Failure Analysis of Ni-8YSZ Electrode under Reoxidation Based on the Real Microstructure
Abstract
:1. Introduction
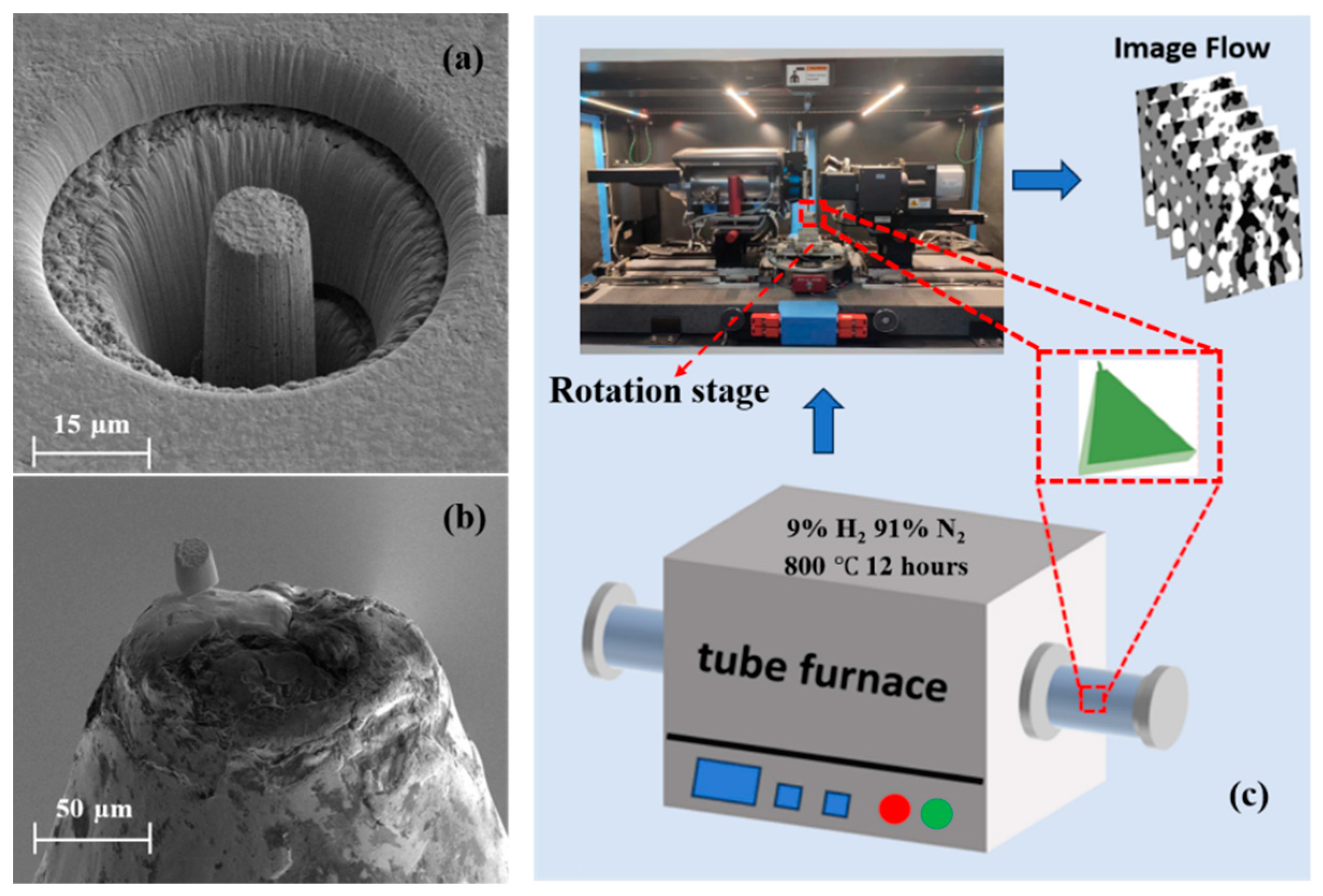
2. Experiment by Nano-CT
2.1. Sample Preparation
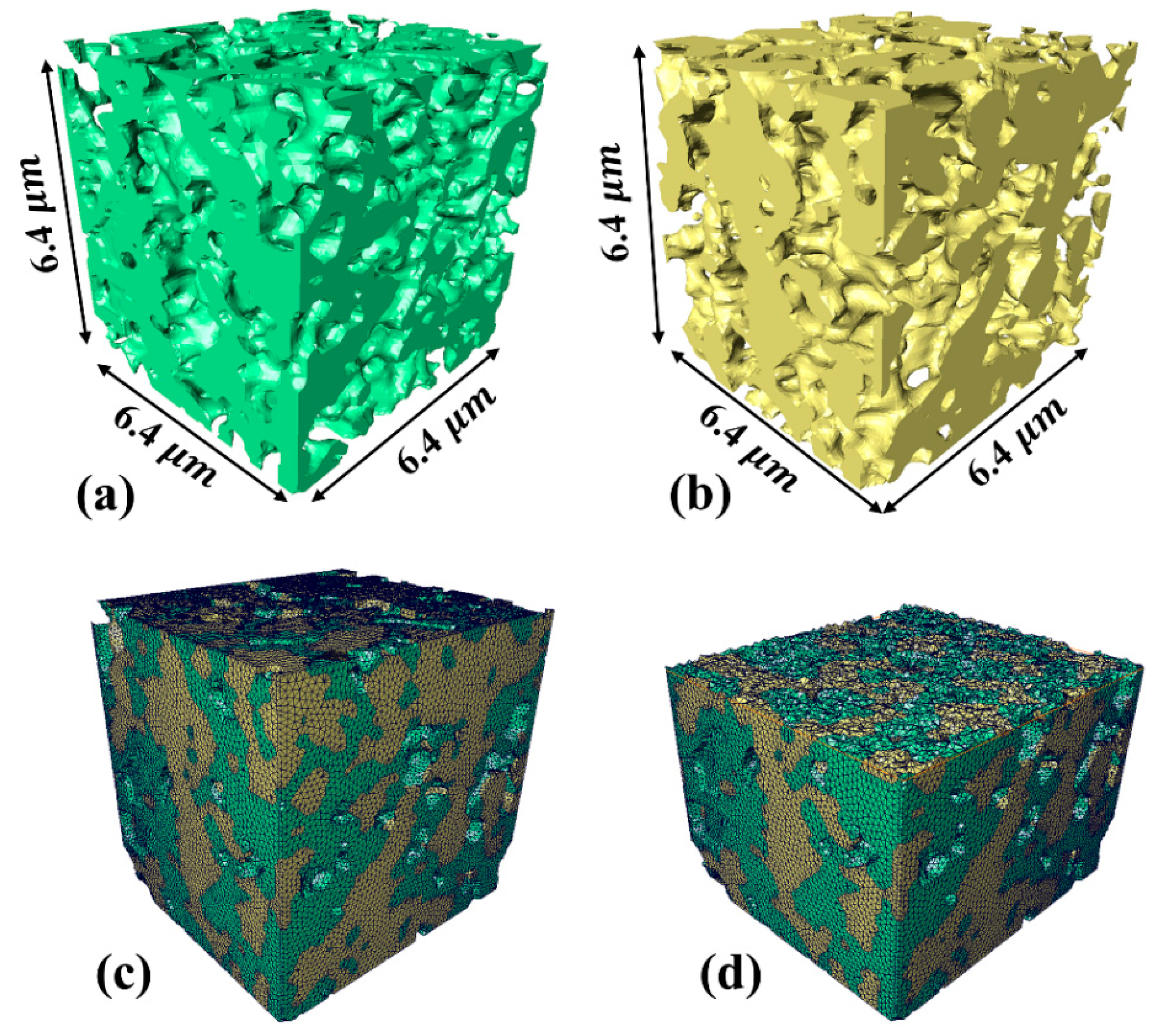
2.2. Three-Phase Segmentation and 3D Reconstruction
3. Reoxidation Model
3.1. Reoxidation Strain
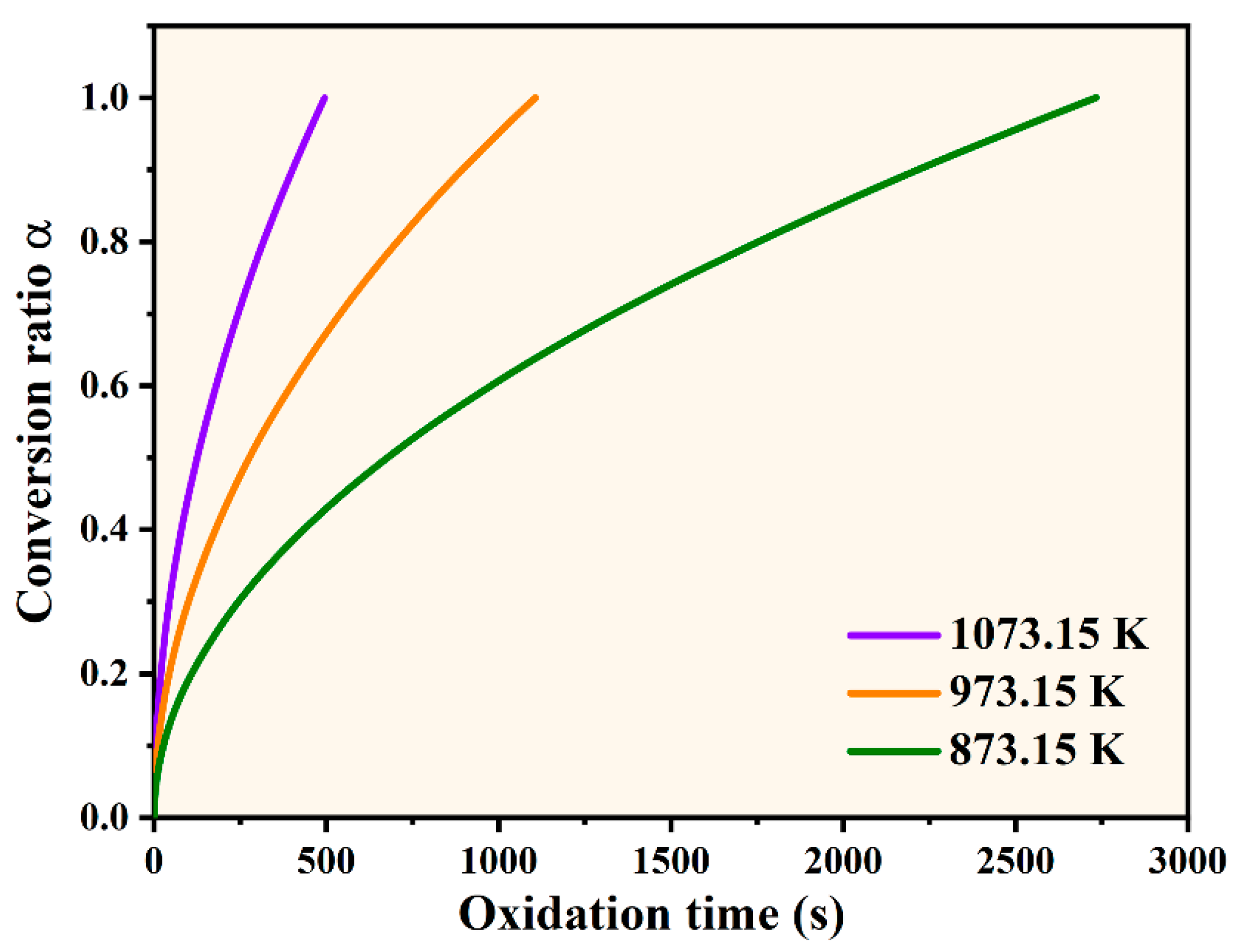
3.2. Oxidation Kinetics
3.3. Thermo-Mechanical Model
4. Results and Discussion
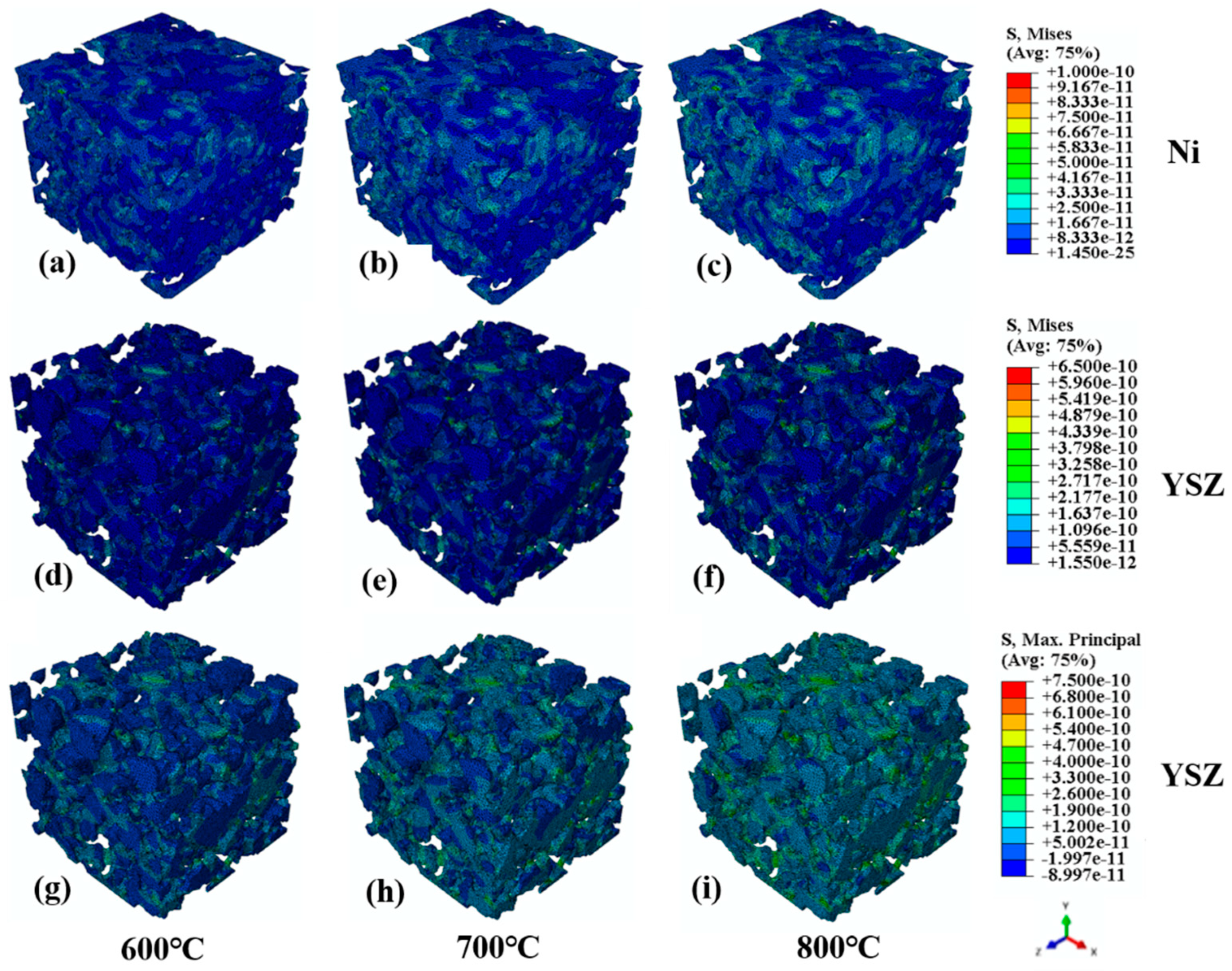
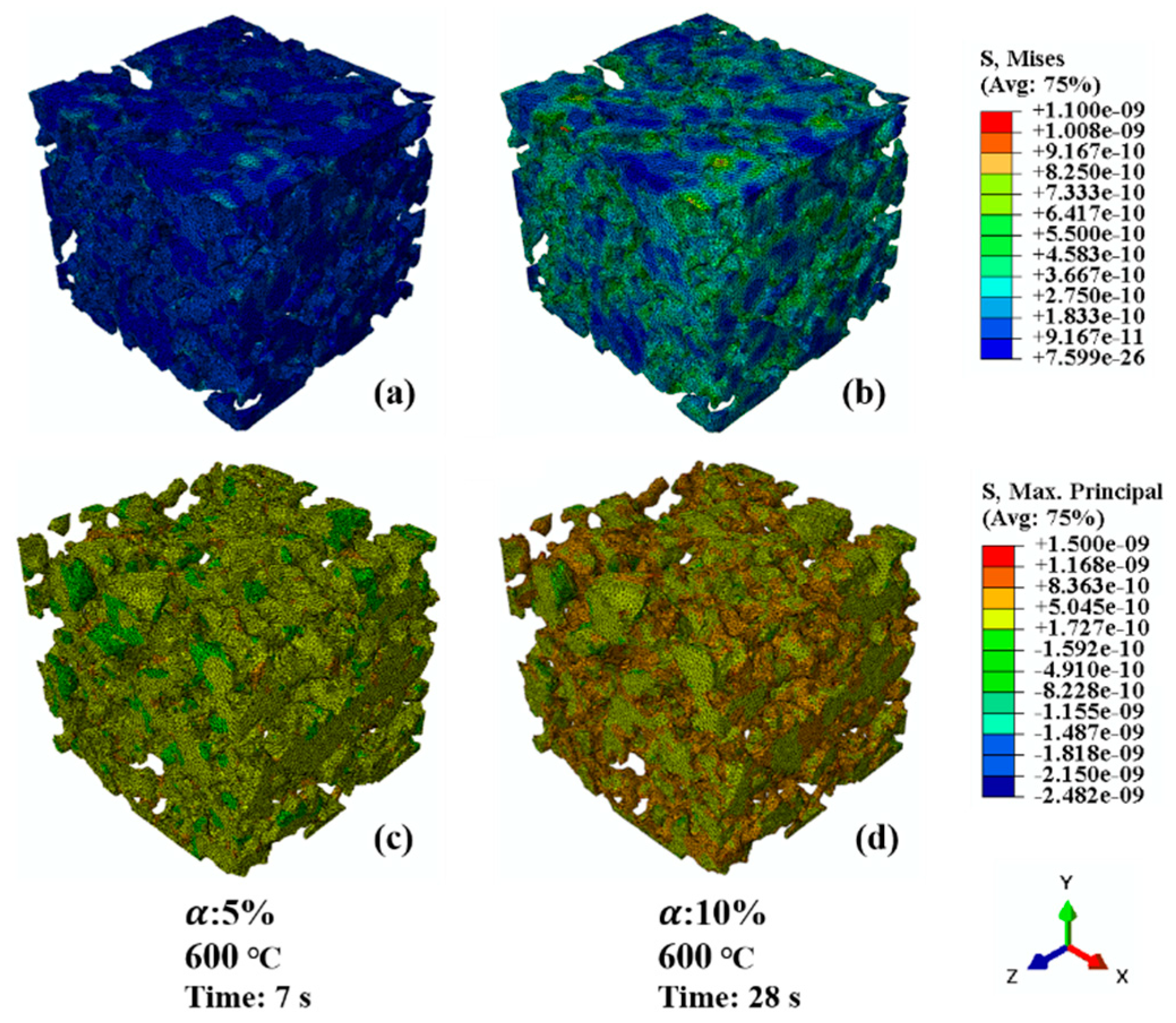
4.1. Stress Distribution in Microstructures
4.2. Model Validation
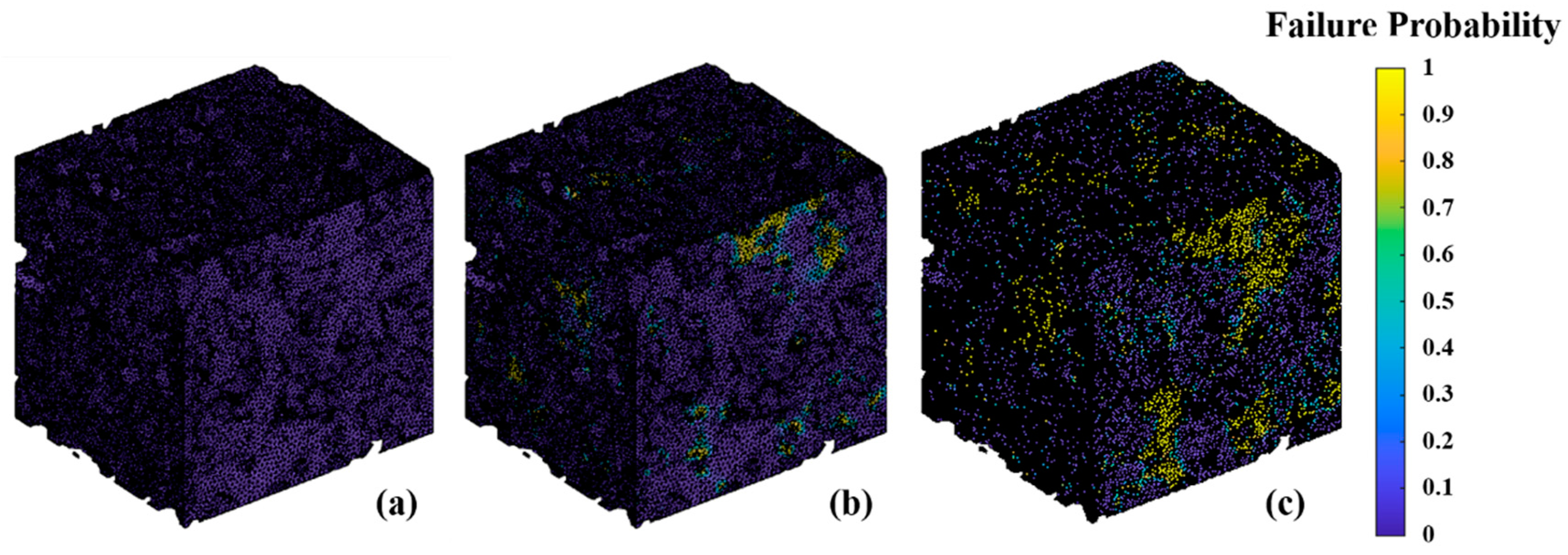
4.3. Failure Probability Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef] [PubMed]
- Subotić, V.; Hochenauer, C. Analysis of solid oxide fuel and electrolysis cells operated in a real-system environment: State-of-the-health diagnostic, failure modes, degradation mitigation and performance regeneration. Prog. Energy Combust. Sci. 2022, 93, 101011. [Google Scholar] [CrossRef]
- Shmatko, V.; Leontyeva, D.; Nevzorova, N.; Smirnova, N.; Brzhezinskaya, M.; Yalovega, G. Interaction between NiOx and MWCNT in NiOx/MWCNTs composite: XANES and XPS study. J. Electron Spectrosc. Relat. Phenom. 2017, 220, 76–80. [Google Scholar] [CrossRef]
- Yang, T.; Fan, Y.; Liu, J.; Finklea, H.; Lee, S.; Guan, B.; Abernathy, H.W.; Kalapos, T.L.; Hackett, G.A. Multiphysics modeling of SOFC performance degradation caused by interface delamination and active layer cracking. Int. J. Hydrogen Energy 2022, 47, 41124–41137. [Google Scholar] [CrossRef]
- Yang, S.; Lu, Y.; Wang, K.; Zhao, X.; Wang, F. Defect transport and thermal mismatch induced fracture in planar solid oxide fuel cell. Int. J. Electrochem. Sci. 2024, 19, 100678. [Google Scholar] [CrossRef]
- Moçoteguy, P.; Brisse, A. A review and comprehensive analysis of degradation mechanisms of solid oxide electrolysis cells. Int. J. Hydrogen Energy 2013, 38, 15887–15902. [Google Scholar] [CrossRef]
- Vladikova, D.; Burdin, B.; Sheikh, A.; Piccardo, P.; Krapchanska, M.; Montinaro, D. Redox Cycling for SOFC Accelerated Degradation. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2022; Volume 334. [Google Scholar] [CrossRef]
- Laurencin, J.; Delette, G.; Lefebvre-Joud, F.; Dupeux, M. A numerical tool to estimate SOFC mechanical degradation: Case of the planar cell configuration. J. Eur. Ceram. Soc. 2008, 28, 1857–1869. [Google Scholar] [CrossRef]
- Sarantaridis, D.; Chater, R.J.; Atkinson, A. Changes in Physical and Mechanical Properties of SOFC Ni–YSZ Composites Caused by Redox Cycling. J. Electrochem. Soc. 2008, 155, B467–B472. [Google Scholar] [CrossRef]
- Sarantaridis, D.; Rudkin, R.; Atkinson, A. Oxidation failure modes of anode-supported solid oxide fuel cells. J. Power Sources 2008, 180, 704–710. [Google Scholar] [CrossRef]
- Shang, S.; Lu, Y.; Cao, X.; Song, X.; Shi, M.; Wang, F. A model for oxidation-induced stress analysis of Ni-based anode-supported planar solid oxide fuel cell. Int. J. Hydrogen Energy 2019, 44, 16956–16964. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Song, M.; Luo, Y.; Tu, S.-T. Effect of inhomogeneous oxidation on the mechanical degradation of anode supported solid oxide fuel cell. J. Power Sources 2020, 450, 227663. [Google Scholar] [CrossRef]
- Wilson, J.R.; Kobsiriphat, W.; Mendoza, R.; Chen, H.-Y.; Hiller, J.M.; Miller, D.J.; Thornton, K.; Voorhees, P.W.; Adler, S.B.; Barnett, S.A. Three-dimensional reconstruction of a solid-oxide fuel-cell anode. Nat. Mater. 2006, 5, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Bae, K.; Kim, H.J.; Son, J.-W.; Kim, N.; Stenfelt, S.; Prinz, F.B.; Shim, J.H. Three-dimensional thermal stress analysis of the re-oxidized Ni-YSZ anode functional layer in solid oxide fuel cells. J. Alloys Compd. 2018, 752, 148–154. [Google Scholar] [CrossRef]
- Pecho, O.M.; Stenzel, O.; Iwanschitz, B.; Gasser, P.; Neumann, M.; Schmidt, V.; Prestat, M.; Hocker, T.; Flatt, R.J.; Holzer, L. 3D Microstructure Effects in Ni-YSZ Anodes: Prediction of Effective Transport Properties and Optimization of Redox Stability. Materials 2015, 8, 5554–5585. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhong, Z.; Jiao, Z. An adaptive thermo-mechanical peridynamic model for crack analysis in anode-supported solid oxide fuel cell. J. Power Sources 2022, 547, 231998. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Che, Q.; Li, J.; Lu, Y.; Shang, S.; Zhang, H. Quantitative characterization of nickel migration in solid oxide fuel cells under redox cycling. J. Alloys Compd. 2022, 921, 166085. [Google Scholar] [CrossRef]
- Song, B.; Ruiz-Trejo, E.; Bertei, A.; Brandon, N.P. Quantification of the degradation of Ni-YSZ anodes upon redox cycling. J. Power Sources 2018, 374, 61–68. [Google Scholar] [CrossRef]
- Pecho, O.M.; Mai, A.; Münch, B.; Hocker, T.; Flatt, R.J.; Holzer, L. 3D Microstructure Effects in Ni-YSZ Anodes: Influence of TPB Lengths on the Electrochemical Performance. Materials 2015, 8, 7129–7144. [Google Scholar] [CrossRef]
- Shearing, P.; Bradley, R.; Gelb, J.; Tariq, F.; Withers, P.; Brandon, N. Exploring microstructural changes associated with oxidation in Ni–YSZ SOFC electrodes using high resolution X-ray computed tomography. Solid State Ionics 2012, 216, 69–72. [Google Scholar] [CrossRef]
- Sainju, R.; Rathnayake, D.; Tan, H.; Bollas, G.; Dongare, A.M.; Suib, S.L.; Zhu, Y. In Situ Studies of Single-Nanoparticle-Level Nickel Thermal Oxidation: From Early Oxide Nucleation to Diffusion-Balanced Oxide Thickening. ACS Nano 2022, 16, 6468–6479. [Google Scholar] [CrossRef]
- Clague, R.; Shearing, P.; Lee, P.; Zhang, Z.; Brett, D.; Marquis, A.; Brandon, N. Stress analysis of solid oxide fuel cell anode microstructure reconstructed from focused ion beam tomography. J. Power Sources 2011, 196, 9018–9021. [Google Scholar] [CrossRef]
- Xiang, Y.; Da, Y.; Zhong, Z.; Shikazono, N.; Jiao, Z. Thermo-mechanical stress analyses of solid oxide fuel cell anode based on three-dimensional microstructure reconstruction. Int. J. Hydrogen Energy 2020, 45, 19791–19800. [Google Scholar] [CrossRef]
- Toros, S. Microstructural finite element modeling of redox behavior of Ni–YSZ based ceramic SOFC anodes. Ceram. Int. 2016, 42, 8915–8924. [Google Scholar] [CrossRef]
- Mozdzierz, M.; Berent, K.; Kimijima, S.; Szmyd, J.S.; Brus, G. A Multiscale Approach to the Numerical Simulation of the Solid Oxide Fuel Cell. Catalysts 2019, 9, 253. [Google Scholar] [CrossRef]
- Shearing, P.R.; Bradley, R.S.; Gelb, J.; Lee, S.N.; Atkinson, A.; Withers, P.J.; Brandon, N.P. Using Synchrotron X-Ray Nano-CT to Characterize SOFC Electrode Microstructures in Three-Dimensions at Operating Temperature. Electrochem. Solid-State Lett. 2011, 14, B117–B120. [Google Scholar] [CrossRef]
- Gunda, N.S.K.; Choi, H.-W.; Berson, A.; Kenney, B.; Karan, K.; Pharoah, J.G.; Mitra, S.K. Focused ion beam-scanning electron microscopy on solid-oxide fuel-cell electrode: Image analysis and computing effective transport properties. J. Power Sources 2011, 196, 3592–3603. [Google Scholar] [CrossRef]
- Wang, H.; Yu, W.; Shen, S. Chemo-mechanical coupling effect in the high-temperature oxidation of metal materials: A review. Sci. China Technol. Sci. 2019, 62, 1246–1254. [Google Scholar] [CrossRef]
- Sato, K.; Watanabe, S.; Huang, Y.; Miyasaka, T.; Matsui, T.; Yashiro, K.; Kawada, T.; Amezawa, K.; Kumada, K.; Eguchi, K. Relationship between microstructure and deformation of porous Ni-based cermets under redox cycling. SN Appl. Sci. 2021, 3, 810. [Google Scholar] [CrossRef]
- Railsback, J.G.; Johnston-Peck, A.C.; Wang, J.; Tracy, J.B. Size-Dependent Nanoscale Kirkendall Effect During the Oxidation of Nickel Nanoparticles. ACS Nano 2010, 4, 1913–1920. [Google Scholar] [CrossRef]
- Haugsrud, R. On the high-temperature oxidation of nickel. Corros. Sci. 2003, 45, 211–235. [Google Scholar] [CrossRef]
- Elder, J.P. The ‘E–ln(A)–f(α)’ triplet in non-isothermal reaction kinetics analysis. Thermochim. Acta 1998, 318, 229–238. [Google Scholar] [CrossRef]
- Gao, P.; Wang, H.; Jin, Z. Study of oxidation properties and decomposition kinetics of three-dimensional (3-D) braided carbon fiber. Thermochim. Acta 2004, 414, 59–63. [Google Scholar] [CrossRef]
- Waldbillig, D.; Wood, A.; Ivey, D.G. Thermal analysis of the cyclic reduction and oxidation behaviour of SOFC anodes. Solid State Ionics 2005, 176, 847–859. [Google Scholar] [CrossRef]
- Karmhag, R.; Niklasson, G.A.; Nygren, M. Oxidation kinetics of small nickel particles. J. Appl. Phys. 1999, 85, 1186–1191. [Google Scholar] [CrossRef]
- Kiss, A.M.; Harris, W.M.; Wang, S.; Vila-Comamala, J.; Deriy, A.; Chiu, W.K.S. In-situ observation of nickel oxidation using synchrotron based full-field transmission X-ray microscopy. Appl. Phys. Lett. 2013, 102, 053902. [Google Scholar] [CrossRef]
- Celik, S.; Ibrahimoglu, B.; Toros, S.; Mat, M.D. Three dimensional stress analysis of solid oxide fuel cell anode micro structure. Int. J. Hydrogen Energy 2014, 39, 19119–19131. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, W.; Zhang, S.; Zhang, Q.; Su, S. Residual stress analysis of a micro-tubular solid oxide fuel cell. Int. J. Hydrogen Energy 2016, 41, 16173–16180. [Google Scholar] [CrossRef]
- Pihlatie, M.; Frandsen, H.; Kaiser, A.; Mogensen, M. Continuum mechanics simulations of NiO/Ni–YSZ composites during reduction and re-oxidation. J. Power Sources 2009, 195, 2677–2690. [Google Scholar] [CrossRef]
- Li, J.; Lin, Z. Effects of electrode composition on the electrochemical performance and mechanical property of micro-tubular solid oxide fuel cell. Int. J. Hydrogen Energy 2012, 37, 12925–12940. [Google Scholar] [CrossRef]
- Watanabe, S.; Sato, K.; Iguchi, F.; Yashiro, K.; Hashida, T.; Kawada, T. Mechanical Strength Evaluation of YSZ, GDC and LSCF under SOFC Operating Conditions. ECS Trans. 2017, 78, 2181–2190. [Google Scholar] [CrossRef]
- De Angelis, S.; Jørgensen, P.S.; Esposito, V.; Tsai, E.H.R.; Holler, M.; Kreka, K.; Abdellahi, E.; Bowen, J.R. Ex-situ tracking solid oxide cell electrode microstructural evolution in a redox cycle by high resolution ptychographic nanotomography. J. Power Sources 2017, 360, 520–527. [Google Scholar] [CrossRef]
- Faes, A.; Hessler-Wyser, A.; Zryd, A.; Van Herle, J. A Review of RedOx Cycling of Solid Oxide Fuel Cells Anode. Membranes 2012, 2, 585–664. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Lin, Z. Numerical study on the mechanical stress and mechanical failure of planar solid oxide fuel cell. Appl. Energy 2018, 229, 63–68. [Google Scholar] [CrossRef]
- Fleischhauer, F.; Bermejo, R.; Danzer, R.; Mai, A.; Graule, T.; Kuebler, J. High temperature mechanical properties of zirconia tapes used for electrolyte supported solid oxide fuel cells. J. Power Sources 2015, 273, 237–243. [Google Scholar] [CrossRef]
- Vedasri, V.; Young, J.; Birss, V. A possible solution to the mechanical degradation of Ni–yttria stabilized zirconia anode-supported solid oxide fuel cells due to redox cycling. J. Power Sources 2010, 195, 5534–5542. [Google Scholar] [CrossRef]

| 600 °C | 700 °C | 800 °C | |
|---|---|---|---|
| K(T) (m·s−1) | 9.14 × 10−5 | 2.25 × 10−4 | 5.00 × 10−4 |
| Ea (kJ·mol−1) | 65.0 | 70.6 | 75.4 |
| E (GPa) | Possion’s Ratio ν | TEC α (10−6·K−1) | Yield/Fracture Stress (MPa) | |||||
|---|---|---|---|---|---|---|---|---|
| 8YSZ | 600 °C | 700 °C | 800 °C | 0.313 | 10.5 | 600 °C | 700 °C | 800 °C |
| 126.9 | 132.2 | 141.7 | 395.3 | 412.4 | 433.2 | |||
| Ni | 5.9 | 0.310 | 13.5 | – | ||||
| NiO | 600 °C | 700 °C | 800°C | 0.330 | 11.8 | 395 | ||
| 104.9 | 107.4 | 110 | ||||||
| This Study | Celik et al. [37] | Clague et al. [22] | |
|---|---|---|---|
| Boundary condition | Roller support at three planes | – | Point constraint and roller support of one cutting surface |
| Reference temperature | Room temperature | Room temperature | Room temperature |
| Size (µm) | 6.40 × 6.40 × 6.40 | 10 × 10 × 10 | 6.68 × 5.04 × 1.50 |
| Mesh quantity | 2,499,970 | 300,000 | 285,000 |
| Stress (MPa) | 69.6–90.9 | 40 | ≥59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Chen, Z.; Zhang, H.; Li, J.; Zhao, X.; Hao, W.; Xie, J.; Wang, F. Failure Analysis of Ni-8YSZ Electrode under Reoxidation Based on the Real Microstructure. Materials 2024, 17, 4599. https://doi.org/10.3390/ma17184599
Yang S, Chen Z, Zhang H, Li J, Zhao X, Hao W, Xie J, Wang F. Failure Analysis of Ni-8YSZ Electrode under Reoxidation Based on the Real Microstructure. Materials. 2024; 17(18):4599. https://doi.org/10.3390/ma17184599
Chicago/Turabian StyleYang, Sen, Zhipeng Chen, Hongye Zhang, Jinzhi Li, Xiang Zhao, Wenqian Hao, Jiamiao Xie, and Fenghui Wang. 2024. "Failure Analysis of Ni-8YSZ Electrode under Reoxidation Based on the Real Microstructure" Materials 17, no. 18: 4599. https://doi.org/10.3390/ma17184599






