The Effects of Metakaolin on the Properties of Magnesium Sulphoaluminate Cement
Abstract
:1. Introduction
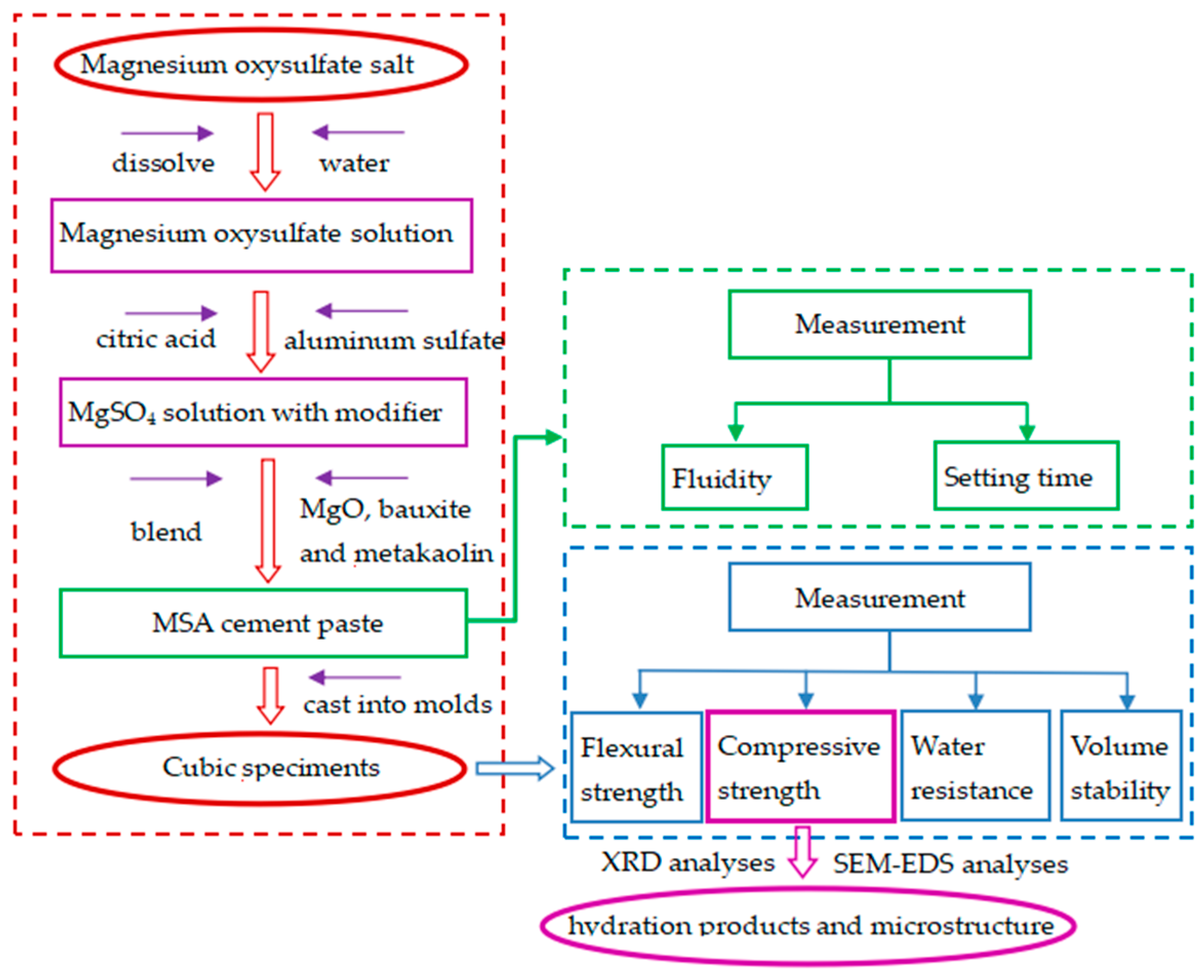
2. Materials and Methods
2.1. Materials
2.1.1. Magnesium Oxide
2.1.2. Bauxite and Metakaolin
2.1.3. Magnesium Oxysulfate and Modifier Additive
2.2. Mix Ratio Design
2.3. Testing Methods
3. Results and Discussion
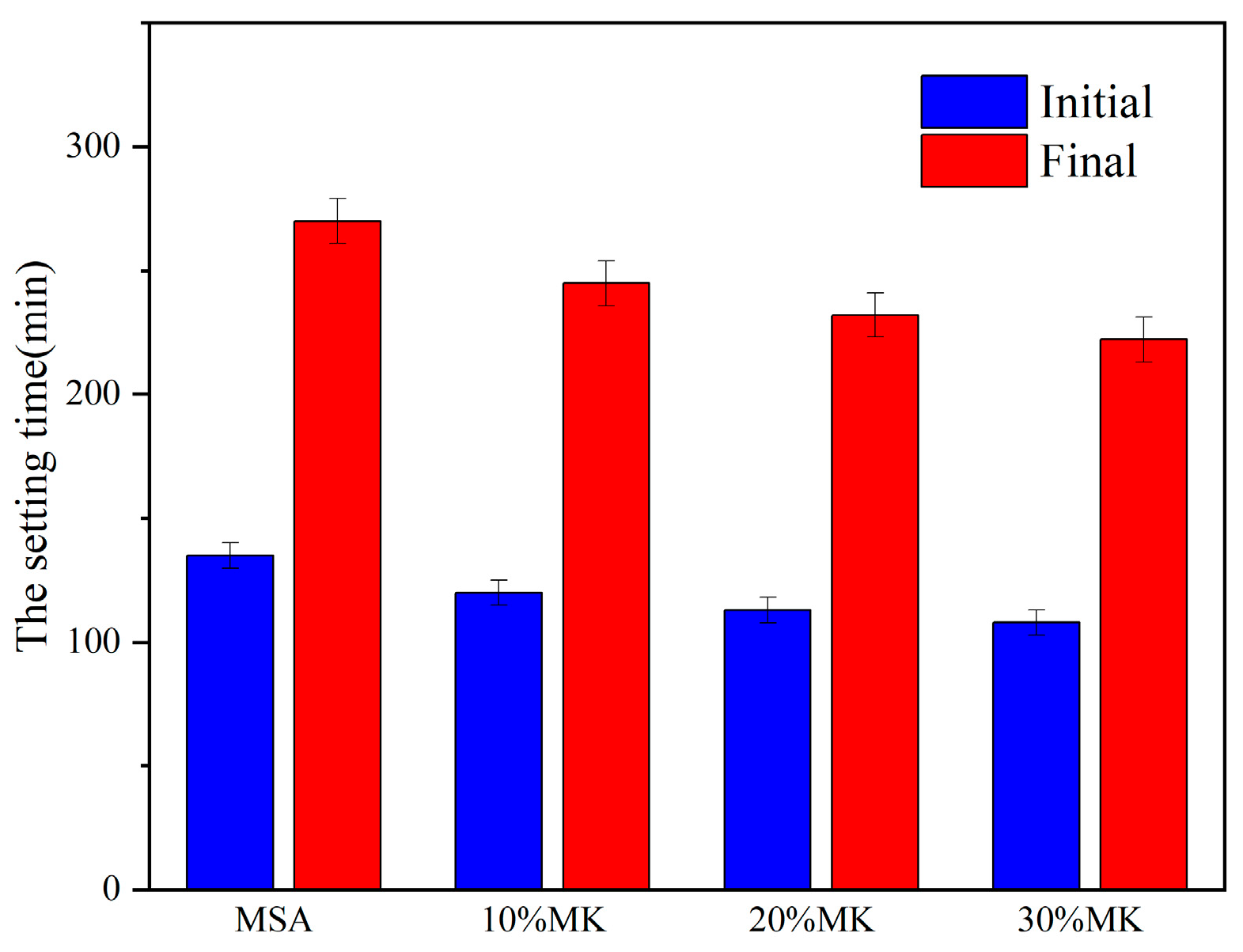
3.1. Effect of Metakaolin on the Fluidity and Setting Time of Magnesian Sulphoaluminate Cement
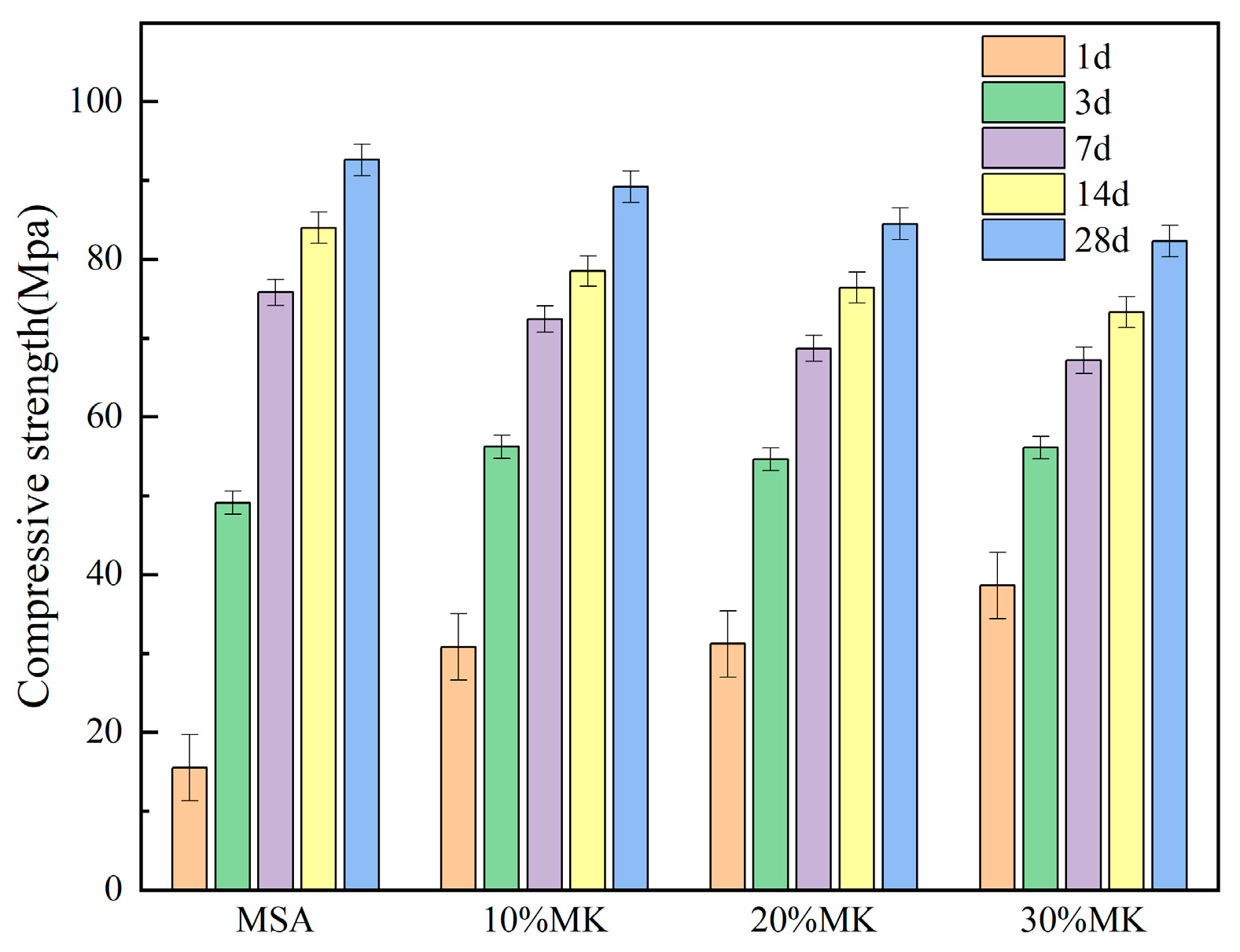
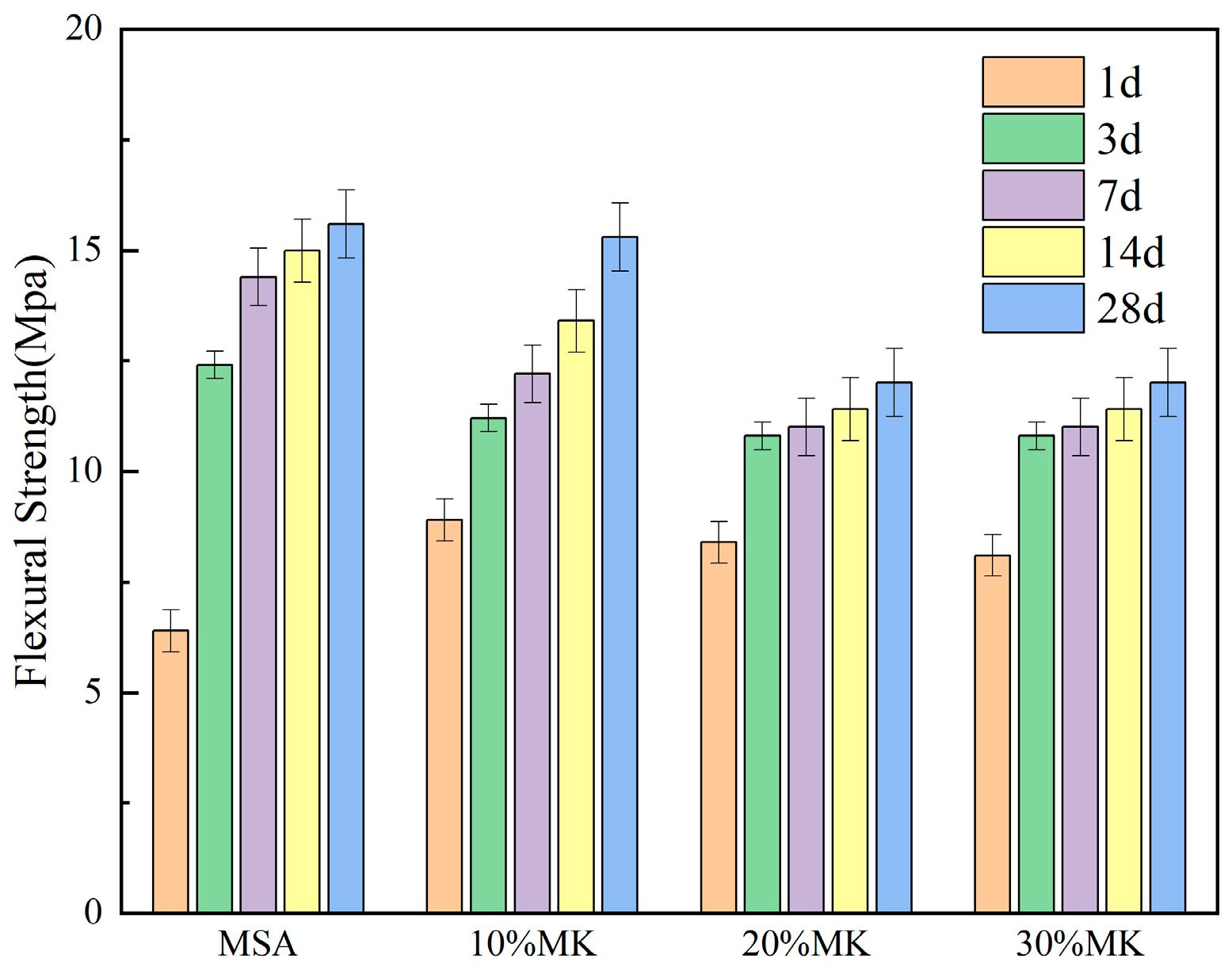
3.2. Effect of Metakaolin on the Strength of Magnesian Sulphoaluminate Cement
3.3. Effect of Metakaolin on the Water Resistance of Magnesian Sulphoaluminate Cement
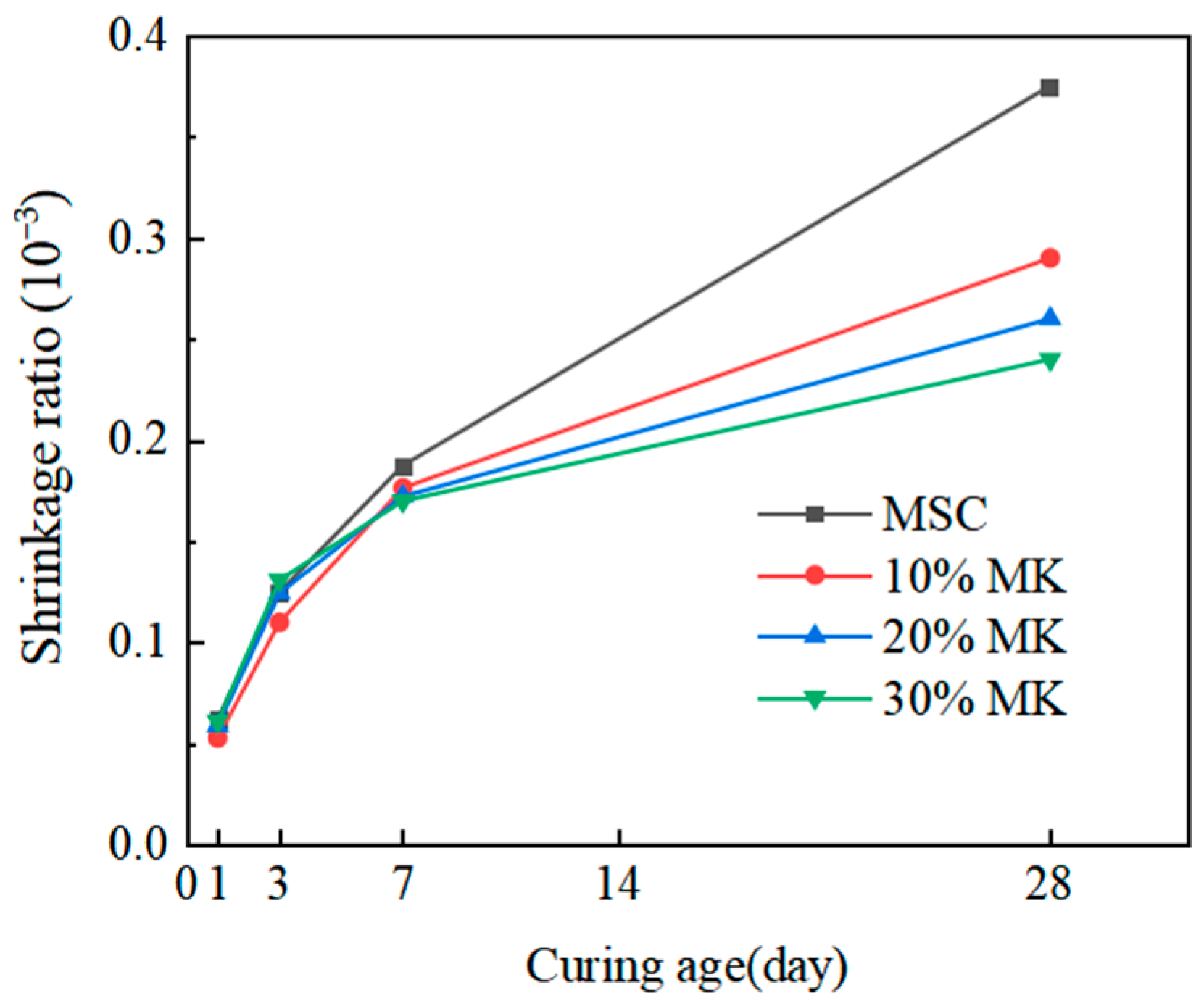
3.4. Effect of Metakaolin on the Volume Stability of Magnesian Sulphoaluminate Cement
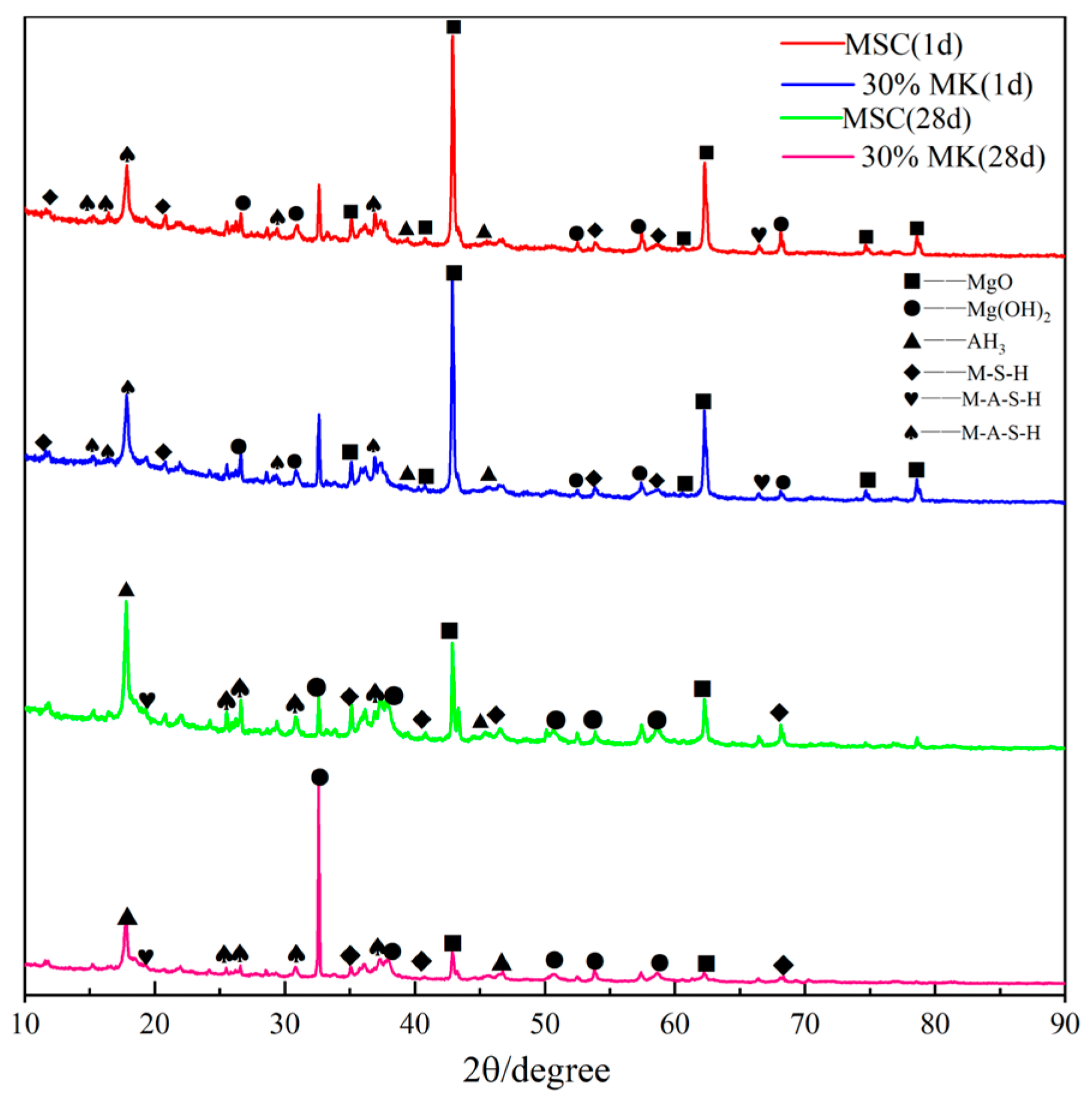
3.5. XRD Analysis
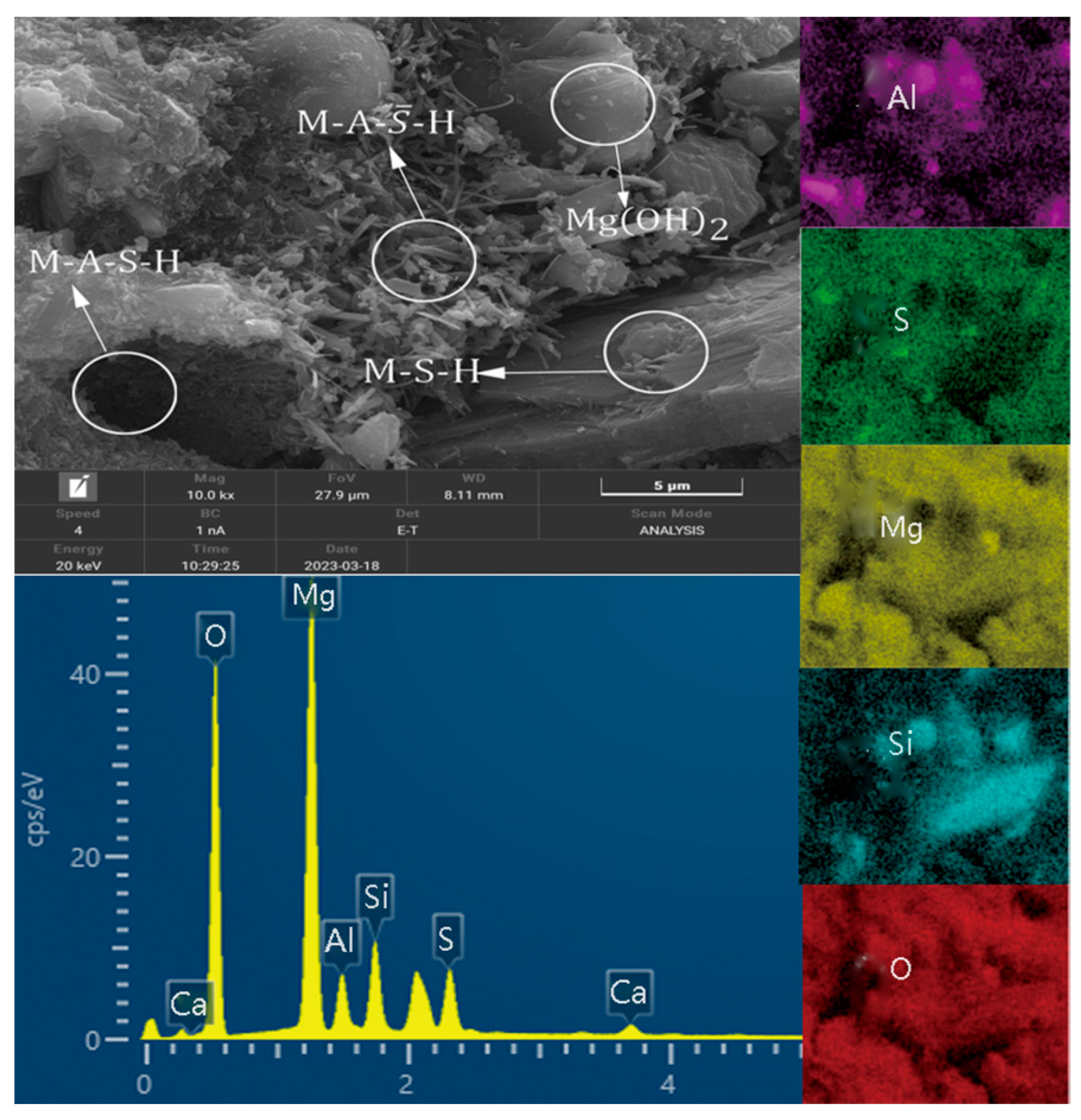
3.6. SEM-EDS Analysis
4. Conclusions
- (1)
- The addition of metakaolin reduces the fluidity and shortens the setting time of MSA cement. With the addition of 10–30% metakaolin, the fluidity was reduced by 6.6–16% compared with the control sample due to the irregular morphology of the grains. The initial setting time and final setting time shortened by 15–27 min and 25–48 min, respectively, for the metakaolin additions in the hydration reaction earlier.
- (2)
- The early strength of the MSA cement is significantly improved by the suitable addition of metakaolin. At a 1 day curing age of MSA cement with a dosage of 10–30% metakaolin, the compressive strength and flexural strength were enhanced by 98% and 39%, respectively, because metakaolin accelerates the early hydration reaction and generates more gel-like hydration products. Nevertheless, the strength decreased slightly when the curing age was 7 d, 14 d, and 28 d.
- (3)
- The water resistance of the MSA cement was improved by the addition of 10–20% metakaolin within the first 3 d and decreased slightly at other curing ages. However, the softening coefficients of the mixtures with 10% and 20% metakaolin were all higher than 0.85 before the 28 d soaking period. The incorporation of 30% metakaolin reduced water resistance at all ages. The addition of 10–30% metakaolin caused an improvement on the volume stability of the MSA cement with increasing dosages before 28 d of age. At a curing age of 1 day, the shrinkage reductions in the MSA cement with 10–30% metakaolin were 15.1%, 5.6% and 4.2% compared to the control sample, which may be due to the more rational phase composition and microstructure. The mechanism of shrinkage reduction of the MSA cement with metakaolin needs to be further studied.
- (4)
- The results show that addition of 10% metakaolin could shorten the setting time and significantly improve the compressive strength, flexural strength, water resistance, and volume stability of MSA cement at1 d of age, while also maintaining good long-term performance. This finding may promote the application of MSA cement as an inorganic bonding agent for repairing materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Demediuk, T.; Cole, W.F. Study of magnesium oxysulphates. Aust. J. Chem. 1957, 10, 287–294. [Google Scholar] [CrossRef]
- Plekhanova, T.A.; Keriene, J.; Gailius, A.; Yakovlev, G.I. Structural, physical and mechanical properties of modified wood-magnesia composite. Constr. Build. Mater. 2007, 21, 1833–1838. [Google Scholar] [CrossRef]
- Guo, T.; Wang, H.; Yang, H. The mechanical properties of magnesium oxysulfate cementenhanced with 517 phase magnesium oxysulfate whiskers. Constr. Build. Mater. 2017, 150, 844–850. [Google Scholar] [CrossRef]
- Beaudio, J.J.; Ramachandran, V.S.; Beaudoin, J.J.; Ramachandran, V.S. Strength development in magnesium oxysulfate cement. Cem. Concr. Res. 1978, 8, 103–112. [Google Scholar]
- Urwong, L.; Sorrell, C.A. Phase relations in magnesium oxysulfate cements. J. Am. Ceram. Soc. 1980, 63, 523–526. [Google Scholar] [CrossRef]
- Wu, C.; Yu, H.; Dong, J. Effects of Phosphoric Acid and phosphates on magnesium oxysulfate cement. Mater. Struct. 2013, 48, 907–917. [Google Scholar] [CrossRef]
- Runcevski, T.; WU, C.; YU, H. Structural characterization of a new magnesium oxysulphate hydrate cement phase and its surface reactions with atmospheric carbon dioxide. J. Am. Ceram. Soc. 2013, 96, 3609–3616. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Zhang, H. The hydration mechanism and performance of modified magnesium oxysulfate cement by tartaric acid. Constr. Build. Mater. 2017, 144, 516–524. [Google Scholar] [CrossRef]
- Wang, N.; Yu, H.; Bi, W. Effects of sodium citrate and citric acid on the properties of magnesium oxysulfate cement. Constr. Build. Mater. 2018, 169, 697–704. [Google Scholar] [CrossRef]
- Wang, N.; Yu, H.; Bi, W.; Guan, Y. The improvement effects of NaH2PO4 and KH2PO4 on the properties of magnesium oxysulfate cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2021, 36, 50–57. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Jiang, L. Effect of additives on the properties of magnesium oxysulfate cement. J. Intell. Fuzzy. Syst. 2017, 33, 3021–3025. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Li, W. Modification of magnesium oxysulfate cement by incorporating weak acids. J. Mater. Civ. Eng. 2018, 30, 04018209. [Google Scholar] [CrossRef]
- Virginia, B.; Magdalena, L.G.; Tiziano, M. Hydration kinetics and microstructural development of a magnesium oxysulfate cement modified by macromolecules. Constr. Build. Mater. 2020, 248, 118624. [Google Scholar]
- Zhou, W.; Zhang, A.; Cao, J. Performance improvement of magnesium oxysulfate cement by the combination of additives. Constr. Build. Mater. 2023, 408, 133683. [Google Scholar] [CrossRef]
- Jin, K.; Wu, C.; Xiong, Z. Impact of Cl− on the performance of magnesium oxysulfate (MOS) cement. Constr. Build. Mater. 2024, 430, 136472. [Google Scholar] [CrossRef]
- Chen, M.; Xiong, R.; Xi, K. Sulfur magnesium oxychloride cement and magnesia sulphoaluminate cement. China Build. Mater. Sci. Tec. 2013, 5, 19–22. [Google Scholar]
- Chen, C.; Wu, C.; Zhang, H. Effect of superplasticizers and their mechanisms of action on magnesium oxysulfate cement properties. Adv. Cem. Res. 2018, 32, 225–233. [Google Scholar] [CrossRef]
- José, T.; Markssuel, T.; Carlos, M. Recycling of calcined clay as an alternative precursor in geopolymers: A study of durability. J. Mater. Res. Technol. 2024, 30, 9213–9220. [Google Scholar]
- Dong, J.; Yu, H.; Zhang, L. Study on experimental conditions of hydration methods of determining active magnesium oxide content. J. Salt. Lake. Res. 2010, 18, 38–41. [Google Scholar]
- GB/T 8077-2012; Methods for testing uniformity of concrete admixture. China National Standardization Administration Committee: Beijing, China, 2012.
- GB/T 1346-2011; Test methods for water requirement of normal consistency, setting time and soundness of the Portland cement. China National Standardization Administration Committee: Beijing, China, 2011.
- GB/T 17671-2021; Test method of cement mortar strength (ISO method). China National Standardization Administration Committee: Beijing, China, 2021.
- Lu, X.; Chen, B. Experimental study of magnesium phosphate cements modified by metakaolin. Constr. Build. Mater. 2016, 1123, 719–726. [Google Scholar] [CrossRef]
- Gong, W.; Wang, N.; Zhang, N. Effect of fly ash and metakaolin on the macroscopic and microscopic characterizations of magnesium oxychloride cement. Constr. Build. Mater. 2020, 267, 120957. [Google Scholar] [CrossRef]
- Liu, R.; Wang, W.; Cui, Y. Impact of metakaolin on hydration properties of magnesium phosphate cement. Case. Stud. Constr. Mater. 2024, 20, e02840. [Google Scholar] [CrossRef]
- Qin, Z.; Zhou, S.; Ma, C. Roles of metakaolin in magnesium phosphate cement: Effect of the replacement ratio of magnesia by metakaolin with different particle sizes. Constr. Build. Mater. 2019, 227, 116675. [Google Scholar] [CrossRef]
- Qiao, Z.; Ding, Z.; Wang, J. Enhanced mechanical properties and environmental erosion resistance with metakaolin in a kind of Chinese traditional Lime-based mortar. Constr. Build. Mater. 2022, 317, 126110. [Google Scholar] [CrossRef]
- Cassagnabère, F.; Mouret, M.; Escadeillas, G. Early hydration of clinker–slag–metakaolin combination in steam curing conditions, relation with mechanical properties. Cem. Concr. Res. 2009, 39, 1164–1173. [Google Scholar] [CrossRef]
- Yi, G.; Ma, C.; Long, G. Effects of metakaolin on a novel aerated magnesium phosphate cement with high early strength. Constr. Build. Mater. 2018, 187, 1130–1133. [Google Scholar] [CrossRef]
- Frías, M.; Cabrera, J. Pore size distribution and degree of hydration of metakaolin-cement pastes. Cem. Concr. Res. 2000, 30, 561–569. [Google Scholar] [CrossRef]
- Gong, W.; Wang, N.; Zhang, N. Effect of Metakaolin on Water Resistance of Magnesium Oxychloride Cement. Aci. Mater. J. 2022, 119, 47–57. [Google Scholar]
- Zhang, N.; Yu, H.; Wang, N. Effects of low- and high-calcium fly ash on magnesium oxysulfate cement. Constr. Build. Mater. 2019, 215, 162–170. [Google Scholar] [CrossRef]
- Zhang, B.; Ji, T.; Ma, Y. Effect of metakaolin and magnesium oxide on flexural strength of ultra-high performance concrete. Cem. Concr. Compos. 2022, 131, 104582. [Google Scholar] [CrossRef]
- Håkon, A.; Depan, H.; Ivar, U. Formation of natural magnesium silica hydrate (M-S-H) and magnesium alumina silica hydrate (M-A-S-H) cement. Materials 2024, 17, 994. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, C.; Li, L. Effect of Fly Ash and Metakaolin on Properties and Microstructure of Magnesium Oxysulfate Cement. Materials 2022, 15, 1334. [Google Scholar] [CrossRef] [PubMed]
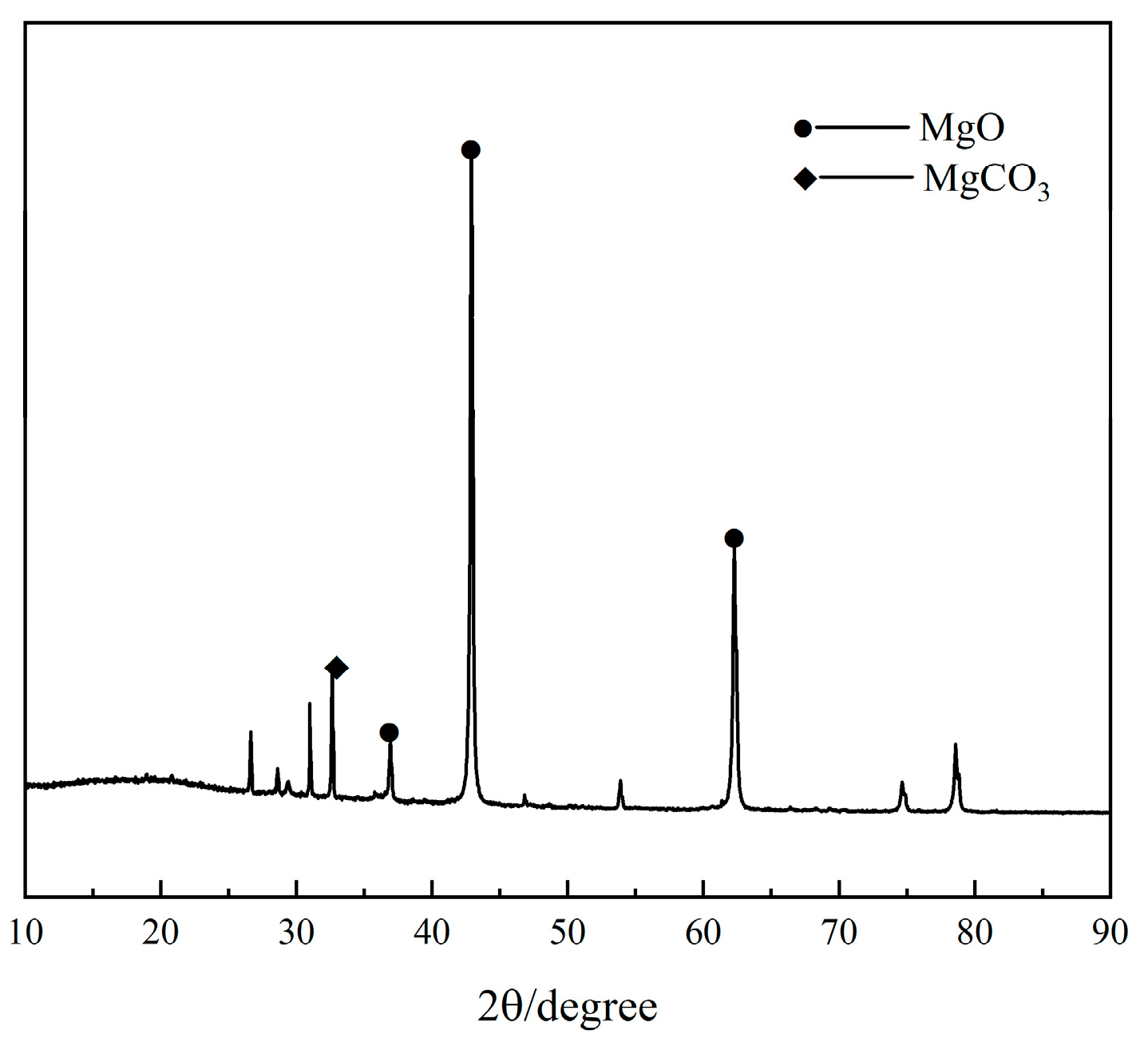
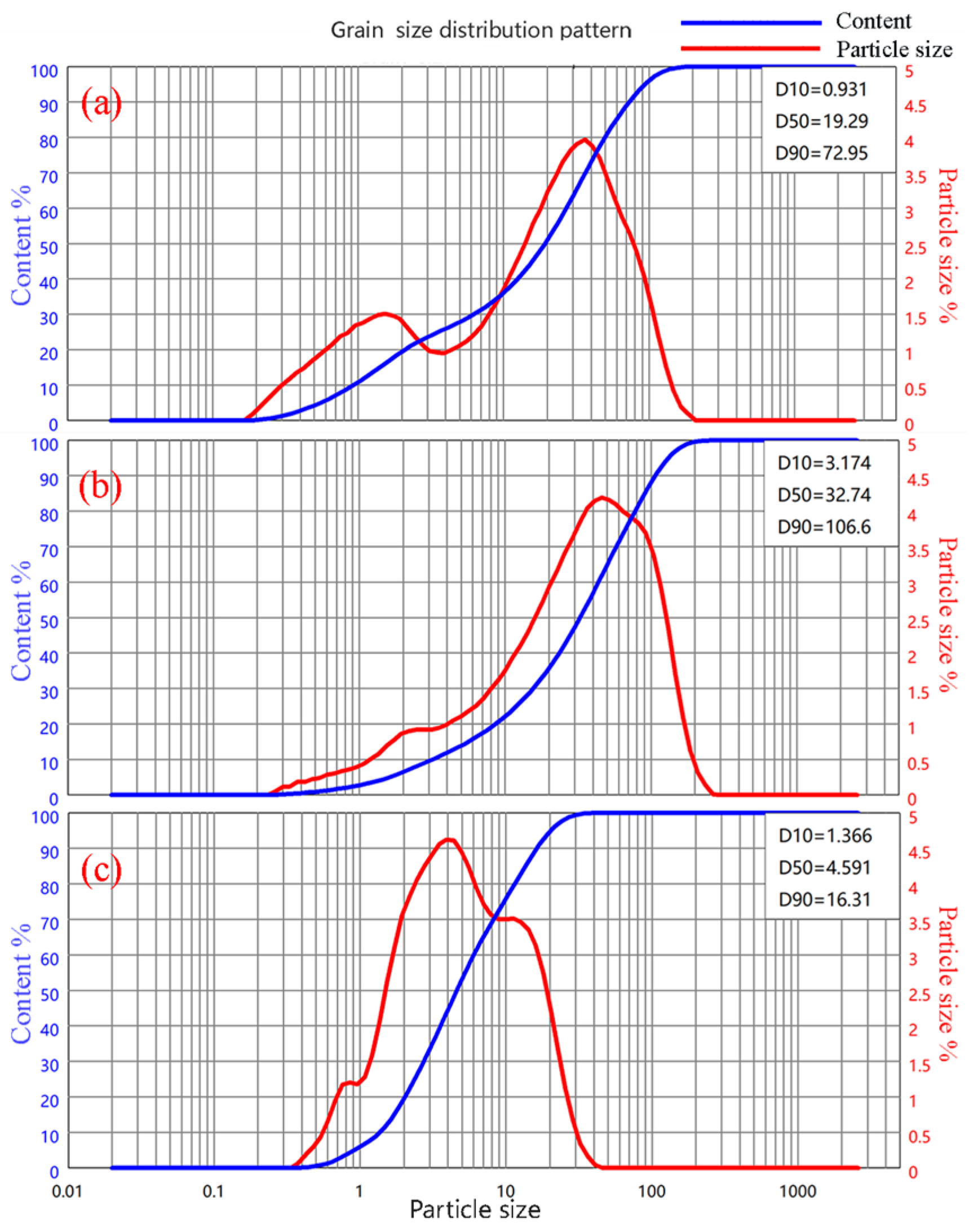
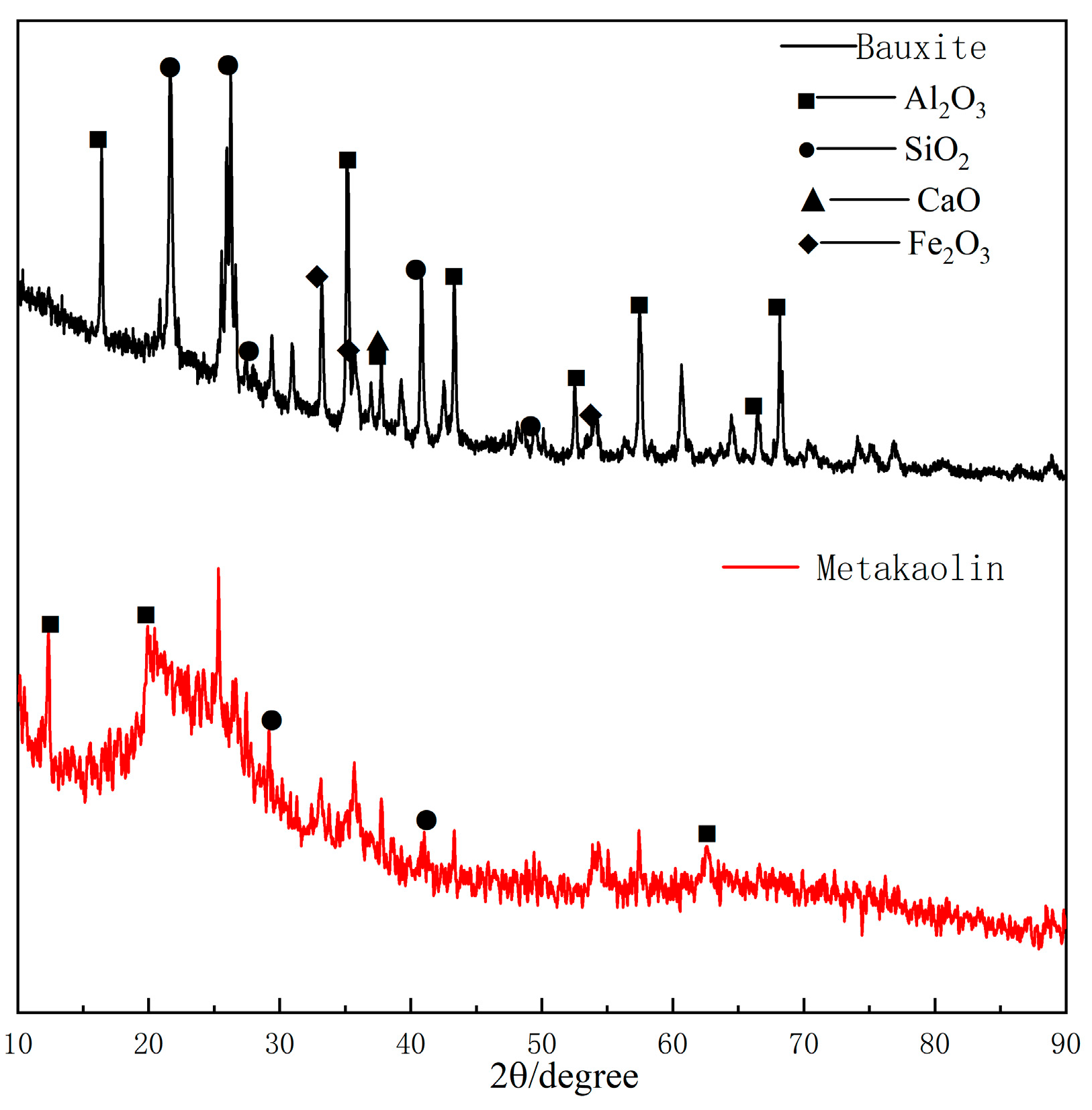




| Component | MgO | SiO2 | CaO | Al2O3 | Fe2O3 | Ignition Loss | Other |
|---|---|---|---|---|---|---|---|
| Content (%) | 88.12 | 3.6 | 1.3 | 0.77 | 0.68 | 1.6 | 3.93 |
| Component | SiO2 | Al2O3 | CaO | Na2O | Fe2O3 | MgO | SO3 | TiO2 | K2O | |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | Bauxite | 39.48 | 53.33 | 0.92 | 0.13 | 1.75 | 0.35 | 0.89 | 1.8 | 0.41 |
| MK | 49.94 | 43.88 | 0.27 | 0.00 | 0.51 | 2.66 | 0.14 | 1.89 | 0.23 | |
| Elements | Apparent Concentration | K Ratio | wt.% | wt.% Sigma | At% |
|---|---|---|---|---|---|
| O | 53.58 | 0.180 | 59.79 | 0.11 | 70.60 |
| Mg | 19.34 | 0.128 | 26.11 | 0.08 | 20.29 |
| Al | 2.29 | 0.016 | 4.01 | 0.04 | 2.81 |
| Si | 3.61 | 0.028 | 5.38 | 0.04 | 3.62 |
| S | 2.96 | 0.025 | 3.94 | 0.04 | 2.32 |
| Ca | 0.75 | 0.006 | 0.77 | 0.02 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Li, Z.; Li, Z.; Wang, D. The Effects of Metakaolin on the Properties of Magnesium Sulphoaluminate Cement. Materials 2024, 17, 4567. https://doi.org/10.3390/ma17184567
Jiang L, Li Z, Li Z, Wang D. The Effects of Metakaolin on the Properties of Magnesium Sulphoaluminate Cement. Materials. 2024; 17(18):4567. https://doi.org/10.3390/ma17184567
Chicago/Turabian StyleJiang, Lili, Zhuhui Li, Zhenguo Li, and Dongye Wang. 2024. "The Effects of Metakaolin on the Properties of Magnesium Sulphoaluminate Cement" Materials 17, no. 18: 4567. https://doi.org/10.3390/ma17184567
APA StyleJiang, L., Li, Z., Li, Z., & Wang, D. (2024). The Effects of Metakaolin on the Properties of Magnesium Sulphoaluminate Cement. Materials, 17(18), 4567. https://doi.org/10.3390/ma17184567





