Fast, Simple, and Sensitive Voltammetric Measurements of Acyclovir in Real Samples via Boron-Doped Diamond Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Reagents and Solutions
2.3. Preparation of Tablets and Municipal Wastewater Samples
2.4. DPV Procedure Parameters
3. Results and Discussion
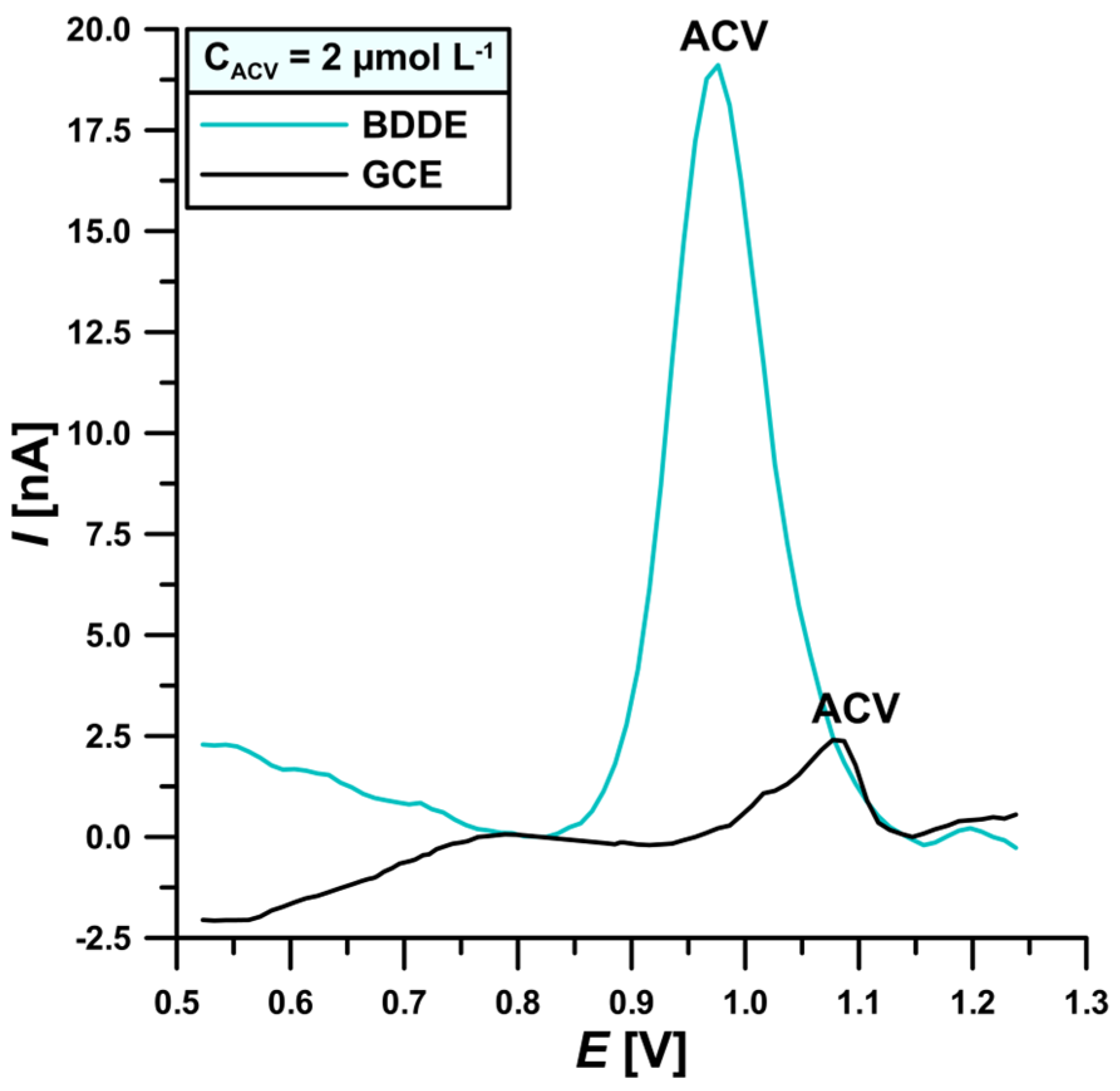
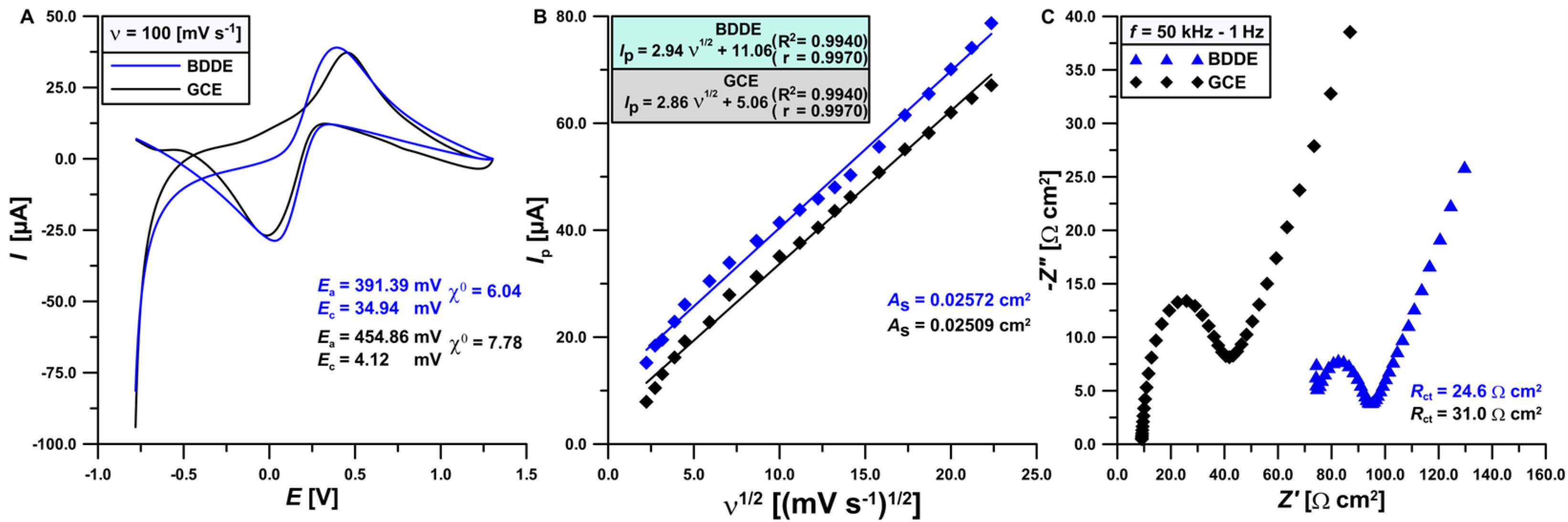
3.1. Comparison of the Electrochemical Properties of BDDE and GCE
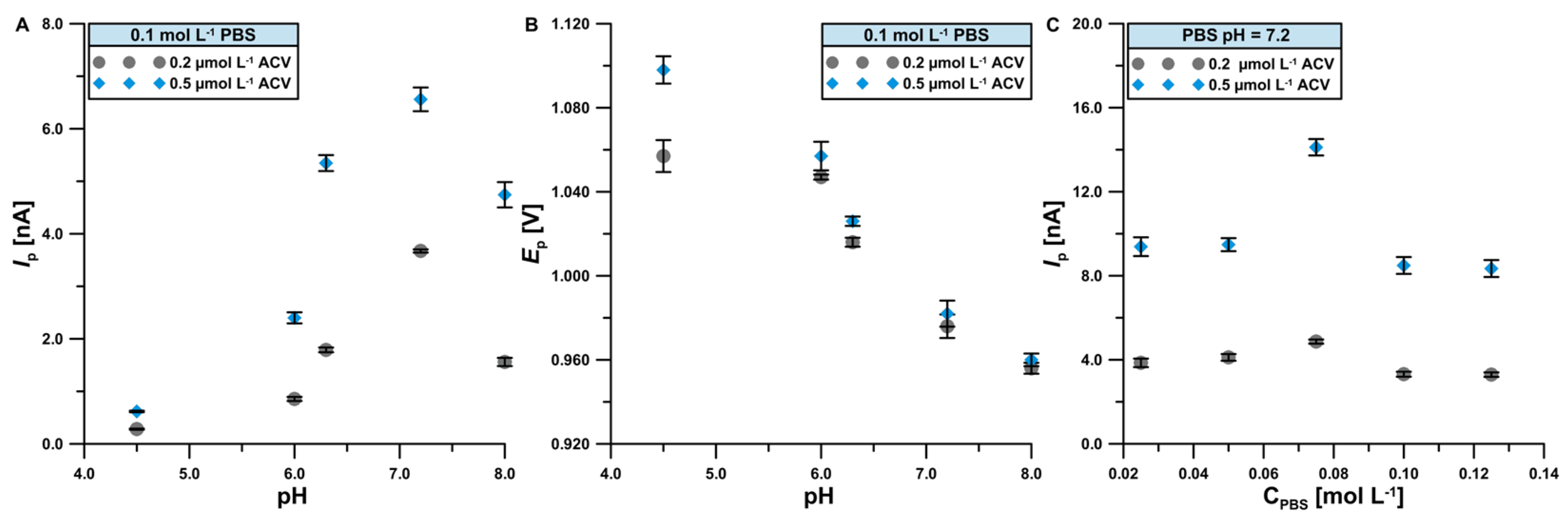
3.2. Optimization of the Basic Electrolyte Composition and Study of the ACV Electrode Process
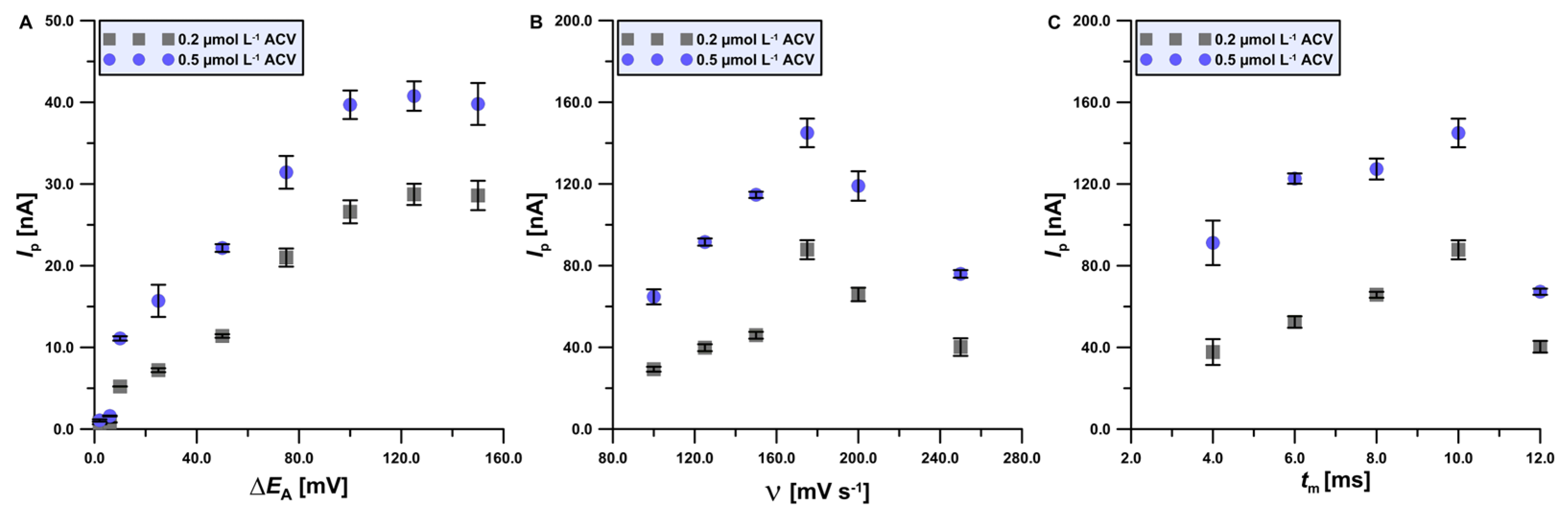
3.3. Procedure Optimization
3.4. Analytical Parameters of the Procedure and the Influence of Interference Species
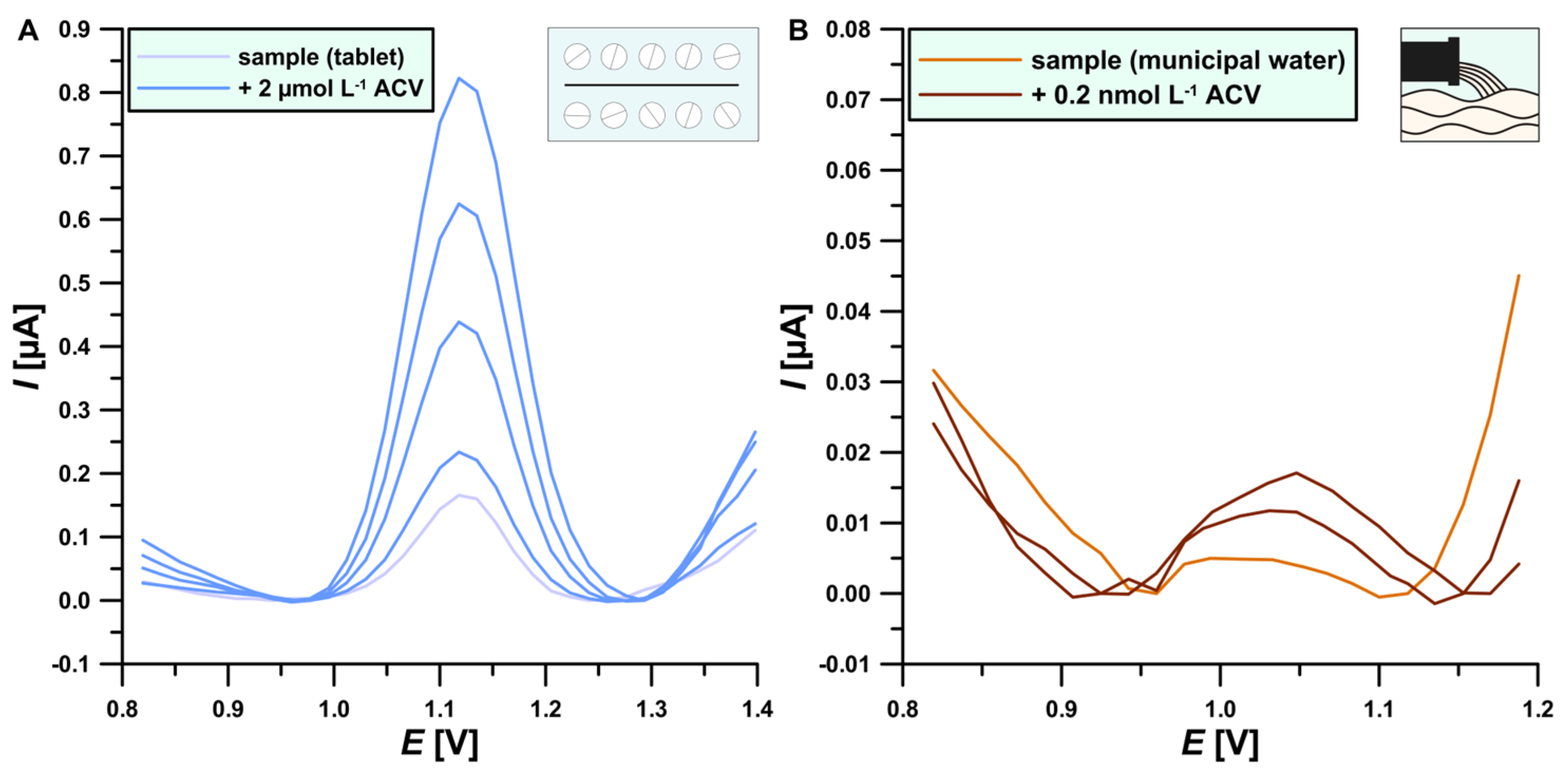
3.5. Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shetti, N.P.; Malode, S.J.; Nandibewoor, S.T. Electrochemical Behavior of an Antiviral Drug Acyclovir at Fullerene-C60-Modified Glassy Carbon Electrode. Bioelectrochemistry 2012, 88, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Azimzadeh, M.; Amini, M.K. Modification of Glassy Carbon Electrode with a Bilayer of Multiwalled Carbon Nanotube/Tiron-Doped Polypyrrole: Application to Sensitive Voltammetric Determination of Acyclovir. Mater. Sci. Eng. C 2015, 53, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Suazo, P.A.; Tognarelli, E.I.; Kalergis, A.M.; González, P.A. Herpes Simplex Virus 2 Infection: Molecular Association with HIV and Novel Microbicides to Prevent Disease. Med. Microbiol. Immunol. 2015, 204, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Sadikoglu, M.; Saglikoglu, G.; Yagmur, S.; Orta, E.; Yilmaz, S. Voltammetric Determination of Acyclovir in Human Urine Using Ultra Trace Graphite and Glassy Carbon Electrodes. Curr. Anal. Chem. 2011, 7, 130–135. [Google Scholar] [CrossRef]
- Ericson, J.E.; Gostelow, M.; Autmizguine, J.; Hornik, C.P.; Clark, R.H.; Benjamin, D.K.; Smith, P.B. Safety of High-Dose Acyclovir in Infants with Suspected and Confirmed Neonatal Herpes Simplex Virus Infections. Pediatr. Infect. Dis. J. 2017, 36, 369–373. [Google Scholar] [CrossRef]
- Dorraji, P.S.; Fotouhi, L. Electropolymerized Film of L-Cysteine in the Presence of Deep Eutectic Solvent on NaOH Nanorods Glassy Carbon Electrode for Sensitive Determination of Acyclovir in Biological Fluids. IEEE Sens. J. 2021, 21, 1324–1331. [Google Scholar] [CrossRef]
- Castro, A.A.; Cordoves, A.I.P.; Farias, P.A.M. Determination of the Antiretroviral Drug Acyclovir in Diluted Alkaline Electrolyte by Adsorptive Stripping Voltammetry at the Mercury Film Electrode. Anal. Chem. Insights 2013, 8, ACI.S11608. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Chen, X.; Hu, S. Studies on Electrochemical Behaviors of Acyclovir and Its Voltammetric Determination with Nano-Structured Film Electrode. Anal. Chim. Acta 2006, 576, 17–22. [Google Scholar] [CrossRef]
- Gupta, A.; Vyas, R.K.; Gupta, A.B. Occurrence of Acyclovir in the Aquatic Environment, Its Removal and Research Perspectives: A Review. J. Water Process Eng. 2021, 39, 101855. [Google Scholar] [CrossRef]
- Yu, L.; Xiang, B. Quantitative Determination of Acyclovir in Plasma by near Infrared Spectroscopy. Microchem. J. 2008, 90, 63–66. [Google Scholar] [CrossRef]
- Mustafa, A.A.; Abdel-Fattah, S.A.; Toubar, S.S.; Sultan, M.A. Spectrophotometric Determination of Acyclovir and Amantadine Hydrochloride through Metals Complexation. J. Anal. Chem. 2004, 59, 33–38. [Google Scholar] [CrossRef]
- Ayad, M.M.; Abdellatef, H.E.; El-Henawee, M.M.; El-Sayed, H.M. Spectrophotometric and Spectrofluorimetric Methods for Analysis of Acyclovir and Acebutolol Hydrochloride. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 66, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Chen, F. Flow Injection-Chemiluminescence Determination of Acyclovir: FI-CL Determination of Acyclovir. Luminescence 2012, 27, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.R.; Balfour, H.H.; Vezina, H.E. Simultaneous Determination of Acyclovir, Ganciclovir, and (R)-9-[4-Hydroxy-2-(Hydroxymethyl)Butyl]Guanine in Human Plasma Using High-Performance Liquid Chromatography. Biomed. Chromatogr. 2009, 23, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Dowling, T.C.; Haidar, S.H.; Yu, L.X.; Polli, J.E.; Kane, M.A. Quantification of Acyclovir in Human Plasma by Ultra-High-Performance Liquid Chromatography—Heated Electrospray Ionization—Tandem Mass Spectrometry for Bioequivalence Evaluation. J. Anal. Bioanal. Tech. 2012, 3, 1000139. [Google Scholar] [CrossRef]
- Jin, L.; Wei, G.; Lu, W.-Y.; Xu, L.-J.; Pan, J. Quantitative Determination of Acyclovir in Aqueous Humor by LC-MS. Chromatographia 2006, 63, 239–242. [Google Scholar] [CrossRef]
- Martínez-Rojas, F.; Del Valle, M.A.; Isaacs, M.; Ramírez, G.; Armijo, F. Electrochemical Behaviour Study and Determination of Guanine, 6-Thioguanine, Acyclovir and Gancyclovir on Fluorine-Doped SnO2 Electrode. Application in Pharmaceutical Preparations. Electroanalysis 2017, 29, 2888–2895. [Google Scholar] [CrossRef]
- Dorraji, P.S.; Jalali, F. Differential Pulse Voltammetric Determination of Nanomolar Concentrations of Antiviral Drug Acyclovir at Polymer Film Modified Glassy Carbon Electrode. Mater. Sci. Eng. C 2016, 61, 858–864. [Google Scholar] [CrossRef]
- Dilgin, D.G.; Karakaya, S. Differential Pulse Voltammetric Determination of Acyclovir in Pharmaceutical Preparations Using a Pencil Graphite Electrode. Mater. Sci. Eng. C 2016, 63, 570–576. [Google Scholar] [CrossRef]
- Shetti, N.P.; Nayak, D.S.; Malode, S.J.; Kulkarni, R.M. Nano Molar Detection of Acyclovir, an Antiviral Drug at Nanoclay Modified Carbon Paste Electrode. Sens. Bio-Sens. Res. 2017, 14, 39–46. [Google Scholar] [CrossRef]
- Carolina Ordoñez, H.; José Espitia, H.; Harold Díaz, S.; Rojas, G.; Alonso Jaramillo, A. Voltammetric Analysis of Acyclovir at Glassy Carbon/Oppy/Templated Electrode. J. Phys. Conf. Ser. 2018, 1119, 012008. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, L.; Wu, Y.; Liu, X.; Feng, J.; Ding, J.; Li, K.; He, Q. Ultrasensitive Electrochemical Detection for Nanomolarity Acyclovir at Ferrous Molybdate Nanorods and Graphene Oxide Composited Glassy Carbon Electrode. Colloids Surf. Physicochem. Eng. Asp. 2022, 641, 128601. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Khorablou, Z.; Mehdikhani, S. Preparation of a Double-Step Modified Carbon Paste Electrode for Trace Quantification of Acyclovir Using TiO2 Nanoparticle and β-Cyclodextrin. Electroanalysis 2018, 30, 2908–2915. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Li, J.; Kong, F.-Y.; Wei, M.-J.; Zhang, P.; Li, Y.; Fang, H.-L.; Wang, W. Improved Performance for the Electrochemical Sensing of Acyclovir by Using the rGO–TiO2–Au Nanocomposite-Modified Electrode. Front. Chem. 2022, 10, 892919. [Google Scholar] [CrossRef]
- Joseph, R.; Kumar, K.G. Electrochemical Sensing of Acyclovir at a Gold Electrode Modified with 2-Mercaptobenzothiazole–[5,10,15,20-Tetrakis-(3-Methoxy-4-Hydroxyphenyl)Porphyrinato]Copper(II). Anal. Sci. 2011, 27, 67–72. [Google Scholar] [CrossRef][Green Version]
- Saleh, G.A.; Askal, H.F.; Refaat, I.H.; Abdel-aal, F.A.M. Adsorptive Square Wave Voltammetric Determination of Acyclovir and Its Application in a Pharmacokinetic Study Using a Novel Sensor of β-Cyclodextrin Modified Pencil Graphite Electrode. Bull. Chem. Soc. Jpn. 2015, 88, 1291–1300. [Google Scholar] [CrossRef]
- Naghian, E.; Marzi Khosrowshahi, E.; Sohouli, E.; Pazoki-Toroudi, H.R.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ahmadi, F. Electrochemical Oxidation and Determination of Antiviral Drug Acyclovir by Modified Carbon Paste Electrode with Magnetic CdO Nanoparticles. Front. Chem. 2020, 8, 689. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, Z.; Gholivand, M.B.; Shamsipur, M.; Mirzaei, M. An Electrochemical Sensor Based on Ag Nanoparticles Decorated on Cadmium Sulfide Nanowires/Reduced Graphene Oxide for the Determination of Acyclovir. J. Alloys Compd. 2022, 903, 163912. [Google Scholar] [CrossRef]
- Hamtak, M.; Fotouhi, L.; Hosseini, M.; Dorraji, P.S. Sensitive Determination of Acyclovir in Biological and Pharmaceutical Samples Based on Polymeric Film Decorated with Nanomaterials on Nanoporous Glassy Carbon Electrode. J. Electrochem. Soc. 2018, 165, B632–B637. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Sarkary, A.; Khorablou, Z.; Dorraji, P.S. Synergistic Effect of ZnO Nanoparticles and Carbon Nanotube and Polymeric Film on Electrochemical Oxidation of Acyclovir. Iran. J. Pharm. Res. 2018, 17, 52–62. [Google Scholar]
- Saleh, G.A.; Askal, H.F.; Refaat, I.H.; Abdel-aal, F.A.M. A New Electrochemical Method for Simultaneous Determination of Acyclovir and Methotrexate in Pharmaceutical and Human Plasma Samples. Anal. Bioanal. Electrochem. 2016, 8, 691–716. [Google Scholar]
- Abedini, S.; Rafati, A.A.; Ghaffarinejad, A. A Simple and Low-Cost Electrochemical Sensor Based on a Graphite Sheet Electrode Modified by Carboxylated Multiwalled Carbon Nanotubes and Gold Nanoparticles for Detection of Acyclovir. New J. Chem. 2022, 46, 20403–20411. [Google Scholar] [CrossRef]
- Ghadirinataj, M.; Hassaninejad-Darzi, S.K.; Emadi, H. An Electrochemical Nanosensor for Simultaneous Quantification of Acetaminophen and Acyclovir by ND@Dy2O3-IL/CPE. Electrochim. Acta 2023, 450, 142274. [Google Scholar] [CrossRef]
- Can, S.; Yilmaz, S.; Saglikoglu, G.; Sadikoglu, M.; Menek, N. Electrocatalytic Oxidation of Acyclovir on Poly(p-Aminobenzene Sulfonic Acid) Film Modified Glassy Carbon Electrode. Electroanalysis 2015, 27, 2431–2438. [Google Scholar] [CrossRef]
- Tarlekar, P.; Khan, A.; Chatterjee, S. Nanoscale Determination of Antiviral Drug Acyclovir Engaging Bifunctionality of Single Walled Carbon Nanotubes—Nafion Film. J. Pharm. Biomed. Anal. 2018, 151, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shaidarova, L.G.; Gedmina, A.V.; Poadnyak, A.A.; Chelnokova, I.A.; Budnikov, H.C. Selective Voltammetric and Flow-Injection Amperometric Determination of Acyclovir and Valacyclovir on an Electrode with a Reduced Graphene Oxide–Polyglycine Film Composite. J. Anal. Chem. 2022, 77, 681–687. [Google Scholar] [CrossRef]
- Yanik, S.; Emre, D.; Alp, M.; Algi, F.; Yilmaz, S.; Bilici, A.; Ozkan-Ariksoysal, D. A Novel Electrochemical Biosensor Based on Palladium Nanoparticles Decorated on Reduced Graphene Oxide-Polyaminophenol Matrix for the Detection and Discrimination of Mitomycin C-DNA and Acyclovir-DNA Interaction. J. Pharm. Biomed. Anal. 2023, 234, 115524. [Google Scholar] [CrossRef]
- Skrzypek, S.; Ciesielski, W.; Yilmaz, S. Voltammetric Study of Aciclovir Using Controled Grow Mercury Drop Electrode. Chem. Anal. 2007, 52, 1071–1078. [Google Scholar]
- Lalei, M.; Zarei, K. Fabrication of RuNPs/TBA/PGE and Its Application for the Electrochemical Determination of Trace Amounts of Acyclovir. Microchem. J. 2023, 190, 108667. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ahmed, Y.M. New Strategy for Determination of Anti-Viral Drugs Based on Highly Conductive Layered Composite of MnO2/Graphene/Ionic Liquid Crystal/Carbon Nanotubes. J. Electroanal. Chem. 2019, 838, 107–118. [Google Scholar] [CrossRef]
- El Henawee, M.; Saleh, H.; Attia, A.K.; Hussien, E.M.; Derar, A.R. Carbon Nanotubes Bulk Modified Printed Electrochemical Sensor for Green Determination of Vortioxetine Hydrobromide by Linear Sweep Voltammetry. Measurement 2021, 177, 109239. [Google Scholar] [CrossRef]
- Alinejad, T.; Chen, C.; Shamsipur, M.; Bagher Gholivand, M.; Paimard, G. Electrochemical Evaluation and Determination of Antiretroviral Drug Ganciclovir Based on Fe-Cu/TiO2/Multi-Walled Carbon Nanotubes Sensor. Measurement 2023, 214, 112846. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Naik, R.R.; Kuchinad, G.T.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Hetero-Nanostructured Iron Oxide and Bentonite Clay Composite Assembly for the Determination of an Antiviral Drug Acyclovir. Microchem. J. 2020, 155, 104727. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Staniec, K.; Gorylewski, D.; Keller, A. First Acyclovir Determination Procedure via Electrochemically Activated Screen-Printed Carbon Electrode Coupled with Well-Conductive Base Electrolyte. Sensors 2024, 24, 1125. [Google Scholar] [CrossRef]
- Compton, R.G.; Foord, J.S.; Marken, F. Electroanalysis at Diamond-Like and Doped-Diamond Electrodes. Electroanalysis 2003, 15, 1349–1363. [Google Scholar] [CrossRef]
- Vinokur, N.; Miller, B.; Avyigal, Y.; Kalish, R. Electrochemical Behavior of Boron-Doped Diamond Electrodes. J. Electrochem. Soc. 1996, 143, L238–L240. [Google Scholar] [CrossRef]
- Oliveira, S.C.B.; Oliveira-Brett, A.M. Voltammetric and electrochemical impedance spectroscopy characterization of a cathodic and anodic pre-treated boron doped diamond electrode. Electrochim. Acta 2010, 55, 4599–4605. [Google Scholar] [CrossRef]
- Einaga, Y. Boron-Doped Diamond Electrodes: Fundamentals for Electrochemical Applications. Acc. Chem. Res. 2022, 55, 3605–3615. [Google Scholar] [CrossRef]
- Matvieiev, O.; Šelešovská, R.; Vojs, M.; Marton, M.; Michniak, P.; Hrdlička, V.; Hatala, M.; Janíková, L.; Chýlková, J.; Skopalová, J.; et al. Novel Screen-Printed Sensor with Chemically Deposited Boron-Doped Diamond Electrode: Preparation, Characterization, and Application. Biosensors 2022, 12, 241. [Google Scholar] [CrossRef]
- Muzyka, K.; Sun, J.; Fereja, T.H.; Lan, Y.; Zhang, W.; Xu, G. Boron-doped diamond: Current progress and challenges in view of electroanalytical applications. Anal. Methods 2019, 11, 397–414. [Google Scholar] [CrossRef]
- Baluchová, S.; Daňhel, A.; Dejmková, H.; Ostatná, V.; Fojta, M.; Schwarzová-Pecková, K. Recent progress in the applications of boron doped diamond electrodes in electroanalysis of organic compounds and biomolecules—A review. Anal. Chim. Acta 2019, 1077, 30–66. [Google Scholar] [CrossRef] [PubMed]
- Hrdlička, V.; Matvieiev, O.; Navrátil, T.; Šelešovská, R. Recent advances in modified boron-doped diamond electrodes: A review. Electrochim. Acta 2023, 456, 142435. [Google Scholar] [CrossRef]
- Sousa, C.P.; Ribeiro, F.W.P.; Oliveira, T.M.B.F.; Salazar-Banda, G.R.; de Lima Neto, P.; Morais, S.; Correia, A.N. Electroanalysis of Pharmaceuticals on Boron-Doped Diamond Electrodes: A Review. ChemElectroChem 2019, 6, 2350–2378. [Google Scholar] [CrossRef]
- Coldibeli, B.; Sartori, E.R. Development of environmentally friendly voltammetric methods for the individual and simultaneous determination of antihypertensives: Validation and application. Diam. Relat. Mater. 2024, 142, 110787. [Google Scholar] [CrossRef]
- Ali, H.S.; Yardım, Y. Simultaneous estimation of total phenolic and alkaloid contents in the tea samples by utilizing the catechin and caffeine oxidation signals through the square-wave voltammetry. Food Chem. 2024, 441, 138262. [Google Scholar] [CrossRef]
- Beck, F. Cyclic Voltammetry—Simulation and Analysis of Reaction Mechanisms. Electroanalysis 1995, 7, 298. [Google Scholar] [CrossRef]
- Özok, H.İ.; Kıran, M.; Yunusoğlu, O.; Yardım, Y. The First Electroanalytical Study of Umifenovir (Arbidol) Used as a Potential Antiviral Drug for the Treatment of SARS-CoV-2: A Voltammetric Quantification on the Boron-Doped Diamond Electrode by Using Anionic Surfactant Media. J. Electrochem. Soc. 2023, 170, 016501. [Google Scholar] [CrossRef]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G. A Statistical Overview of Standard (IUPAC and ACS) and New Procedures for Determining the Limits of Detection and Quantification: Application to Voltammetric and Stripping Techniques. Pure Appl. Chem. 1997, 69, 297–328. [Google Scholar] [CrossRef]



| Technique (Sensor) | Linear Range (µmol L−1) | LOD (µmol L−1) | Matrix Type | Ref. |
|---|---|---|---|---|
| Spectroscopic Methods | ||||
| PLS—NIRS | – | 0.22–9.55 | plasma samples | [10] |
| Spectrophotometry | 497.31–7193.29 | – | pharmaceutical samples | [11] |
| Spectrophotometry | 8.88–35.52 | 1.13 | pharmaceutical samples | [12] |
| Spectrofluorimetry | 1.13–11.10 | 0.09 | pharmaceutical samples | [12] |
| FI–CL | 0.89–355.22 | 0.27 | pharmaceutical samples | [13] |
| Chromatographic Methods | ||||
| HPLC–UV | – | 0.018 | plasma samples | [14] |
| UHPLC–HESI–MS/MS | 0.0044–8.88 | 0.0022 | plasma samples | [15] |
| LC–ESI–MS | 0.022–0.22 | 0.0044 | aqueous humor | [16] |
| Voltammetric Methods | ||||
| LCAdSV (MWNTs-DHP film-coated GCE) | 0.08–10.0 | 0.03 | tablets | [8] |
| DPAdSV (C60/GCE) | 0.09–6.0 | 0.0148 | pharmaceutical samples, human urine, and blood plasma | [1] |
| LCAdSV (GCE/TFM) | 0.09–0.53 | 0.001 | synthetic sample that contains antiretroviral drugs or ATP and DNA | [7] |
| SWV (FTO) | 4.0–40.0 | 1.25 | pharmaceutical samples | [17] |
| DPAdSV (Cysteic acid (DES)/nano-NaOH/GCE) | 0.03–0.1 0.1–3.2 | 0.008 | tablets and biological fluids | [6] |
| DPV (UTGE and GCE) | (UTGE) 4–70 (GCE) 2–100 | (UTGE) 1.0 (GCE) 0.35 | spiked human urine | [5] |
| DPV (PEBT/GCE) | 0.03–0.3 0.3–1.5 | 0.012 | human blood serum, pharmaceutical formulations | [18] |
| LSAdSV (OPPY/CNT/GCE) | 0.03–10.0 | 0.01 | pharmaceutical and clinical preparations | [2] |
| DPV (PGE) | 1.0–100.0 | 0.3 | pharmaceutical formulations | [19] |
| SWAdSV (NC/GPE) | 0.05–1.0 | 0.0002 | pharmaceutical and biological samples | [20] |
| SWV (GC/OPPy/Acy) | 0.5–10.0 | 0.2 | pharmaceutical samples | [21] |
| LSAdSV (FeMoO4-GO/GCE) | 0.1–10.0 10.0–100 | 0.02 | drug samples | [22] |
| DPV (β-CD/TiO2 NPs/CPE) | 0.09–2.98 2.98–47.61 | 0.021 | blood serum samples | [23] |
| LSAdSV (rGO–TiO2–Au/GCE) | 1.0–100 | 0.3 | tablet samples | [24] |
| SWV (MBZ/TMHPP Cu(II)-modified GE) | 0.01–1000.0 | 0.01 | pharmaceutical formulations and urine samples | [25] |
| SWAdSV (β-CD/EPPGE) | 0.05–0.6 1.0–9.0 | 0.00759 | tablets, human urine | [26] |
| DPV (CdO/Fe3O4/CPE) | 1.0–100.0 | 0.3 | tablets, blood serum, and urine samples | [27] |
| DPAASV (Ag NPs/CdS NWs/RG/GCE) | 0.01–4.0 4.0–40.0 | 0.0033 | blood serum, tablet, and topical cream samples | [28] |
| DPAASV (CS-MWCNTs+TiO2 NPs/PCC/nanoporous GCE) | 0.03–1.0 | 0.01 | human fluid and tablet samples | [29] |
| DPV (P-OAP/MWCNTs-ZnO NPs-CPE) | 0.399–35.36 | 0.3 | pharmaceutical formulations | [30] |
| SWAdSV (EPPEG) | 0.5–3.0 | 0.0607 | pharmaceutical formulations and human plasma | [31] |
| LSV (AuNPs/CNTs-COOH/GSE) | 0.192–52.0 52.0–200.0 | 0.057 | pharmaceutical preparations | [32] |
| SWV (ND@Dy2O3-IL/CPE) | 0.097–116.6 | 0.029 | human serum sample | [33] |
| DPV (p-ABSA-GCE) | 0.2–9.0 | 0.0557 | tablets | [34] |
| SWV (SWNT/Naf/GCE) | 0.01–30.0 | 0.0018 | human urine sample | [35] |
| CV (polyGly-GOred-GCE) | 5.0–5000.0 | – | pharmaceutical preparations | [36] |
| DPV (rGO/Pd@PACP/PGE) | 0.1–0.5 | 0.0513 | – | [37] |
| SWV (CGMDE) | 0.2–2.0 | 0.07 | – | [38] |
| DPAdSV (RuNPs/TBA/PGE) | 0.003–0.030 0.030–3.0 | 0.0008 | tablet and urine samples | [39] |
| DPV (GC/CNT/ILC/RGO/MnO2) | 0.01–30.0 | 0.000843 | human serum | [40] |
| SWV (γ-Fe2O3-Bent/CPE) | 0.5–8.0 | 0.00155 | pharmaceutical and urine samples | [43] |
| DPAdSV (aSPCE) | 0.0005–0.05 0.005–1.0 | 0.00012 | tablets | [44] |
| DPV (BDDE) | 0.0001–0.001 0.001–0.01 0.01–50.0 | 0.0000299 | municipal water samples and tablets | This work |
| Parameter | Value |
|---|---|
| Electrochemical cleaning step | 1.4 V (5 s) |
| Analytical signal recording range | from 0.1 V to 1.4 V |
| Scan rate (ν) | 175 mV s−1 |
| Amplitude (ΔEA) | 125 mV |
| Modulation time (tm) | 10 ms |
| Equilibrium time | 5 s |
| Electrochemical Cleaning Step | RSD (%) |
|---|---|
| - | 10.2 |
| 1.2 V (10 s) | 13.1 |
| 1.4 V (10 s) | 6.64 |
| 1.4 V (5 s) | 3.99 |
| Parameter | Measurement Method | ||
|---|---|---|---|
| DPV LOD: 0.0299 nmol L−1 LOQ: 0.0995 nmol L−1 | UHPLC-DAD LOD: 32 nmol L−1 | UHPLC-MS (TOF) LOD: 1.1 nmol L−1 | |
| Tablet (Declared Value = 200 mg) | |||
| Found ± SD (n = 3) | 188.93 ± 9.45 mg | 192.51 ± 5.2 mg | 191.92 ± 5.7 mg |
| Recovery ± Coefficient of variation | 94.47 ± 5.00% | 96.26 ± 2.58% | 95.96 ± 2.97% |
| Parameter | Purified municipal wastewater | ||
| Found ± SD (n = 3) | 1.34 ± 0.0659 nmol L−1 | >LOD >LOQ | Detected >LOQ |
| Recovery * ± Coefficient of variation | 92.27 ± 2.59% | 93.42 ± 8.10% | 95.21 ± 8.61% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorylewski, D.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I. Fast, Simple, and Sensitive Voltammetric Measurements of Acyclovir in Real Samples via Boron-Doped Diamond Electrode. Materials 2024, 17, 4480. https://doi.org/10.3390/ma17184480
Gorylewski D, Tyszczuk-Rotko K, Wójciak M, Sowa I. Fast, Simple, and Sensitive Voltammetric Measurements of Acyclovir in Real Samples via Boron-Doped Diamond Electrode. Materials. 2024; 17(18):4480. https://doi.org/10.3390/ma17184480
Chicago/Turabian StyleGorylewski, Damian, Katarzyna Tyszczuk-Rotko, Magdalena Wójciak, and Ireneusz Sowa. 2024. "Fast, Simple, and Sensitive Voltammetric Measurements of Acyclovir in Real Samples via Boron-Doped Diamond Electrode" Materials 17, no. 18: 4480. https://doi.org/10.3390/ma17184480
APA StyleGorylewski, D., Tyszczuk-Rotko, K., Wójciak, M., & Sowa, I. (2024). Fast, Simple, and Sensitive Voltammetric Measurements of Acyclovir in Real Samples via Boron-Doped Diamond Electrode. Materials, 17(18), 4480. https://doi.org/10.3390/ma17184480







