One-Step Air Spraying of Structural Coating on Cu Alloy as Superhydrophobic Surface for Enhanced Corrosion Resistance and Anti-Icing Performance
Abstract
1. Introduction
2. Sample Preparation and Characterization
2.1. Materials
2.2. Preparation
2.3. Characterization
3. Results and Discussion
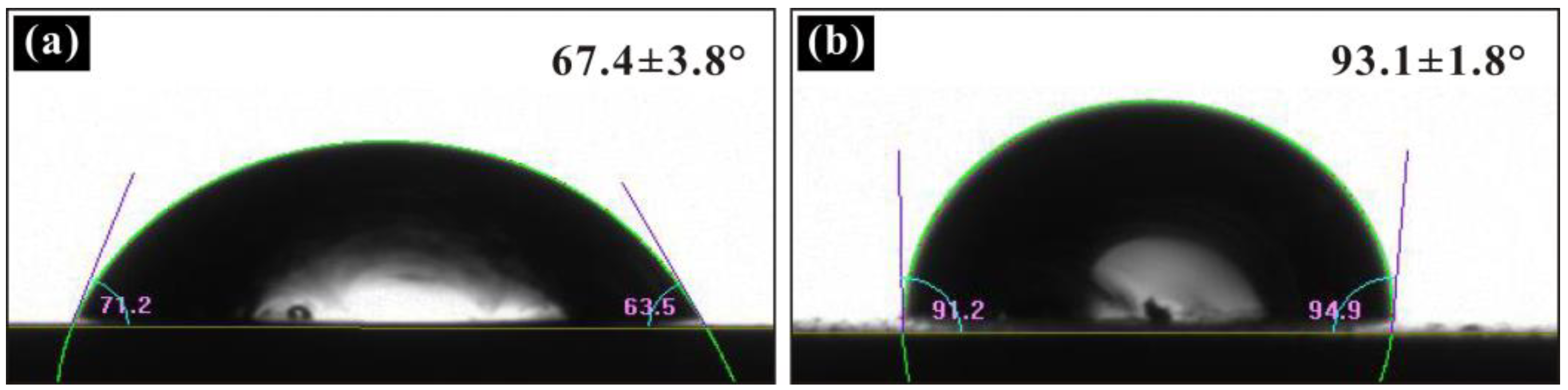
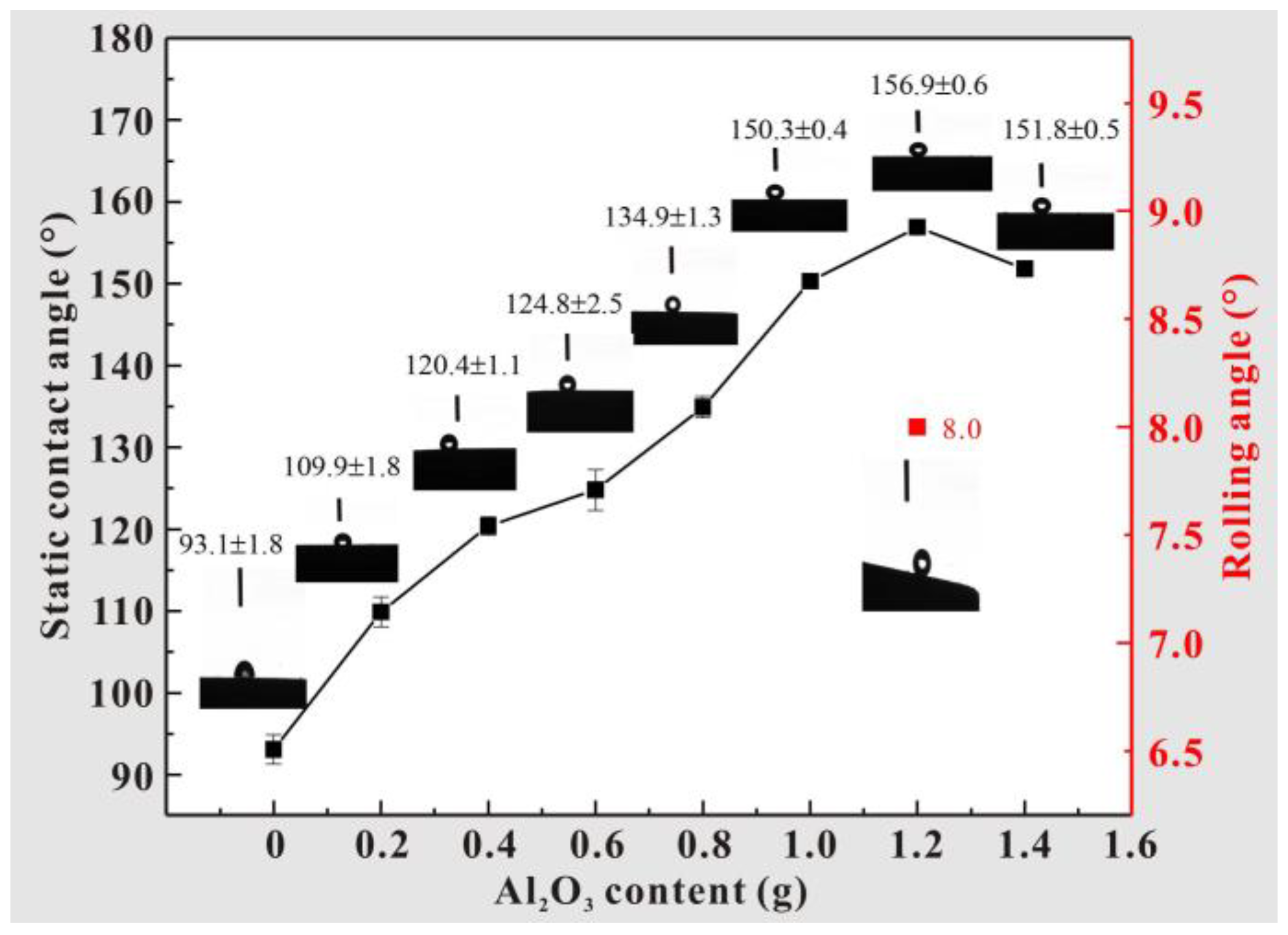
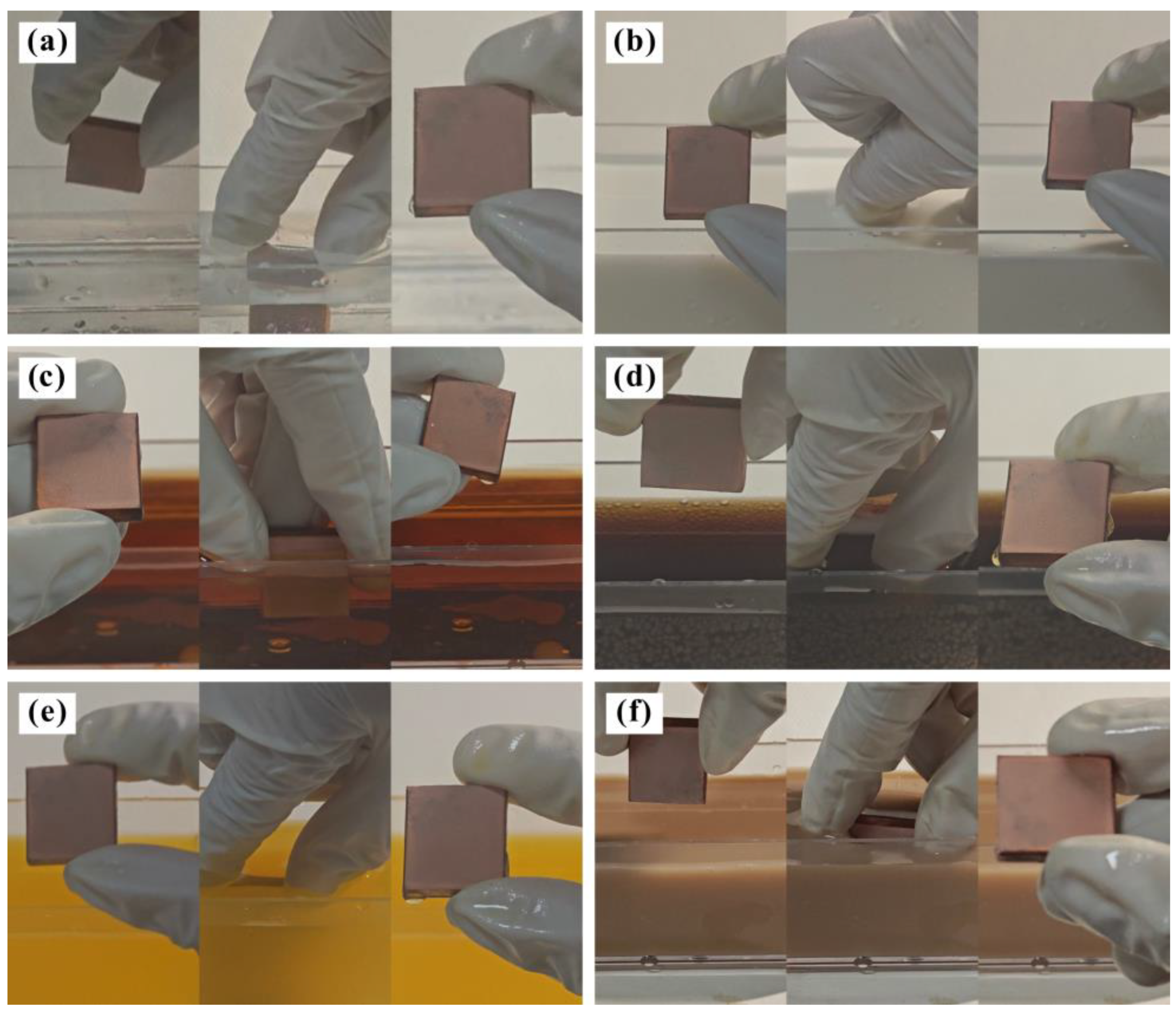
3.1. Coating Wettability Test
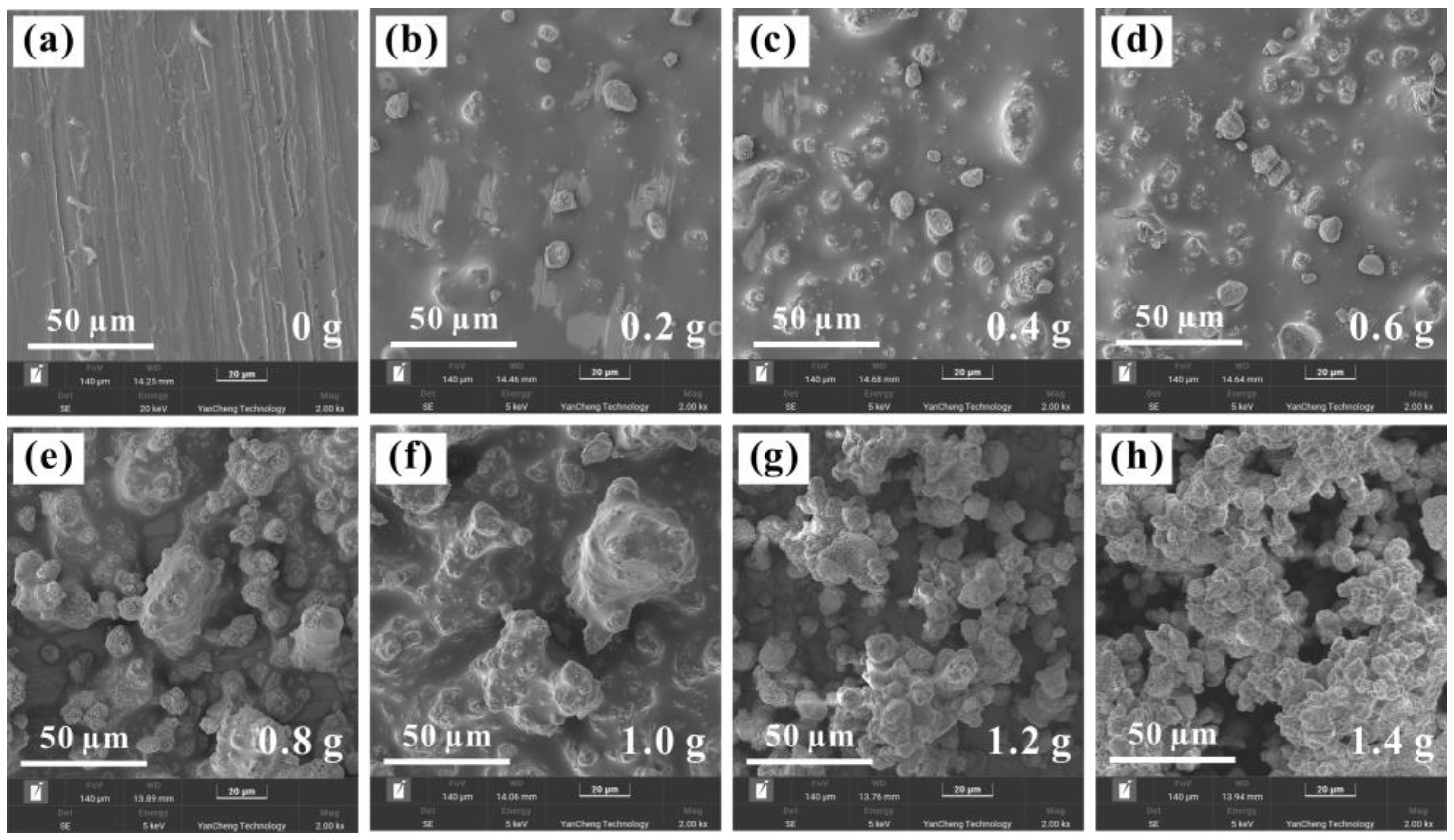
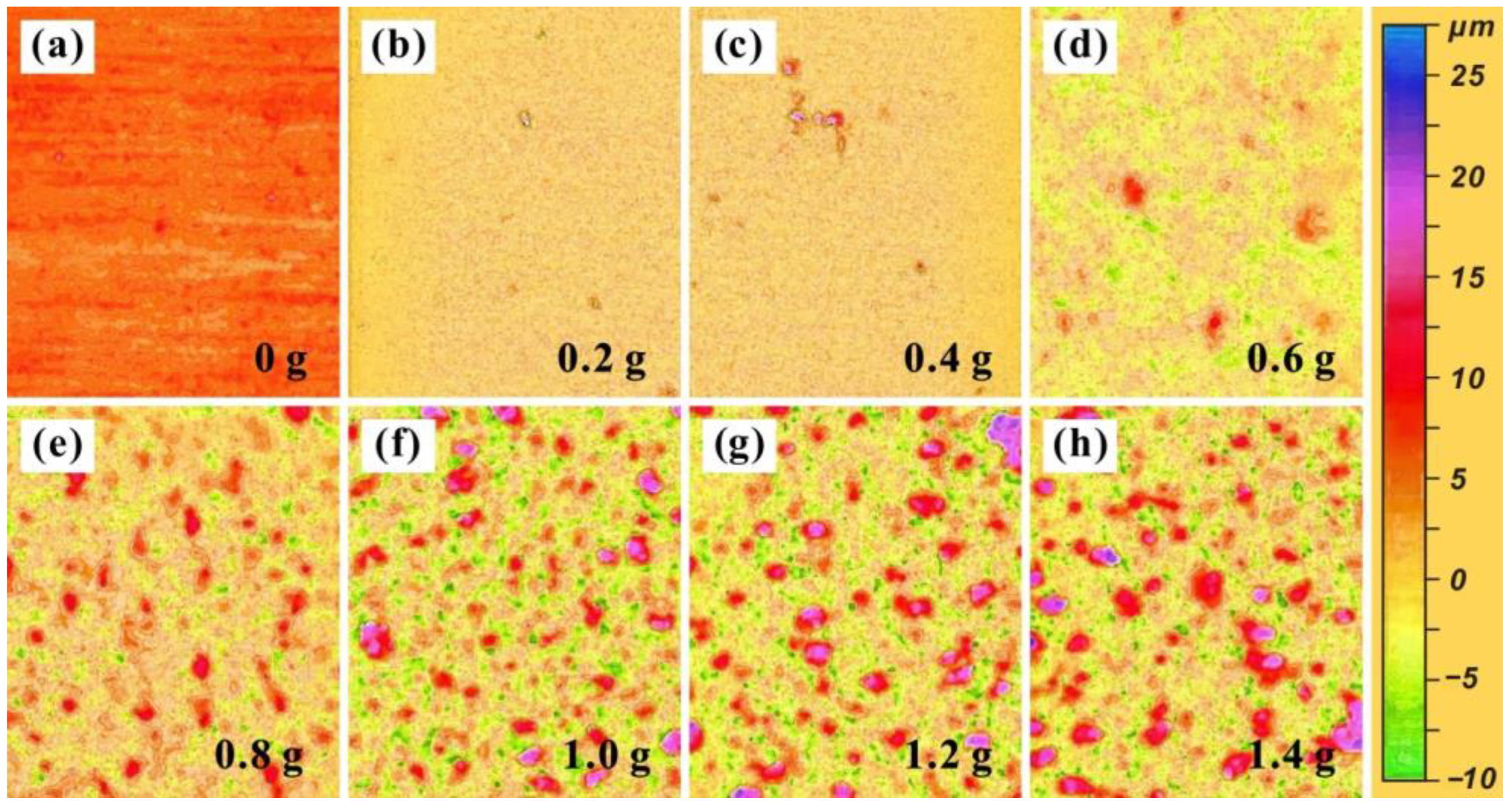
3.2. Coating Morphology Analysis
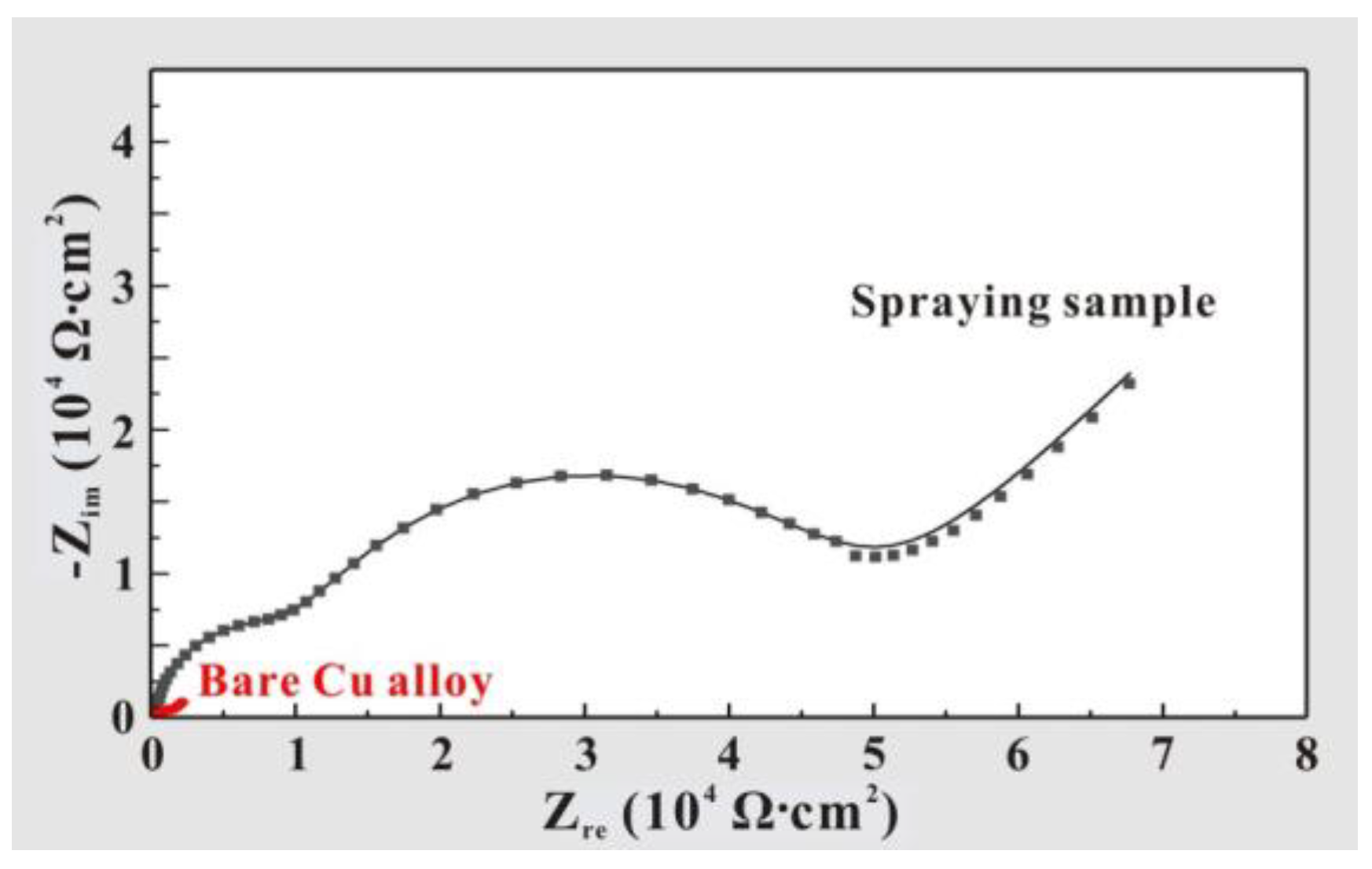
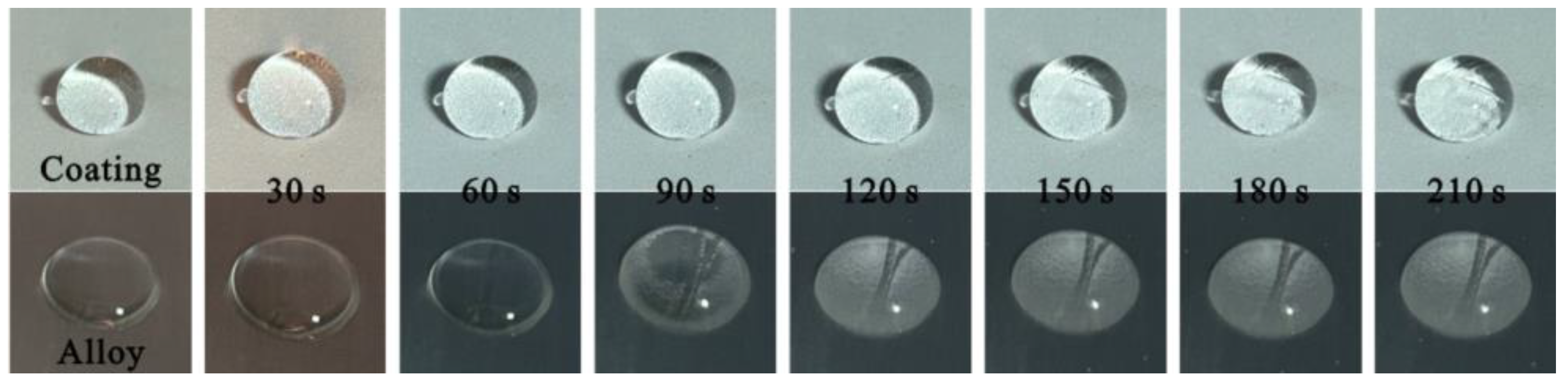
3.3. Multifunctional Behavior Testing
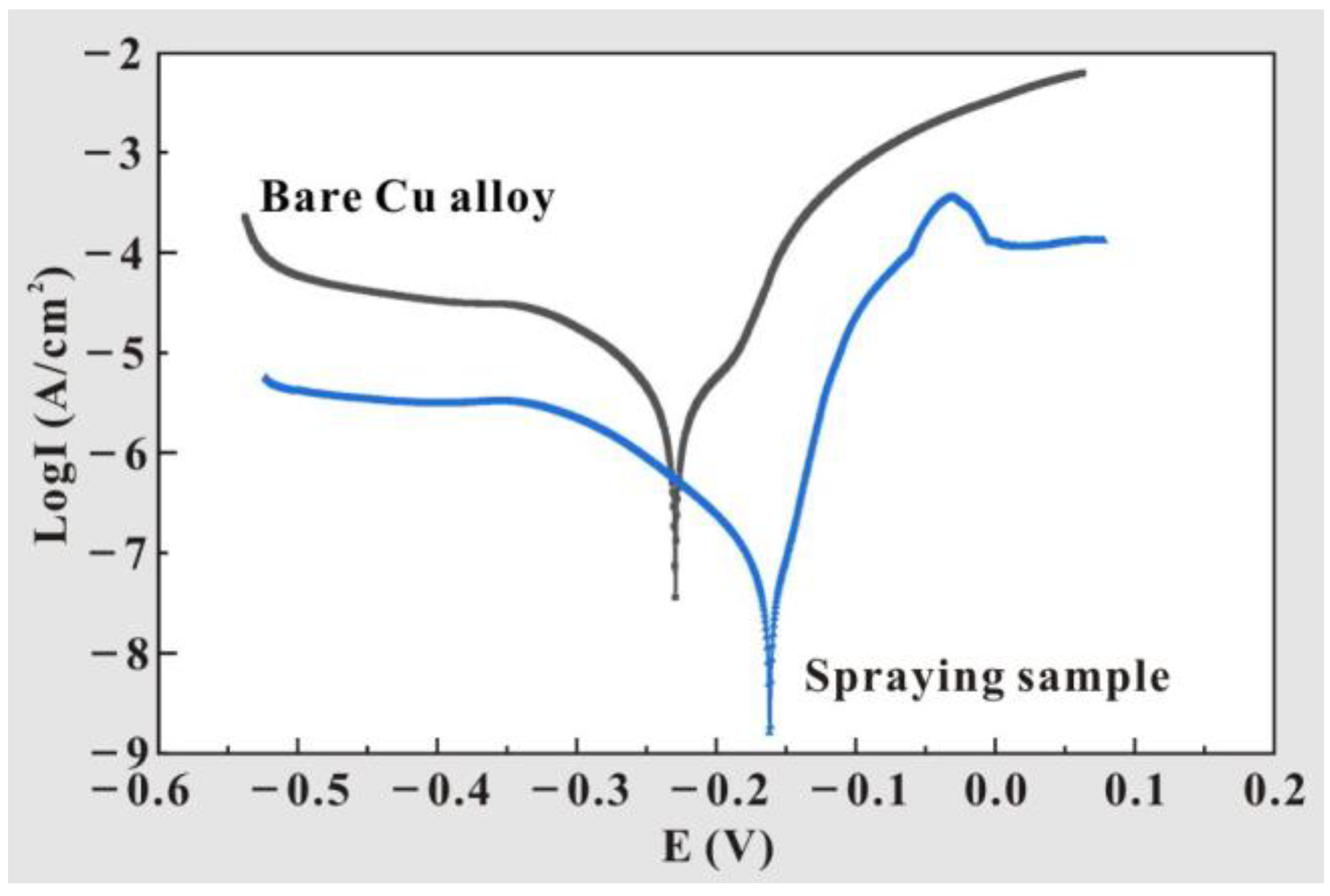
| Samples | Corrosion Potential (V) | Corrosion Current Density (A/cm2) |
|---|---|---|
| T2 Cu alloy | −0.23 | 3.61 × 10−8 |
| Al2O3-PDMS composite coating | −0.16 | 1.60 × 10−9 |
4. Conclusions
- (1)
- Using air spraying technology, a micro–nano-structured coating with layered characteristics was prepared on the surface of a T2 Cu alloy. By adjusting the content of Al2O3, the wettability of the coating surface was optimized. When the content of Al2O3 reaches 1.2 g, the contact angle obtains a maximum value of 156.9 ± 0.6°, and the rolling angle is less than 10°, thus exhibiting excellent superhydrophobic characteristics.
- (2)
- The structural coating prepared by air spraying changed the state of the high-viscosity solid–liquid contact interface. Instead, the interface characteristic is a composite three-phase contact state of solid–gas–liquid. Under this condition, the prepared micro–nano-structure can capture more air phases and then produce an “air cushion” effect to hold up the liquid. Ultimately, the viscous resistance of the coating to water nearly disappears, thus exhibiting excellent superhydrophobic characteristics, anti-pollution behavior, and corrosion resistance.
- (3)
- The water droplet on the Cu alloy substrate completely solidifies and freezes at 150 s. However, the water droplet on the Al2O3-PDMS composite coating does not fully freeze until 210 s. This is because the prepared micro–nano-structure can capture a large amount of air, which makes the heat transfer coefficient of the coating surface much lower than that of the Cu alloy substrate, thus achieving a delay effect in the icing process.
- (4)
- For future work, it is necessary to gain a deeper understanding of the anti-icing mechanism of as-prepared superhydrophobic coatings by using numerical simulation. A new simulation model should also be developed to predict the anti-icing performance of as-prepared coatings under different conditions, thus providing valuable theoretical guidance for experimental research. Meanwhile, the fractional description of thermal transport could be useful if such as-prepared nanoscale samples are considered for future research [44].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bident, A.; Delange, F.; Labrugere, J.F. Fabrication and characterization of copper and copper alloys reinforced with graphene. J. Compos. Mater. 2024, 58, 109–117. [Google Scholar] [CrossRef]
- Amalia, L.; Li, Y.; Bei, H. Copper effects on the microstructures and deformation mechanisms of CoCrFeNi high entropy alloys. Appl. Phys. Lett. 2024, 124, 141901. [Google Scholar] [CrossRef]
- Danladi, F.I.; Rawat, A.; Adak, A.K. Synthesis and Tuning of Electronic, Optical, and Photoelectrochemical Properties of Copper Metavanadate Alloys via Alkaline Earth Metal Substitution. ECS J. Solid State Sci. Technol. 2024, 13, 073010. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Ma, G. Laser cladding Mo-based coatings on copper alloys to improve the current-carrying tribological properties of Cu/Al friction pairs. Surf. Coat. Technol. 2024, 487, 130989. [Google Scholar] [CrossRef]
- Izaguirre, I.; De, P.J.; Rosero-Romo, J.J. Exploration and optimization of copper-based alloys incorporating amorphizing elements for heat transfer applications. Mater. Charact. 2024, 208, 113675–113684. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Xu, L. Magnetic field-assisted surface engineering technology for active regulation of Fe3O4 medium to enable the laccase electrochemical biosensing of catechol with visible stripe patterns. Anal. Chim. Acta 2024, 1311, 342739. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qi, W.; Yu, M. Synthesis of Gemini-type imidazoline quaternary ammonium salt using by-product fatty acid as corrosion inhibitor for Q235 steel. Sci. Rep. 2024, 14, 13854. [Google Scholar] [CrossRef]
- Niu, Z.B.; Li, D.; Jia, D.Y. Comparative study on the microstructure evolution and crystallization behavior of precursor-derived and mechanical alloying derived SiBCN. J. Eur. Ceram. Soc. 2024, 44, 668–678. [Google Scholar] [CrossRef]
- Hochman, L.G. Laser treatment of onychomycosis using a novel 0.65-millisecond pulsed Nd:YAG 1064-nm laser. J. Cutan. Laser Ther. 2011, 13, 2–5. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. 2015, 24, 811–820. [Google Scholar] [CrossRef]
- Nandan, R.; Debroy, T.; Bhadeshia, H.K.D.H. Recent advances in friction-stir welding—Process, weldment structure and properties. Prog. Mater. Sci. 2008, 53, 980–1023. [Google Scholar] [CrossRef]
- Cheng, J.; Luo, X.; Shen, Z. Study on Vegetation Concrete Technology for Slope Protection and Greening Engineering. Pol. J. Environ. Stud. 2020, 29, 4017–4028. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, L.; Lei, S. Effect of lotus seed resistant starch on the bioconversion pathway of taurocholic acid by regulating the intestinal microbiota. Int. J. Biol. Macromol. 2024, 266, 131174. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, A.B.N.; Norfazilah, W.I.W.; Farzuhana, H.M.Z.N. Synthesis of a water-based TEOS–PDMS sol–gel coating for hydrophobic cotton and polyester fabrics. New J. Chem. 2024, 48, 933–950. [Google Scholar]
- Wang, J.; Yu, S.; Yin, X. Fabrication of cross-like ZIF-L structures with water repellency and self-cleaning property via a simple in-situ growth strategy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126731. [Google Scholar] [CrossRef]
- Schutzius, T.M.; Bayer, I.S.; Qin, J. Water-Based, Nonfluorinated Dispersions for Environmentally Benign, Large-Area, Superhydrophobic Coatings. ACS Appl. Mater. Interfaces 2013, 5, 13419–13425. [Google Scholar] [CrossRef]
- Mcbride, S.; Lake, J.; Varanasi, K. Self-ejection of salts and other foulants from superhydrophobic surfaces to enable sustainable anti-fouling. J. Chem. Phys. 2023, 158, 134721. [Google Scholar] [CrossRef]
- Li, X.W.; Ma, C.H.; Shi, T. Waterborne robust superhydrophobic PFDTES@TiO2-PU coating with stable corrosion resistance, long-term environmental adaptability, and delayed icing functions on Al-Li alloy. J. Mater. Res. Technol. 2024, 32, 3357–3370. [Google Scholar] [CrossRef]
- Lee, H.; Yang, J.; Kim, D. Anti-frosting characteristics of superhydrophobic-hydrophilic wettability switchable surfaces. Int. J. Heat Mass Transf. 2024, 221, 125035. [Google Scholar] [CrossRef]
- Stratakis, E.; Mateescu, A.; Barberoglou, M. From superhydrophobicity and water repellency to superhydrophilicity: Smart polymer-functionalized surfaces. Chem. Commun. 2010, 46, 4136–4138. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Sun, S. Tunable Adhesive Self-Cleaning Coating with Superhydrophobicity and Photocatalytic Activity. Nanomaterials 2021, 11, 1486. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Ajmal, Z.; Heng, S.L. Fabrication of a sustainable superhydrophobic surface of Ag-NPs@SA on copper alloy for corrosion resistance, photocatalysis, and simulated distribution of Ag atoms. Analyst 2024, 149, 3245–3262. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.G.; Cao, H.J.; Min, Y.L. Airborne hydrocarbon induced multifunctional coating towards bouncing characteristics, icing delaying, hot-water repellency, and anti-corrosion on copper. Vacuum 2023, 216, 112447. [Google Scholar] [CrossRef]
- Rajput, A.; Mishra, A.; Ali, A. Transparent superhydrophobic coating for copper metal and study of surface morphology and antimicrobial characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133067. [Google Scholar] [CrossRef]
- Ma, F.; Shen, L.; Zeng, Z. Fabricating a slippery porous nickel coating based on the hydrogen bubble template method for corrosion protection with self-healing property. Surf. Topogr. Metrol. Prop. 2022, 10, 045004. [Google Scholar] [CrossRef]
- Liu, L.; Ma, F.; Liu, L. Fluorine-free superhydrophobic coating with mechanical interlocking and high corrosion resistance. Prog. Org. Coat. 2022, 168, 106871. [Google Scholar]
- Li, H.; Sun, Y.; Wang, Z. Constructing Superhydrophobic Surface on Copper Substrate with Dealloying-Forming and Solution-Immersion Method. Materials 2022, 15, 4816. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, D.; Liu, K. An all-in-one bio-inspired superhydrophobic coating with mechanical/chemical/physical robustness. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128803. [Google Scholar] [CrossRef]
- Ye, Y.; Kang, Z.; Wang, F. Achieving hierarchical structure with superhydrophobicity and enhanced anti-corrosion via electrochemical etching and chemical vapor deposition. Appl. Surf. Sci. 2023, 610, 155362. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Guo, W. Effect of TiO2 content on the thermal control properties of Al2O3-xTiO2 composite coatings prepared by supersonic plasma spraying technology. J. Mater. Res. Technol. 2024, 32, 3582–3593. [Google Scholar] [CrossRef]
- Younes, G.; Price, G.; Dandurand, Y. Study of Moisture-Curable Hybrid NIPUs Based on Glycerol with Various Diamines: Emergent Advantages of PDMS Diamines. ACS Omega 2020, 5, 30657–30670. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, Y.; Zhang, Y. The effect of micro/nanostructures formed by laser ablation on the superhydrophobicity of AZ31B magnesium alloy. J. Mater. Res. 2024, 39, 850–863. [Google Scholar] [CrossRef]
- Yang, C.; Hao, H. The apparent contact angle of water droplet on the micro-structured hydrophobic surface. Sci. China Chem. 2010, 53, 912–916. [Google Scholar] [CrossRef]
- Vuellers, F.; Peppou-Chapman, S.; Kavalenka, M.N. Effect of repeated immersions and contamination on plastron stability in superhydrophobic surfaces. Phys. Fluids 2019, 31, 012102. [Google Scholar] [CrossRef]
- Mollaamin, F.; Shahriari, S.; Monajjemi, M. Influence of Transition Metals for Emergence of Energy Storage in Fuel Cells through Hydrogen Adsorption on the MgAl Surface. Russ. J. Phys. Chem. B 2024, 18, 398–418. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, S.; Su, W. Construction of a durable superhydrophobic surface based on the oxygen inhibition layer of organosilicon resins. Thin Solid Film. 2021, 717, 138467. [Google Scholar] [CrossRef]
- Wu, S.W.; Jiang, Q.T.; Yuan, S. Environmentally friendly expanded graphite-doped ZnO superhydrophobic coating with good corrosion resistance in marine environment. Rare Met. 2023, 42, 3075–3087. [Google Scholar] [CrossRef]
- Li, X.; Su, H.; Li, H. Photothermal superhydrophobic surface with good corrosion resistance, anti-/de-icing property and mechanical robustness fabricated via multiple-pulse laser ablation. Appl. Surf. Sci. 2024, 646, 158944. [Google Scholar] [CrossRef]
- Wang, P.; Cai, D. Preparation of graphene-modified anticorrosion coating and study on its corrosion resistance mechanism. Int. J. Photoenergy 2020, 2020, 8846644. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, J.F.; Wang, W.L. Wear and corrosion properties of Cu-AlN composite coatings deposited by cold spray. J. Mater. Res. Technol. 2024, 30, 3986–3995. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Z.; Tao, J. Spraying preparation of eco-friendly superhydrophobic coatings with ultralow water adhesion for effective anticorrosion and antipollution. ACS Appl. Mater. Interfaces 2020, 12, 25484–25493. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhao, H.; Ju, D. Study on Material Design and Corrosion Resistance Based on Multi-Principal Component Alloying Theory. Materials 2023, 16, 1939. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhou, C.; Liu, R. Fabrication of superhydrophobic surface with enhanced corrosion resistance on H62 brass substrate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124475. [Google Scholar] [CrossRef]
- Hernández-Acosta, M.A.; Martines-Arano, H.; Soto-Ruvalcaba, L. Fractional thermal transport and twisted light induced by an optical two-wave mixing in single-wall carbon nanotubes. Int. J. Therm. Sci. 2020, 147, 106136. [Google Scholar] [CrossRef]







| Element | Cu | Ag | O | Sb | As | Fe | Pb | S | Others |
|---|---|---|---|---|---|---|---|---|---|
| Mass fraction (wt%) | 99.90 | 0.001 | 0.06 | 0.002 | 0.002 | 0.005 | 0.005 | 0.005 | 0.02 |
| Materials | Specifications |
|---|---|
| Aluminum oxide particles | 50 nm, 99.8% |
| Polydimethylsiloxane | SYLGARD™ 184 |
| Silicone rubber curing agent | SYLGARD™ 184 |
| N-hexane | 97.0% |
| Liquid | Static Contact Angle (°) | Rolling Angle (°) |
|---|---|---|
| Water | 156.3 ± 0.6 | 8.0 ± 0.2 |
| Tea | 156.4 ± 0.3 | 8.5 ± 1.0 |
| Milk | 151.2 ± 2.0 | 8.9 ± 1.1 |
| Soy milk | 154.4 ± 1.4 | 8.5 ± 0.8 |
| Cola | 155.7 ± 0.4 | 8.1 ± 0.5 |
| Samples | Electrolyte | Rct (Ω⋅cm 2) | Reference |
|---|---|---|---|
| Graphene coating | 3.5 wt% NaCl solution | 3768 | [39] |
| Cu-AlN coating | 3.5 wt% NaCl solution | 3200 | [40] |
| Bio-based coating | 3.5 wt% NaCl solution | 10,670 | [41] |
| Al2O3-PDMS coating | 3.5 wt% NaCl solution | 12,000 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Li, X. One-Step Air Spraying of Structural Coating on Cu Alloy as Superhydrophobic Surface for Enhanced Corrosion Resistance and Anti-Icing Performance. Materials 2024, 17, 4485. https://doi.org/10.3390/ma17184485
Li B, Li X. One-Step Air Spraying of Structural Coating on Cu Alloy as Superhydrophobic Surface for Enhanced Corrosion Resistance and Anti-Icing Performance. Materials. 2024; 17(18):4485. https://doi.org/10.3390/ma17184485
Chicago/Turabian StyleLi, Ben, and Xuewu Li. 2024. "One-Step Air Spraying of Structural Coating on Cu Alloy as Superhydrophobic Surface for Enhanced Corrosion Resistance and Anti-Icing Performance" Materials 17, no. 18: 4485. https://doi.org/10.3390/ma17184485
APA StyleLi, B., & Li, X. (2024). One-Step Air Spraying of Structural Coating on Cu Alloy as Superhydrophobic Surface for Enhanced Corrosion Resistance and Anti-Icing Performance. Materials, 17(18), 4485. https://doi.org/10.3390/ma17184485






