Synthesis of Fe2O3/TiO2 Photocatalytic Composites for Methylene Blue Degradation as a Novel Strategy for High-Value Utilisation of Iron Scales
Abstract
1. Introduction
- (1)
- Differences in research purpose and motivation: This paper focuses on the high-value utilisation of Iron Scales (ISs). According to the estimate of global crude steel production in 2022 (1.885 billion tons), iron scale production is as high as 37.7 million tons, and this study proposes to use it as a raw material to prepare Fe2O3/TiO2 photocatalytic composite materials and treat liquid waste (organic pollutants). This is not only an innovation in the recycling method of iron scale but also significantly improves its added value.
- (2)
- Innovation in raw material source and preparation technology: Although the existing research mainly uses iron nitrate (Fe(NO3)3) as a precursor to prepare Fe2O3 by hydrometallurgy, this study uses a dry process to convert ISs into high-purity (greater than 98.5wt.%), porous Fe2O3 particles. This study not only realised the efficient conversion of traditional waste represented by iron scale for the first time but also provided an additional site for the adsorption of TiO2 nanoparticles, thereby improving the efficiency of Fe2O3 as a carrier of catalytic materials and opening up a new way of low-cost preparation.
2. Materials and Methods
2.1. Experimental Materials and Preparation Process
2.2. Performance Testing and Characterisation
3. Results
3.1. Characterization of Fe2O3/TiO2 Photocatalytic Composites
3.1.1. XRD Patterns
3.1.2. SEM-EDS Analysis
3.1.3. FTIR Analysis
3.1.4. XPS Analysis
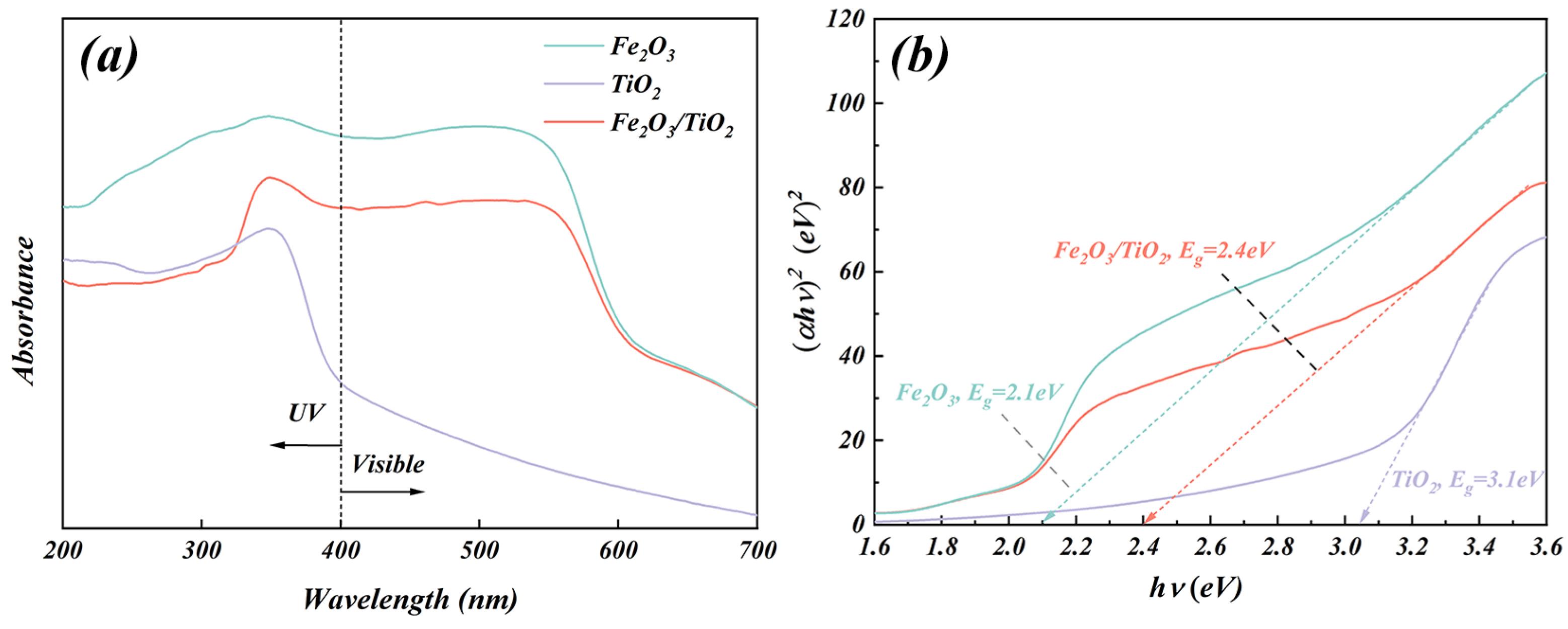
3.1.5. UV-Vis Diffuse Reflection Spectroscopy (DRS) Analysis
3.1.6. PL Analysis
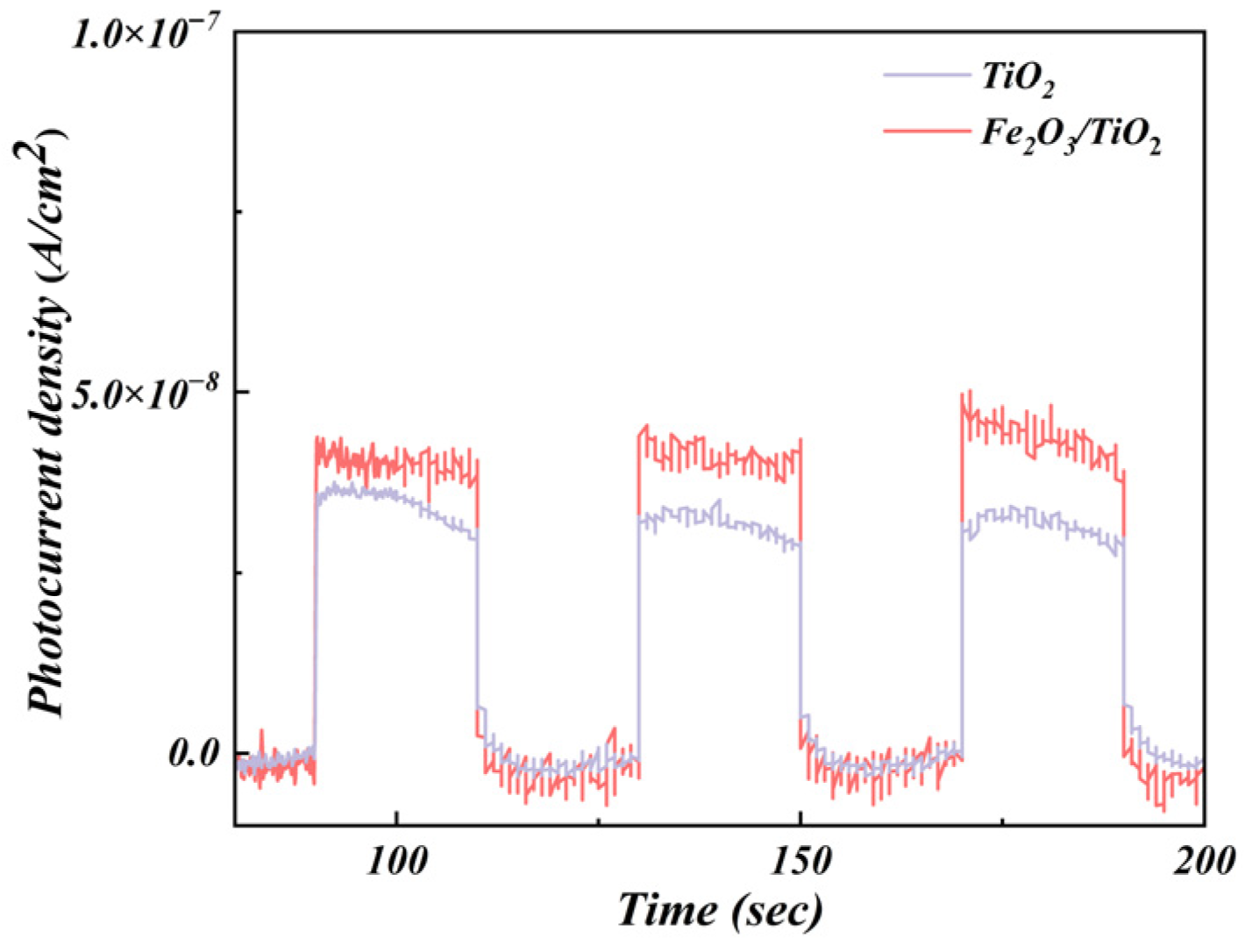
3.1.7. Photocurrent Density Analysis
3.2. Analysis of the Photodegradation of MB
3.2.1. Effect of Reaction Time
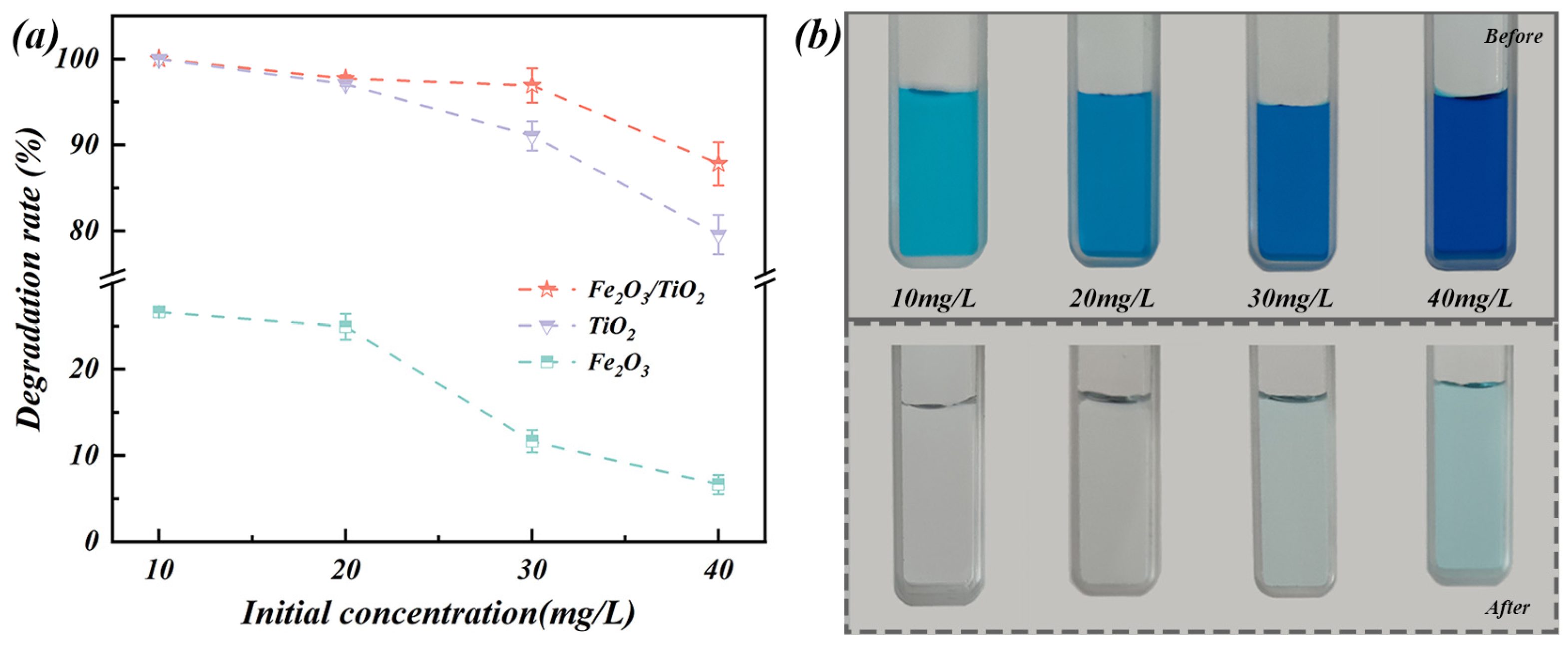
3.2.2. Effect of Initial Concentration
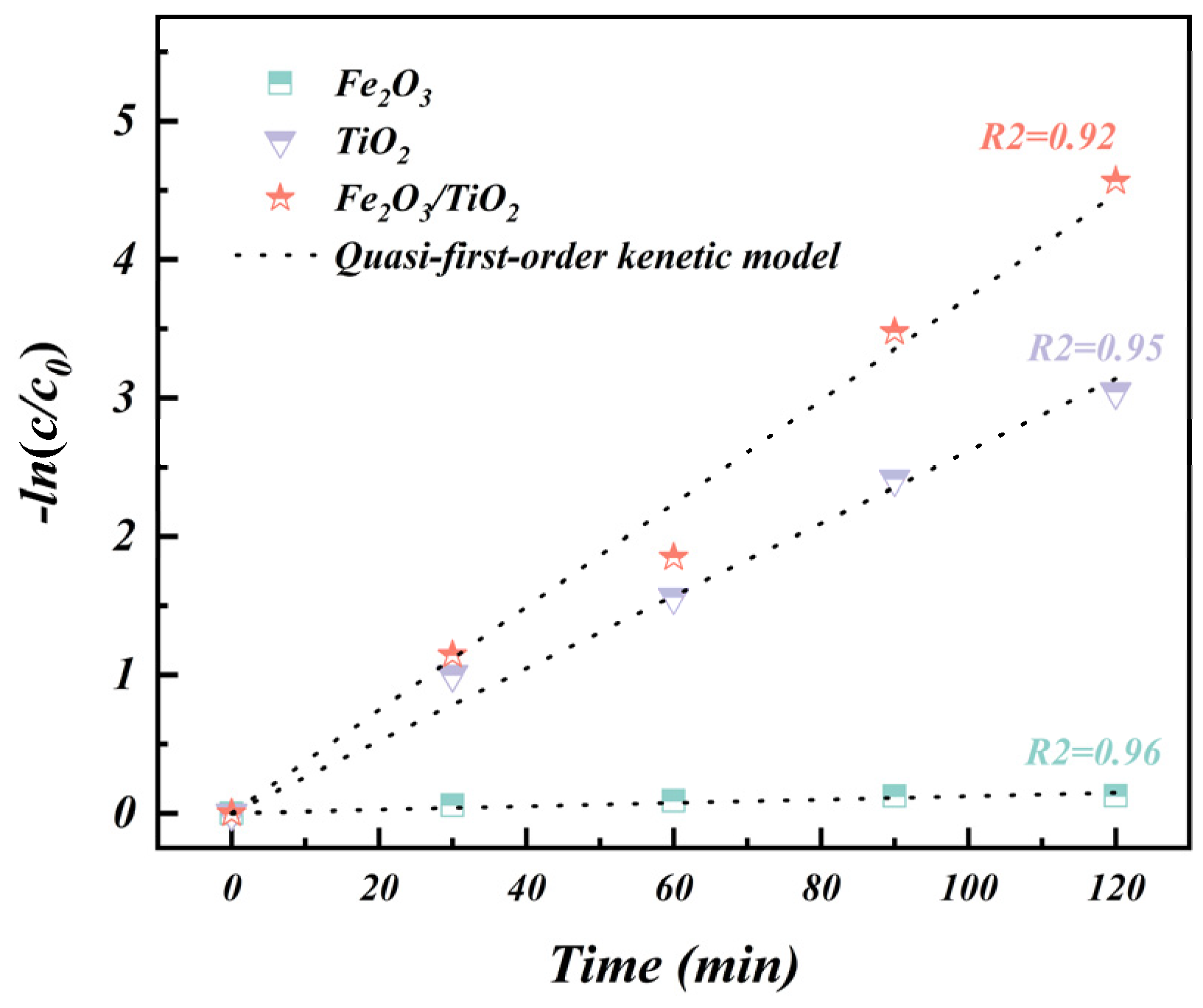
3.2.3. Reaction Kinetics Study
3.2.4. Material Reusability
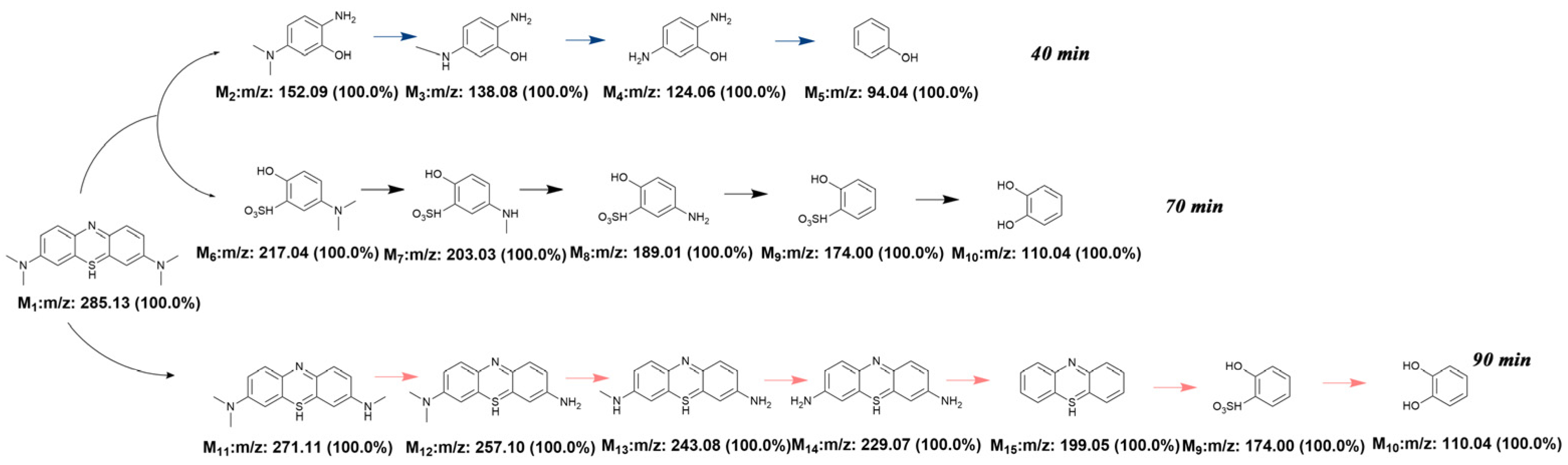
3.3. Degradation Pathways of MB Dye
4. Discussion
5. Conclusions
- (1)
- The oxidation roasting process successfully transforms ISs into highly pure, porous Fe2O3 particles, providing additional sites for the adsorption of the TiO2 nanoparticles on the composites. This enables the Fe2O3 particles to act as effective carriers for the catalytic materials. In the sol-gel process, a photocatalytic composite comprising Fe2O3 particles and anatase TiO2 nanoparticles as the core and shell, respectively, is formed. The loading of Fe2O3 barely affects the crystal structure and grain size of anatase TiO2.
- (2)
- The Fe2O3/TiO2 photocatalytic composites exhibit outstanding removal performance of MB organic pollutants. Under optimised experimental conditions, the MB removal rate reaches an impressive 97.71% with good reusability after four photocatalytic cycles. Additionally, during dye degradation, the reaction process follows a quasi-first-order kinetic model with a rate constant of 0.038 min−1, which is 1.5 times higher than that of the unloaded TiO2 particles (k = 0.026 min−1) and 38 times higher than that of the Fe2O3 particles (k = 0.001 min−1).
- (3)
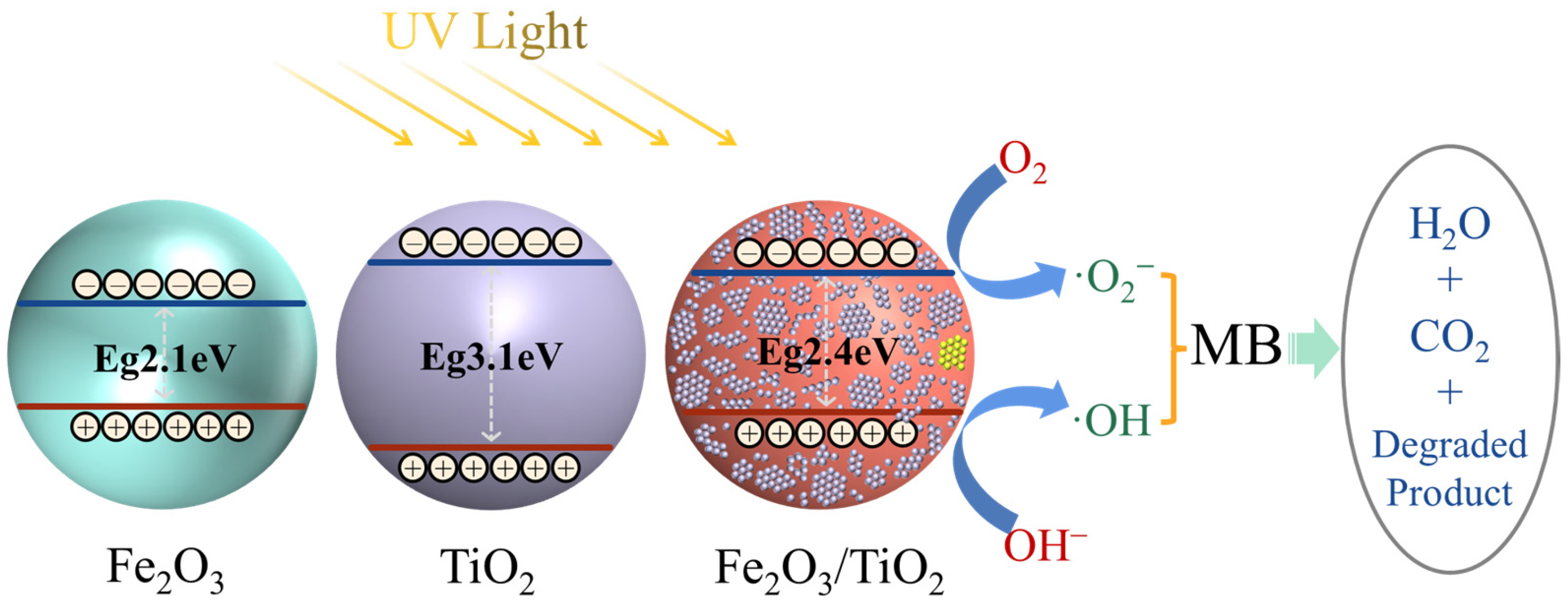
- The incorporation of Fe2O3 as a support enhances the separation of the electron-hole pairs in TiO2 and improves the transfer rate of the photogenerated carriers. Furthermore, it extends the lifetime of the photogenerated electrons and holes. The presence of Fe2O3 further reduces the bandgap (2.4 eV) of the Fe2O3/TiO2 photocatalytic composites, considerably contributing to its high photocatalytic activity.
6. Prospect
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, J.; Xiong, Y. Electrochemical oxidation technology: A review of its application in high-efficiency treatment of wastewater containing persistent organic pollutants. J. Water Process Eng. 2021, 44, 102308. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Alhaz Uddin, M.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Ye, S.; Chen, Y.; Yao, X.; Zhang, J. Simultaneous removal of organic pollutants and heavy metals in wastewater by photoelectrocatalysis: A review. Chemosphere 2021, 273, 128503. [Google Scholar] [CrossRef]
- Nordin, N.; Ho, L.N.; Ong, S.A.; Ibrahim, A.H.; Rani, A.L.A.; Lee, S.L.; Ong, Y.P. Hydroxyl radical formation in the hybrid system of photocatalytic fuel cell and peroxi-coagulation process affected by iron plate and UV light. Chemosphere 2020, 244, 125459. [Google Scholar] [CrossRef]
- Gowthaman, K.; Gowthaman, P.; Venkatachalam, M.; Saroja, M.; Kutraleeswaran, M.; Dhinesh, S. Design and synthesis of TiO2/ZnO nanocomposite with enhanced oxygen vacancy: Better photocatalytic removal of MB dye under visible light-driven condition. Inorg. Chem. Commun. 2022, 146, 110197. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Duan, B.; Zhang, M. Modified sol-gel synthesis of carbon nanotubes supported titania composites with enhanced visible light induced photocatalytic activity. J. Nanomater. 2016, 2016, 3967156. [Google Scholar] [CrossRef]
- Yildiz, A.; Yesilbas, Ö.F.; Nas, M.S.; Calimli, M.H.; Bayat, R.; Sen, F. In situ preparation of TiO2/f-MWCNT catalyst using Pluronic F127 assisted sol-gel process for sonocatalytic degradation of methylene blue. Environ. Res. 2023, 231, 115972. [Google Scholar] [CrossRef]
- Ardani, M.R.; Pang, A.L.; Pal, U.; Haniff, M.A.S.M.; Ismail, A.G.; Hamzah, A.A.; Khanday, W.A.; Ahmadipour, M. Ultrasonic-assisted of TiO2-MWCNT nanocomposite with advanced photocatalytic efficiency for elimination of dye pollutions. Diam. Relat. Mater. 2023, 137, 110066. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Bouziani, A.; Park, J.; Ozturk, A. Synthesis of α-Fe2O3/TiO2 heterogeneous composites by the sol-gel process and their photocatalytic activity. J. Photochem. Photobiol. A Chem. 2020, 400, 112718. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Montini, T.; Bontempi, E.; Zafaeiratos, S.; Jaén, J.J.D.; Fornasiero, P. Synthesis and photocatalytic application of visible-light active β-Fe2O3/g-C3N4 hybrid nanocomposites. Appl. Catal. B Environ. 2016, 187, 171–180. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, K.; Gao, G.; Ren, J.; Duan, R.; Fang, Y.; Hu, X. Singlet oxygen generation boosted by Ag–Pt nanoalloy combined with disordered surface layer over TiO2 nanosheet for improving the photocatalytic activity. Appl. Surf. Sci. 2021, 538, 147944. [Google Scholar] [CrossRef]
- Moulahoum, H.; Ghorbanizamani, F.; Sakarya, S.; Timur, S. Lightless catalytic layered chitosan coating film using doped TiO2@ metal ions nanoparticles for highly efficient dye degradation in aqueous media and disinfection applications. Prog. Org. Coat. 2022, 169, 106923. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Sattar, M.A.; Jahirul, M.I. Phenol degradation of waste and stormwater on a flat plate photocatalytic reactor with TiO2 on glass slide: An experimental and modelling investigation. J. Water Process Eng. 2022, 47, 102769. [Google Scholar] [CrossRef]
- Guo, M.Z.; Li, J.S.; Poon, C.S. Improved photocatalytic nitrogen oxides removal using recycled glass-nano-TiO2 composites with NaOH pre-treatment. J. Clean. Prod. 2019, 209, 1095–1104. [Google Scholar] [CrossRef]
- Gu, S.; Liu, X.; Wang, H.; Liu, Z.; Xing, H.; Yu, L. Preparation and characterization of TiO2 photocatalytic composites supported by blast furnace slag fibres for wastewater degradation. Ceram. Int. 2023, 49, 5180–5188. [Google Scholar] [CrossRef]
- Valášková, M.; Kočí, K.; Madejová, J.; Matějová, L.; Pavlovský, J.; Barrocas, B.T.; Klemencová, K. α-Fe2O3 Nanoparticles/Iron-Containing Vermiculite Composites: Structural, Textural, Optical and Photocatalytic Properties. Minerals 2022, 12, 607. [Google Scholar] [CrossRef]
- Curcio, M.S.; Canela, M.C.; Waldman, W.R. Selective surface modification of TiO2-coated polypropylene by photodegradation. Eur. Polym. J. 2018, 101, 177–182. [Google Scholar] [CrossRef]
- Sheng, W.; Shi, J.L.; Hao, H.; Li, X.; Lang, X. Polyimide-TiO2 hybrid photocatalysis: Visible light-promoted selective aerobic oxidation of amines. Chem. Eng. J. 2020, 379, 122399. [Google Scholar] [CrossRef]
- Mansor, E.S.; Abdallah, H.; Shaban, A.M. Development of TiO2/polyvinyl alcohol-cellulose acetate nanocomposite reverse osmosis membrane for groundwater-surface water interfaces purification. Mater. Sci. Eng. B 2023, 289, 116222. [Google Scholar] [CrossRef]
- Dell’Edera, M.; Porto, C.L.; De Pasquale, I.; Petronella, F.; Curri, M.L.; Agostiano, A.; Comparelli, R. Photocatalytic TiO2-based coatings for environmental applications. Catal. Today 2021, 380, 62–83. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Lai, Q.; Fan, M. Review of the progress in preparing nano TiO2: An important environmental engineering material. J. Environ. Sci. 2014, 26, 2139–2177. [Google Scholar] [CrossRef]
- Song, J.; Guan, R.; Xie, M.; Dong, P.; Yang, X.; Zhang, J. Advances in electrospun TiO2 nanofibers: Design, construction, and applications. Chem. Eng. J. 2022, 431, 134343. [Google Scholar] [CrossRef]
- Song, Q.; Zhao, H.; Jia, J.; Yang, L.; Lv, W.; Bao, J.; Shu, X.; Gu, Q.; Zhang, P. Pyrolysis of municipal solid waste with iron-based additives: A study on the kinetic, product distribution and catalytic mechanisms. J. Clean. Prod. 2020, 258, 120682. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Tang, X.; Wang, Y.; An, H.; Yi, H. Decarbonization pathways of China’s iron and steel industry toward carbon neutrality. Resour. Conserv. Recycl. 2023, 194, 106994. [Google Scholar] [CrossRef]
- Nur-A-Tomal, M.S.; Biswal, S.; Pahlevani, F.; Sahajwalla, V. Recycling tannery solid wastes as an alternative carbon resource for ironmaking. J. Mater. Cycles Waste Manag. 2022, 24, 2030–2040. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Shamsabadipour, A.; Mahtabian, S.; Ahmadi, M.; Rahdar, A.; Díez-Pascual, A.M. Role of iron oxide (Fe2O3) nanocomposites in advanced biomedical applications: A state-of-the-art review. Nanomaterials 2022, 12, 3873. [Google Scholar] [CrossRef] [PubMed]
- Trenczek-Zajac, A.; Synowiec, M.; Zakrzewska, K.; Zazakowny, K.; Kowalski, K.; Dziedzic, A.; Radecka, M. Scavenger-Supported photocatalytic evidence of an extended Type I electronic structure of the TiO2@Fe2O3 interface. ACS Appl. Mater. Interfaces 2022, 14, 38255–38269. [Google Scholar] [CrossRef] [PubMed]
- Etshindo, L.A.; Sousa, C.; Tamiasso-Martinhon, P.; Colaço, M.V.; Camara, A.R.; Rocha, A.S. SnO2-TiO2 materials for photocatalytic degradation of cationic dye under UV and visible light and a chitosan composite film investigation. Catal. Today 2025, 444, 114995. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ahmed, S. Easy and green synthesis of TiO2 (Anatase and Rutile): Estimation of crystallite size using Scherrer equation, Williamson-Hall plot, Monshi-Scherrer Model, size-strain plot, Halder- Wagner Model. Results Mater. 2023, 20, 100492. [Google Scholar] [CrossRef]
- Liebig, F.; Thünemann, A.F.; Koetz, J. Ostwald ripening growth mechanism of gold nanotriangles in vesicular template phases. Langmuir 2016, 32, 10928–10935. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Ni, G.; Lu, J.; Shi, S.; Li, M.; Ye, Q. Spectroscopic (FTIR, 1H NMR) and SEM investigation of physicochemical structure changes of coal subjected to microwave-assisted oxidant stimulation. Fuel 2022, 317, 123473. [Google Scholar] [CrossRef]
- Van Viet, P.; Van Chuyen, D.; Hien, N.Q.; Duy, N.N.; Thi, C.M. Visible-Light-Induced photo-Fenton degradation of rhodamine B over Fe2O3-diatomite materials. J. Sci. Adv. Mater. Devices 2020, 5, 308–315. [Google Scholar] [CrossRef]
- Abega, A.V.; Ngomo, H.M.; Nongwe, I.; Mukaya, H.E.; Sone, P.M.A.K.; Mbianda, X.Y. Easy and convenient synthesis of CNT/TiO2 nanohybrid by in-surface oxidation of Ti3+ ions and application in the photocatalytic degradation of organic contaminants in water. Synth. Met. 2019, 251, 1–14. [Google Scholar] [CrossRef]
- Sleigh, C.; Pijpers, A.P.; Jaspers, A.; Coussens, B.; Meier, R.J. On the determination of atomic charge via ESCA including application to organometallics. J. Electron Spectrosc. Relat. Phenom. 1996, 77, 41–57. [Google Scholar] [CrossRef]
- Sanjines, R.; Tang, H.; Berger, H.; Gozzo, F.; Margaritondo, G.; Lévy, F. Electronic structure of anatase TiO2 oxide. J. Appl. Phys. 1994, 75, 2945–2951. [Google Scholar] [CrossRef]
- Wang, X.; Qin, M.; Fang, F.; Jia, B.; Wu, H.; Qu, X.; Volinsky, A.A. Effect of glycine on one-step solution combustion synthesis of magnetite nanoparticles. J. Alloys Compd. 2017, 719, 288–295. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina. Chem. Mater. 1990, 2, 186–191. [Google Scholar] [CrossRef]
- Haukka, S.; Lakomaa, E.L.; Jylha, O.; Vilhunen, J.; Hornytzkyj, S. Dispersion and distribution of titanium species bound to silica from titanium tetrachloride. Langmuir 1993, 9, 3497–3506. [Google Scholar] [CrossRef]
- Allen, G.C.; Curtis, M.T.; Hooper, A.J.; Tucker, P.M. X-ray photoelectron spectroscopy of iron–oxygen systems. J. Chem. Soc. Dalton Trans. 1974, 14, 1525–1530. [Google Scholar] [CrossRef]
- Wang, X.; Hu, C.; An, H.; Zhu, D.; Zhong, Y.; Wang, D.; Tang, C.; Sun, L.; Zhou, H. Photocatalytic removal of MB and hydrogen evolution in water by (Sr0.6Bi0.305)2Bi2O7/TiO2 heterostructures under visible-light irradiation. Appl. Surf. Sci. 2021, 544, 148920. [Google Scholar] [CrossRef]
- Zha, R.; Nadimicherla, R.; Guo, X. Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J. Mater. Chem. A 2015, 3, 6565–6574. [Google Scholar] [CrossRef]
- Bilal Tahir, M.; Nadeem Riaz, K.; Asiri, A.M. Boosting the performance of visible light-driven WO3/g-C3N4 anchored with BiVO4 nanoparticles for photocatalytic hydrogen evolution. Int. J. Energy Res. 2019, 43, 5747–5758. [Google Scholar] [CrossRef]
- Apopei, P.; Catrinescu, C.; Teodosiu, C.; Royer, S. Mixed-Phase TiO2 photocatalysts: Crystalline phase isolation and reconstruction, characterization and photocatalytic activity in the oxidation of 4-chlorophenol from aqueous effluents. Appl. Catal. B Environ. 2014, 160, 374–382. [Google Scholar] [CrossRef]
- Li, J.; Ren, J.; Hao, Y.; Zhou, E.; Wang, Y.; Wang, X.J.; Su, R.; Liu, Y.; Qi, X.H.; Li, F.T. Construction of β-Bi2O3/Bi2O2CO3 heterojunction photocatalyst for deep understanding the importance of separation efficiency and valence band position. J. Hazard. Mater. 2021, 401, 123262. [Google Scholar] [CrossRef]
- Jing, B.; Ao, Z.; Zhao, W.; Xu, Y.; Chen, Z.; An, T. Evaluation procedure of photocatalysts for VOCs degradation from the view of density functional theory calculations: G-C3N4 dots/graphene as an example. J. Mater. Chem. A 2020, 8, 20363–20372. [Google Scholar] [CrossRef]
- Chun, W.J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, Y.; Domen, K. Conduction and valence band positions of Ta2O5, TaON, and Ta3N5 by UPS and electrochemical methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, Y.F.; Du, X.J.; Huang, Q.; Chen, Z. Promoted photo-Fenton reactivity through electron transfer between non-contacted Au nanoparticles and Fe2O3 nanowires in a confined space. Environ. Sci. Nano 2023, 10, 1482–1493. [Google Scholar] [CrossRef]
- Duan, F.; Liu, C.; Liu, X.; Wang, L.; Zhang, S.; Liu, X. TiO2 immobilized on polyarylene ether nitrile/Fe3+ complex for efficient adsorption and photocatalytic degradation towards methylene blue. J. Alloys Compd. 2021, 875, 159951. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, W.; Sun, B.; Li, H.; Qiao, P.; Xu, Y.; Wu, J.; Lin, K.; Fu, H. Defects-Engineering of magnetic γ-Fe2O3 ultrathin nanosheets/mesoporous black TiO2 hollow sphere heterojunctions for efficient charge separation and the solar-driven photocatalytic mechanism of tetracycline degradation. Appl. Catal. B Environ. 2019, 240, 319–328. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, D.; Wang, D.; Kong, X.; Huang, J.; Wang, F.; Wu, Y. Size dependent photocatalytic activity of ZnS nanostructures prepared by a facile precipitation method. Mater. Sci. Eng. B 2016, 208, 15–21. [Google Scholar] [CrossRef]
- Cheng, N.; Zhang, L.; Wang, M.; Shu, J.; Shao, P.; Yang, L.; Meng, X.; Fan, Y.; Li, M. Highly effective recovery of palladium from a spent catalyst by an acid-and oxidation-resistant electrospun fibers as a sorbent. Chem. Eng. J. 2023, 466, 143171. [Google Scholar] [CrossRef]
- Feng, C.; Jiang, F.; Xiong, G.; Chen, C.; Wang, Z.; Pan, Y.; Fei, Z.; Lu, Y.; Li, X.; Zhang, R.; et al. Revelation of Mn4+-Osur-Mn3+ active site and combined Langmuir-Hinshelwood mechanism in propane total oxidation at low temperature over MnO2. Chem. Eng. J. 2023, 451, 138868. [Google Scholar] [CrossRef]
- Razdan, N.K.; Bhan, A. Catalytic site ensembles: A context to reexamine the Langmuir-Hinshelwood kinetic description. J. Catal. 2021, 404, 726–744. [Google Scholar] [CrossRef]
- Gao, X.; Yin, H.; Guo, C.; Yan, B.; Li, M.; Xin, L.; Wu, Z. Comprehensive removal of various dyes by thiourea modified chitosan/nano ZnS composite via enhanced photocatalysis: Performance and mechanism. Int. J. Biol. Macromol. 2023, 247, 125677. [Google Scholar] [CrossRef]
- Munguti, L.; Dejene, F. Influence of annealing temperature on structural, optical and photocatalytic properties of ZnO–TiO2 composites for application in dye removal in water. Nano-Struct. Nano-Objects 2020, 24, 100594. [Google Scholar] [CrossRef]
- Salgado, B.C.B.; Valentini, A. Evaluation of the photocatalytic activity of SiO2@ TiO2 hybrid spheres in the degradation of methylene blue and hydroxylation of benzene: Kinetic and mechanistic study. Braz. J. Chem. Eng. 2020, 36, 1501–1518. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Karthikeyan, P.; Park, C.M. Boosted insights of novel accordion-like (2D/2D) hybrid photocatalyst for the removal of cationic dyes: Mechanistic and degradation pathways. J. Environ. Manag. 2020, 273, 111125. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, J.; Hu, Z. Nano-Sized g-C3N4 thin layer@ CeO2 sphere core-shell photocatalyst combined with H2O2 to degrade doxycycline in water under visible light irradiation. Sep. Purif. Technol. 2019, 227, 115665. [Google Scholar] [CrossRef]
- Damdinova, S.; Liu, W.; Wang, S.; Dugarov, B.; Su, Z.; Cheng, Z. Preparation of coral-like Ag2MoO4–TiO2 heterostructure and its photocatalytic properties. Mater. Chem. Phys. 2019, 235, 121765. [Google Scholar] [CrossRef]
- Liu, B.; Wen, L.; Zhao, X. The photoluminescence spectroscopic study of anatase TiO2 prepared by magnetron sputtering. Mater. Chem. Phys. 2007, 106, 350–353. [Google Scholar] [CrossRef]
- Akhavan, O.; Azimirad, R. Photocatalytic property of Fe2O3 nanograin chains coated by TiO2 nanolayer in visible light irradiation. Appl. Catal. A Gen. 2009, 369, 77–82. [Google Scholar] [CrossRef]
- Rocha, H.F.; Silva, V.; Lima, D.L.; Calisto, V. Evaluation of the impact of photodegradation processes on the environmental persistence of amoxicillin. Case Stud. Chem. Environ. Eng. 2024, 9, 100724. [Google Scholar] [CrossRef]
- Kadıoğlu, E.N.; Öztürk, H.; Eroğlu, H.A.; Akbal, F.; Kuleyin, A.; Özkaraova, E.B. Artificial neural network modeling of Fenton-based advanced oxidation processes for recycling of textile wastewater. J. Ind. Eng. Chem. 2024, 136, 542–553. [Google Scholar] [CrossRef]
- Barrocas, B.; Chiavassa, L.D.; Oliveira, M.C.; Monteiro, O.C. Impact of Fe, Mn co-doping in titanate nanowires photocatalytic performance for emergent organic pollutants removal. Chemosphere 2020, 250, 126240. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Osawa, R.; Oliveira, M.C.; Monteiro, O.C. Enhancing Removal of Pollutants by Combining Photocatalysis and Photo-Fenton Using Co, Fe-Doped Titanate Nanowires. Materials 2023, 16, 2051. [Google Scholar] [CrossRef]
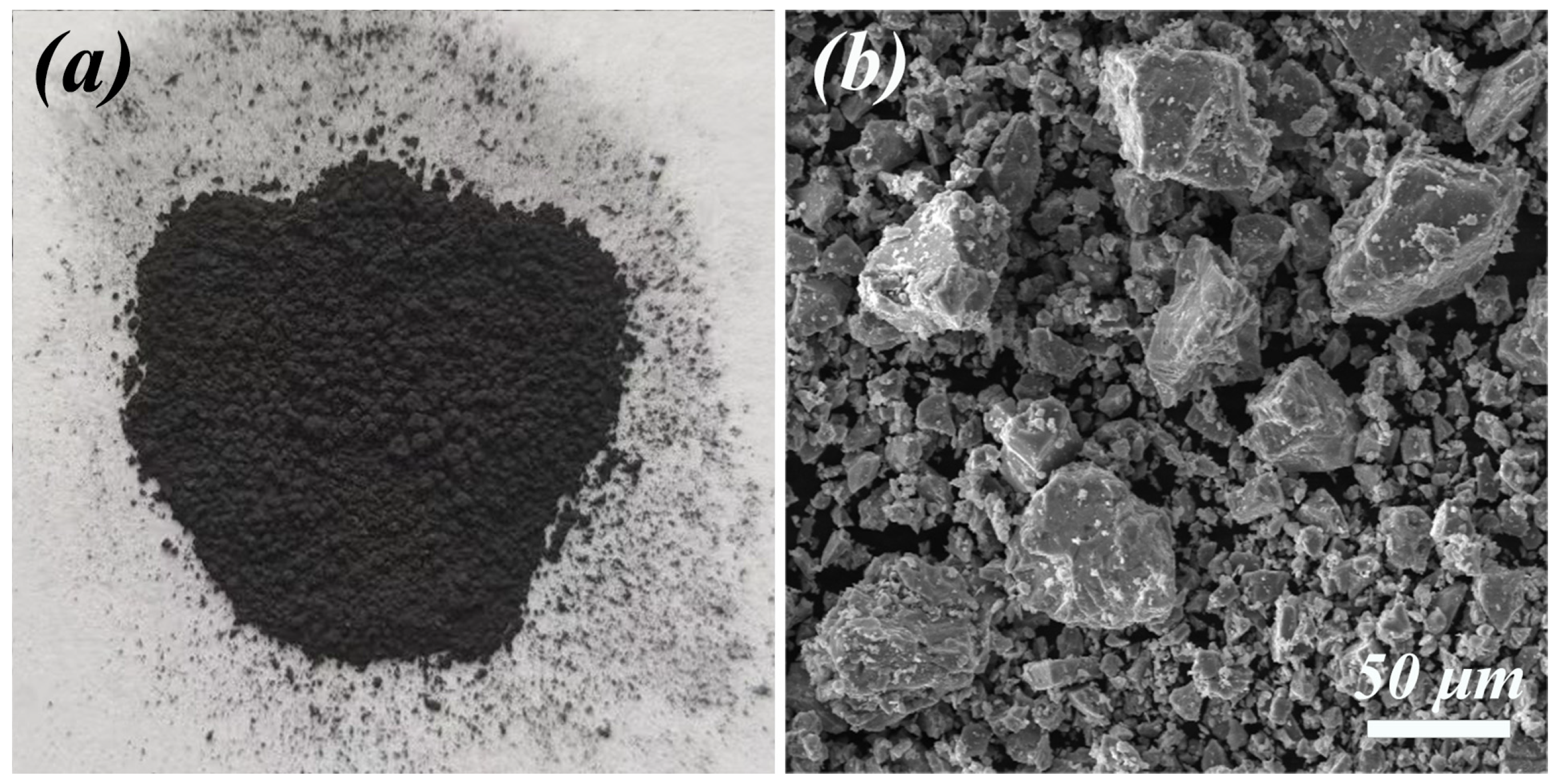
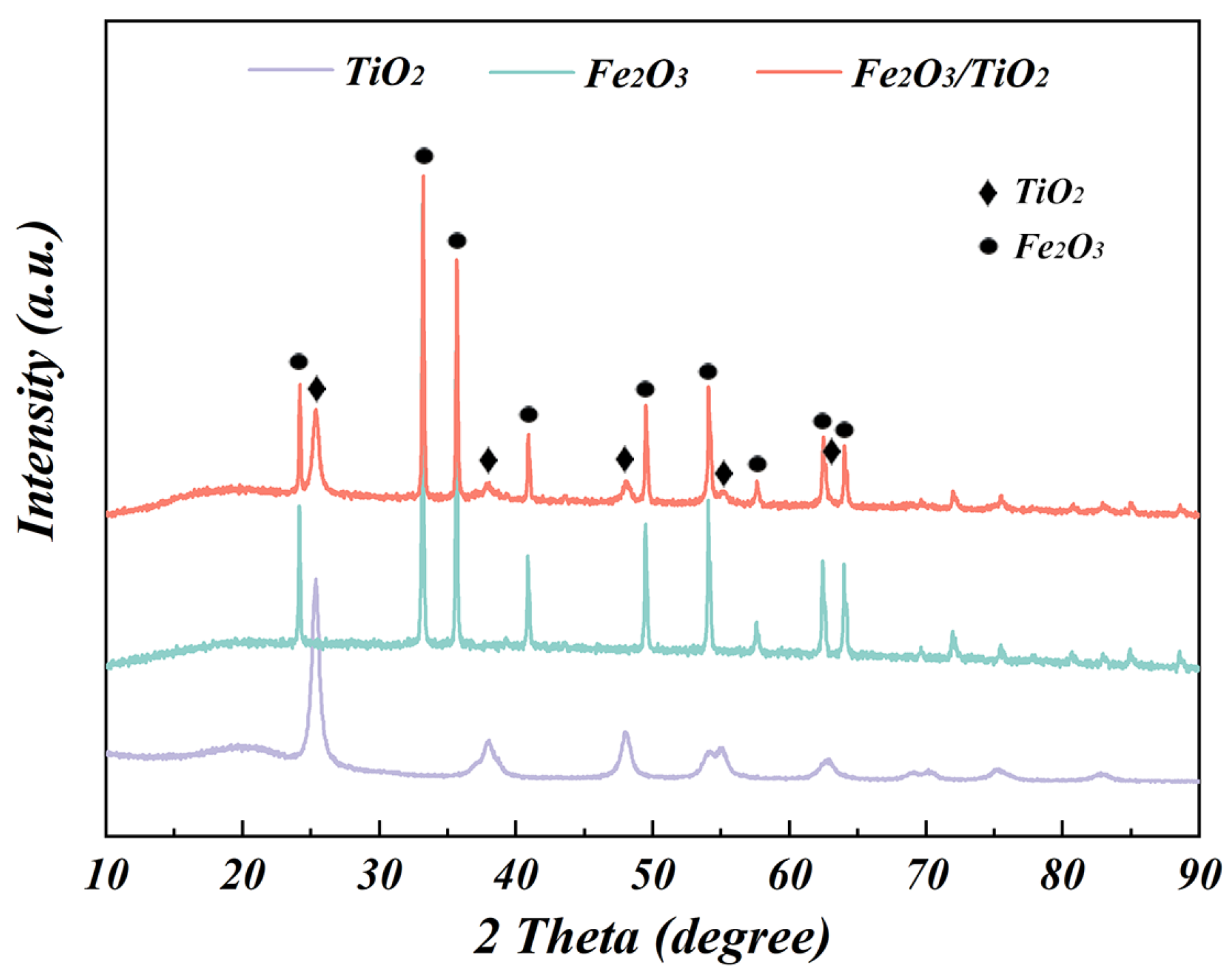
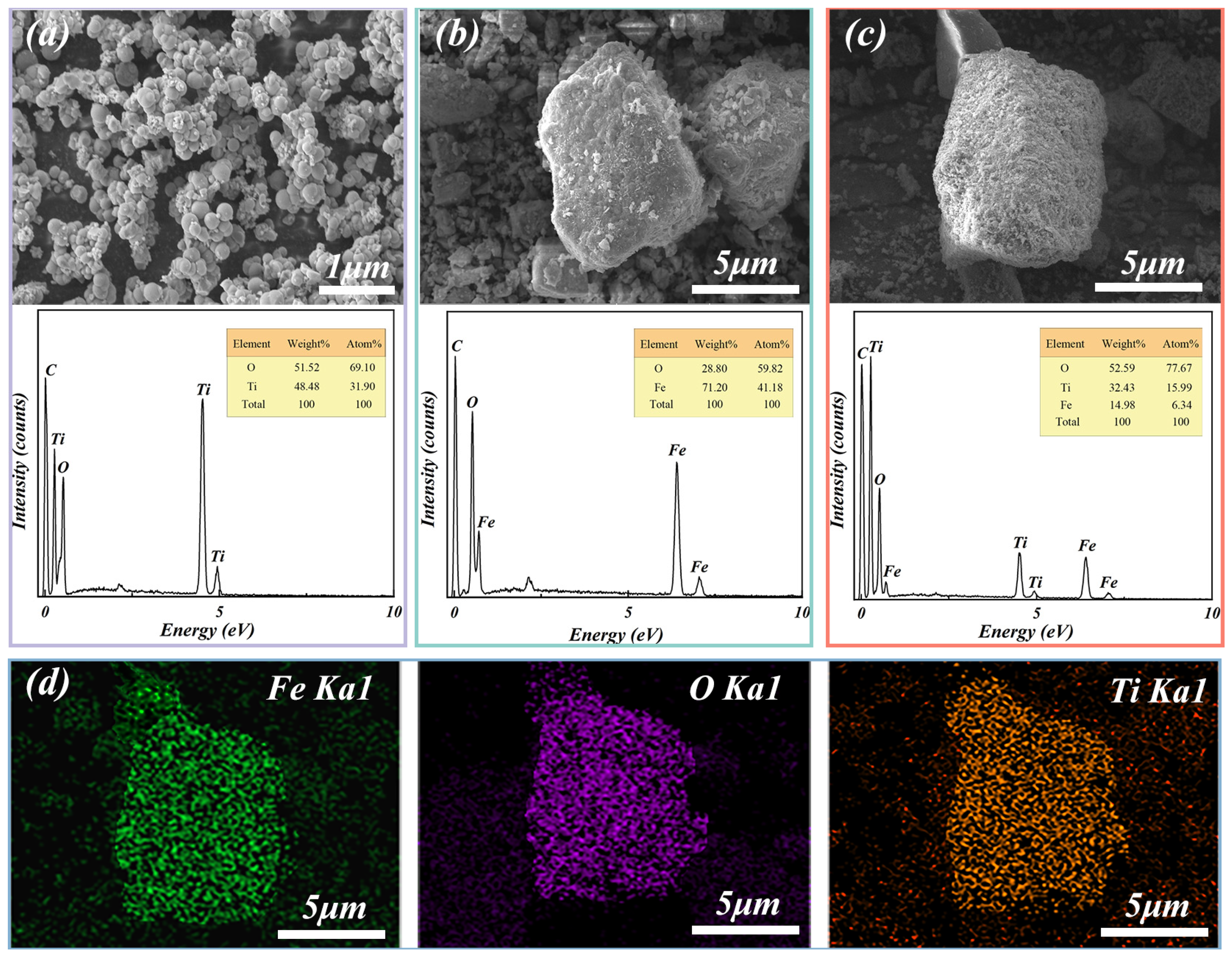
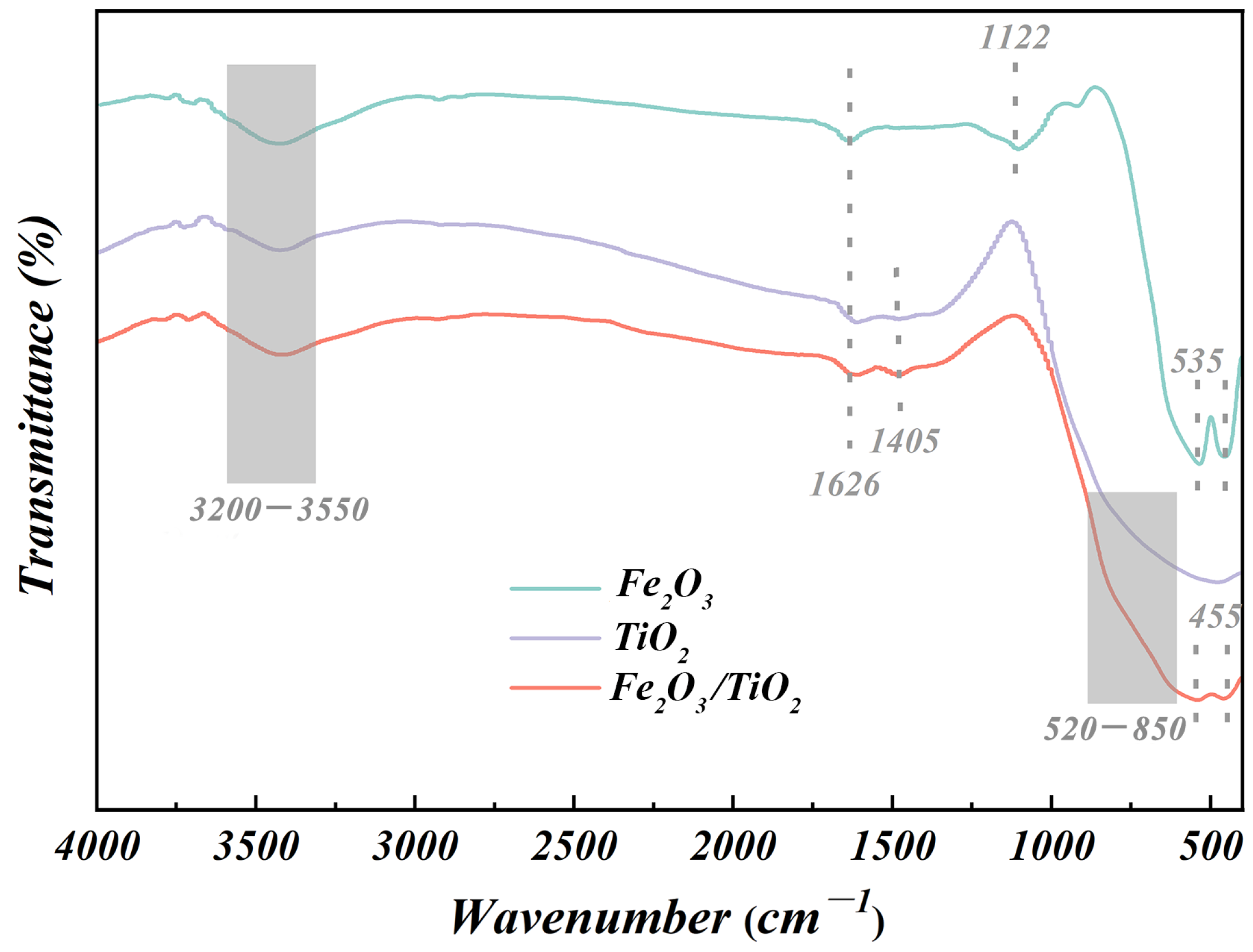




| Composition | Fe | C | S | P2O5 | SiO2 | MnO | Al2O3 | CaO | MgO | Diameter (μm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Weight | 73.85 | 0.016 | 0.009 | 0.051 | 0.058 | 0.347 | 0.008 | 0.131 | 0.017 | 5–100 |
| Sample | Absolute Electronegativity (eV) | Bandgap Energy (eV) | Calculated Conduction Band Position (eV) | Calculated Valence Band Position (eV) |
|---|---|---|---|---|
| Fe2O3 | 5.88 | 2.10 | 0.33 | 2.43 |
| TiO2 | 5.81 | 3.10 | −0.24 | 2.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Cui, Z.; Feng, B.; Sui, M.; Huang, H.; Wu, Z. Synthesis of Fe2O3/TiO2 Photocatalytic Composites for Methylene Blue Degradation as a Novel Strategy for High-Value Utilisation of Iron Scales. Materials 2024, 17, 4546. https://doi.org/10.3390/ma17184546
Liu L, Cui Z, Feng B, Sui M, Huang H, Wu Z. Synthesis of Fe2O3/TiO2 Photocatalytic Composites for Methylene Blue Degradation as a Novel Strategy for High-Value Utilisation of Iron Scales. Materials. 2024; 17(18):4546. https://doi.org/10.3390/ma17184546
Chicago/Turabian StyleLiu, Li, Zhenghao Cui, Bo Feng, Mengjing Sui, Huaqin Huang, and Zhaoyang Wu. 2024. "Synthesis of Fe2O3/TiO2 Photocatalytic Composites for Methylene Blue Degradation as a Novel Strategy for High-Value Utilisation of Iron Scales" Materials 17, no. 18: 4546. https://doi.org/10.3390/ma17184546
APA StyleLiu, L., Cui, Z., Feng, B., Sui, M., Huang, H., & Wu, Z. (2024). Synthesis of Fe2O3/TiO2 Photocatalytic Composites for Methylene Blue Degradation as a Novel Strategy for High-Value Utilisation of Iron Scales. Materials, 17(18), 4546. https://doi.org/10.3390/ma17184546






