In Vitro Proliferation of MG-63 Cells in Additively Manufactured Ti-6Al-4V Biomimetic Lattice Structures with Varying Strut Geometry and Porosity
Abstract
1. Introduction
2. Materials and Methods
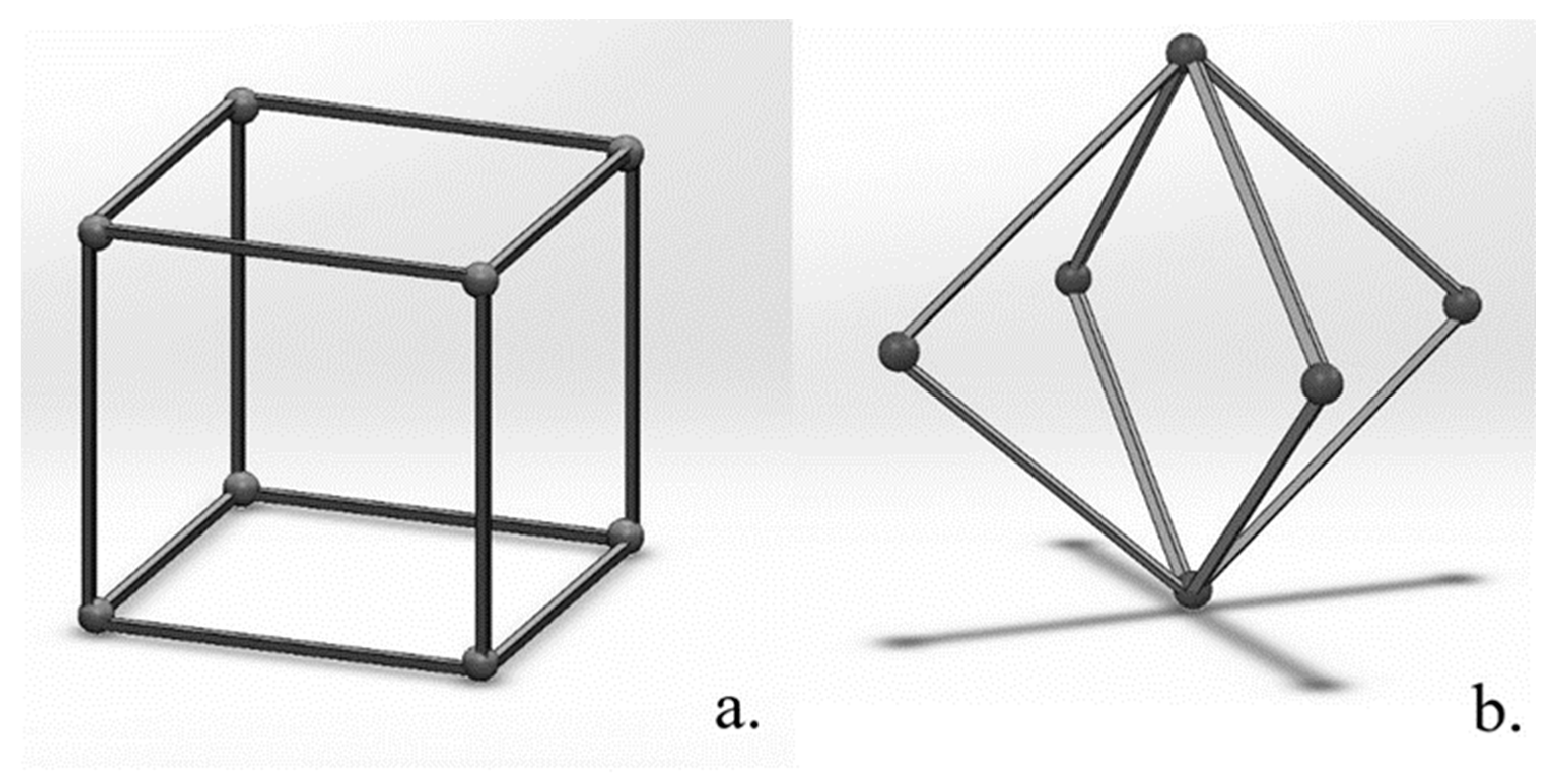
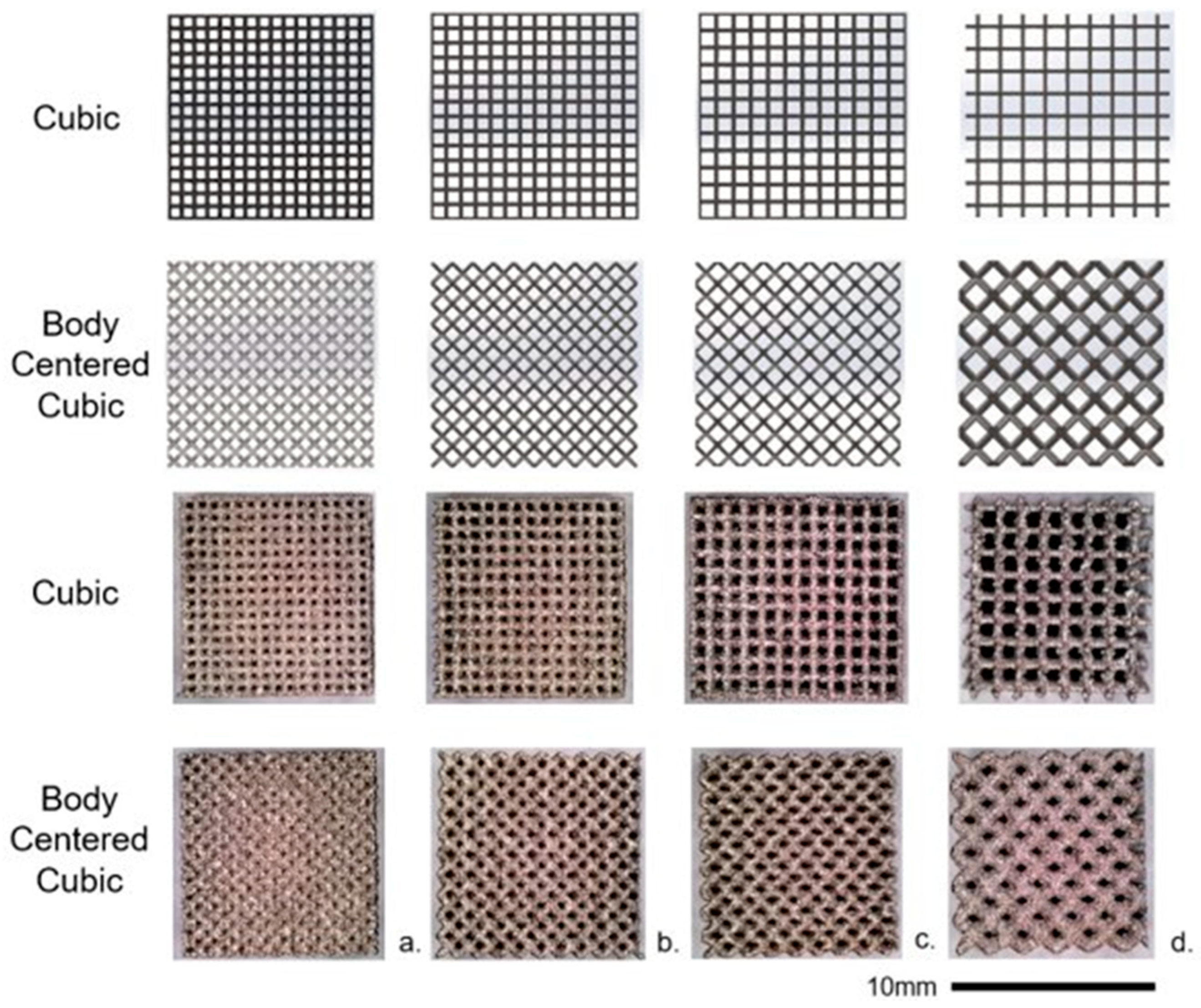
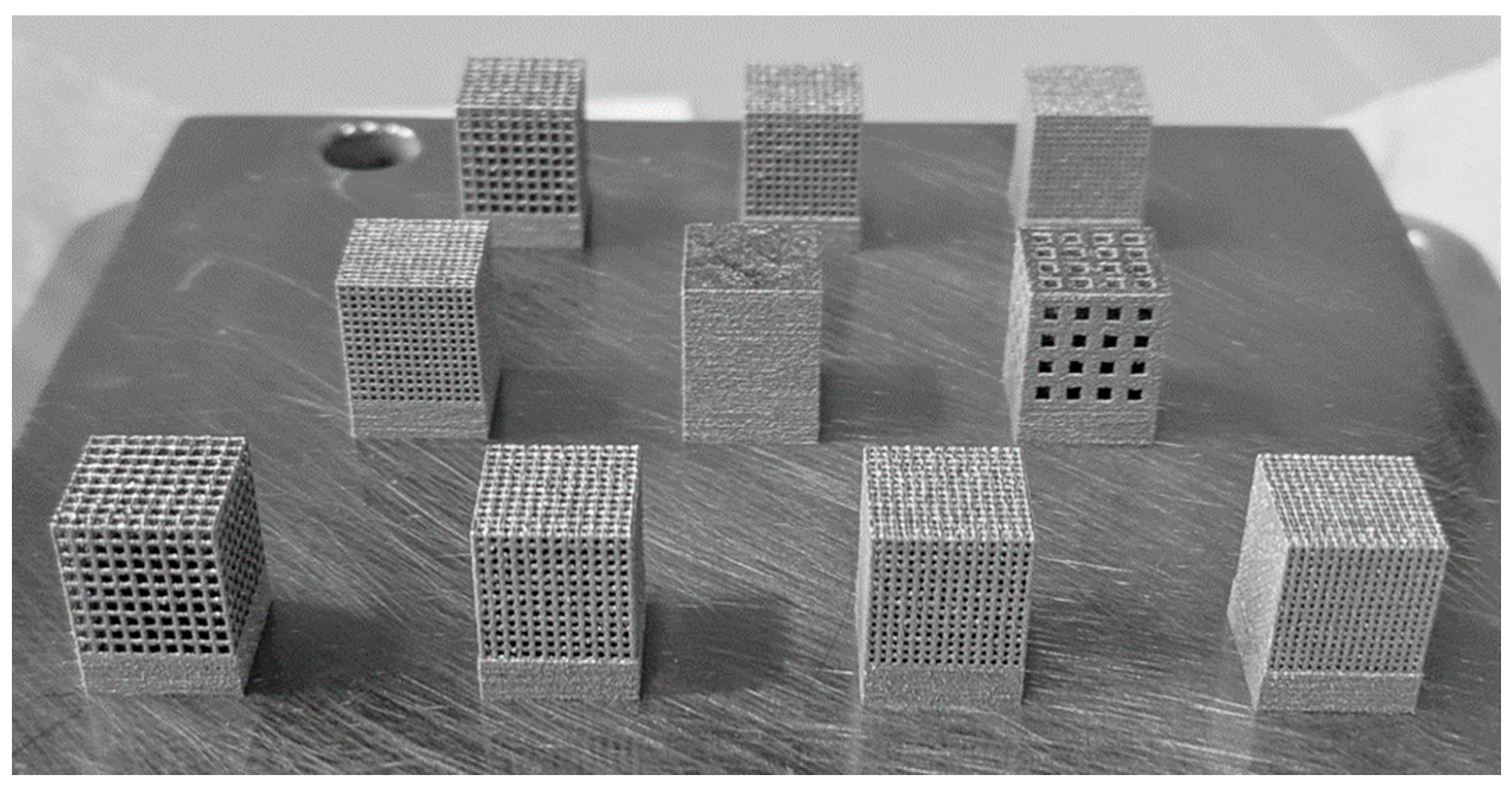
2.1. 3D-Printed Ti-6Al-4V Lattice Structures
2.2. MG-63 Cell Culture Preparation
2.3. Cell Culture Tests
2.3.1. Glucose Assay
2.3.2. ALP Assay
2.3.3. Cell Count
3. Results
3.1. Glucose Concentration in Cell Culture Media Surrounding Titanium Lattices
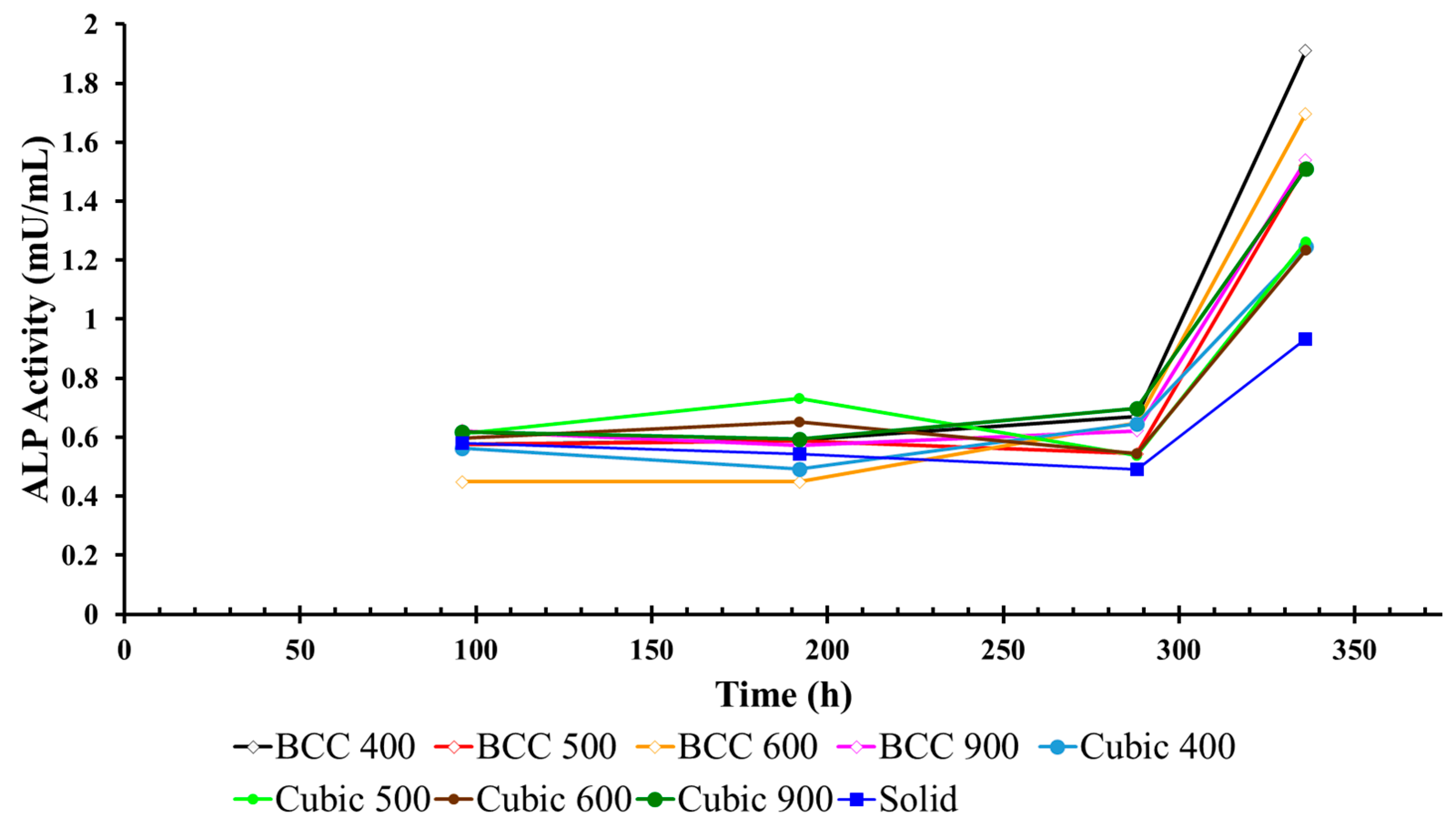
3.2. ALP Activity in Cell Culture Media Surrounding Titanium Lattices
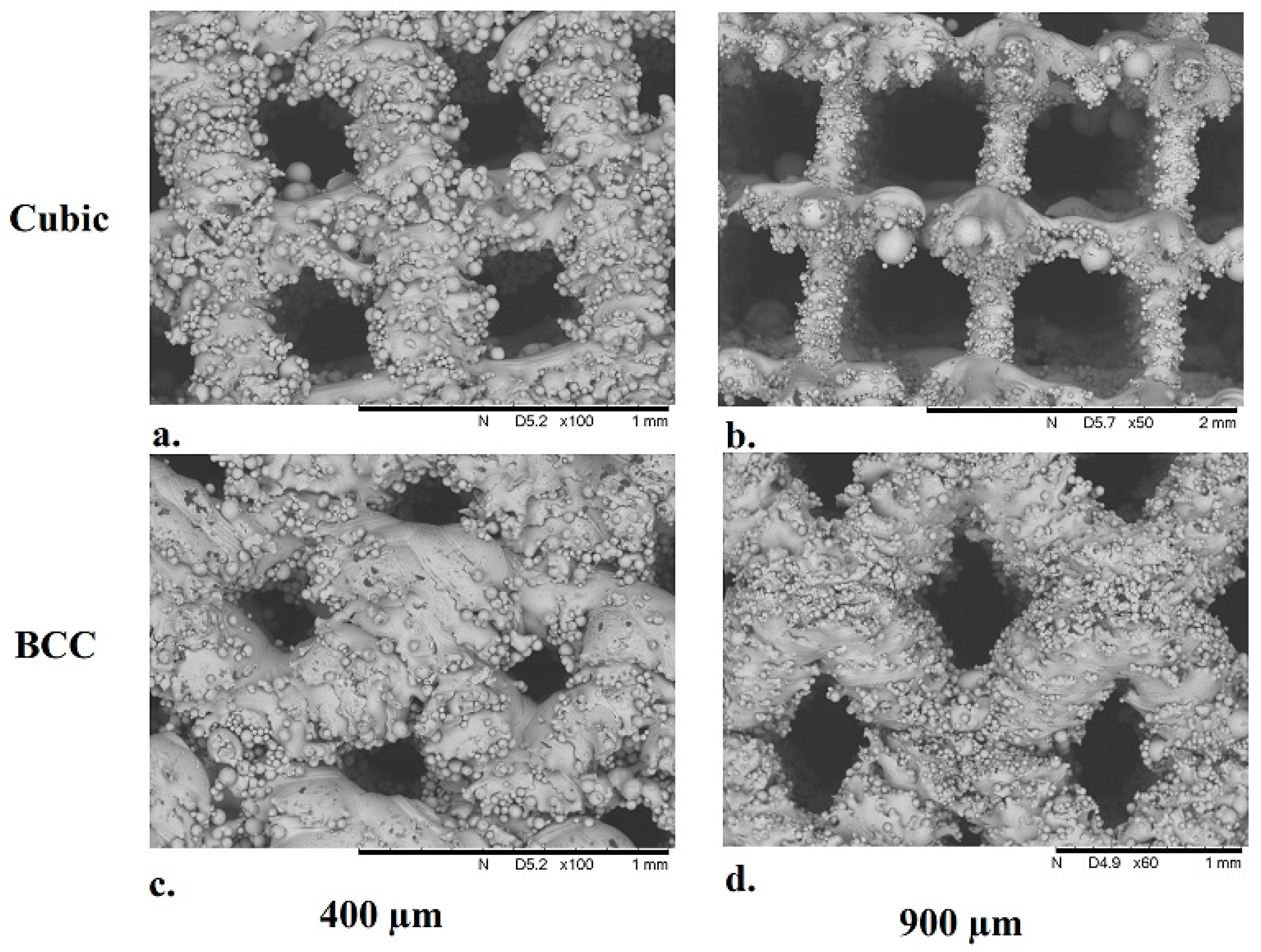
3.3. Structural Characterization of Cubic and Body-Centered Cubic Titanium Lattices
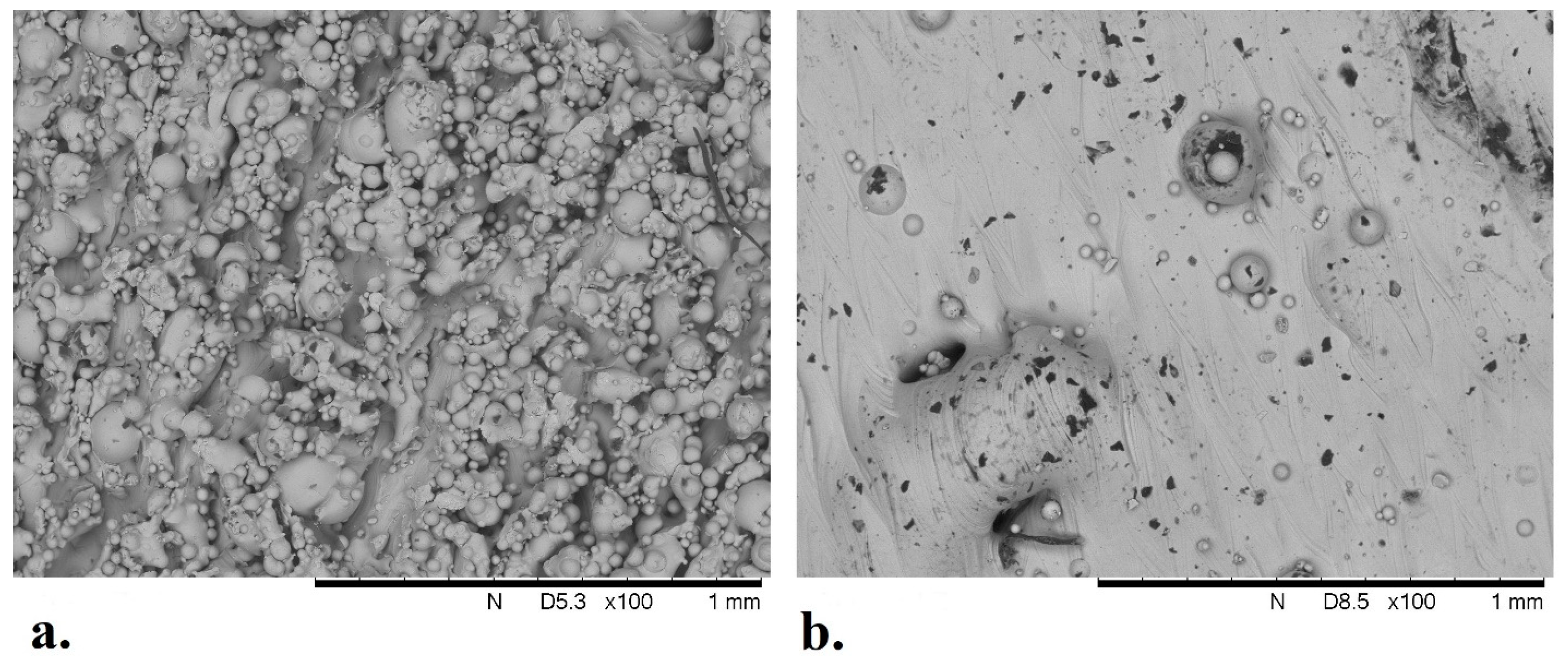
3.4. Analysis of Solid Titanium Specimen
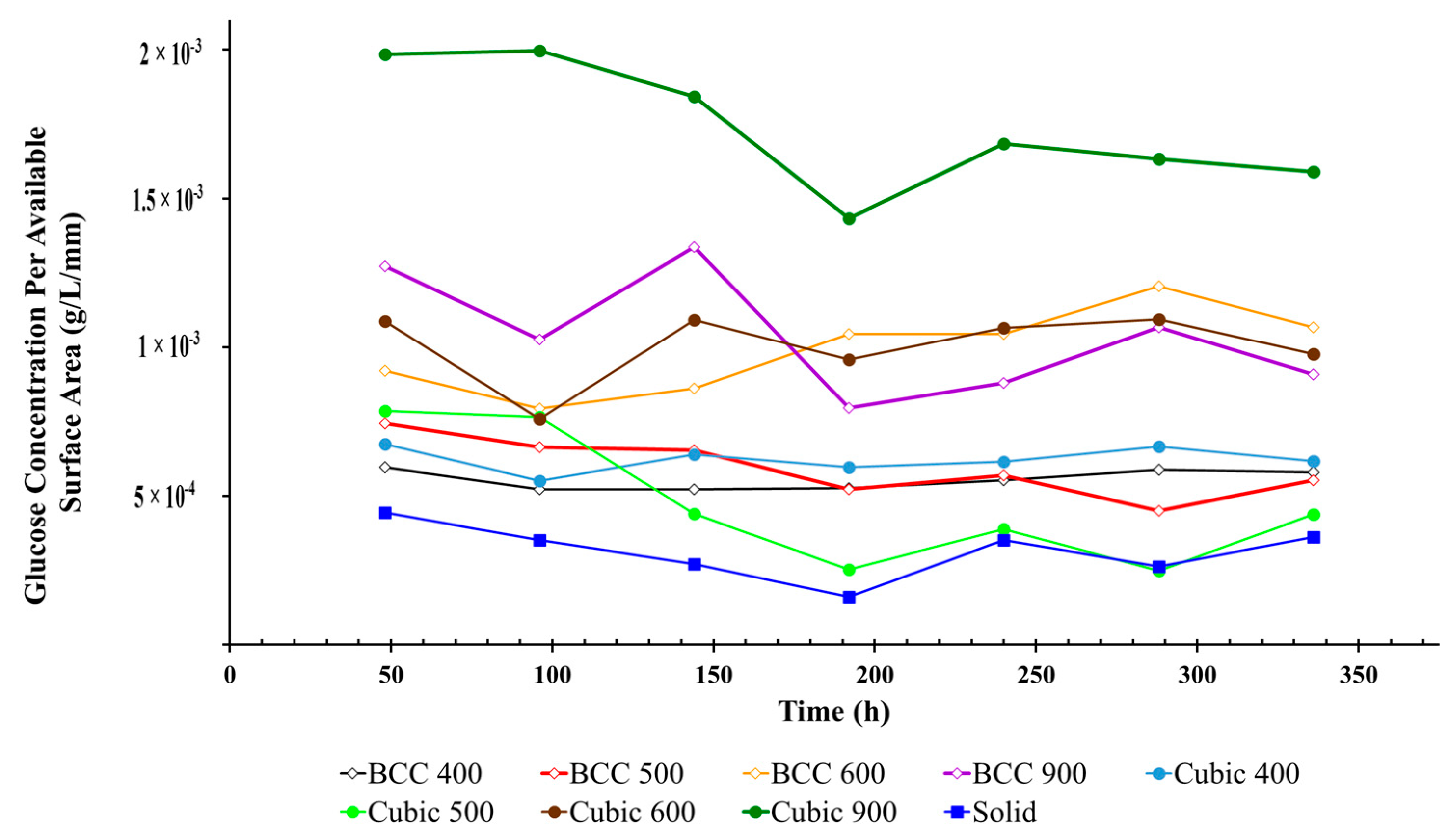
3.5. Glucose Concentration Per Available Surface Area of Titanium Lattices
3.6. Cell Count in Media Surrounding Titanium Lattices
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Technical Considerations for Additive Manufactured Medical Devices. Silver Spring. 2017. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/technical-considerations-additive-manufactured-medical-devices (accessed on 19 April 2024).
- Spinal Elements® Announces FDA 510(k) Clearance of Lucent® 3D Additive Manufactured Interbody Devices. 2021. Available online: https://www.businesswire.com/news/home/20210420005407/en/Spinal-Elements%C2%AE-Announces-FDA-510-k-Clearance-of-Lucent%C2%AE-3D-Additive-Manufactured-Interbody%20Devices/?feedref=JjAwJuNHiystnCoBq_hl%20WFAllVCLJFCqzlmaJ8DKHU4plfPZtlGYRDUHCSmgbii6XkLWuQZD%20HgGRnjQvCyg3iCfEFIaJW7-otp9V1XQiK2eHTJy3ZqOEt7kKgLu20J (accessed on 19 April 2024).
- US FDA Grants 510K Clearance for Aurora Spine’s Interbody Fusion Device. 2022. Available online: https://www.medicaldevice-network.com/news/fda-510k-aurora-spine-interbody-fusion-device/ (accessed on 19 April 2024).
- Stoffelen, D.V.C.; Eraly, K.; Debeer, P. The use of 3D printing technology in reconstruction of a severe glenoid defect: A case report with 2.5 years of follow-up. J. Shoulder Elb. Surg. 2015, 24, e218–e222. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qu, X.; Mao, Y.; Dai, K.; Zhu, Z. Custom Acetabular Cages Offer Stable Fixation and Improved Hip Scores for Revision THA With Severe Bone Defects. Clin. Orthop. Relat. Res. 2016, 474, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Kumta, S.M.; Geel, N.V.; Demol, J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput. Aided Surg. 2015, 20, 14–23. [Google Scholar] [CrossRef]
- Ridzwan, M.I.Z.; Shuib, S.; Hassan, A.Y.; Shokri, A.A.; Mohamad Ibrahim, M.N. Problem of Stress Shielding and Improvement to the Hip Implant Designs: A Review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar] [CrossRef]
- Lane, J.M.; Mait, J.E.; Unnanuntana, A.; Hirsch, B.P.; Shaffer, A.D.; Shonuga, O.A. 6.616—Materials in Fracture Fixation; Ducheyne PBT-CB, Ed.; Elsevier: Oxford, UK, 2011; pp. 219–235. [Google Scholar] [CrossRef]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and mechanobiology of trabecular bone: A review. J. Biomech. Eng. 2015, 137, 010802–01080215. [Google Scholar] [CrossRef]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Srivas, P.K.; Kapat, K.; Dadhich, P.; Pal, P.; Dutta, J.; Datta, P.; Dhara, S. Osseointegration assessment of extrusion printed Ti6Al4V scaffold towards accelerated skeletal defect healing via tissue in-growth. Bioprinting 2017, 6, 8–17. [Google Scholar] [CrossRef]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Yan, X.; Li, Q.; Yin, S.; Chen, Z.; Jenkins, R.; Chen, C.; Wang, J.; Ma, W.; Bolot, R.; Lupoi, R.; et al. Mechanical and in vitro study of an isotropic Ti6Al4V lattice structure fabricated using selective laser melting. J. Alloys Compd. 2019, 782, 209–223. [Google Scholar] [CrossRef]
- Ataollahi, S. A Review on Additive Manufacturing of Lattice Structures in Tissue Engineering. Bioprinting 2023, 35, e00304. [Google Scholar] [CrossRef]
- Pagani, S.; Liverani, E.; Giavaresi, G.; De Luca, A.; Belvedere, C.; Fortunato, A.; Leardini, A.; Fini, M.; Tomesani, L.; Caravaggi, P. Mechanical and in Vitro Biological Properties of Uniform and Graded Cobalt-Chrome Lattice Structures in Orthopedic Implants. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- De Wild, M.; Schumacher, R.; Mayer, K.; Schkommodau, E.; Thoma, D.; Bredell, M.; Gujer, A.K.; Grätz, K.W.; Weber, F.E. Bone regeneration by the osteoconductivity of porous titanium implants manufactured by selective laser melting: A histological and micro computed tomography study in the rabbit. Tissue Eng. Part A 2013, 19, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fraulob, M.; Haïat, G. Biomechanical behaviours of the bone–implant interface: A review. J. R. Soc. Interface 2019, 16, 20190259. [Google Scholar] [CrossRef]
- Kienapfel, H.; Sprey, C.; Wilke, A.; Griss, P. Implant fixation by bone ingrowth. J. Arthroplast. 1999, 14, 355–368. [Google Scholar] [CrossRef]
- Biemond, J.E.; Aquarius, R.; Verdonschot, N.; Buma, P. Frictional and bone ingrowth properties of engineered surface topographies produced by electron beam technology. Arch. Orthop. Trauma. Surg. 2011, 131, 711–718. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef]
- Obaton, A.-F.; Fain, J.; Djemaï, M.; Meinel, D.; Léonard, F.; Mahé, E.; Lécuelle, B.; Fouchet, J.-J.; Bruno, G. In vivo XCT bone characterization of lattice structured implants fabricated by additive manufacturing. Heliyon 2017, 3, e00374. [Google Scholar] [CrossRef] [PubMed]
- Obaton, A.-F.; Fain, J.; Meinel, D.; Tsamos, A.; Léonard, F.; Lécuelle, B.; Djemaï, M. In Vivo Bone Progression in and around Lattice Implants Additively Manufactured with a New Titanium Alloy. Appl. Sci. 2023, 13, 7282. [Google Scholar] [CrossRef]
- Van der Stok, J.; Van der Jagt, O.P.; Amin Yavari, S.; De Haas, M.F.; Waarsing, J.H.; Jahr, H.; Van Lieshout, E.M.; Patka, P.; Verhaar, J.A.; Zadpoor, A.A.; et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J. Orthop. Res. 2013, 31, 792–799. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Barradas, A.W.C.; van Blitterswijk, C.A.; de Boer, J.; Feijen, J.; Grijpma, D.W. Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomater. 2010, 6, 4208–4217. [Google Scholar] [CrossRef] [PubMed]
- Wally, Z.J.; Haque, A.M.; Feteira, A.; Claeyssens, F.; Goodall, R.; Reilly, G.C. Selective laser melting processed Ti6Al4V lattices with graded porosities for dental applications. J. Mech. Behav. Biomed. Mater. 2019, 90, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, K.; Su, L.; Liang, P.; Ji, P.; Wang, C. The effect of 3D-printed Ti6Al4V scaffolds with various macropore structures on osteointegration and osteogenesis: A biomechanical evaluation. J. Mech. Behav. Biomed. Mater. 2018, 88, 488–496. [Google Scholar] [CrossRef]
- Markhoff, J.; Wieding, J.; Weissmann, V.; Pasold, J.; Jonitz-Heincke, A.; Bader, R. Influence of Different Three-Dimensional Open Porous Titanium Scaffold Designs on Human Osteoblasts Behavior in Static and Dynamic Cell Investigations. Materials 2015, 8, 5490–5507. [Google Scholar] [CrossRef] [PubMed]
- Standard Guide for Powder Reuse Schema in Powder Bed Fusion Processes for Medical Applications for Additive Manufacturing Feedstock Materials (ASTM F3456). Available online: https://www.astm.org/f3456-22.html (accessed on 19 April 2024).
- Meuwly, F.; Papp, F.; Ruffieux, P.-A.; Bernard, A.; Kadouri, A.; von Stockar, U. Use of glucose consumption rate (GCR) as a tool to monitor and control animal cell production processes in packed-bed bioreactors. J. Biotechnol. 2006, 122, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef]
- Yang, L.; Ferrucci, M.; Mertens, R.; Dewulf, W.; Yan, C.; Shi, Y.; Yang, S. An investigation into the effect of gradients on the manufacturing fidelity of triply periodic minimal surface structures with graded density fabricated by selective laser melting. J. Mater. Process. Technol. 2020, 275, 116367. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, J.; Chen, X.; Yi, C.; Chen, R.; Zhang, Z. Configuration Optimization Design of Ti6Al4V Lattice Structure Formed by SLM. Materials 2018, 11, 1856. [Google Scholar] [CrossRef]
- Zani, B.G.; Edelman, E.R. Cellular bridges: Routes for intercellular communication and cell migration. Commun. Integr. Biol. 2010, 3, 215–220. [Google Scholar] [CrossRef]
- Martinez, M.A.F.; Balderrama, Í.d.F.; Karam, P.S.B.H.; de Oliveira, R.C.; de Oliveira, F.A.; Grandini, C.R.; Vicente, F.B.; Stavropoulos, A.; Zangrando, M.S.R.; Sant’ana, A.C.P. Surface roughness of titanium disks influences the adhesion, proliferation and differentiation of osteogenic properties derived from human. Int. J. Implant. Dent. 2020, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.A.; Von Walter, M.; Wirtz, T.; Sellei, R.; Schmidt-Rohlfing, B.; Paar, O.; Erli, H.J. Structural, mechanical and in vitro characterization of individually structured Ti–6Al–4V produced by direct laser forming. Biomaterials 2006, 27, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. Part A 2014, 102, 2636–2643. [Google Scholar] [CrossRef] [PubMed]


| Pore Size (µm) | Strut Geometry | Glucose Concentration (g/L) | Standard Deviation (g/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | Day 14 | |||
| 0 | Solid | 2.66 ± 0.28 | 2.11 ± 0.65 | 1.63 ± 0.38 | 0.96 ± 0.16 | 2.10 ± 0.58 | 1.57 ± 0.57 | 2.17 ± 0.53 | 0.45 |
| 400 | Cubic | 2.60 ± 0.19 | 2.28 ± 0.56 | 2.28 ± 0.51 | 2.30 ± 0.99 | 2.41 ± 0.70 | 2.57 ± 1.39 | 2.53 ± 1.10 | 0.78 |
| BCC | 2.53 ± 0.13 | 2.18 ± 0.47 | 2.37 ± 0.81 | 2.87 ± 0.11 | 2.87 ± 0.13 | 3.31 ± 0.7 | 2.93 ± 0.03 | 0.35 | |
| 500 | Cubic | 2.60 ± 0.11 | 2.13 ± 0.50 | 2.47 ± 0.38 | 2.30 ± 1.05 | 2.38 ± 0.79 | 2.58 ± 1.37 | 2.38 ± 1.49 | 0.81 |
| BCC | 2.65 ± 0.19 | 1.85 ± 0.87 | 2.66 ± 0.47 | 2.33 ± 1.01 | 2.59 ± 0.92 | 2.66 ± 1.50 | 2.38 ± 0.83 | 0.83 | |
| 600 | Cubic | 2.50 ± 0.19 | 2.23 ± 0.48 | 2.20 ± 0.50 | 1.76 ± 0.73 | 1.92 ± 0.72 | 1.51 ± 0.73 | 1.86 ± 0.66 | 0.57 |
| BCC | 2.77 ± 0.38 | 2.23 ± 0.48 | 2.91 ± 0.34 | 1.73 ± 1.28 | 1.91 ± 1.31 | 2.32 ± 1.69 | 1.98 ± 1.38 | 0.98 | |
| 900 | Cubic | 2.46 ± 0.14 | 2.39 ± 0.58 | 1.38 ± 0.22 | 0.79 ± 0.08 | 1.21 ± 0.10 | 0.78 ± 0.09 | 1.37 ± 0.14 | 0.19 |
| BCC | 2.57 ± 0.18 | 2.59 ± 0.36 | 2.39 ± 0.34 | 1.86 ± 0.77 | 2.18 ± 0.78 | 2.11 ± 1.35 | 2.06 ± 0.78 | 0.65 | |
| Pore Size (µm) | Strut Geometry | ALP Activity in Media (mU/mL) | Standard Deviation (mU/mL) | |||
|---|---|---|---|---|---|---|
| Day 4 | Day 8 | Day 12 | Day 14 | |||
| 0 | Solid | 0.57 ± 0.37 | 0.54 ± 0.30 | 0.48 ± 0.10 | 0.93 ± 0.26 | 0.17 |
| 400 | Cubic | 0.56 ± 0.17 | 0.49 ± 0.24 | 0.64 ± 0.26 | 1.24 ± 0.45 | 0.29 |
| BCC | 0.57 ± 0.24 | 0.59 ± 0.29 | 0.66 ± 0.25 | 1.90 ± 0.96 | 0.56 | |
| 500 | Cubic | 0.61 ± 0.12 | 0.73 ± 0.58 | 0.53 ± 0.17 | 1.26 ± 0.50 | 0.28 |
| BCC | 0.57 ± 0.20 | 0.58 ± 0.40 | 0.54 ± 0.21 | 1.52 ± 0.75 | 0.41 | |
| 600 | Cubic | 0.59 ± 0.22 | 0.65 ± 0.37 | 0.54 ± 0.16 | 1.23 ± 0.48 | 0.27 |
| BCC | 0.44 ± 0.26 | 0.44 ± 0.27 | 0.64 ± 0.35 | 1.69 ± 0.84 | 0.51 | |
| 900 | Cubic | 0.61 ± 0.26 | 0.59 ± 0.38 | 0.69 ± 0.27 | 1.50 ± 0.72 | 0.38 |
| BCC | 0.62 ± 0.21 | 0.57 ± 0.32 | 0.62 ± 0.24 | 1.53 ± 0.68 | 0.40 | |
| Pore Size (µm) | Strut Geometry | Averaged Measured Pore Size (µm) | Pore Size Deviation (%) | Averaged Measured Strut Diameter (µm) | Strut Diameter Deviation (%) | Surface Area (mm2) |
|---|---|---|---|---|---|---|
| 400 | Cubic | 374.6 | −6.5 | 310.1 | 55.0 | 3671 |
| BCC | 219.2 | −45.2 | 428.5 | 114.2 | 4181 | |
| 500 | Cubic | 439.2 | −12.1 | 333.8 | 66.9 | 2982 |
| BCC | 411.5 | −17.7 | 344.8 | 72.4 | 3166 | |
| 600 | Cubic | 559.1 | −6.8 | 268.2 | 34.1 | 2330 |
| BCC | 533.6 | −11.0 | 375.5 | 87.6 | 2655 | |
| 900 | Cubic | 849.5 | −5.7 | 312.6 | 56.3 | 1262 |
| BCC | 675.2 | −24.9 | 629.16 | 57.2 | 2167 |
| Pore Size (µm) | Strut Geometry | Average Estimated Cell Count (Cells/mL) | Standard Deviation (Cells/mL) | Percent Difference (%) |
|---|---|---|---|---|
| 0 | Solid | 1.84 × 106 | 7.62 × 105 | - |
| 400 | Cubic | 9.93 × 105 | 5.42 × 105 | 75.2 |
| BCC | 4.50 × 105 | 8.29 × 104 | ||
| 500 | Cubic | 1.15 × 106 | 6.04 × 105 | 34.4 |
| BCC | 8.12 × 105 | 5.79 × 105 | ||
| 600 | Cubic | 8.33 × 105 | 2.33 × 105 | 112.5 |
| BCC | 2.23 × 105 | 1.67 × 105 | ||
| 900 | Cubic | 1.90 × 106 | 6.92 × 105 | 78.1 |
| BCC | 8.33 × 105 | 2.65 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papazoglou, D.P.; Hobbs, L.; Sun, Y.; Neidhard-Doll, A. In Vitro Proliferation of MG-63 Cells in Additively Manufactured Ti-6Al-4V Biomimetic Lattice Structures with Varying Strut Geometry and Porosity. Materials 2024, 17, 4608. https://doi.org/10.3390/ma17184608
Papazoglou DP, Hobbs L, Sun Y, Neidhard-Doll A. In Vitro Proliferation of MG-63 Cells in Additively Manufactured Ti-6Al-4V Biomimetic Lattice Structures with Varying Strut Geometry and Porosity. Materials. 2024; 17(18):4608. https://doi.org/10.3390/ma17184608
Chicago/Turabian StylePapazoglou, Dimitri P., Laura Hobbs, Yvonne Sun, and Amy Neidhard-Doll. 2024. "In Vitro Proliferation of MG-63 Cells in Additively Manufactured Ti-6Al-4V Biomimetic Lattice Structures with Varying Strut Geometry and Porosity" Materials 17, no. 18: 4608. https://doi.org/10.3390/ma17184608
APA StylePapazoglou, D. P., Hobbs, L., Sun, Y., & Neidhard-Doll, A. (2024). In Vitro Proliferation of MG-63 Cells in Additively Manufactured Ti-6Al-4V Biomimetic Lattice Structures with Varying Strut Geometry and Porosity. Materials, 17(18), 4608. https://doi.org/10.3390/ma17184608





