Distribution of Rare Earth Elements in Ash from Lignite Combustion in Polish Power Plants
Abstract
:1. Introduction
2. Selection of Samples and Research Methodology
3. Results
3.1. Fuel Properties
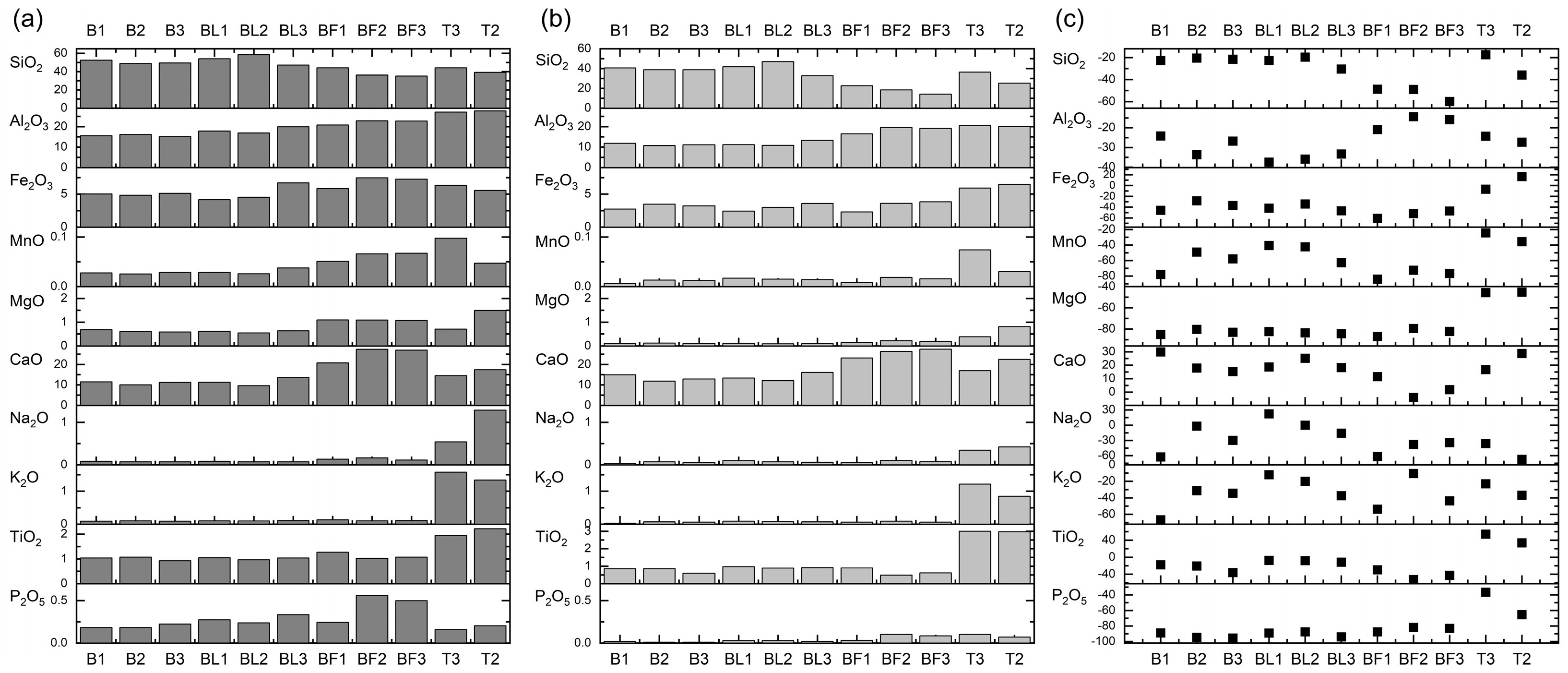
3.2. Chemical Composition
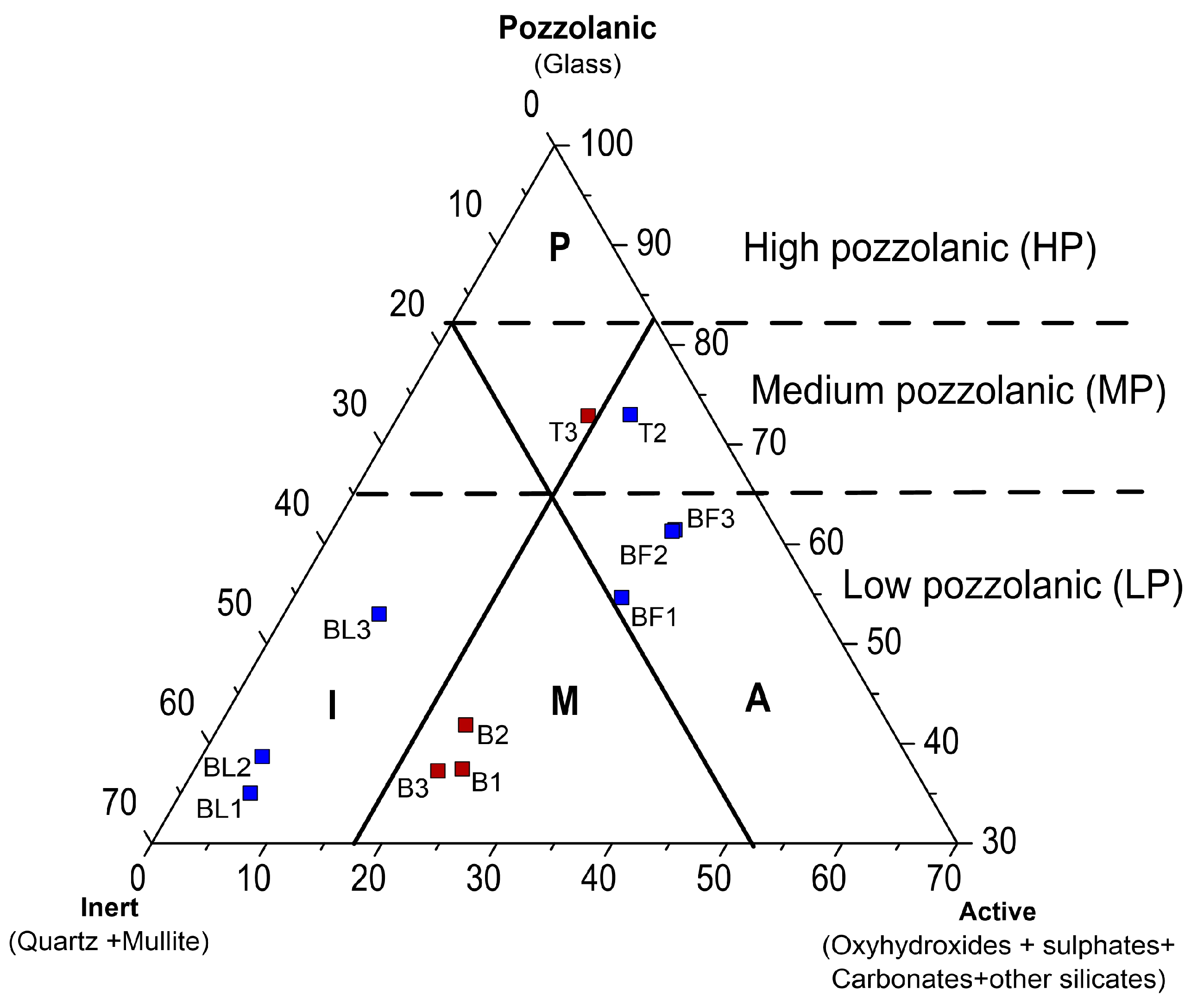
3.3. Mineral Composition of Ash Samples
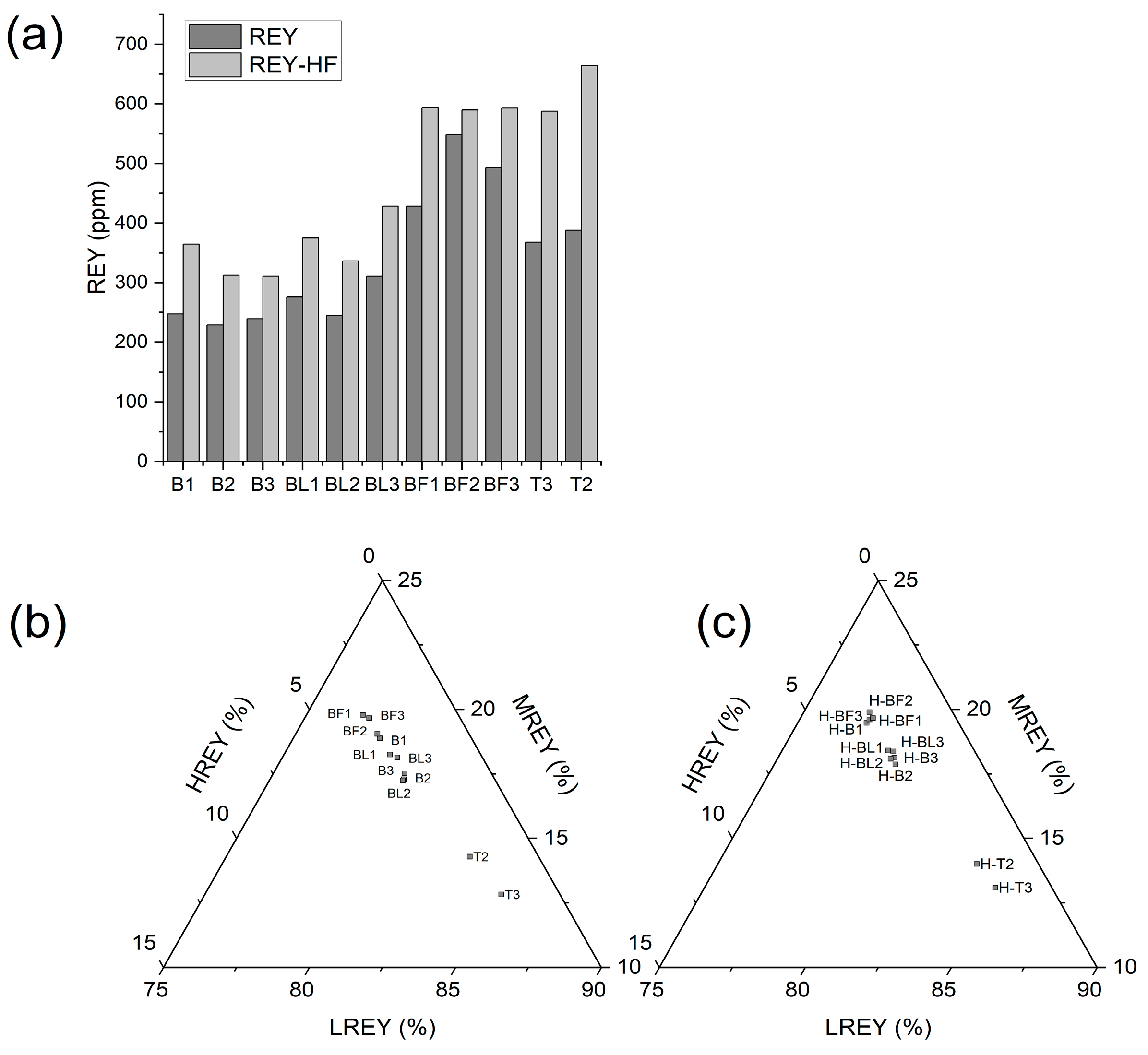
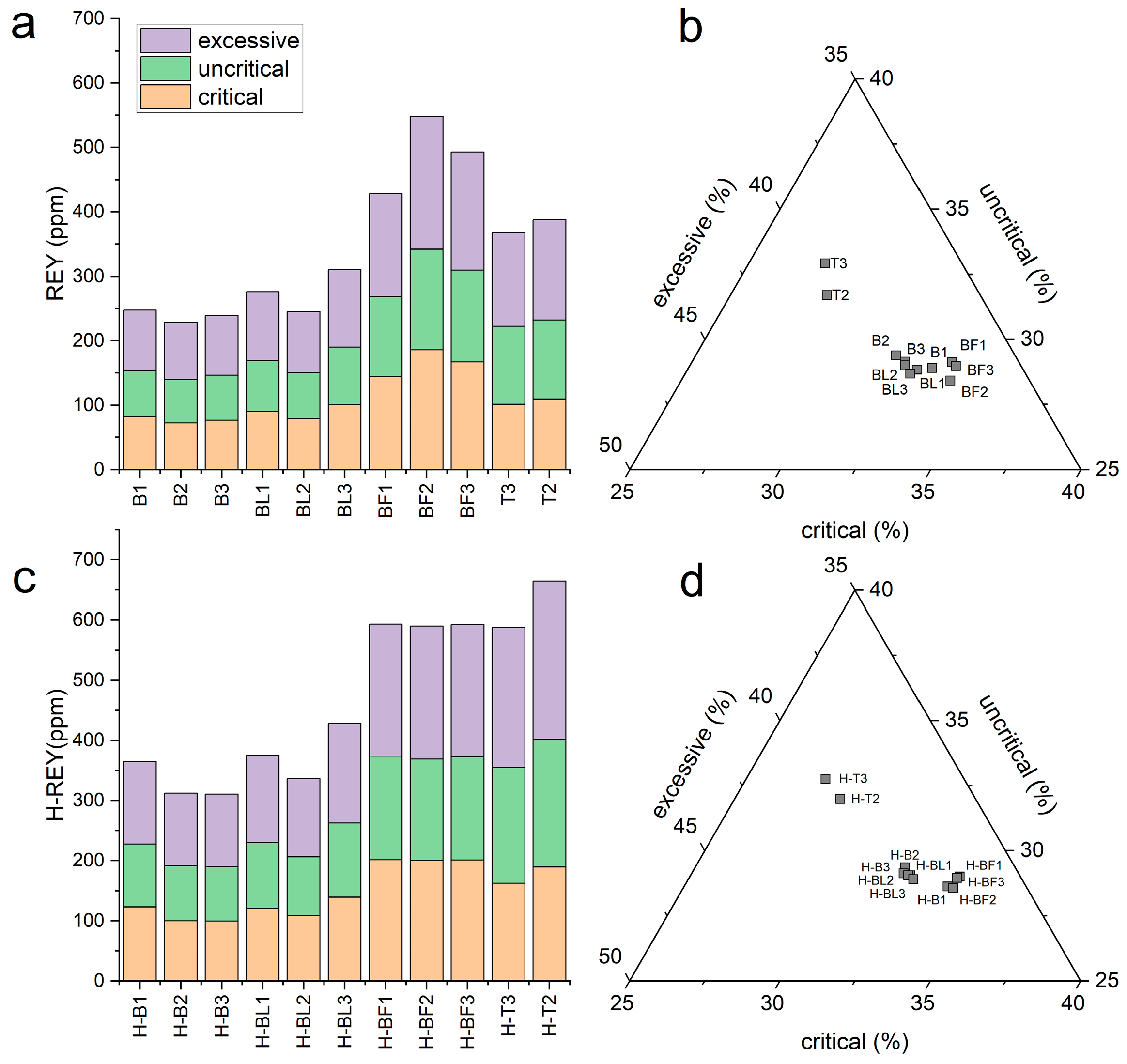
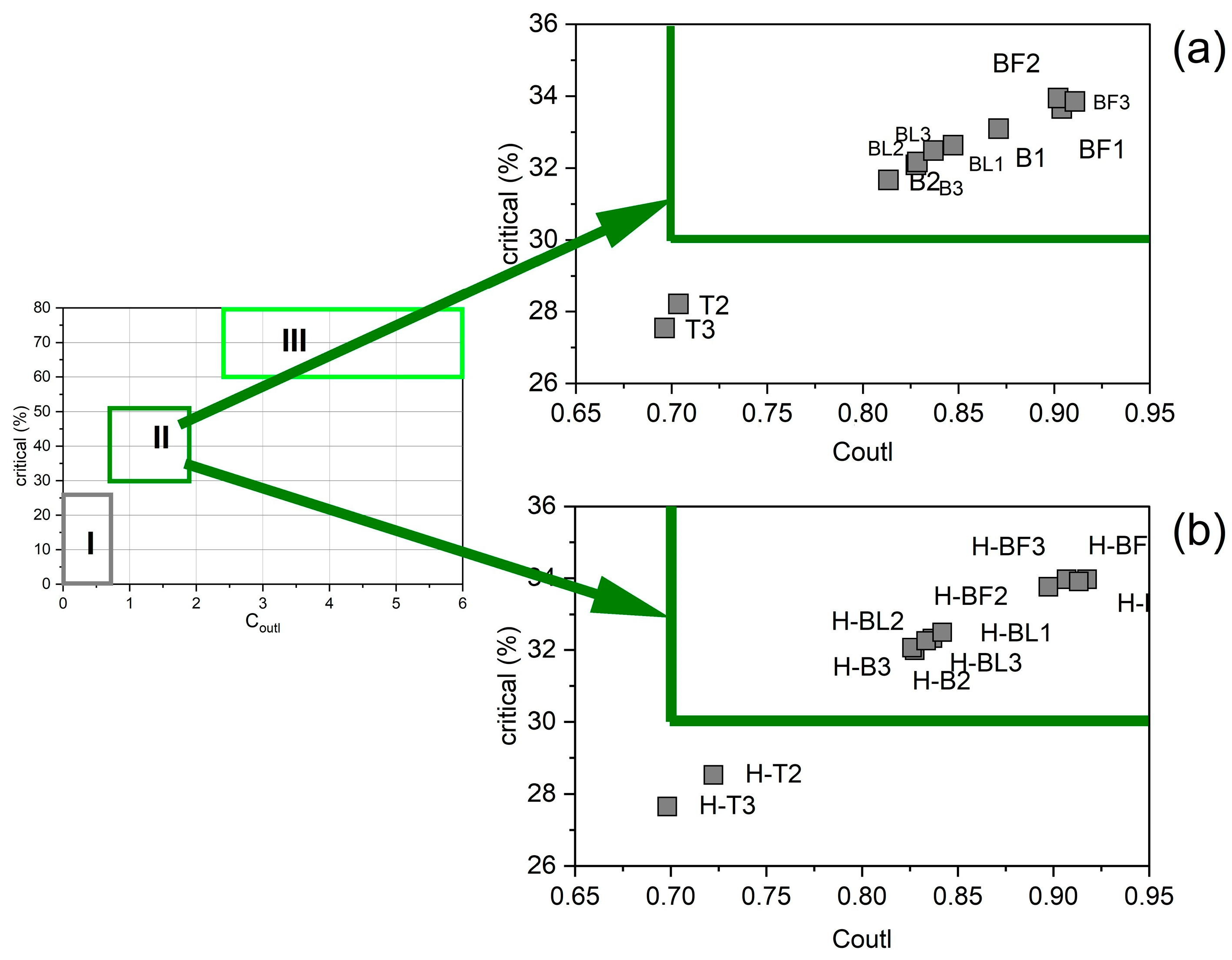
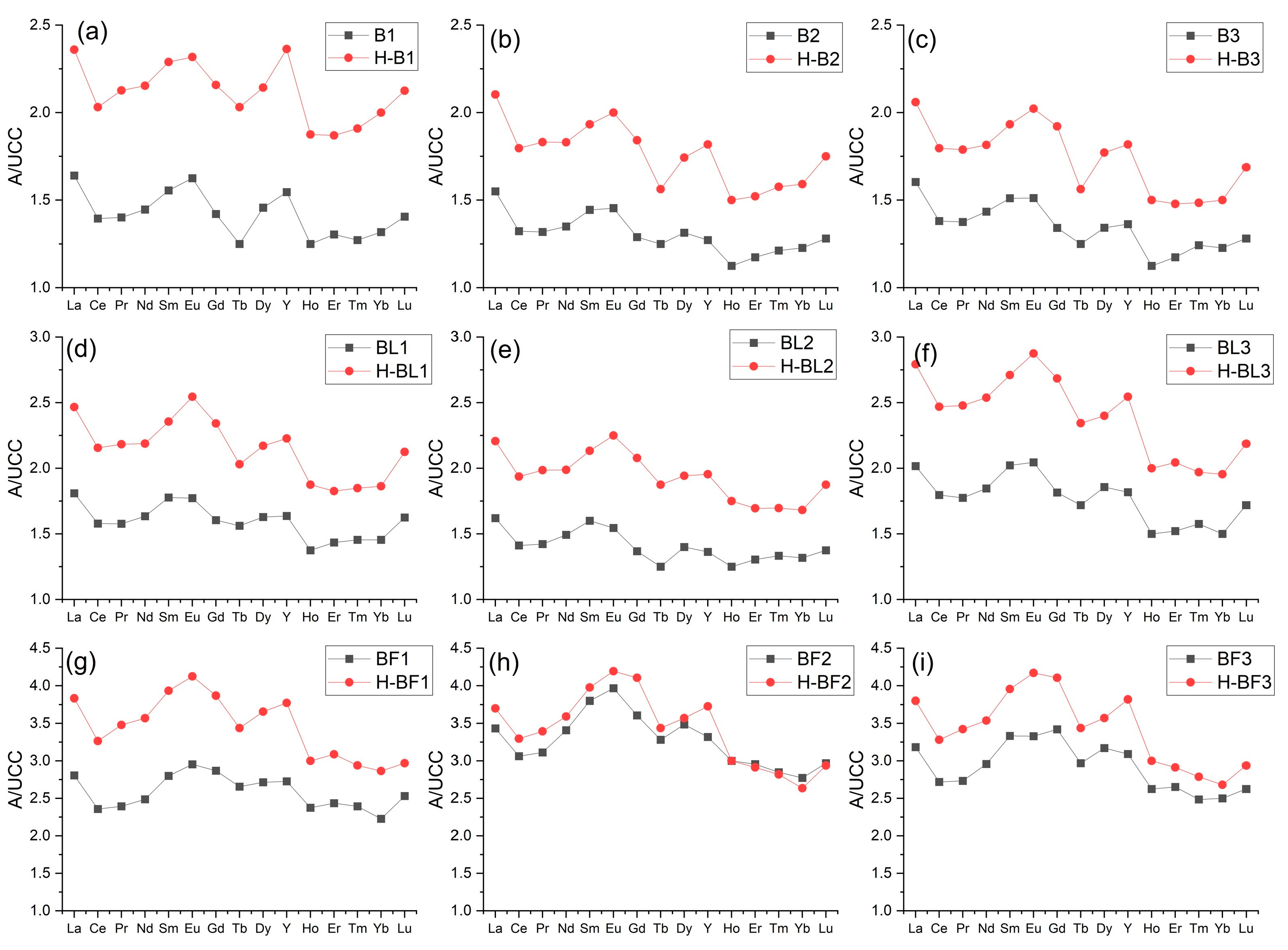
3.4. Rare Earth Elements and Yttrium (REY) in Ash Samples
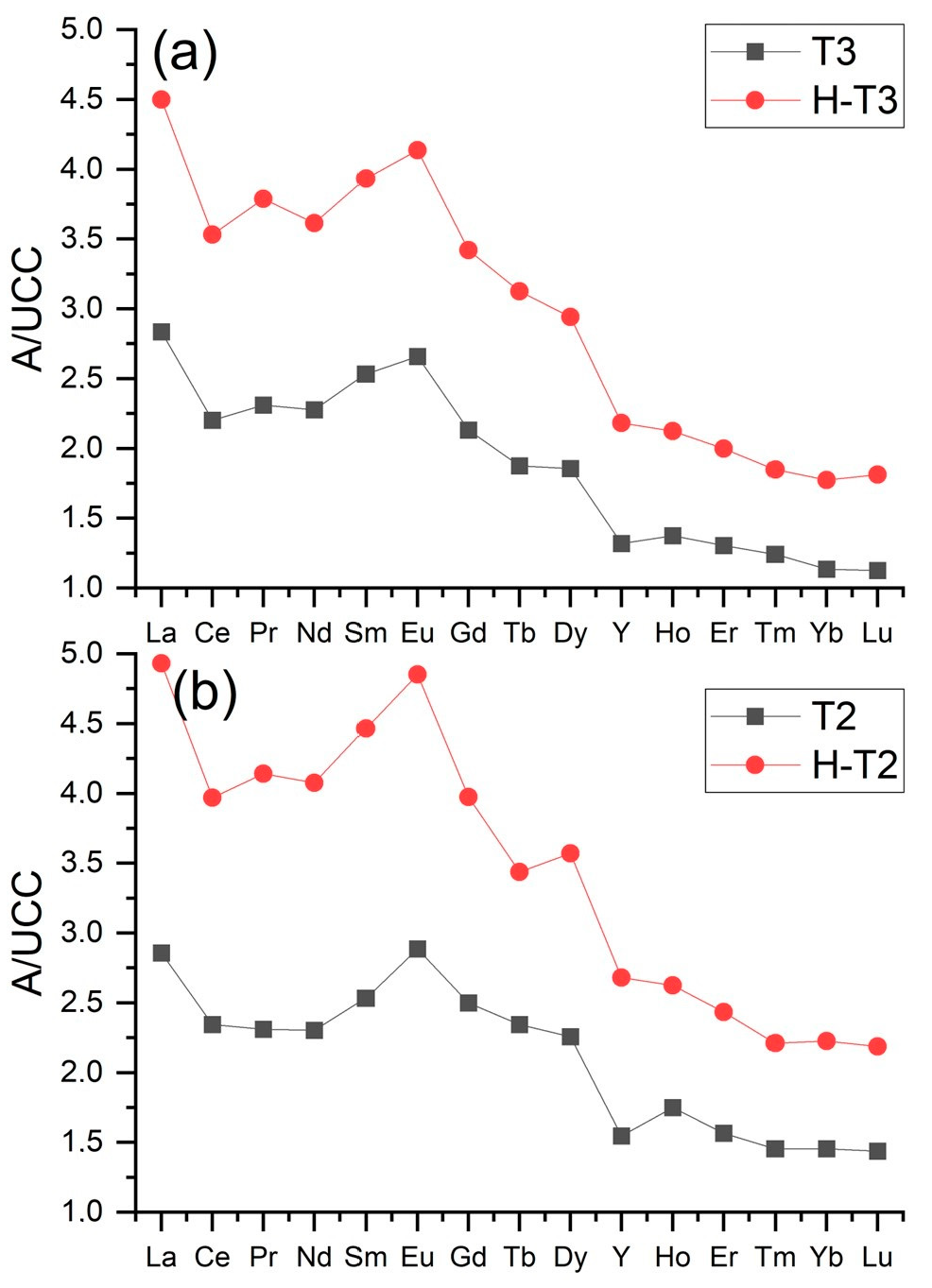
3.5. Characterization of the Residue after Ash Dissolution in 4% HF
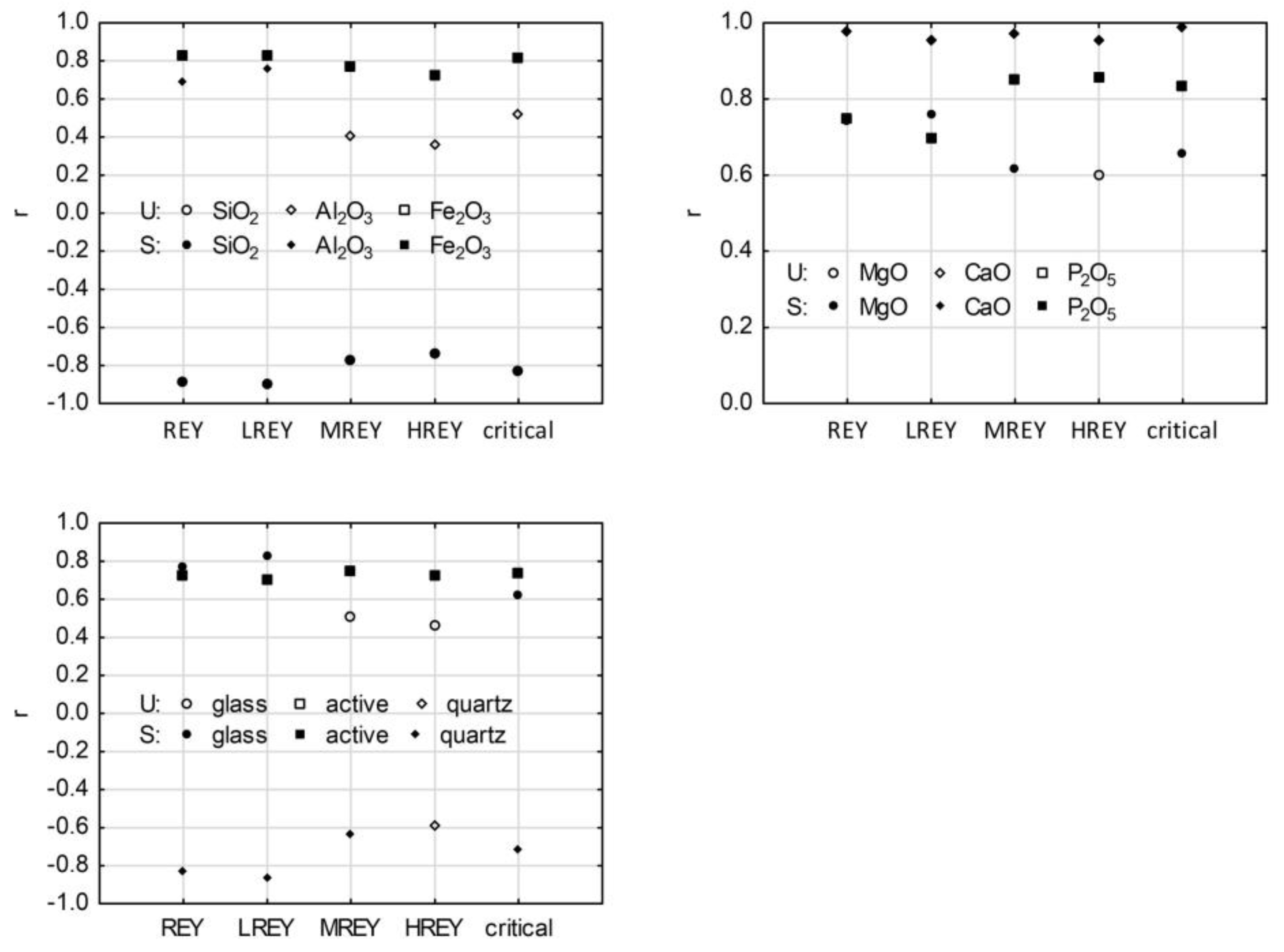
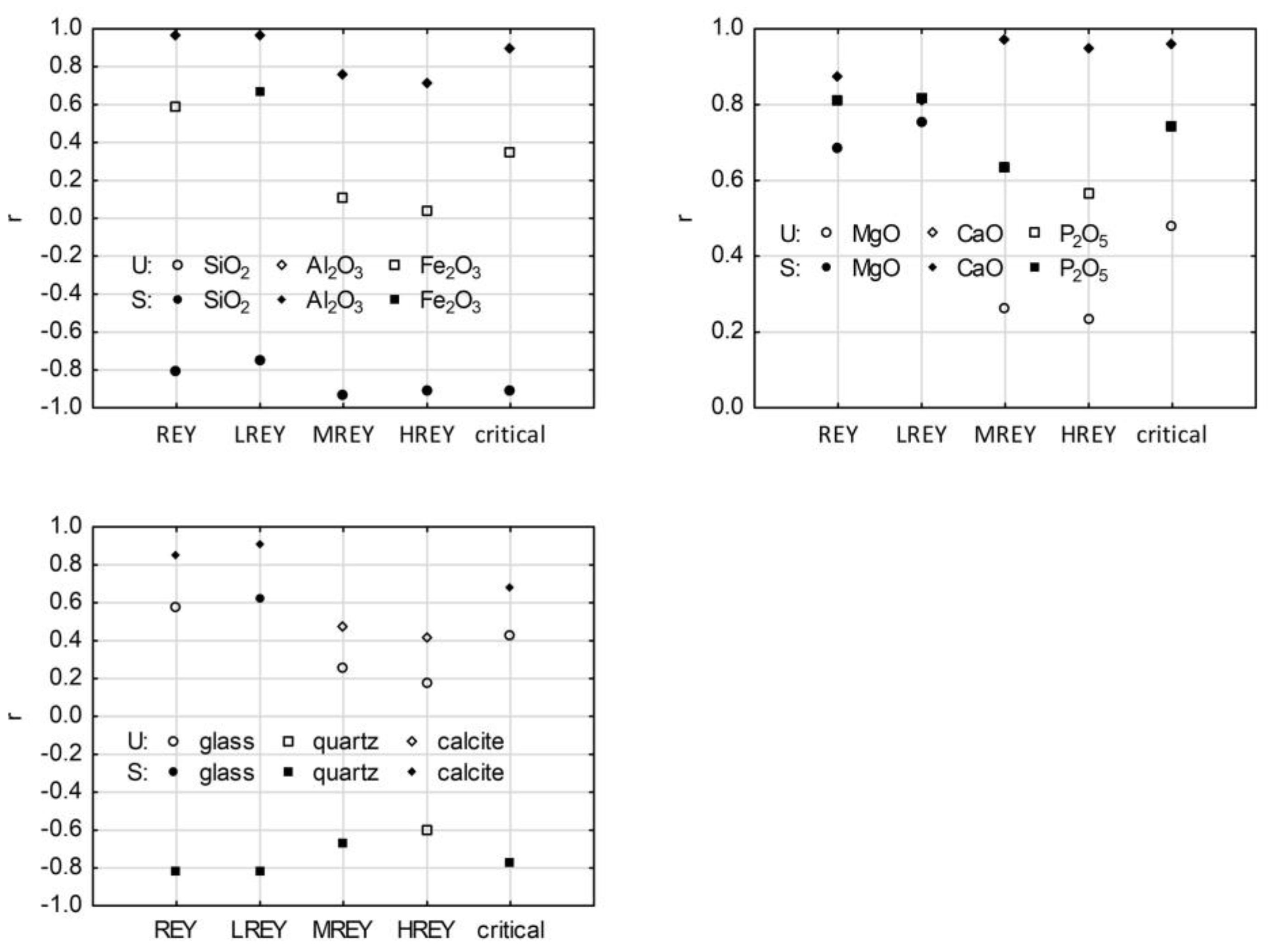
3.6. REY and Its Relation to Main Chemical Components in Raw Ash Samples
3.7. REY and Its Relation to Phase Composition in Raw Ash Samples
3.8. REY and Main Chemical Components in Residue after Ash Dissolution in 4% HF
3.9. REY and Its Relation to Mineral Components in Residue after Ash Dissolution in 4% HF
4. Conclusions
- The REY distribution showed that FA from the electrofilters of Bełchatów contained the highest amounts of these elements (428–548 ppm), and the lowest were found in BA (228–247 ppm). The REY in FA from the flue gas duct was in amounts between the BA and the FA from the electrofilter (245–310 ppm). Ash from Turów did not show any differentiation because it occurred in amounts of 367 and 388 ppm (BA and FA, respectively).
- In the residue, after ash treatment in 4% HF, a high removal efficiency of almost all chemical components was usually observed compared to the raw samples. The exception was CaO because it enriched all ashes. The best presented high removal efficiency was for the main components: (i) SiO2–FA from electrofilters (ca. 50%), (ii) Al2O3–BA and FA from the gas channel (ca. 30%), (iii) Fe2O3–all ashes from power plant Bełchatów (ca. 40%), as well as glass from ashes from power plant Bełchatów (usually < 10%).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bau, M. Controls of the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Seredin, V.V. A new method for primary evaluation of the outlook for rare earth element ores. Geol. Ore Depos. 2010, 52, 428–433. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare Earth Elements: Industrial Applications and Economic Dependency of Europe. In Proceedings of the International Conference on Applied Economics (ICOAE), Kazan, Russia, 2–4 July 2015; Volume 24, pp. 126–135. [Google Scholar] [CrossRef]
- Opare, E.O.; Struhs, E.; Mirkouei, A.A. A comparative state-of-technology review and future directions for rare earth element separation. Renew. Sustain. Energy Rev. 2021, 143, 110917. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs; Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023; Available online: https://data.europa.eu/doi/10.2873/725585 (accessed on 15 May 2024).
- Radwanek-Bąk, B. Zasoby kopalin Polski w aspekcie oceny surowcow krytycznych Unii Europejskiej. Gospod. Surowcami Miner. Miner. Resour. Manag. 2011, 27, 5–19. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-79953805090&origin=inward (accessed on 15 May 2024).
- Smakowski, T. Surowce mineralne—Krytyczne czy deficytowe dla gospodarki UE i Polski. Zesz. Nauk. Inst. Gospod. Surowcami. Miner. Energią Pol. Akad. Nauk 2011, 81, 59–68. [Google Scholar]
- Mayfield, D.B.; Lewis, A.S. Environmental review of coal ash as a resource for rare earth and strategic elements. In Proceedings of the World of Coal Ash Conference (WOCA), Lexington, KY, USA, 22–25 April 2013. [Google Scholar]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef]
- Jarosiński, A. Możliwości pozyskiwania metali ziem rzadkich w Polsce. Zesz. Nauk. Inst. Gospod. Surowcami Miner. Energią Pol Akad. Nauk 2016, 92, 75–88. [Google Scholar]
- Wang, J.; Guo, M.; Liu, M.; Wei, X. Long-term outlook for global rare earth production. Resour. Policy 2020, 65, 101569. [Google Scholar] [CrossRef]
- Liu, S.L.; Fan, H.R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.F.; Li, X.C. Global rare earth elements projects: New developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Woźniak, J.; Gurdziel, M. Rare earth elements in deposited hard coal fly ash in Poland. E3S Web Conf. 2021, 278, 01016. [Google Scholar] [CrossRef]
- Kowalski, P.; Legendre, C. Raw materials critical for the green transition. OECD Trade Policy Pap. 2023, 269, 1–93. [Google Scholar] [CrossRef]
- Kopiński, D. Afrykańskie Surowce Krytyczne i Bezpieczeństwo Ekonomiczne Unii Europejskiej; Polski Instytut Ekonomiczny: Warszawa, Poland, 2023. [Google Scholar]
- Hund, K.; La Porta, D.; Fabregas, T.P.; Laing, T.; Drexhage, J. Minerals for Climate Action: The Mineral Intensity of the Clean Energy Transition; World Bank Publications the World Bank Group: Washington, DC, USA, 2020. [Google Scholar]
- Calus-Moszko, J.; Bialecka, B. Analysis of the possibilities of rare earth elements obtaining from coal and fly ash. Gospod. Surowcami Miner. Miner. Resour. Manag. 2013, 29, 68–80. [Google Scholar] [CrossRef]
- Kashiwakura, S.; Kumagai, Y.; Kubo, H.; Wagatsuma, K. Dissolution of rare earth elements from coal fly ash particles in a dilute H2SO4 solvent. Open J. Phys. Chem. 2013, 3, 69–75. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S.; Sun, Y.; Chekryzhov, I.Y. Coal deposits as promising sources of rare metals for alternative power and energy-efficient technologies. Appl. Geochem. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Henke, K.R.; Hood, M.M.; Eble, C.F.; Honaker, R.Q.; Zhang, W.; Qian, D. Notes on the potential for the concentration of rare earth elements and yttrium in coal combustion fly ash. Minerals 2015, 5, 356–366. [Google Scholar] [CrossRef]
- Hower, J.C.; Granite, E.J.; Mayfield, D.B.; Lewis, A.S.; Finkelman, R.B. Notes on contributions to the science of rare earth element enrichment in coal and coal combustion byproducts. Minerals 2016, 6, 32. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Henke, K.R.; Graham, U.M.; Hood, M.M.; Joshi, P.; Preda, D.V. Ponded and landfilled fly ash as a source of rare earth elements from a Kentucky power plant. Coal Comb. Gasific. Prod. 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Blissett, R.S.; Smalley, N.; Rowson, N.A. An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content. Fuel 2014, 119, 236–239. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Komorek, J.; Lewandowska, M.; Nowak, J.; Białecka, B.; Całusz-Moszko, J.; Klupa, A. Ashes from bituminous coal burning in fluidized bed boilers as a potential source of rare earth elements. Gospod. Surowcami Miner. Miner. Resour. Manag. 2018, 34, 21–36. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Komorek, J.; Lewandowska, M. The high temperature ashes (HTA) from bituminous coal combustion as a potential resource of rare earth elements. Gospod. Surowcami Miner. Miner. Resour. Manag. 2018, 34, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Komorek, J.; Białecka, B.; Nowak, J.; Klupa, A. Assessment of the potential of polish fly ashes as a source of rare earth elements. Ore Geol. Rev. 2020, 124, 103638. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Komorek, J.; Kokowska-Pawłowska, M.; Nowak, J. Distribution of rare-earth elements in ashes produced in the coal combustion process from power boilers. Energies 2023, 16, 2696. [Google Scholar] [CrossRef]
- Lin, R.; Soong, Y.; Granite, E.J. Evaluation of trace elements in U.S. coals using the USGS COALQUAL database version 3.0. Part I: Rare earth elements and yttrium (REY). Int. J. Coal Geol. 2018, 192, 1–13. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, C.; Tang, M.; Cao, S.; Liu, C.; Zhang, N.; Wen, M.; Luo, Y.; Hu, T.; Ji, W. Study on the modes of occurrence of rare earth elements in coal fly ash by statistics and a sequential chemical extraction procedure. Fuel 2019, 237, 555–565. [Google Scholar] [CrossRef]
- Virolainen, S.; Repo, E.; Sainio, T. Recovering rare earth elements from phosphogypsum using a resin-in-leachprocess: Selection of resin, leaching agent, and eluent. Hydrometallurgy 2019, 189, 105–125. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, S.; Zou, J.; French, D.; Graham, I.T. Rare earth elements and yttrium in coal ash from the Luzhou power plant in Sichuan, Southwest China: Concentration, characterization and optimized extraction. Int. J. Coal Geol. 2019, 203, 1–14. [Google Scholar] [CrossRef]
- Costis, S.; Mueller, K.K.; Coudert, L.; Neculita, C.M.; Reynier, N.; Blais, J.F. Recovery potential of rare earth elements from mining and industrial residues: A review and cases studies. J. Geochem. Explor. 2021, 221, 106699. [Google Scholar] [CrossRef]
- Gaustad, G.; Williams, E.; Leader, A. Rare earth metals from secondary sources: Review of potential supply from waste and byproducts. Resour. Conserv. Recycl. 2021, 167, 105213. [Google Scholar] [CrossRef]
- Amato, A.; Becci, A.; Birloaga, I.; De Michelis, I.; Ferella, F.; Innocenzi, V.; Ippolito, N.M.; Pillar Jimenez Gomez, C.; Vegliò, F.; Beolchini, F. Sustainability analysis of innovative technologies for the rare earth elements recovery. Renew. Sustain. Energy Rev. 2019, 106, 41–53. [Google Scholar] [CrossRef]
- Dev, S.; Sachanb, A.; Dehghanib, F.; Ghosha, T.; Briggsc, B.R.; Aggarwal, S. Mechanisms of biological recovery of rare-earth elements from industrial and electronic wastes: A review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Szponder, D.K.; Trybalski, K. Określanie właściwości popiołów lotnych przy użyciu różnych metod i urządzeń badawczych. Górnictwo Geoinżynieria 2009, 33, 287–298. [Google Scholar]
- Olszewski, P.; Świnder, H.; Klupa, A. Possibilities of management of selected wastes from the processes of clean coal technologies. Pr. Nauk. GIG Górnictwo Środowisko 2012, 4, 123–136. [Google Scholar]
- Galos, K.; Uliasz-Bocheńczyk, A. Źródła i użytkowanie popiołów lotnych ze spalania węgli w Polsce. Gospod. Surowcami Miner. Miner. Resour. Manag. 2005, 21, 23–42. [Google Scholar]
- Pyssa, J. Odpady z energetyki—Przemysłowe zagospodarowanie odpadów z kotłów fluidalnych. Gospod. Surowcami Miner. Miner. Resour. Manag. 2005, 21, 83–92. [Google Scholar]
- Sahoo, P.K.; Kim, K.; Powell, M.A.; Equeenuddin, S.M. Recovery of metals and other beneficial products from coal fly ash: A sustainable approach for fly ash management. Int. J. Coal Sci. Technol. 2016, 3, 267–283. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, Z.; Sun, F.; He, S.; He, M.; Wang, B.; Wang, Y. Recovery of alumina from coal fly ash by CaCl2 calcination followed by H2SO4 leaching. J. Environ. Anal. Toxicol. 2017, 7, 427. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Dai, S.; Yan, X.; Ward, C.R.; Hower, J.C.; Zhao, L.; Wang, X.; Zhao, L.; Ren, D.; Finkelman, R.B. Valuable elements in Chinese coals: A review. Int. Geol. Rev. 2018, 60, 590–620. [Google Scholar] [CrossRef]
- Gorai, S. Utilization of Fly ash for sustainable environment management. J. Mater. Environ. Sci. 2018, 9, 385–393. [Google Scholar]
- Jarosiński, A.; Kulczycka, J. Ocena możliwości pozyskiwania niektórych surowców krytycznych w Polsce w związku z realizacją koncepcji gospodarki o obiegu zamkniętym. Inżynieria Miner. J. Pol. Miner. Eng. Soc. 2018, 19, 315–324. [Google Scholar]
- Unver, I.K.; Terzi, M. Distribution of trace elements in coal and coal fly ash and their recovery with mineral processing practices: A review. J. Min. Environ. 2018, 9, 641–655. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andrés, J.M.; Stanton, K.; Towler, M.; Nugteren, H.; Janssen-Jurkovicová, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Hower, J.; Qian, D.; Briot, N.; Henke, K.; Hood, M.; Taggart, R.; Hsu-Kim, H. Rare earth element associations in the Kentucky State University stoker ash. Int. J. Coal Geol. 2018, 189, 75–82. [Google Scholar] [CrossRef]
- PN-ISO-7404; Methods of Petrographic Analysis of Coal. ISO: Geneva, Switzerland, 2016.
- PN-ISO 1171:2002; Solid Mineral Fuels—Determination of Ash Content. ISO: Geneva, Switzerland, 2002.
- PN-ISO 687:2005; Brown Coals and Lignites—Determination of Moisture Content—Indirect Gravimetric Method. ISO: Geneva, Switzerland, 2005.
- PN-ISO 1928:2020-05; Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value. ISO: Geneva, Switzerland, 2020.
- ASTM D7348-21; Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues. ASTM: West Conshohocken, PA, USA, 2021.
- Alpern, B.; de Sousa, M.J.L. Documented international enquiry on solid sedimentary fossil fuels; coal: Definitions, classifications, reserves-resources, and energy potential. Int. J. Coal Geol. 2002, 50, 3–41. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Baxter, D.; Andersen, L.K. A new approach for the combined chemical and mineral classification of the inorganic matter in coal. 2. Potential applications of the classification systems. Fuel 2009, 88, 246–254. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.H. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; pp. 1–312. [Google Scholar]
- Hower, J.C.; Groppo, J.G.; Joshi, P.; Dai, s.; Moecher, D.P.; Johnston, M.N. Location of cerium in coal–combustion fly ashes: Implications for recovery of lanthanides. Coal Combust. Gasif. Prod. 2013, 5, 73–78. [Google Scholar] [CrossRef]
- Kolker, A.; Scott, C.; Hower, J.C.; Vazquez, J.A.; Lopano, C.L.; Dai, S. Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMPRG ion micro probe. Int. J. Coal Geol. 2017, 184, 1–10. [Google Scholar] [CrossRef]
- Taggart, R.K.; Hower, J.C.; Dwyer, G.S.; Hsu-Kim, H. Trends in the rare earth element content of U.S.-based coal combustion fly ashes. Environ. Sci. Technol. 2016, 50, 5919–5926. [Google Scholar] [CrossRef] [PubMed]


| Power Plant | Sample Type (Waste Type) | Sample Symbol |
|---|---|---|
| Bełchatów | Lignite for power units 9–12 | BW1 |
| Lignite for power units 5–8 | BW2 | |
| Lignite for power units 1–4 | BW3 | |
| Bottom ash—power unit 12 | B1 | |
| Bottom ash—power unit 8 | B2 | |
| Bottom ash—power unit 4 | B3 | |
| Fly ash gas duct—power unit 12 | BL1 | |
| Fly ash gas duct—power unit 8 | BL2 | |
| Fly ash—gas duct—power unit 4 | BL3 | |
| Fly ash—electrofilter—power unit 12 | BF1 | |
| Fly ash—electrofilter—power unit 8 | BF2 | |
| Fly ash—electrofilter—power unit 4 | BF3 | |
| Turów | Lignite | TW4 |
| Bottom ash | T3 | |
| Fly ash—electrofilter | T2 |
| Sample | Ma | Ad | Qsaf | H | L | I | SM | Hmmf | Lmmf | Immf |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (MJ/kg) | (%) | |||||||
| BW1 | 14.95 | 16.86 | 20.0 | 69 | 5 | 4 | 22 | 89 | 6 | 5 |
| BW2 | 14.91 | 18.52 | 20.0 | 71 | 5 | 6 | 19 | 87 | 6 | 7 |
| BW3 | 15.32 | 22.32 | 19.4 | 69 | 1 | 6 | 23 | 90 | 2 | 8 |
| TW4 | 8.56 | 22.94 | 23.5 | 56 | 6 | 5 | 33 | 84 | 9 | 7 |
| Sample | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | LOI | DAI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | 52.49 | 15.57 | 5.03 | 0.03 | 0.68 | 11.47 | 0.08 | 0.09 | 1.04 | 0.18 | 13.34 | 3.91 | 3.37 | 70.35 | 16.85 | 1.13 |
| B2 | 48.81 | 16.13 | 4.83 | 0.03 | 0.61 | 10.02 | 0.07 | 0.10 | 1.07 | 0.18 | 18.15 | 3.79 | 3.03 | 61.76 | 16.50 | 1.43 |
| B3 | 49.42 | 15.17 | 5.12 | 0.03 | 0.59 | 11.17 | 0.07 | 0.09 | 0.92 | 0.22 | 17.19 | 3.71 | 3.26 | 72.44 | 18.98 | 1.29 |
| BL1 | 54.00 | 17.84 | 4.15 | 0.03 | 0.62 | 11.21 | 0.08 | 0.10 | 1.05 | 0.27 | 10.64 | 3.95 | 3.03 | 64.72 | 18.10 | 1.25 |
| BL2 | 58.49 | 16.88 | 4.52 | 0.03 | 0.55 | 9.62 | 0.07 | 0.10 | 0.96 | 0.24 | 8.56 | 4.63 | 3.47 | 60.29 | 17.64 | 1.43 |
| BL3 | 47.13 | 19.90 | 6.71 | 0.04 | 0.64 | 13.57 | 0.07 | 0.11 | 1.04 | 0.34 | 10.47 | 3.06 | 2.37 | 77.72 | 21.21 | 1.57 |
| BF1 | 44.17 | 20.81 | 5.84 | 0.05 | 1.10 | 20.72 | 0.13 | 0.13 | 1.27 | 0.24 | 5.54 | 2.20 | 2.12 | 82.69 | 18.91 | 1.00 |
| BF2 | 36.12 | 22.82 | 7.46 | 0.07 | 1.09 | 27.39 | 0.16 | 0.10 | 1.02 | 0.56 | 3.21 | 1.45 | 1.58 | 107.73 | 25.18 | 0.63 |
| BF3 | 35.10 | 22.72 | 7.27 | 0.07 | 1.07 | 26.94 | 0.11 | 0.11 | 1.07 | 0.50 | 5.02 | 1.49 | 1.54 | 125.00 | 25.19 | 1.00 |
| T3 | 44.06 | 27.09 | 6.33 | 0.10 | 0.71 | 14.51 | 0.54 | 1.58 | 1.94 | 0.16 | 2.95 | 3.45 | 1.63 | 7.20 | 20.49 | 2.93 |
| T2 | 39.21 | 27.64 | 5.55 | 0.05 | 1.49 | 17.43 | 1.29 | 1.34 | 2.22 | 0.20 | 3.60 | 2.42 | 1.42 | 7.21 | 11.68 | 1.04 |
| Sample | UC | G | Q | Mu | Ge | Cc | Ah | An | Mgh | He | Li |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | 13.34 | 31.8 | 27.8 | 5.5 | 6.0 | 13.7 | 1.9 | ||||
| B2 | 18.15 | 34.0 | 23.8 | 6.0 | 4.6 | 12.7 | 0.8 | ||||
| B3 | 17.19 | 30.1 | 26.8 | 6.7 | 5.3 | 11.8 | 2 | ||||
| BL1 | 10.64 | 31.4 | 32.7 | 20.0 | 2.3 | 3.1 | |||||
| BL2 | 8.56 | 35.4 | 37.3 | 14.0 | 1.9 | 2.9 | |||||
| BL3 | 10.47 | 47.5 | 22.7 | 12.0 | 3.5 | 3.9 | |||||
| BF1 | 5.54 | 51.7 | 15.9 | 7.5 | 3.3 | 5.1 | 11.1 | ||||
| BF2 | 3.21 | 59.5 | 8.6 | 12.1 | 3.1 | 3.2 | 10.4 | ||||
| BF3 | 5.02 | 58.3 | 8.7 | 10.2 | 4.1 | 4.9 | 8.9 | ||||
| T3 | 2.95 | 70.7 | 10.3 | 5.4 | 10.6 | ||||||
| T2 | 3.60 | 66.3 | 6.3 | 5.8 | 12.4 | 3.08 | 2.45 |
| Sample | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | |||||||||||||||
| B1 | 34 | 49.2 | 89.3 | 9.95 | 37.6 | 7 | 1.43 | 5.4 | 0.8 | 5.1 | 1 | 3 | 0.42 | 2.9 | 0.45 |
| B2 | 28 | 46.5 | 84.7 | 9.36 | 35.1 | 6.5 | 1.28 | 4.9 | 0.8 | 4.6 | 0.9 | 2.7 | 0.4 | 2.7 | 0.41 |
| B3 | 30 | 48.1 | 88.4 | 9.77 | 37.3 | 6.8 | 1.33 | 5.1 | 0.8 | 4.7 | 0.9 | 2.7 | 0.41 | 2.7 | 0.41 |
| BL1 | 36 | 54.3 | 101 | 11.2 | 42.5 | 8 | 1.56 | 6.1 | 1 | 5.7 | 1.1 | 3.3 | 0.48 | 3.2 | 0.52 |
| BL2 | 30 | 48.6 | 90.4 | 10.1 | 38.8 | 7.2 | 1.36 | 5.2 | 0.8 | 4.9 | 1 | 3 | 0.44 | 2.9 | 0.44 |
| BL3 | 40 | 60.5 | 115 | 12.6 | 48 | 9.1 | 1.8 | 6.9 | 1.1 | 6.5 | 1.2 | 3.5 | 0.52 | 3.3 | 0.55 |
| BF1 | 60 | 84.2 | 151 | 17 | 64.7 | 12.6 | 2.6 | 10.9 | 1.7 | 9.5 | 1.9 | 5.6 | 0.79 | 4.9 | 0.81 |
| BF2 | 73 | 103 | 196 | 22.1 | 88.6 | 17.1 | 3.49 | 13.7 | 2.1 | 12.2 | 2.4 | 6.8 | 0.94 | 6.1 | 0.95 |
| BF3 | 68 | 95.5 | 174 | 19.4 | 76.9 | 15 | 2.93 | 13 | 1.9 | 11.1 | 2.1 | 6.1 | 0.82 | 5.5 | 0.84 |
| T3 | 29 | 85.1 | 141 | 16.4 | 59.2 | 11.4 | 2.34 | 8.1 | 1.2 | 6.5 | 1.1 | 3 | 0.41 | 2.5 | 0.36 |
| T2 | 34 | 85.7 | 150 | 16.4 | 59.9 | 11.4 | 2.54 | 9.5 | 1.5 | 7.9 | 1.4 | 3.6 | 0.48 | 3.2 | 0.46 |
| Sample | REY | LREY | MREY | HREY | LREY | MREY | HREY | Critical | Uncritical | Excessive | Critical | Uncritical | Excessive | Coutl | |
| (ppm) | (%) | (ppm) | (%) | ||||||||||||
| B1 | 247.6 | 193.1 | 46.7 | 7.8 | 78.0 | 18.9 | 3.1 | 81.9 | 71.6 | 94.1 | 33.1 | 28.9 | 38.0 | 0.87 | |
| B2 | 228.9 | 182.2 | 39.6 | 7.1 | 79.6 | 17.3 | 3.1 | 72.5 | 67.3 | 89.1 | 31.7 | 29.4 | 38.9 | 0.81 | |
| B3 | 239.4 | 190.4 | 41.9 | 7.1 | 79.5 | 17.5 | 3.0 | 76.8 | 69.8 | 92.8 | 32.1 | 29.1 | 38.8 | 0.83 | |
| BL1 | 276.0 | 217.0 | 50.4 | 8.6 | 78.6 | 18.2 | 3.1 | 90.1 | 79.6 | 106.3 | 32.6 | 28.8 | 38.5 | 0.85 | |
| BL2 | 245.1 | 195.1 | 42.3 | 7.8 | 79.6 | 17.2 | 3.2 | 78.9 | 71.1 | 95.2 | 32.2 | 29.0 | 38.8 | 0.83 | |
| BL3 | 310.6 | 245.2 | 56.3 | 9.1 | 79.0 | 18.1 | 2.9 | 100.9 | 89.1 | 120.6 | 32.5 | 28.7 | 38.8 | 0.84 | |
| BF1 | 428.2 | 329.5 | 84.7 | 14.0 | 77.0 | 19.8 | 3.3 | 144.1 | 124.7 | 159.4 | 33.7 | 29.1 | 37.2 | 0.90 | |
| BF2 | 548.5 | 426.8 | 104.5 | 17.2 | 77.8 | 19.1 | 3.1 | 186.2 | 155.9 | 206.4 | 33.9 | 28.4 | 37.6 | 0.90 | |
| BF3 | 493.1 | 380.8 | 96.9 | 15.4 | 77.2 | 19.7 | 3.1 | 166.9 | 142.9 | 183.3 | 33.9 | 29.0 | 37.2 | 0.91 | |
| T3 | 367.6 | 313.1 | 47.1 | 7.4 | 85.2 | 12.8 | 2.0 | 101.2 | 121.0 | 145.4 | 27.5 | 32.9 | 39.5 | 0.70 | |
| T2 | 388.0 | 323.4 | 55.4 | 9.1 | 83.4 | 14.3 | 2.4 | 109.4 | 123.0 | 155.5 | 28.2 | 31.7 | 40.1 | 0.70 | |
| Sample | SiO2 | REY | Glass | H-SiO2 | H-REY | H-Glass | R-SiO2 | E-REY | R-Glass |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (ppm) | (%) | (%) | (ppm) | (%) | (%) | (%) | (%) | |
| B1 | 52.49 | 247.6 | 31.8 | 40.55 | 364.8 | 6.52 | 22.74 | 47.34 | 79.48 |
| B2 | 48.81 | 228.9 | 34.0 | 38.82 | 312.5 | 7.29 | 20.46 | 36.57 | 78.52 |
| B3 | 49.42 | 239.4 | 30.1 | 38.78 | 310.6 | 9.18 | 21.53 | 29.73 | 69.49 |
| BL1 | 54 | 276 | 31.4 | 41.75 | 375.1 | 8.77 | 22.68 | 35.94 | 72.03 |
| BL2 | 58.49 | 245.1 | 35.4 | 47.04 | 336.6 | 5.07 | 19.57 | 37.33 | 85.70 |
| BL3 | 47.13 | 310.6 | 47.5 | 32.79 | 428.2 | 5.98 | 30.43 | 37.87 | 87.41 |
| BF1 | 44.17 | 428.2 | 51.7 | 22.62 | 593.3 | 5.68 | 48.78 | 38.55 | 89.01 |
| BF2 | 36.12 | 548.5 | 59.5 | 18.41 | 590.2 | 20.45 | 49.01 | 7.6 | 65.62 |
| BF3 | 35.1 | 493.1 | 58.3 | 14.07 | 592.9 | 9.04 | 59.9 | 20.25 | 84.49 |
| T3 | 44.06 | 367.6 | 70.7 | 36.37 | 587.9 | 24.73 | 17.46 | 59.93 | 65.04 |
| T2 | 39.21 | 388 | 66.3 | 25.16 | 664.6 | 14.61 | 35.83 | 71.3 | 77.96 |
| Sample | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | |||||||||||||||
| B1 | 52 | 70.8 | 130 | 15.1 | 56 | 10.3 | 2.04 | 8.2 | 1.3 | 7.5 | 1.5 | 4.3 | 0.63 | 4.4 | 0.68 |
| B2 | 40 | 63.1 | 115 | 13 | 47.6 | 8.7 | 1.76 | 7 | 1 | 6.1 | 1.2 | 3.5 | 0.52 | 3.5 | 0.56 |
| B3 | 40 | 61.8 | 115 | 12.7 | 47.2 | 8.7 | 1.78 | 7.3 | 1 | 6.2 | 1.2 | 3.4 | 0.49 | 3.3 | 0.54 |
| BL1 | 49 | 74 | 138 | 15.5 | 56.9 | 10.6 | 2.24 | 8.9 | 1.3 | 7.6 | 1.5 | 4.2 | 0.61 | 4.1 | 0.68 |
| BL2 | 43 | 66.2 | 124 | 14.1 | 51.7 | 9.6 | 1.98 | 7.9 | 1.2 | 6.8 | 1.4 | 3.9 | 0.56 | 3.7 | 0.6 |
| BL3 | 56 | 83.8 | 158 | 17.6 | 66 | 12.2 | 2.53 | 10.2 | 1.5 | 8.4 | 1.6 | 4.7 | 0.65 | 4.3 | 0.7 |
| BF1 | 83 | 115 | 209 | 24.7 | 92.8 | 17.7 | 3.63 | 14.7 | 2.2 | 12.8 | 2.4 | 7.1 | 0.97 | 6.3 | 0.95 |
| BF2 | 82 | 111 | 211 | 24.1 | 93.4 | 17.9 | 3.69 | 15.6 | 2.2 | 12.5 | 2.4 | 6.7 | 0.93 | 5.8 | 0.94 |
| BF3 | 84 | 114 | 210 | 24.3 | 92 | 17.8 | 3.67 | 15.6 | 2.2 | 12.5 | 2.4 | 6.7 | 0.92 | 5.9 | 0.94 |
| T3 | 48 | 135 | 226 | 26.9 | 94 | 17.7 | 3.64 | 13 | 2 | 10.3 | 1.7 | 4.6 | 0.61 | 3.9 | 0.58 |
| T2 | 59 | 148 | 254 | 29.4 | 106 | 20.1 | 4.27 | 15.1 | 2.2 | 12.5 | 2.1 | 5.6 | 0.73 | 4.9 | 0.7 |
| Sample | REY | LREY | MREY | HREY | LREY | MREY | HREY | Critical | Uncritical | Excessive | Critical | Uncritical | Excessive | Coutl | |
| (ppm) | (%) | (ppm) | (%) | ||||||||||||
| B1 | 364.8 | 282.2 | 71.0 | 11.5 | 77.4 | 19.5 | 3.2 | 123.1 | 104.4 | 137.2 | 33.8 | 28.6 | 37.6 | 0.90 | |
| B2 | 312.5 | 247.4 | 55.9 | 9.3 | 79.2 | 17.9 | 3.0 | 100.0 | 91.8 | 120.8 | 32.0 | 29.4 | 38.6 | 0.83 | |
| B3 | 310.6 | 245.4 | 56.3 | 8.9 | 79.0 | 18.1 | 2.9 | 99.6 | 90.5 | 120.5 | 32.1 | 29.1 | 38.8 | 0.83 | |
| BL1 | 375.1 | 295.0 | 69.0 | 11.1 | 78.6 | 18.4 | 3.0 | 121.2 | 109.0 | 144.9 | 32.3 | 29.1 | 38.6 | 0.84 | |
| BL2 | 336.6 | 265.6 | 60.9 | 10.2 | 78.9 | 18.1 | 3.0 | 108.6 | 97.8 | 130.3 | 32.3 | 29.1 | 38.7 | 0.83 | |
| BL3 | 428.2 | 337.6 | 78.6 | 12.0 | 78.8 | 18.4 | 2.8 | 139.1 | 123.8 | 165.3 | 32.5 | 28.9 | 38.6 | 0.84 | |
| BF1 | 593.3 | 459.2 | 116.3 | 17.7 | 77.4 | 19.6 | 3.0 | 201.5 | 172.1 | 219.6 | 34.0 | 29.0 | 37.0 | 0.92 | |
| BF2 | 590.2 | 457.4 | 116.0 | 16.8 | 77.5 | 19.7 | 2.8 | 200.5 | 168.6 | 221.1 | 34.0 | 28.6 | 37.5 | 0.91 | |
| BF3 | 592.9 | 458.1 | 118.0 | 16.9 | 77.3 | 19.9 | 2.8 | 201.1 | 171.7 | 220.2 | 33.9 | 29.0 | 37.1 | 0.91 | |
| T3 | 587.9 | 499.6 | 76.9 | 11.4 | 85.0 | 13.1 | 1.9 | 162.5 | 192.6 | 232.8 | 27.6 | 32.8 | 39.6 | 0.70 | |
| T2 | 664.6 | 557.5 | 93.1 | 14.0 | 83.9 | 14.0 | 2.1 | 189.6 | 212.6 | 262.4 | 28.5 | 32.0 | 39.5 | 0.72 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, Z.; Komorek, J.; Kokowska-Pawłowska, M.; Nowak, J. Distribution of Rare Earth Elements in Ash from Lignite Combustion in Polish Power Plants. Materials 2024, 17, 4477. https://doi.org/10.3390/ma17184477
Adamczyk Z, Komorek J, Kokowska-Pawłowska M, Nowak J. Distribution of Rare Earth Elements in Ash from Lignite Combustion in Polish Power Plants. Materials. 2024; 17(18):4477. https://doi.org/10.3390/ma17184477
Chicago/Turabian StyleAdamczyk, Zdzisław, Joanna Komorek, Magdalena Kokowska-Pawłowska, and Jacek Nowak. 2024. "Distribution of Rare Earth Elements in Ash from Lignite Combustion in Polish Power Plants" Materials 17, no. 18: 4477. https://doi.org/10.3390/ma17184477






