Effects of Dolomitic Limestone on the Properties of Magnesium Oxysulfate Cement
Abstract
1. Introduction
2. Materials and Methods
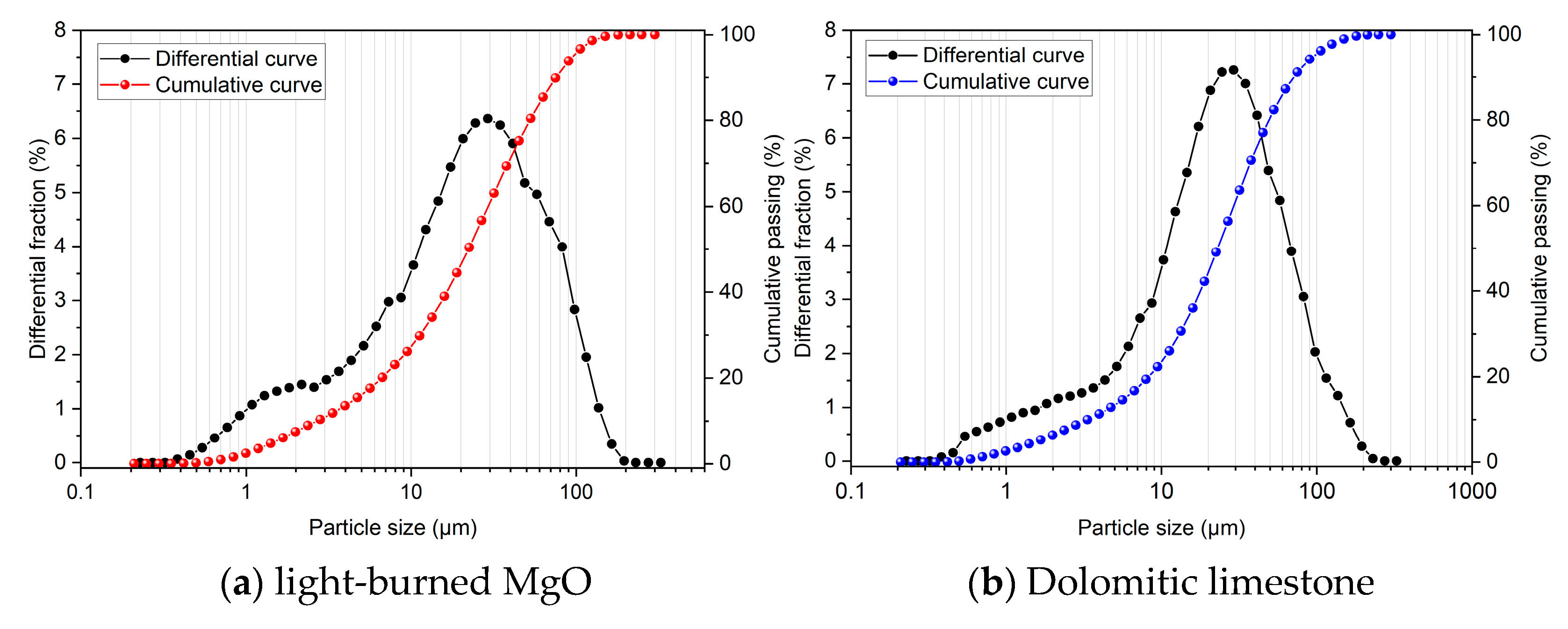
2.1. Raw Materials
2.2. Specimen Preparation
2.3. Analytical Methods
3. Results and Discussion
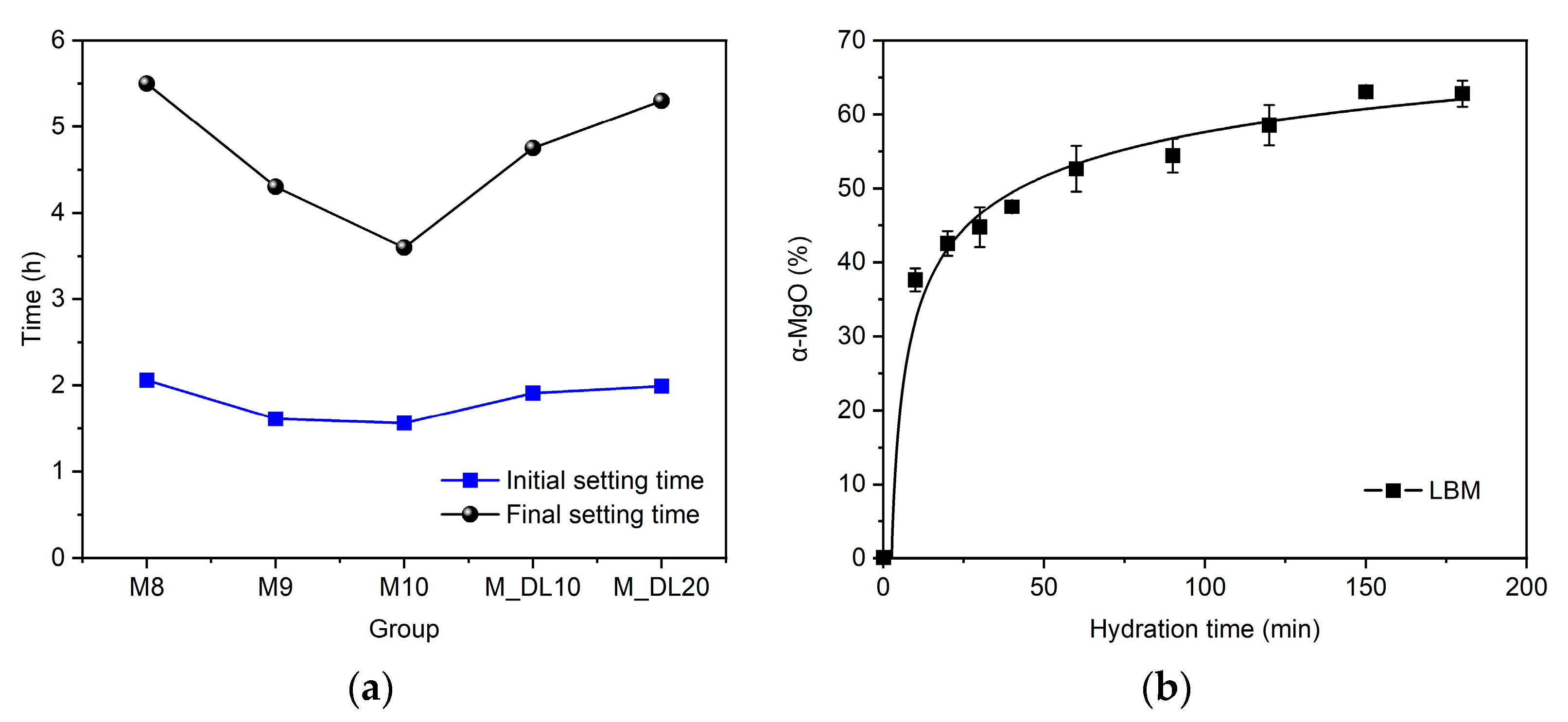
3.1. Effects of Material Ratio and DL on the Setting Time of MOS Cement
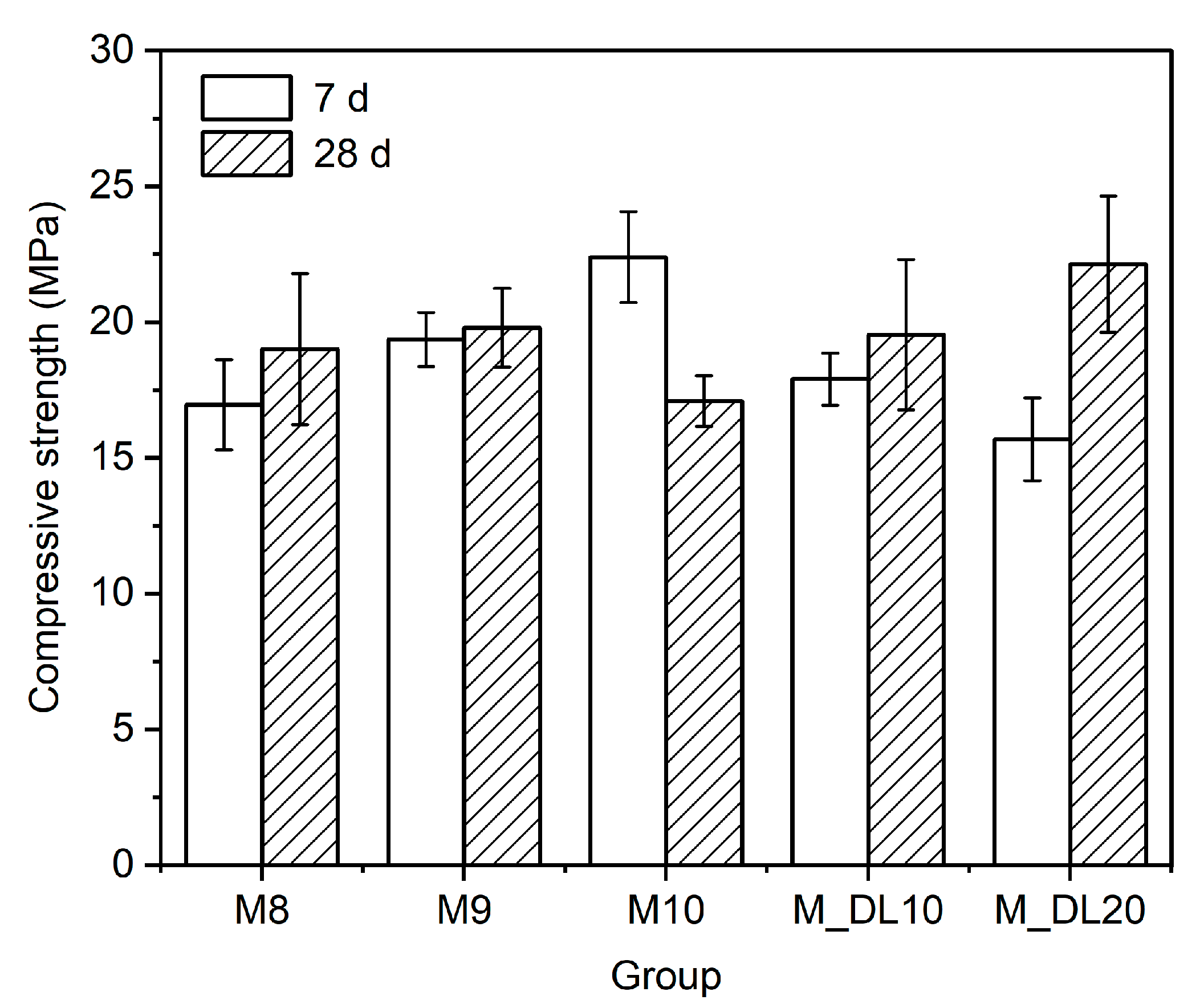
3.2. Compressive Strength of MOS Cement Pastes
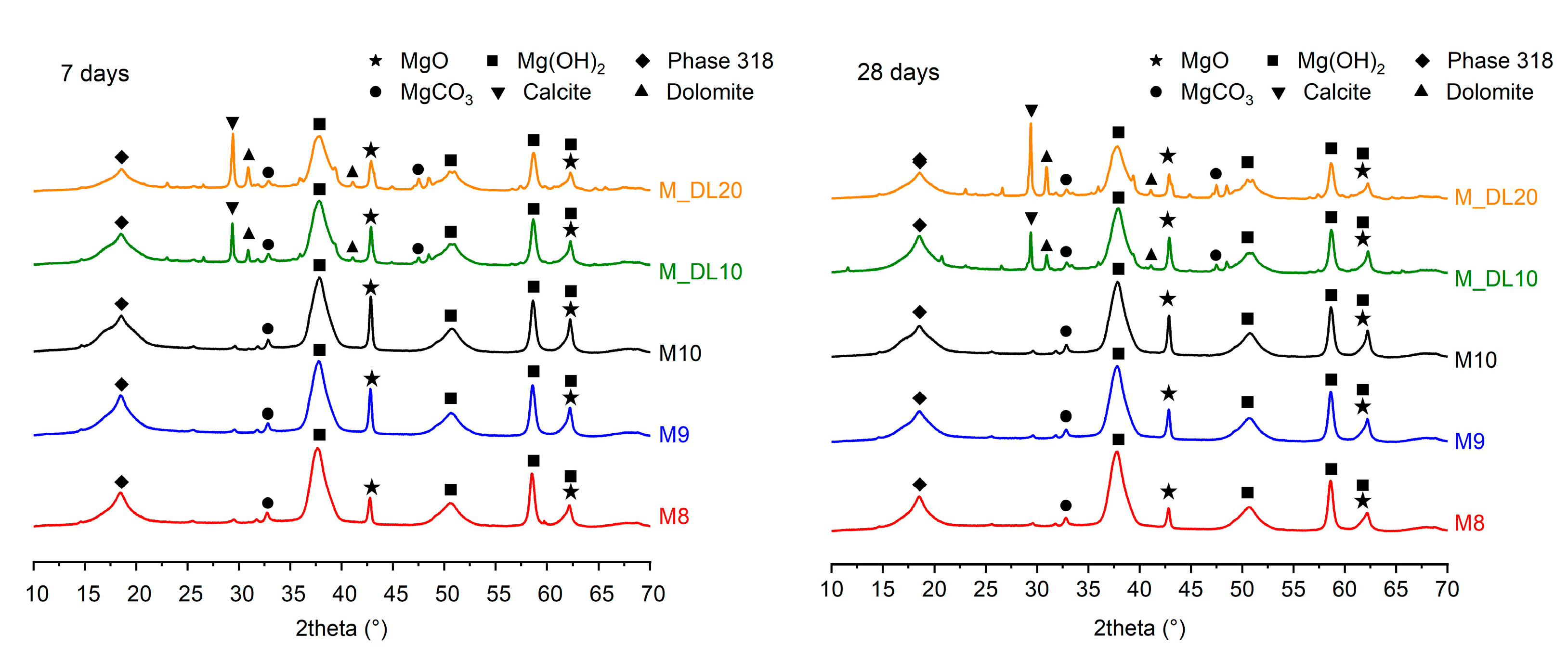
3.3. X-ray Diffraction Analysis (XRD)
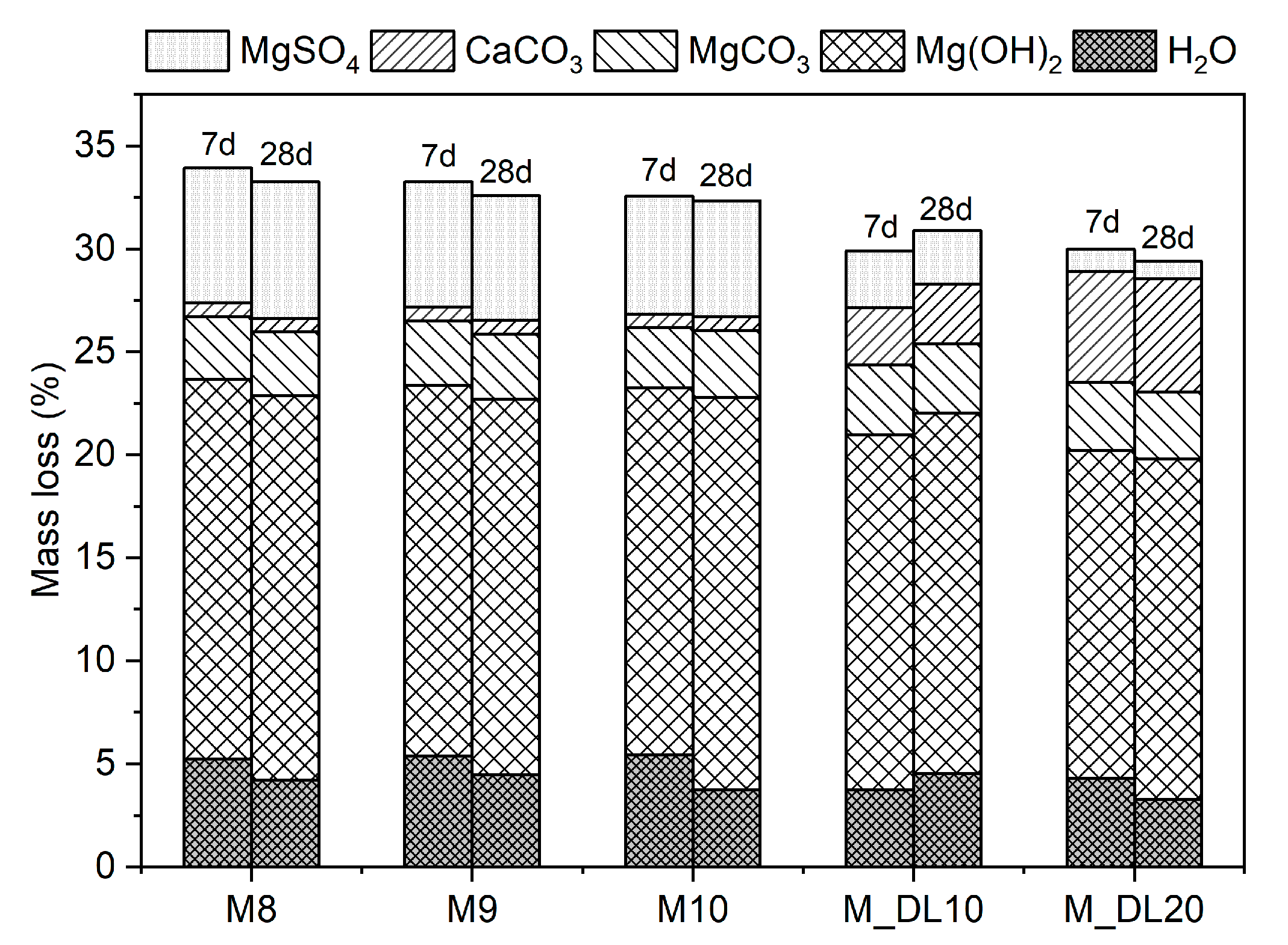
3.4. TG/DTG Analysis
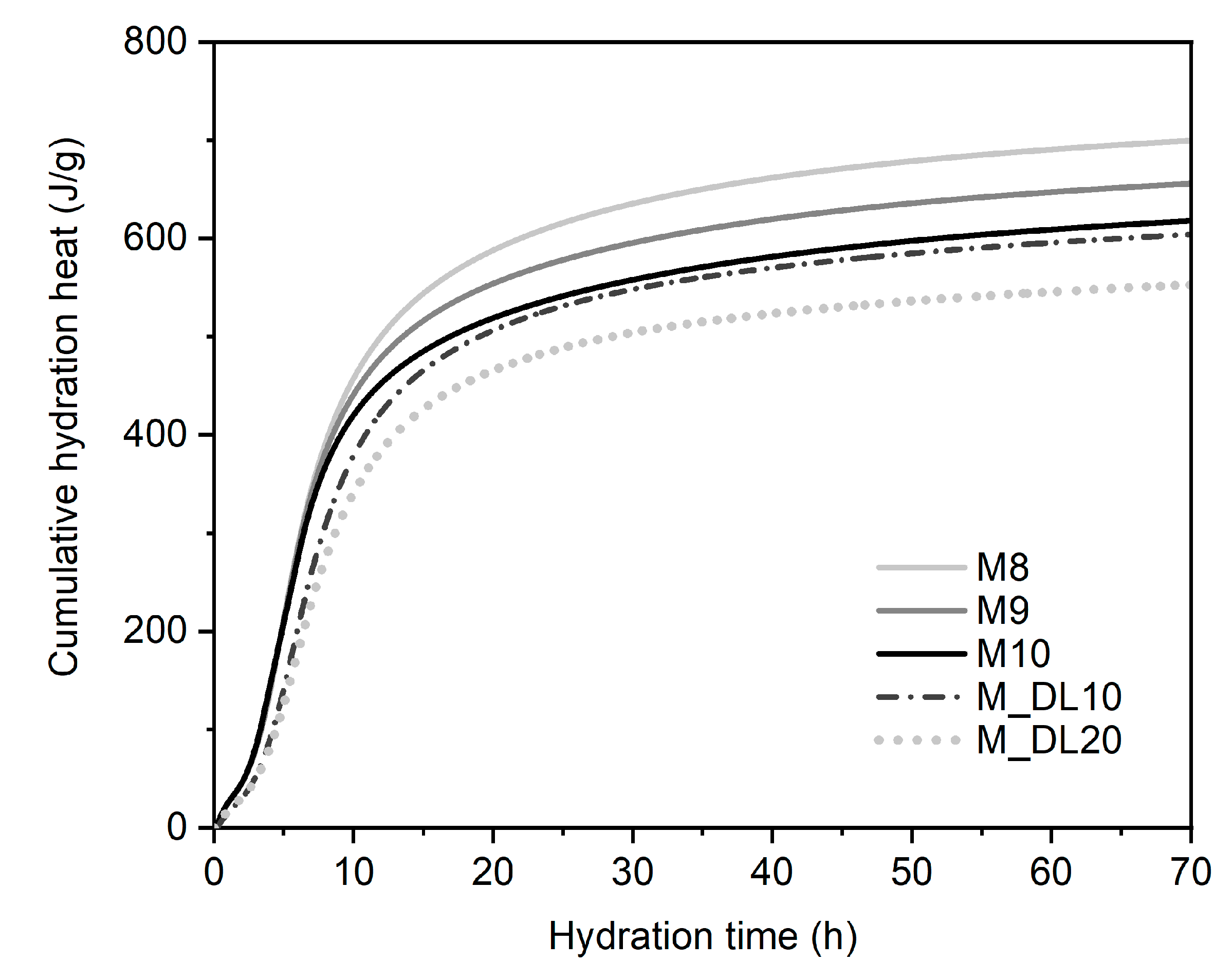
3.5. Hydration Heat Evolution
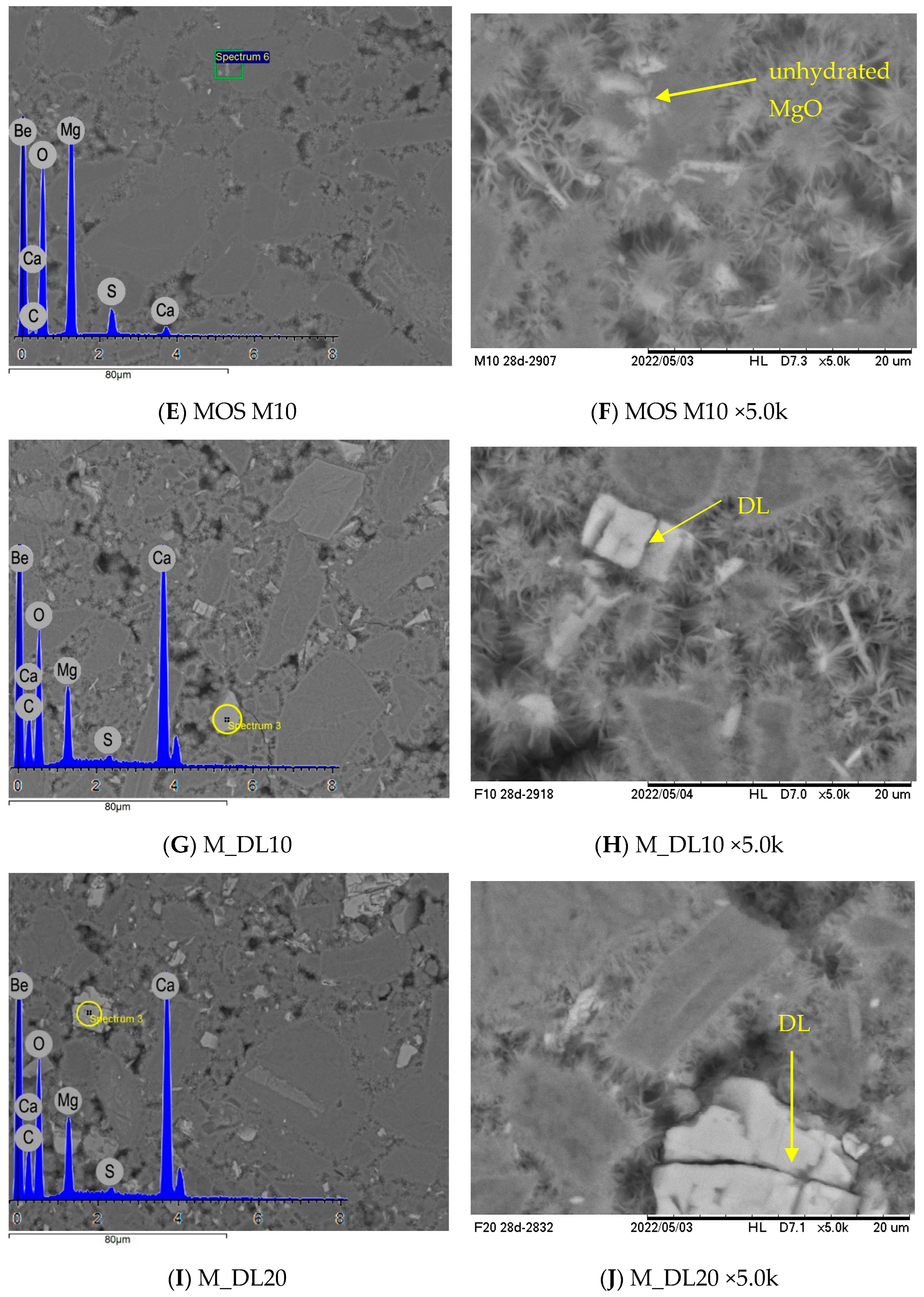
3.6. Microstructural Analysis
4. Conclusions
- The substitution of MgO with dolomitic limestone decreases the heat release rate generated, prolongs the hydration process of the MOS cement, and reduces the amount of unhydrated MgO and late Mg(OH)2 formation;
- The decreasing content of the hydration product, the 318 phase, and greater brucite formation at longer curing times decrease the mechanical strength of MOS cement prepared without chemical additives;
- A lower MgO content in the paste composition delays and prolongs the hydration process and final setting time in MOS cement, while the initial setting time of the pastes, for an equal molar ratio of MgSO4/H2O, depends mainly on the content of active MgO and the hydration kinetics of LBM;
- Microstructural analyses demonstrate the formation of 318 phase crystals, as well as the presence of dolomitic limestone embedded within the cementitious matrix. This can be associated with the filler effect, which results in the improved physical and mechanical performance of the materials after extended curing periods.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shand, M.A. Magnesium Oxysulfate Cement. Magnes. Cem. 2020, 75–83. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed]
- Ba, M.; Xue, T.; He, Z.; Wang, H.; Liu, J. Carbonation of magnesium oxysulfate cement and its influence on mechanical performance. Constr. Build. Mater. 2019, 223, 1030–1037. [Google Scholar] [CrossRef]
- Gomes, C.E.M.; Camarini, G. Magnesium Oxysulfate Fibercement. Key Eng. Mater. 2014, 600, 308–318. [Google Scholar] [CrossRef]
- Tan, Y.; Wu, C.; Yu, H.; Li, Y.; Wen, J. Review of reactive magnesia-based cementitious materials: Current developments and potential applicability. J. Build. Eng. 2021, 40, 102342. [Google Scholar] [CrossRef]
- Yu, W.; Yu, H.; Ma, H.; Shi, T.; Wen, J.; Ma, H.; Li, L.; Chen, X. Basic magnesium sulfate cement products exposed to air at various exposure ages: Phase composition, microstructure, and mechanical characteristics. J. Build. Eng. 2023, 79, 107799. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, H.; Yang, D.; Feng, T. Basic magnesium sulfate cement: Autogenous shrinkage evolution and mechanism under various chemical admixtures. Cem. Concr. Compos. 2022, 128, 104412. [Google Scholar] [CrossRef]
- Marmorato, G.C.E.; Gladis, C. Magnesium Oxysulfate Fibercement. In Proceedings of the Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014; Volume 600, pp. 308–318. [Google Scholar]
- Wang, L.; Chen, S.S.; Tsang, D.C.; Poon, C.-S.; Shih, K. Recycling contaminated wood into eco-friendly particleboard using green cement and carbon dioxide curing. J. Clean. Prod. 2016, 137, 861–870. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Chen, T. Recycling of raw rice husk to manufacture magnesium oxysulfate cement based lightweight building materials. J. Clean. Prod. 2018, 191, 220–232. [Google Scholar] [CrossRef]
- Nobre, J.; Ahmed, H.; Bravo, M.; Evangelista, L.; de Brito, J. Magnesia (MgO) Production and Characterization, and Its Influence on the Performance of Cementitious Materials: A Review. Materials 2020, 13, 4752. [Google Scholar] [CrossRef]
- Sun, Q.; Ba, M.; Zhang, D.; Zhou, S.; Zhang, N.; Wang, F. Effects of citrate acid, solidum silicate and their compound on the properties of magnesium oxysulfate cement under carbonation condition. Constr. Build. Mater. 2022, 330, 127136. [Google Scholar] [CrossRef]
- Chau, C.; Chan, J.; Li, Z. Influences of fly ash on magnesium oxychloride mortar. Cem. Concr. Compos. 2009, 31, 250–254. [Google Scholar] [CrossRef]
- Gomes, C.M.; de Oliveira, A.D.S. Chemical phases and microstructural analysis of pastes based on magnesia cement. Constr. Build. Mater. 2018, 188, 615–620. [Google Scholar] [CrossRef]
- Liska, M.; Al-Tabbaa, A.; Carter, K.; Fifield, J. Scaled-up commercial production of reactive magnesium cement pressed masonry units. Part I: Production. Proc. Inst. Civ. Eng. Constr. Mater. 2012, 165, 211–223. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2022; U.S. Geological Survey: Asheville, NC, USA, 2022.
- Demediuk, T.; Cole, W. A study of Mangesium Oxysulphates. Aust. J. Chem. 1957, 10, 287–294. [Google Scholar] [CrossRef]
- Ba, M.; Gao, Q.; Ma, Y.; Zhu, J.; Du, Y. Improved hydration and properties of magnesium oxysulfate (MOS) cement using sodium silicate as an additive. Constr. Build. Mater. 2021, 267, 120988. [Google Scholar] [CrossRef]
- Gomes, C.M.; Cheung, N.; Gomes, G.M.; Sousa, A.K.; Peruzzi, A.P. Improvement of water resistance in magnesia cements with renewable source silica. Constr. Build. Mater. 2021, 272, 121650. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, J.; Cai, R.; Wei, C.; Chen, J.; Chen, E. In situ monitoring of hydration of magnesium oxysulfate cement paste: Effect of MgO/MgSO4 ratio. Constr. Build. Mater. 2020, 251, 119003. [Google Scholar] [CrossRef]
- Tang, S.; Wei, C.; Cai, R.; Huang, J.; Chen, E.; Yuan, J. In situ monitoring of pore structure of magnesium oxysulfate cement paste: Effect of MgSO4/H2O ratio. J. Ind. Eng. Chem. 2020, 83, 387–400. [Google Scholar] [CrossRef]
- John, V.M.; Damineli, B.L.; Quattrone, M.; Pileggi, R.G. Fillers in cementitious materials—Experience, recent advances and future potential. Cem. Concr. Res. 2018, 114, 65–78. [Google Scholar] [CrossRef]
- Damineli, B.L.; Pileggi, R.G.; Lagerblad, B.; John, V.M. Effects of filler mineralogy on the compressive strength of cementitious mortars. Constr. Build. Mater. 2021, 299, 124363. [Google Scholar] [CrossRef]
- Luong, V.-T.; Amal, R.; Scott, J.A.; Ehrenberger, S.; Tran, T. A comparison of carbon footprints of magnesium oxide and magnesium hydroxide produced from conventional processes. J. Clean. Prod. 2018, 202, 1035–1044. [Google Scholar] [CrossRef]
- ISO 8336; Fibre-Cement Flat Sheets—Product Specification and Test Methods. International Organization for Standardization: Geneva, Switzerland, 2017.
- Ruan, S.; Unluer, C. Influence of supplementary cementitious materials on the performance and environmental impacts of reactive magnesia cement concrete. J. Clean. Prod. 2017, 159, 62–73. [Google Scholar] [CrossRef]
- Li, Q.; Su, A.; Gao, X. Preparation of durable magnesium oxysulfate cement with the incorporation of mineral admixtures and sequestration of carbon dioxide. Sci. Total. Environ. 2022, 809, 152127. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hua, Y.; Chang, Z.; Yue, Y.; Qin, J.; Qian, J. Hydration, carbonation and strength development of reactive MgO cement blended with lime (CaO) under different curing conditions. J. Build. Eng. 2023, 76, 107082. [Google Scholar] [CrossRef]
- Gomes, C.M.; de Oliveira, A.K.D.S. Effects of filler carbonates on magnesium-oxide based pastes. Constr. Build. Mater. 2020, 262, 119913. [Google Scholar] [CrossRef]
- Pereira, I.d.S.; Bamberg, A.L.; de Sousa, R.O.; Monteiro, A.B.; Martinazzo, R.; Silveira, C.A.P.; Silveira, A.d.O. Agricultural use and pH correction of anaerobic sewage sludge with acid pH. J. Environ. Manag. 2020, 275, 111203. [Google Scholar] [CrossRef]
- Palma, D.; Fuess, L.T.; de Lima-Model, A.N.; da Conceição, K.Z.; Cereda, M.P.; Tavares, M.H.F.; Gomes, S.D. Using dolomitic limestone to replace conventional alkalinization in the biodigestion of rapid acidification cassava processing wastewater. J. Clean. Prod. 2017, 172, 2942–2953. [Google Scholar] [CrossRef]
- WB/T 1019-2002; Caustic Burned Magnesia for Magnesium Oxychloride Cement Products. State Commission for Economic and Trade: Beijing, China, 2002.
- Dong, J.M.; Yu, H.F.; Zhang, L.M. Study on Experimental Conditions of Hydration Methods of Determining Active Magnesium Oxide Content. J. Salt Lake Res. 2010, 18, 38–41. [Google Scholar]
- ASTM C807-21; Standard Test Method for Time of Setting of Hydraulic Cement Mortar by Modified Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C150/C150M-20; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM C109/C109M; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-Inch or [50-Mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2020.
- Xing, Z.; Bai, L.; Ma, Y.; Wang, D.; Li, M. Mechanism of Magnesium Oxide Hydration Based on the Multi-Rate Model. Materials 2018, 11, 1835. [Google Scholar] [CrossRef]
- Du, H.; Li, J.; Ni, W.; Hou, C.; Liu, W. The hydration mechanism of magnesium oxysulfate cement prepared by magnesium desulfurization byproducts. J. Mater. Res. Technol. 2022, 17, 1211–1220. [Google Scholar] [CrossRef]
- Chen, C.; Wu, C.; Zhang, H.; Chen, Y.; Niu, J.; Chen, F.; Yu, H.; Magarotto, R.; Moratti, F.; Zeminian, N.; et al. Effect of superplasticisers and their mechanisms of action on magnesium oxysulfate cement properties. Adv. Cem. Res. 2020, 32, 225–233. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Pannach, M.; Freyer, D. 3Mg(OH)2·MgSO4·8H2O: A Metastable Phase in the System Mg(OH)2-MgSO4-H2O. Z. Fur Anorg. Und Allg. Chem. 2013, 639, 1827–1833. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, Z.; Zhang, Z.; Chang, J.; Bi, W.; Cheeseman, C.R.; Zhang, T. Effect of hydromagnesite addition on the properties and water resistance of magnesium oxysulfate (MOS) cement. Cem. Concr. Res. 2021, 143, 106387. [Google Scholar] [CrossRef]
- Vandeperre, L.; Liska, M.; Al-Tabbaa, A. Microstructures of reactive magnesia cement blends. Cem. Concr. Compos. 2008, 30, 706–714. [Google Scholar] [CrossRef]
- Gu, K.; Chen, B.; Yu, H.; Zhang, N.; Bi, W.; Guan, Y. Characterization of magnesium-calcium oxysulfate cement prepared by replacing MgSO4 in magnesium oxysulfate cement with untreated desulfurization gypsum. Cem. Concr. Compos. 2021, 121, 104091. [Google Scholar] [CrossRef]
- Dung, N.; Lesimple, A.; Hay, R.; Celik, K.; Unluer, C. Formation of carbonate phases and their effect on the performance of reactive MgO cement formulations. Cem. Concr. Res. 2019, 125, 105894. [Google Scholar] [CrossRef]
- Dung, N.; Hooper, T.; Unluer, C. Enhancing the performance of MgO-activated slag-fly ash mixes by accelerated carbonation. J. CO2 Util. 2020, 42, 101356. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.; Yu, H.; Chen, W.; Chen, C.; Zheng, S.; Chen, F. Study of using light-burned dolomite ores as raw material to produce magnesium oxysulfate cement. Adv. Cem. Res. 2018, 30, 437–450. [Google Scholar] [CrossRef]
- Wang, R.; Qin, L.; Gao, X. Mechanical strength and water resistance of magnesium oxysulfate cement based lightweight materials. Cem. Concr. Compos. 2020, 109, 103554. [Google Scholar] [CrossRef]






| Samples | Mass Fraction (wt. %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MgO | Al2O3 | SiO2 | SO3 | Cl | K2O | CaO | MnO | Fe2O3 | LOI * | |
| LBM | 94.1 | 0.09 | 0.28 | 0.26 | 0.06 | - | 1.47 | 0.18 | 0.91 | 2.62 |
| DL | 7.60 | 1.03 | 3.99 | 0.13 | - | 0.22 | 43.9 | 0.09 | 0.42 | 42.4 |
| Mixtures | Molar Ratio MgO:MgSO4:H2O | Dolomitic Limestone (% by Weight of MgO) | W/C Ratio |
|---|---|---|---|
| M8 | 8:1:20 | - | 0.70 |
| M9 | 9:1:20 | - | 0.64 |
| M10 | 10:1:20 | - | 0.59 |
| M_DL10 | 10:1:20 | 10 | 0.59 |
| M_DL20 | 10:1:20 | 20 | 0.59 |
| Mixture | The Maximum Heat Release Rate | Hydration Time | 72 h Cumulative Heat |
|---|---|---|---|
| (J/g)/h | (h) | (J/g of MgO Normalized) | |
| M8 | 49.55 | 7.2 | 701.14 |
| M9 | 48.74 | 7.03 | 657.41 |
| M10 | 47.18 | 6.92 | 619.6 |
| M_DL10 | 38.81 | 8.47 | 605.66 |
| M_DL20 | 34.64 | 8.76 | 554.06 |
| Period | Average Heat Release Rate Decrease (%) * | |
|---|---|---|
| M_DL10 | M_DL20 | |
| (N–I) | 51 | 20 |
| (I–II) | 37 | 31 |
| (II–III) | 34 | 40 |
| (III–IV) | 13 | 22 |
| (IV–V) | 2 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molano, J.C.A.; Azevedo, A.G.S.; Freitas, T.O.G.; Silva, G.C.D.; Savastano, H., Jr. Effects of Dolomitic Limestone on the Properties of Magnesium Oxysulfate Cement. Materials 2024, 17, 4580. https://doi.org/10.3390/ma17184580
Molano JCA, Azevedo AGS, Freitas TOG, Silva GCD, Savastano H Jr. Effects of Dolomitic Limestone on the Properties of Magnesium Oxysulfate Cement. Materials. 2024; 17(18):4580. https://doi.org/10.3390/ma17184580
Chicago/Turabian StyleMolano, Juan Camilo Adrada, Adriano Galvão Souza Azevedo, Taís Oliveira Gonçalves Freitas, Gabriela Casemiro Da Silva, and Holmer Savastano, Jr. 2024. "Effects of Dolomitic Limestone on the Properties of Magnesium Oxysulfate Cement" Materials 17, no. 18: 4580. https://doi.org/10.3390/ma17184580
APA StyleMolano, J. C. A., Azevedo, A. G. S., Freitas, T. O. G., Silva, G. C. D., & Savastano, H., Jr. (2024). Effects of Dolomitic Limestone on the Properties of Magnesium Oxysulfate Cement. Materials, 17(18), 4580. https://doi.org/10.3390/ma17184580










