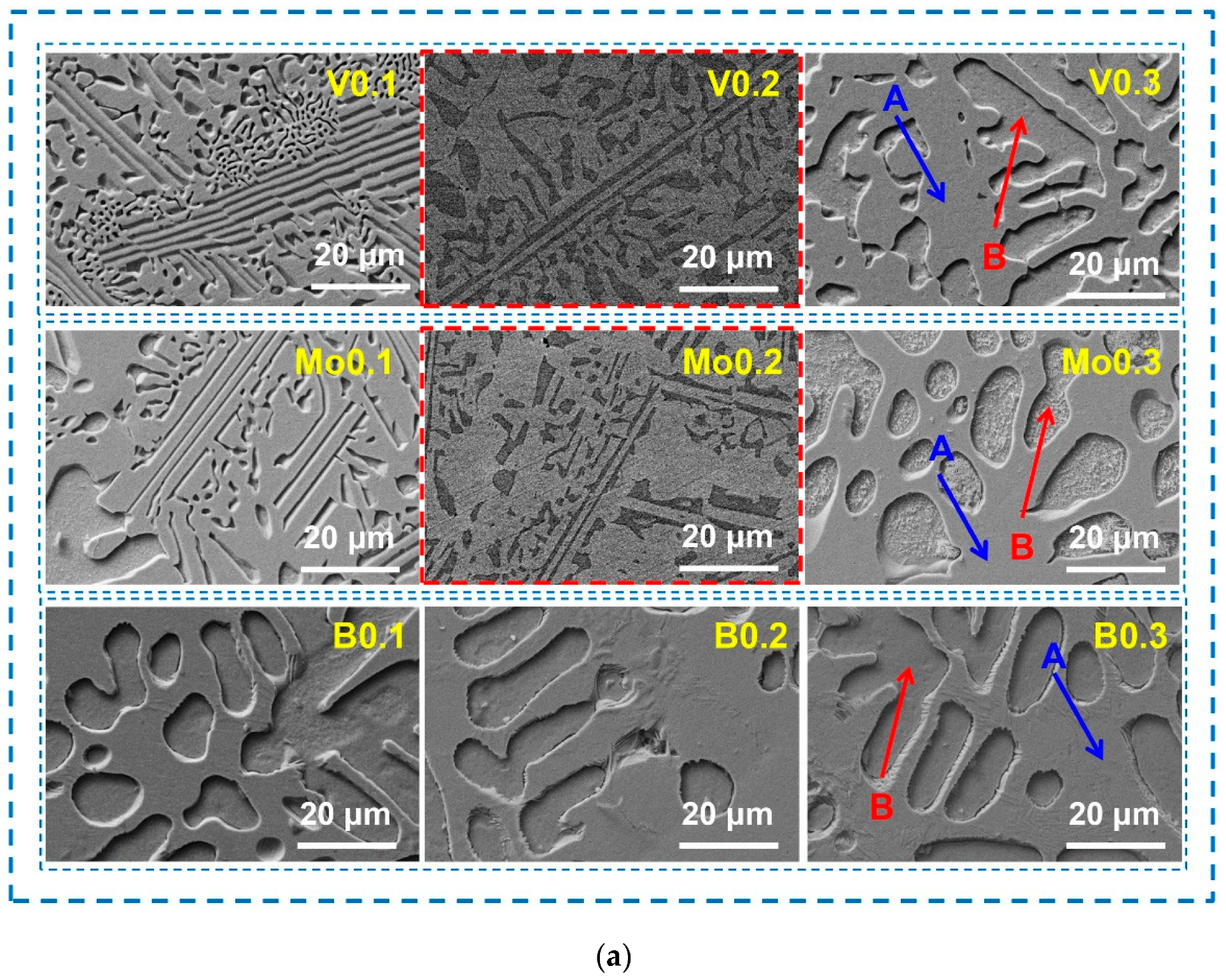
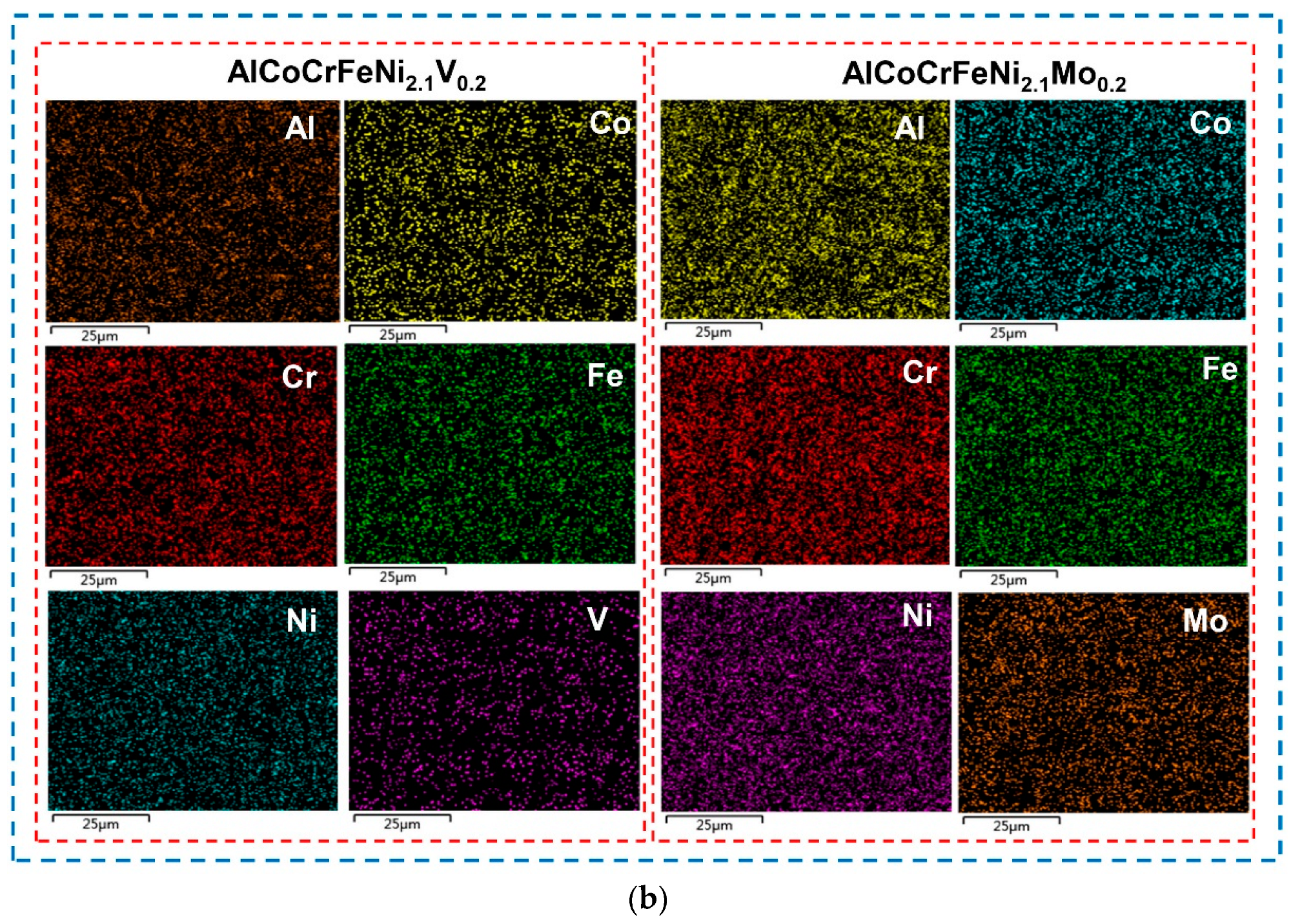

| Alloy | Regions | Al | Co | Cr | Fe | Ni | V/Mo/B |
|---|---|---|---|---|---|---|---|
| V0.1 | Nominal | 16.13 | 16.13 | 16.13 | 16.13 | 33.87 | 1.61 |
| A | 10.06 | 20.00 | 21.49 | 19.26 | 27.72 | 1.46 | |
| B | 21.35 | 15.14 | 12.03 | 13.03 | 37.26 | 1.19 | |
| V0.2 | Nominal | 15.87 | 15.87 | 15.87 | 15.87 | 33.33 | 3.17 |
| A | 10.59 | 19.5 | 19.08 | 18.57 | 29.28 | 2.99 | |
| B | 27.87 | 13.44 | 8.76 | 9.96 | 37.4 | 2.57 | |
| V0.3 | Nominal | 15.63 | 15.63 | 15.63 | 15.63 | 32.81 | 4.69 |
| A | 11.20 | 17.83 | 18.27 | 17.18 | 30.22 | 5.30 | |
| B | 22.15 | 14.92 | 11.57 | 12.83 | 34.27 | 4.26 | |
| Mo0.1 | Nominal | 16.13 | 16.13 | 16.13 | 16.13 | 33.87 | 1.61 |
| A | 11.47 | 18.79 | 18.51 | 17.02 | 32.96 | 1.24 | |
| B | 23.50 | 15.50 | 12.81 | 12.17 | 35.41 | 0.62 | |
| Mo0.2 | Nominal | 15.87 | 15.87 | 15.87 | 15.87 | 33.33 | 3.17 |
| A | 11.05 | 17.64 | 18.68 | 18.36 | 30.46 | 3.82 | |
| B | 28.11 | 12.09 | 10.63 | 10.74 | 36.74 | 1.69 | |
| Mo0.3 | Nominal | 15.63 | 15.63 | 15.63 | 15.63 | 32.81 | 4.69 |
| A | 14.26 | 16.55 | 18.02 | 14.37 | 33.81 | 2.99 | |
| B | 24.03 | 20.50 | 14.89 | 17.33 | 14.34 | 8.91 | |
| B0.1 | Nominal | 16.13 | 16.13 | 16.13 | 16.13 | 33.87 | 1.61 |
| A | 12.86 | 17.92 | 17.72 | 19.16 | 32.33 | 0 | |
| B | 14.08 | 15.72 | 32.73 | 14.88 | 22.58 | 0 | |
| B0.2 | Nominal | 15.87 | 15.87 | 15.87 | 15.87 | 33.33 | 3.17 |
| A | 12.94 | 18.33 | 18.69 | 18.90 | 31.14 | 0 | |
| B | 17.83 | 14.81 | 22.83 | 14.10 | 30.43 | 0 | |
| B0.3 | Nominal | 15.63 | 15.63 | 15.63 | 15.63 | 32.81 | 4.69 |
| A | 22.34 | 12.98 | 19.84 | 10.94 | 33.90 | 0 | |
| B | 14.48 | 19.57 | 20.50 | 17.37 | 28.09 | 0 |
| Alloys | Phase Composition | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| Mo0.2 | FCC + BCC | 1346.3 | 24.6 |
| B0.1 | FCC + BCC | 1160.9 | 14.3 |
| AlCoCrFeNi2.1 [13] | FCC + BCC | 944 | 25.6 |
| AlCrFeNi3 [17] | FCC + BCC | 1200 | 10.1 |
| Al0.9CoFeNi2 [18] | FCC + BCC | 1005 | 6.2 |
| Cu40Al20Ti20V20 [19] | FCC + BCC | 1300 | 6.3 |
| Ti0.15AlCoCrFeNi2.1 [20] | FCC + BCC | 1253 | 12.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.-Y.; Zhang, H.-L.; Nong, Z.-S.; Cui, X.; Gu, Z.-H.; Liu, T.; Li, H.-M.; Arzikulov, E. Effect of Alloying on Microstructure and Mechanical Properties of AlCoCrFeNi2.1 Eutectic High-Entropy Alloy. Materials 2024, 17, 4471. https://doi.org/10.3390/ma17184471
Tian X-Y, Zhang H-L, Nong Z-S, Cui X, Gu Z-H, Liu T, Li H-M, Arzikulov E. Effect of Alloying on Microstructure and Mechanical Properties of AlCoCrFeNi2.1 Eutectic High-Entropy Alloy. Materials. 2024; 17(18):4471. https://doi.org/10.3390/ma17184471
Chicago/Turabian StyleTian, Xue-Yao, Hong-Liang Zhang, Zhi-Sheng Nong, Xue Cui, Ze-Hao Gu, Teng Liu, Hong-Mei Li, and Eshkuvat Arzikulov. 2024. "Effect of Alloying on Microstructure and Mechanical Properties of AlCoCrFeNi2.1 Eutectic High-Entropy Alloy" Materials 17, no. 18: 4471. https://doi.org/10.3390/ma17184471







