Diatom-Based Artificial Anode—Uniform Coating of Intrinsic Carbon to Enhance Lithium Storage
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Composites
2.3. Characterization
2.4. Cell Fabrication and Electrochemical Analysis
2.5. Theoretical Calculations
3. Results and Discussion
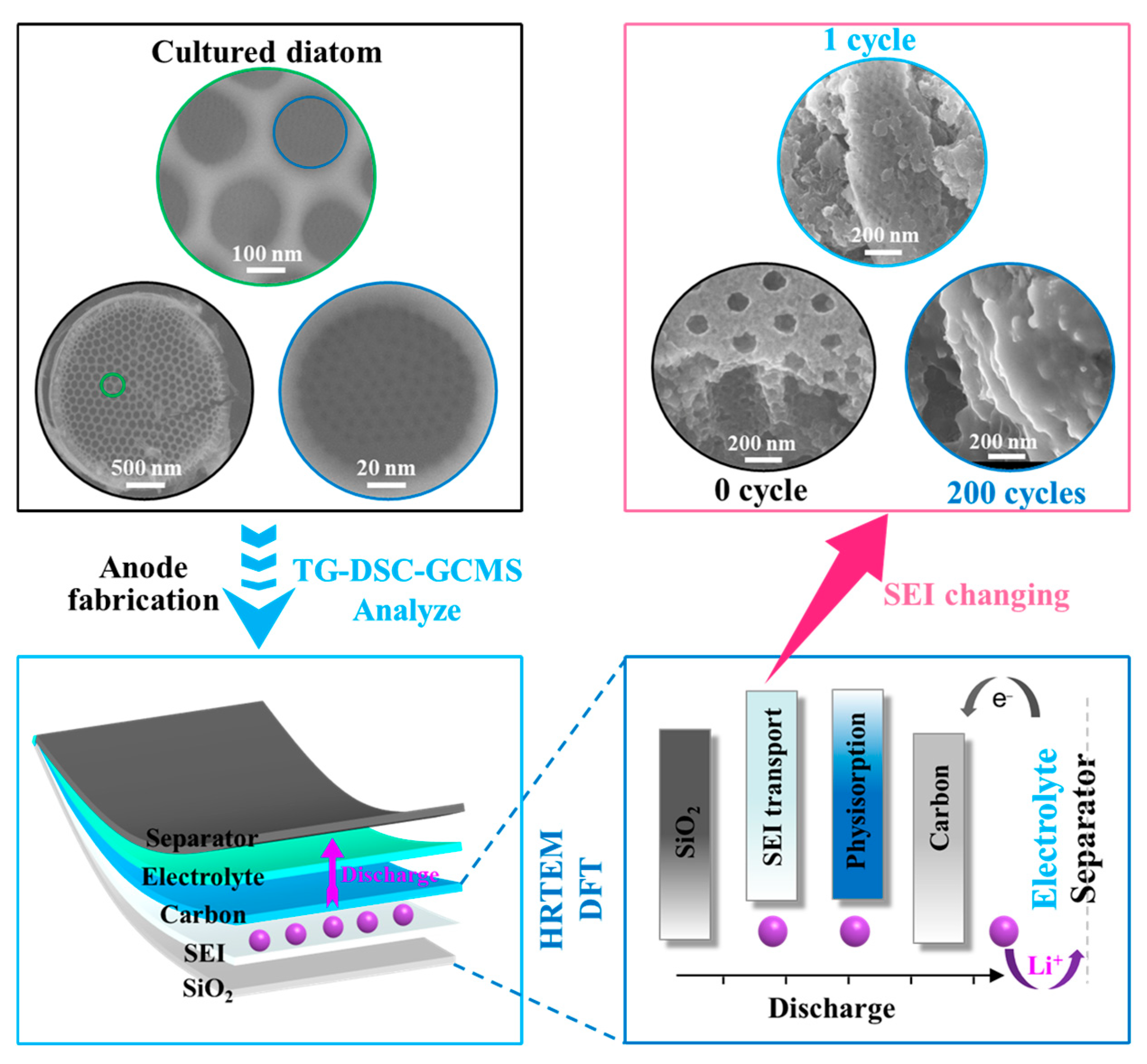
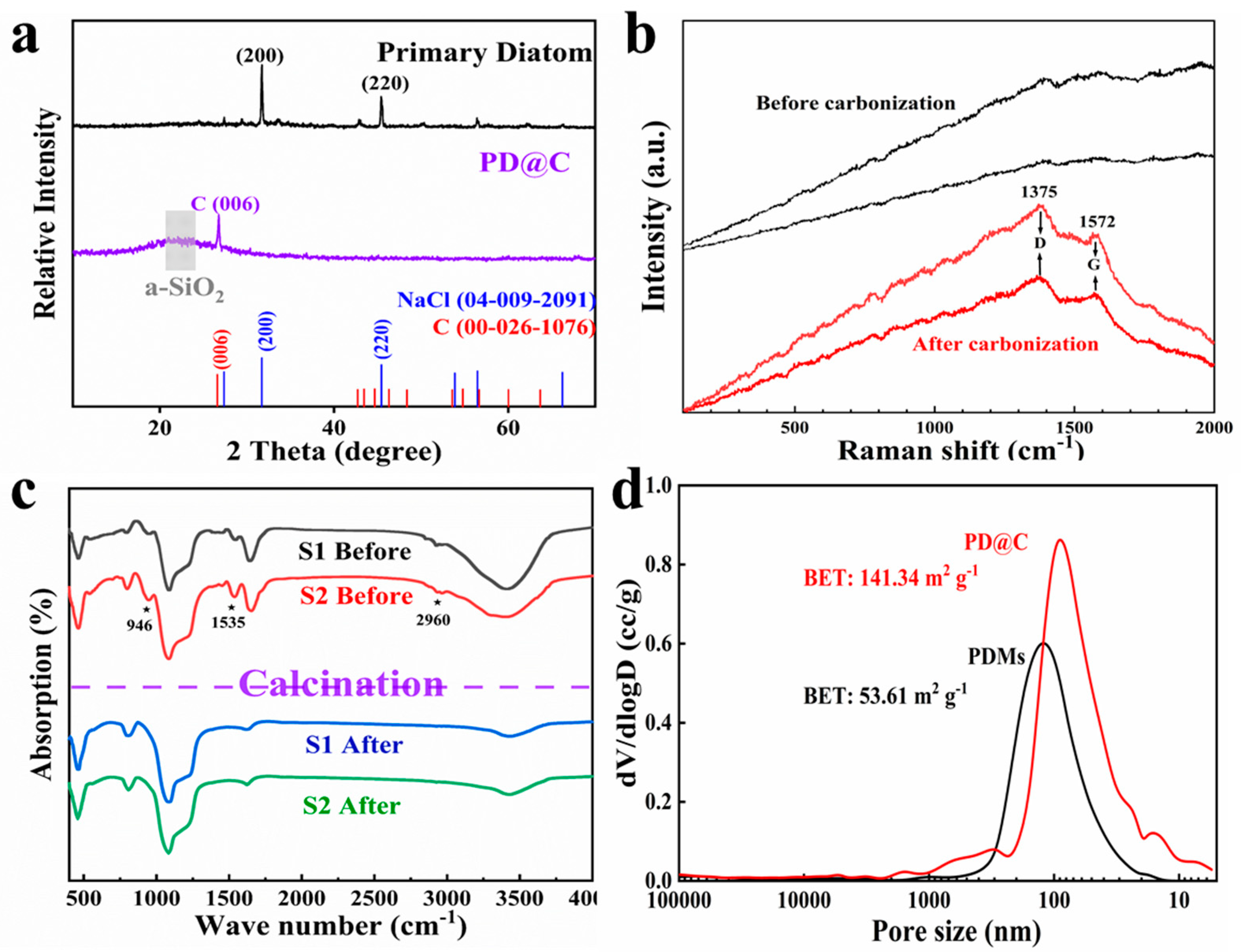
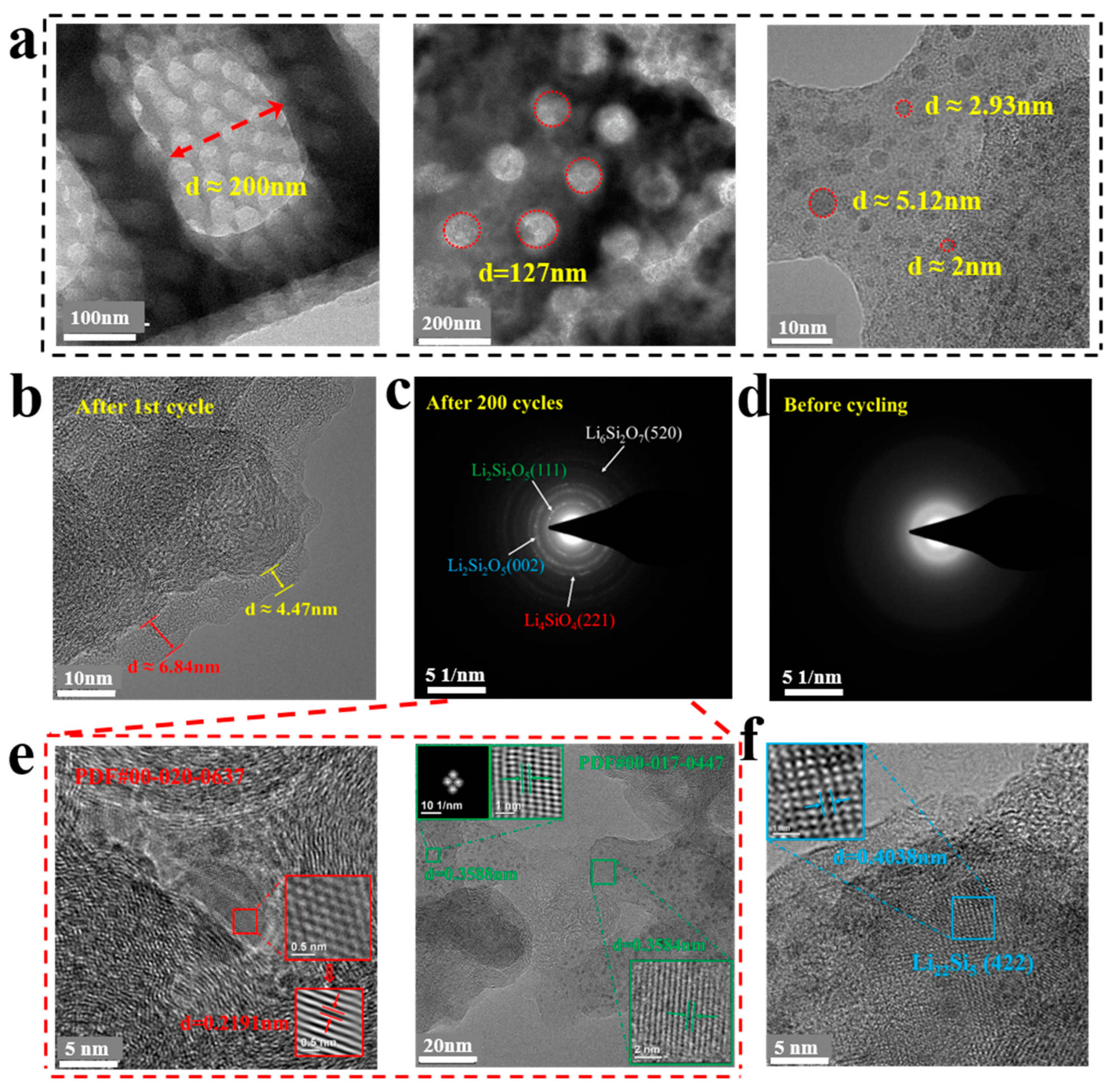
3.1. Characterization of the PD@C Composites
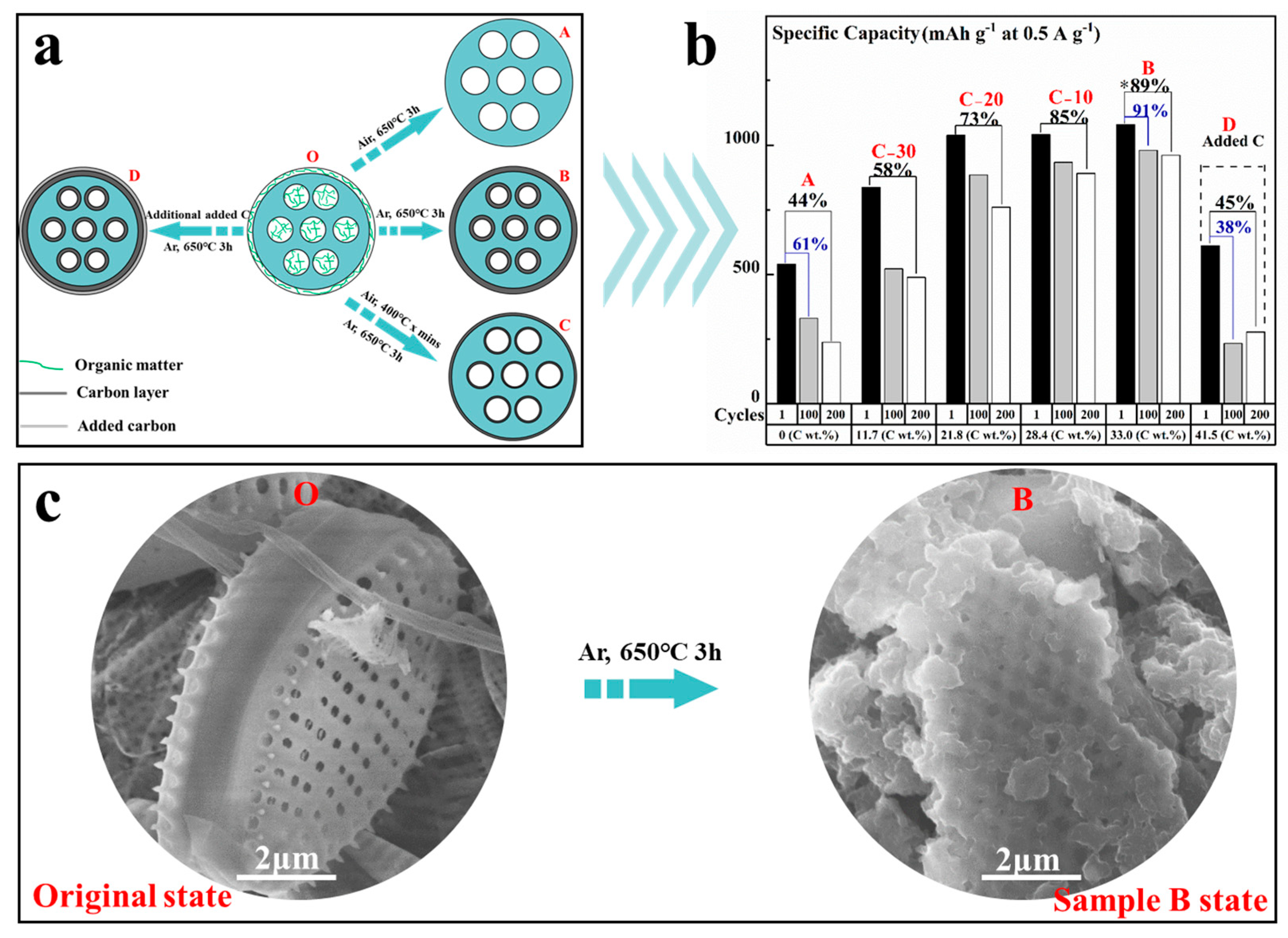
3.1.1. The Formation Process of Carbon Layer
3.1.2. The Composition Analysis of Composites
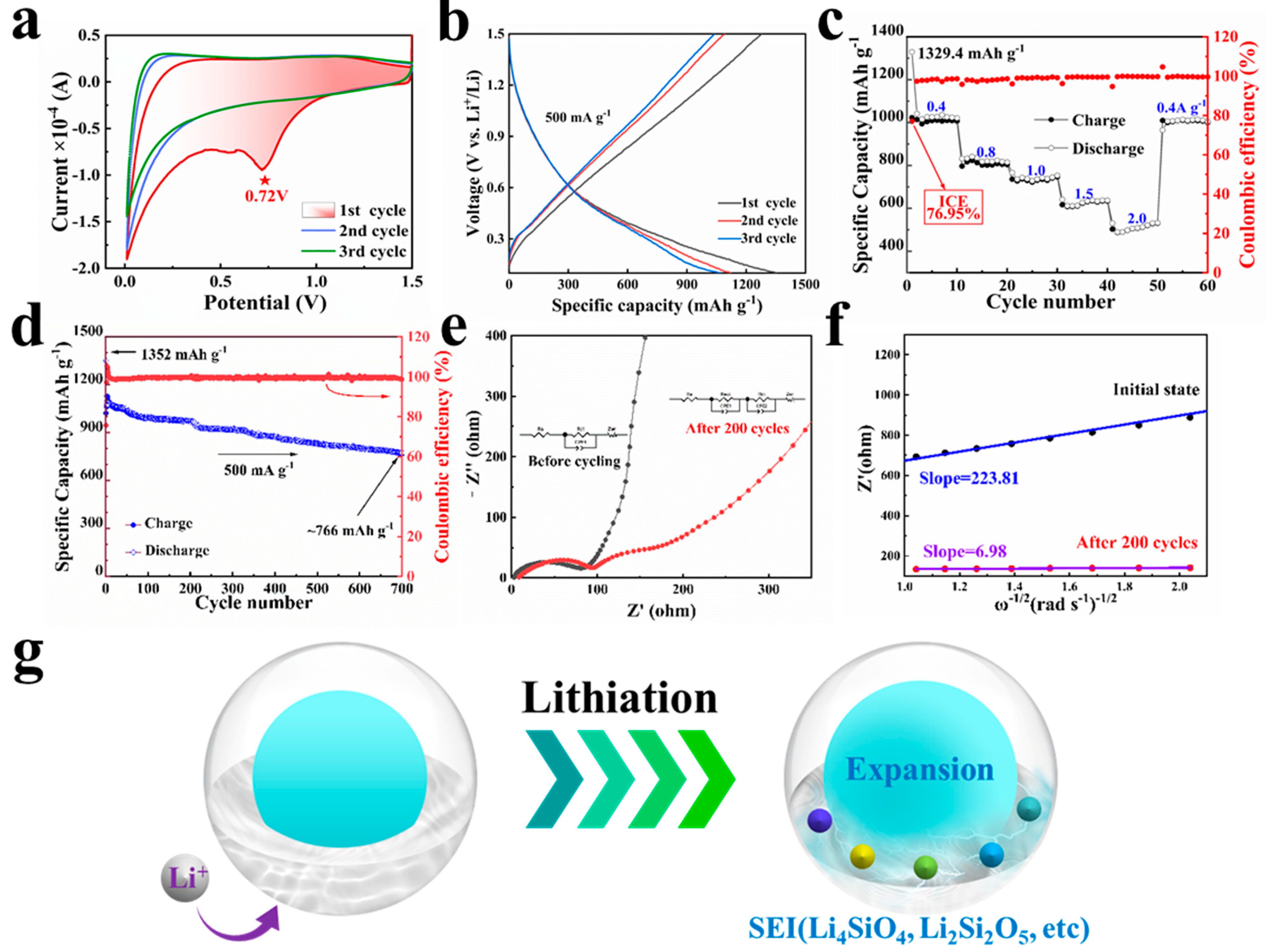
3.2. Anode Application and Its Electrochemical Characterization
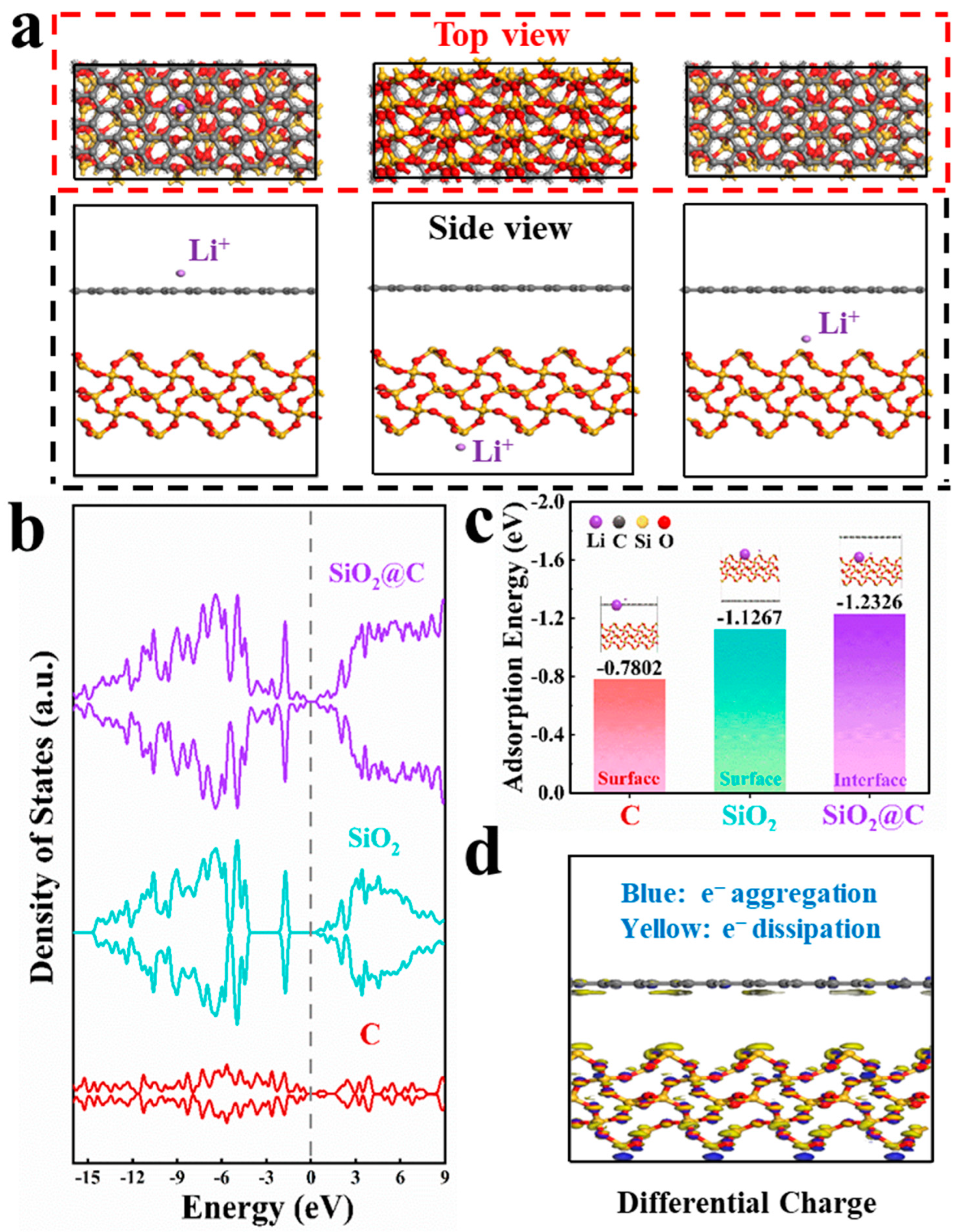
3.3. Theoretical Calculations of C Layer Enhancement Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Ren, Y.; Mu, T.; Yin, X.; Du, C.; Huo, H.; Cheng, X.; Zuo, P.; Yin, G. Engineering molecular polymerization for template-free SiOx/C hollow spheres as ultrastable anodes in Lithium-ion batteries. Adv. Funct. Mater. 2021, 31, 2101145. [Google Scholar] [CrossRef]
- Zhang, K.; Mao, H.; Gu, X.; Song, C.; Yang, J.; Qian, Y. ZIF-Derived Cobalt-Containing N-Doped Carbon-Coated SiOx Nanoparticles for Superior Lithium Storage. ACS Appl. Mater. Interfaces 2020, 12, 7206–7211. [Google Scholar] [CrossRef]
- Gong, D.; Sun, L.L.; Li, X.H.; Zhang, W.Q.; Zhang, D.Y.; Cai, J. Micro/Nanofabrication, Assembly, and Actuation Based onMicroorganisms: Recent Advances and Perspectives. Small Struct. 2023, 4, 2200356. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.A.; Shin, M.; Juang, L.J.; Kastrup, C.J.; Go, G.M.; Lee, H. Diatom Frustule Silica Exhibits Superhydrophilicity and Superhemophilicity. ACS Nano 2020, 14, 4755–4766. [Google Scholar] [CrossRef]
- Yu, J.; Luo, J.D.; Zhang, H.; Zhang, Z.; Wei, J.C.; Cai, J.X.; Yang, Z.Y. Two for One: A Biomass Strategy for Simultaneous Synthesis of MnO2 Microcubes and Porous Carbon Microcubes for High Performance Asymmetric Supercapacitors. ACS Sustain. Chem. Eng. 2020, 8, 6333–6342. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, D.; Cai, J. Diatom Frustule Array for Flow-through Enhancement of Fluorescent Signal in a Microfluidic Chip. Micromachines 2021, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Cai, J.; Wang, Z.; Zhang, W.; Xu, J.; Zhang, D.; Gong, D. Facile fabrication of diatomite biosilica-based nasal drug delivery vehicle for enhanced treatment of allergic rhinitis. Colloids Surf. B Biointerfaces 2024, 234, 113715. [Google Scholar] [CrossRef]
- Ragni, R.; Cicco, S.R.; Vona, D.; Farinola, G.M. Multiple Routes to Smart Nanostructured Materials from Diatom Microalgae: A Chemical Perspective. Adv. Mater. 2018, 30, e1704289. [Google Scholar] [CrossRef]
- Aggrey, P.; Nartey, M.; Kan, Y.; Cvjetinovic, J.; Andrews, A.; Salimon, A.I.; Dragnevski, K.I.; Korsunsky, A.M. On the diatomite based nanostructure preserving materia1 synthesis for energy app1ications. RSC Adv. 2021, 11, 31884–31922. [Google Scholar] [CrossRef]
- Blanco, M.V.; Renman, V.; Vullum-Bruer, F.; Svensson, A.M. Nanostructured diatom earth SiO2 negative electrodes with superior electrochemical performance for lithium ion batteries. RSC Adv. 2020, 10, 33490–33498. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, N.; Sun, Y.; Ke, H.; Cheng, H. Application of diatomite as an effective polysulfides adsorbent for lithiumsulfur batteries. J. Energy Chem. 2017, 26, 1267–1275. [Google Scholar] [CrossRef]
- McMillon-Brown, L.; Mariano, M.; Lin, Y.L.; Li, J.; Hashmi, S.M.; Semichaevsky, A.; Rand, B.P.; Taylor, A.D. Light-trapping in polymer solar cells by processing with nanostructured diatomaceous earth. Org. Electron. 2017, 51, 422–427. [Google Scholar] [CrossRef]
- Jeong, S.-G.; Jeon, J.; Chung, O.; Kim, S.; Kim, S. Evaluation of PCM/diatomite composites using exfoliated graphite nanoplatelets (xGnP) to improve thermal properties. J. Therm. Anal. Calorim. 2013, 114, 689–698. [Google Scholar] [CrossRef]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-Based Anodes for Lithium-Ion Batteries: From Fundamentals to Practical Applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Li, A.; Yu, Y.; Chen, X.; Song, H. Three-Dimensional hierarchical porous structures constructed by two-stage MXene-wrapped Si nanoparticles for Li-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 48718–48728. [Google Scholar] [CrossRef]
- Pan, K.; Zou, F.; Canova, M.; Zhu, Y.; Kim, J.-H. Systematic electrochemical characterizations of Si and SiO anodes for high-capacity Li-Ion batteries. J. Power Sources 2019, 413, 20–28. [Google Scholar] [CrossRef]
- Zhang, K.; Du, W.; Qian, Z.; Lin, L.; Gu, X.; Yang, J.; Qian, Y. SiOx embedded in N-doped carbon nanoslices: A scalable synthesis of high-performance anode material for Lithium-ion batteries. Carbon 2021, 178, 202–210. [Google Scholar] [CrossRef]
- Liu, H.; Huangzhang, E.; Sun, C.; Fan, Y.; Ma, Z.; Zhao, X.; Nan, J. SiOx/C composite anode of Lithium-Ion batteries with enhanced performances using multicomponent binders. ACS Omega 2021, 6, 26805–26813. [Google Scholar] [CrossRef]
- Qiang, Z.; Liu, X.; Zou, F.; Cavicchi, K.A.; Zhu, Y.; Vogt, B.D. Bimodal Porous Carbon-Silica Nanocomposites for Li-Ion Batteries. J. Phys. Chem. C 2017, 121, 16702–16709. [Google Scholar] [CrossRef]
- Gu, Z.; Xia, X.; Liu, C.; Hu, X.; Chen, Y.; Wang, Z.; Liu, H. Yolk structure of porous C/SiO2/C composites as anode for lithium-ion batteries with quickly activated SiO2. J. Alloys Compd. 2018, 757, 265–272. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; Lv, W.; Li, L.; Sun, Z. Towards a low carbon process for lithium recovery from spent lithium-ion batteries by a carbon conversion control strategy of carbothermic reduction. Green Chem. 2022, 24, 9734–9744. [Google Scholar] [CrossRef]
- Song, Z.; Chen, S.; Zhao, Y.; Xue, S.; Qian, G.; Fang, J.; Zhang, T.; Long, C.; Yang, L.; Pan, F. Constructing a Resilient Hierarchical Conductive Network to Promote Cycling Stability of SiOx Anode via Binder Design. Small 2021, 17, 2102256. [Google Scholar] [CrossRef] [PubMed]
- Aberoumand, S.; Dubal, D.; Woodfield, P.; Parsa, S.M.; Mahale, K.; Pham, H.D.; Tung, T.; Nguyen, H.-Q.; Dao, D.V. Enhancement in vanadium redox flow battery performance using reduced graphene oxide nanofluid electrolyte. J. Energy Storage 2023, 72, 108343. [Google Scholar] [CrossRef]
- Parsa, S.M.; Norozpour, F.; Shoeibi, S.; Shahsavar, A.; Aberoumand, S.; Afrand, M.; Said, Z.; Karimi, N. Lithium-ion battery thermal management via advanced cooling parameters: State-of-the-art review on application of machine learning with exergy, economic and environmental analysis. J. Taiwan Inst. Chem. Eng. 2023, 148, 104854. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Liu, S.; Cui, F.; Luo, J.; Wang, Y.; Zhang, S.; Zhang, C.; Yang, X. Cultured diatoms suitable for the advanced anode of lithium ion batteries. ACS Sustain. Chem. Eng. 2021, 9, 844–852. [Google Scholar] [CrossRef]
- Qian, G.; Li, Y.; Chen, H.; Xie, L.; Liu, T.; Yang, N.; Song, Y.; Lin, C.; Cheng, J.; Nakashima, N.; et al. Revealing the aging process of solid electrolyte interphase on SiOx anode. Nat. Commun. 2023, 14, 6048. [Google Scholar] [CrossRef]
- Askaruly, K.; Yeleuov, M.; Taurbekov, A.; Sarsembayeva, B.; Tolynbekov, A.; Zhylybayeva, N.; Azat, S.; Abdisattar, A.; Daulbayev, C. A facile synthesis of graphite-coated amorphous SiO2 from biosources as anode material for libs. Mater. Today Commun. 2023, 34, 105136. [Google Scholar] [CrossRef]
- Singh, S.B.; Dastgheib, S.A. Characteristics of graphene oxide-like materials prepared from different deashed-devolatilized coal chars and comparison with graphite-based graphene oxide, with or without the ultrasonication treatment. Carbon 2024, 228, 119331. [Google Scholar] [CrossRef]
- Kononenko, O.; Brzhezinskaya, M.; Zotov, A.; Korepanov, V.; Levashov, V.; Matveev, V.; Roshchupkin, D. Influence of numerous Moir_e superlattices on transport properties of twisted multilayer graphene. Carbon 2022, 194, 52–61. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Thermally exfoliated graphene oxide for hydrogen storage. Mater. Chem. Phys. 2020, 239, 122102. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkov, M.N.; Stolyarova, D.Y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Diankin, I.D.; et al. Guiding graphene derivatization for covalent immobilization of aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Mishakov, I.V.; Bauman, Y.I.; Shubin, Y.V.; Maksimova, T.A.; Stoyanovskii, V.O.; Gerasimov, E.Y.; Vedyagin, A.A. One-pot functionalization of catalytically derived carbon nanostructures with heteroatoms for toxic-free environment. Appl. Surf. Sci. 2022, 590, 153055. [Google Scholar] [CrossRef]
- Guo, B.; Shu, J.; Wang, Z.; Yang, H.; Shi, L.; Liu, Y.; Chen, L. Electrochemical reduction of nano-SiO2 in hard carbon as anode material for lithium ion batteries. Electrochem. Commun. 2008, 10, 1876–1878. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design. Adv. Sci. 2017, 5, 1700322. [Google Scholar] [CrossRef] [PubMed]
- Wishke, R.; Man, A.; Arad, S. Production of Silver Nanoparticles by the Diatom Phaeodactylum tricornutum; Nanotechnology VIII; International Society for Optics and Photonics: Bellingham WA, USA, 2017; Volume 10248. [Google Scholar]
- Leonardo, S.; Garibo, D.; Fernández-Tejedor, M.; O’sullivan, C.K.; Campàs, M. Addressed immobilization of biofunctionalized diatoms on electrodes by gold electrodeposition. Biofabrication 2017, 9, 015027. [Google Scholar] [CrossRef]
- Rea, I.; Terracciano, M.; Chand, R.; Asekaran, S. Bioengineered silicon diatoms: Adding photonic features to a nanostructured semiconductive material for biomolecular sensing. Nanoscale Res. Lett. 2016, 11, 405. [Google Scholar] [CrossRef]
- Feurtet-Mazel, A.; Mornet, S.; Charron, L.; Mesmer-Dudons, N.; Maury-Brachet, R.; Baudrimont, M. Biosynthesis of gold nanoparticles by the living freshwater diatom Eolimna minima, a species developed in river biofilms. Environ. Sci. Pollut. Res. 2016, 23, 4334–4339. [Google Scholar] [CrossRef]
- Gautam, S.; Kashyap, M.; Gupta, S.; Kumar, V.; Schoefs, B.; Gordon, R.; Jeffryes, C.; Joshi, K.B.; Vinayak, V. Metabolic engineering of TiO2 nanoparticles in Nitzschia palea to form diatom nanotubes: An ingredient for solar cells to produce electricity and biofuel. RSC Adv. 2016, 6, 97276–97284. [Google Scholar] [CrossRef]
- Delalat, B.; Sheppard, V.C.; Ghaemi, S.R.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.-L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted drug delivery using genetically engineered diatom biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef]
- Lang, Y.; del Monte, F.; Collins, L.; Rodriguez, B.J.; Thompson, K.; Dockery, P.; Finn, D.P.; Pandit, A. Functionalization of the living diatom Thalassiosira weissflogii with thiol moieties. Nat. Commun. 2013, 4, 3683. [Google Scholar] [CrossRef] [PubMed]
- Jeffryes, C.; Gutu, T.; Jiao, J.; Rorrer, G.L. Two-stage photobioreactor process for the metabolic insertion of nanostructured germanium into the silica microstructure of the diatom Pinnularia sp. Mater. Sci. Eng. C 2008, 28, 107–118. [Google Scholar] [CrossRef]
- Jeffryes, C.; Gutu, T.; Jiao, J. Metabolic insertion of nanostructured TiO2 into the patterned biosilica of the diatom Pinnularia sp. by a two-stage bioreactor cultivation process. Acs Nano 2008, 2, 2103–2112. [Google Scholar] [CrossRef]
- Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A new energy storage anode material for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6895–6899. [Google Scholar] [CrossRef]
- Yang, X.; Huang, H.; Li, Z.; Zhong, M.; Zhang, G.; Wu, D. Preparation and lithium-storage performance of carbon/silica composite with a unique porous bicontinuous nanostructure. Carbon 2014, 77, 275–280. [Google Scholar] [CrossRef]
- Kim, J.M.; Huh, Y.S.; Kim, H.J. Hollow triple-shelled SiO2/TiO2/polypyrrole nanospheres for enhanced lithium storage capability. Chem. Eng. J. 2014, 237, 380–386. [Google Scholar] [CrossRef]
- Liang, Y.; Cai, L.; Chen, L.; Lin, X.; Fu, R.; Zhang, M.; Wu, D. Silica nanonetwork confined in nitrogen-doped ordered mesoporous carbon framework for high-performance lithium-ion battery anodes. Nanoscale 2015, 7, 3971–3975. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Y.; Xu, C. Ultrafine Nickel-Nanoparticle-Enabled SiO2 Hierarchical Hollow Spheres for High-Performance Lithium Storage. Adv. Funct. Mater. 2018, 28, 1704561. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, X.; Yan, C. Titanosilicate Derived SiO2/TiO2@C Nanosheets with Highly Distributed TiO2 Nanoparticles in SiO2 Matrix as Robust Lithium Ion Battery Anode. ACS Appl. Mater. Interfaces 2018, 10, 44463–44471. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Mu, D.; Wu, B. Microsphere-Like SiO2/MXene Hybrid Material Enabling High Performance Anode for Lithium Ion Batteries. Small 2019, 16, 1905430. [Google Scholar] [CrossRef]
- Norberg, A.N.; Wagner, N.P.; Kaland, H.; Vullum-Bruer, F.; Svensson, A.M. Silica from diatom frustules as anode material for Li-ion batteries. RSC Adv. 2019, 9, 41228–41239. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Cai, J.; Gong, D.; Guo, A.; Wang, J.-K.; Zhang, J. Diatom-Based Artificial Anode—Uniform Coating of Intrinsic Carbon to Enhance Lithium Storage. Materials 2024, 17, 4473. https://doi.org/10.3390/ma17184473
Luo J, Cai J, Gong D, Guo A, Wang J-K, Zhang J. Diatom-Based Artificial Anode—Uniform Coating of Intrinsic Carbon to Enhance Lithium Storage. Materials. 2024; 17(18):4473. https://doi.org/10.3390/ma17184473
Chicago/Turabian StyleLuo, Junlong, Jun Cai, De Gong, Aoping Guo, Jaw-Kai Wang, and Jiangtao Zhang. 2024. "Diatom-Based Artificial Anode—Uniform Coating of Intrinsic Carbon to Enhance Lithium Storage" Materials 17, no. 18: 4473. https://doi.org/10.3390/ma17184473





