Enhanced Water Resistance of TiO2–GO–SMS-Modified Soil Composite for Use as a Repair Material in Earthen Sites
Abstract
:1. Introduction
2. Experimental Section
2.1. Specimen Preparation
2.2. Experimental Method
3. Results and Discussion
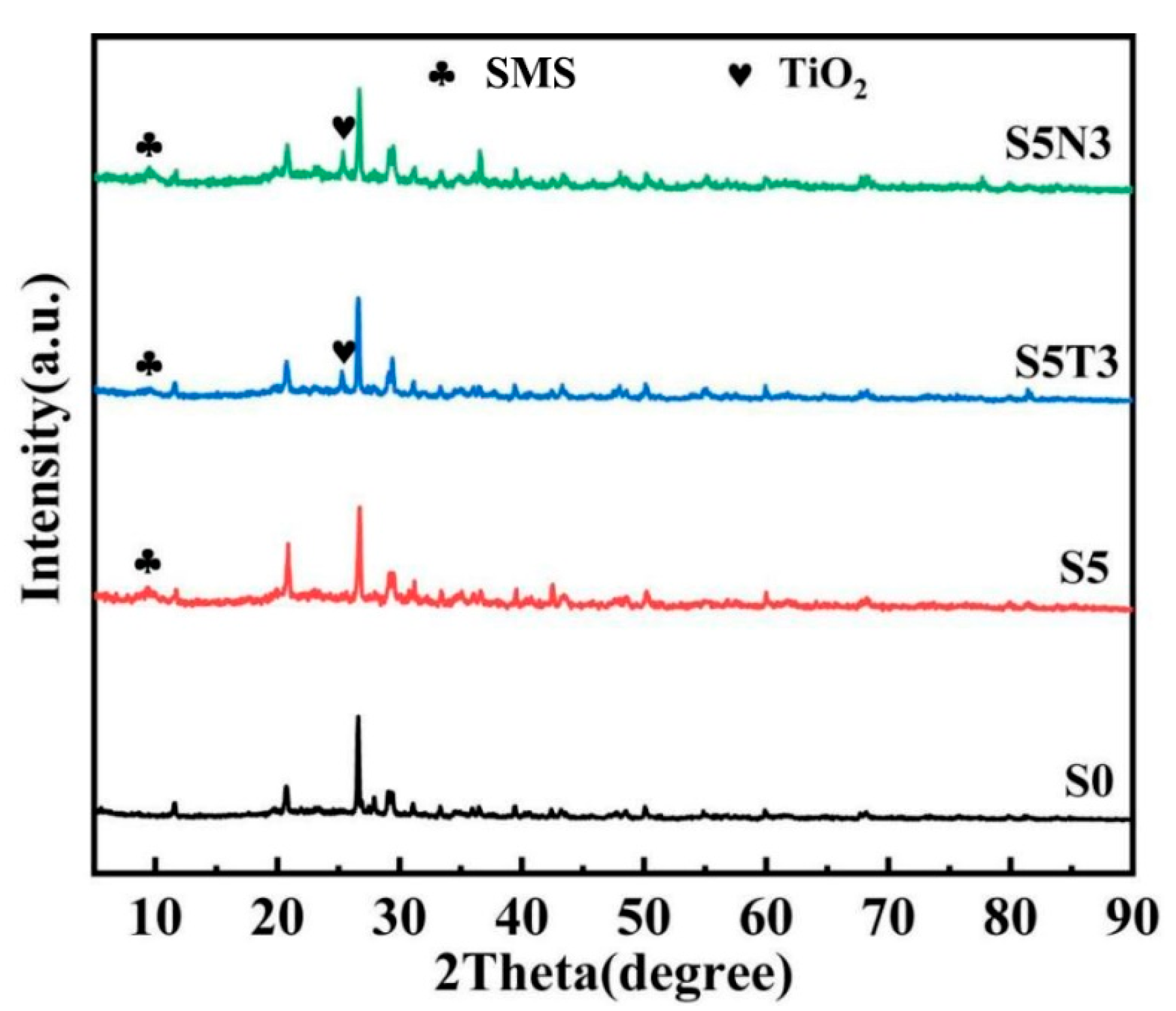
3.1. Microstructure Characterization
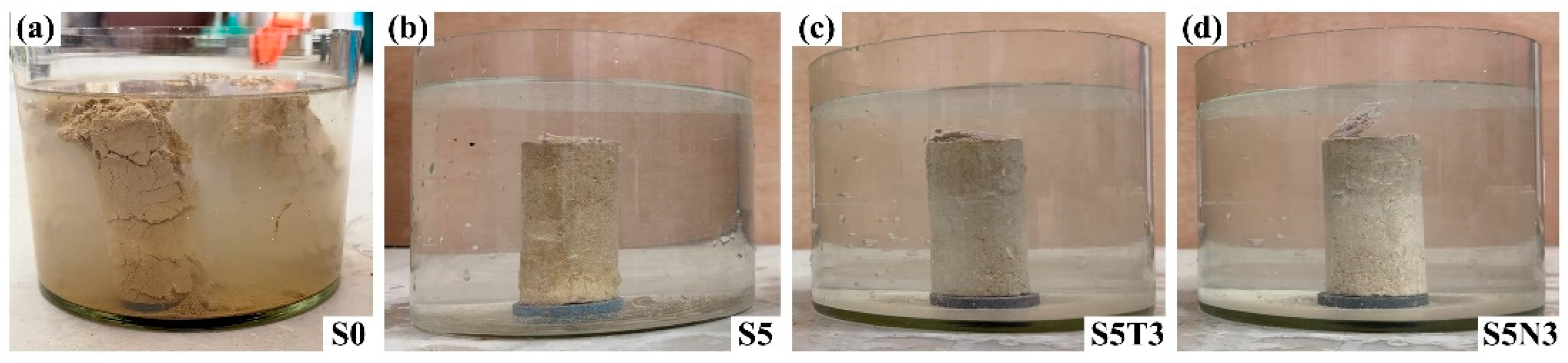
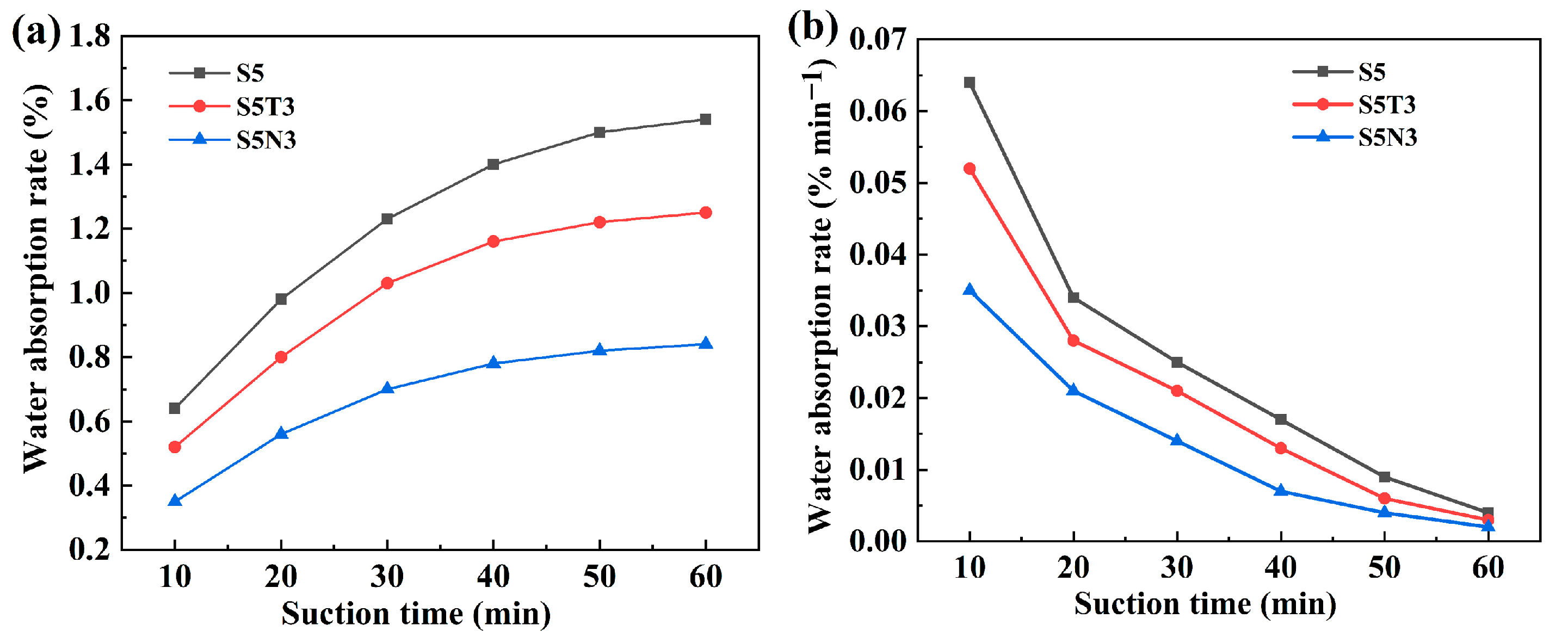
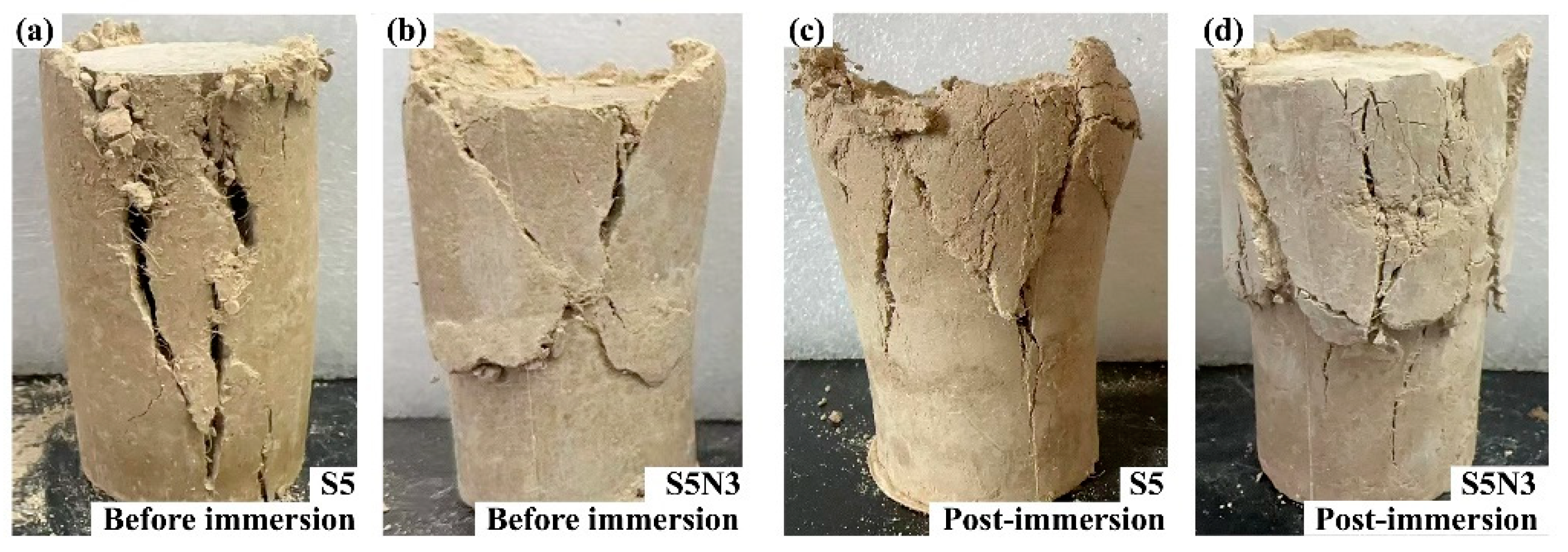
3.2. Water Immersion Resistance Performance
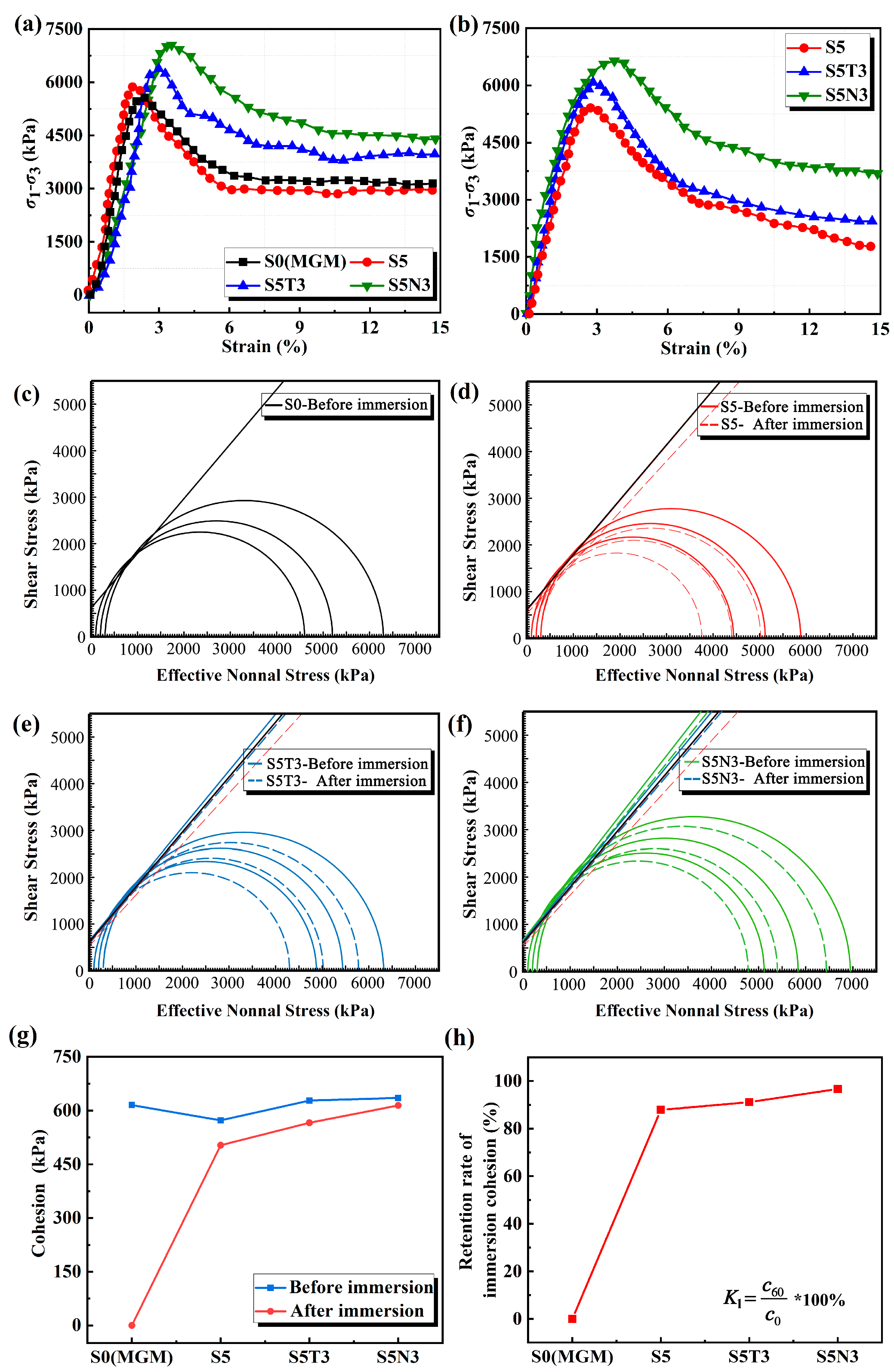
3.3. Mechanical Properties
3.4. Structural Evolution Mechanism of S5N3
3.4.1. The Formation Mechanism of S0 (Original Soil)
3.4.2. S5 Reaction Mechanism
3.4.3. S5T3 Evolution Mechanism
3.4.4. S5N3 Evolution Mechanism
3.4.5. Synergistic Hydration Effect
3.4.6. Ion-Exchange Adsorption Effects
3.4.7. Filling and Water Resistance Effect
4. Conclusions
- (1)
- Compared with S0 (original soil), the water resistance performance was effectively enhanced after the addition of SMS, TiO2, and GO. The water absorption rate of S5N3 was only 0.84% (S0 collapsed structurally after 30 min of immersion). This result was attributed to film formation from SMS, which developed a water-repellent layer on the surface of the restorative soil particles and improved their water resistance.
- (2)
- The addition of SMS, TiO2, and GO effectively enhanced the mechanical properties of S0 (original soil), in which S5N3 retained 93.07% of its post-immersion unconfined compressive strength and 96.54% of its post-immersion cohesion after 28 days of curing. This result was dominated by coupled synergistic hydration, nano-filling, and bridging anti-cracking effects from the [CH3SiO3/2]n/TiO2/GO nano-composited component.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Chen, W.; Fan, W. Protecting earthen sites by soil hydrophobicity under freeze-thaw and dry-wet cycles. Constr. Build. Mater. 2020, 262, 120089. [Google Scholar] [CrossRef]
- Wan, Y.; Hui, X.M.N.; He, X.X.; Xue, J.F.; Feng, D.Z.; Chen, Z.X.; Li, J.S.; Liu, L.; Xue, Q. Utilization of flue gas desulfurization gypsum to produce green binder for dredged soil solidification: Strength, durability, and planting performance. J. Clean. Prod. 2022, 367, 133076. [Google Scholar] [CrossRef]
- Hou, S.W.; Gao, G.L.; Zhang, H.; Lai, Z.W.; Han, J.Y. Influence of spiral anchor composite foundation on seismic vulnerability of raw soil structure. Earthq. Res. Adv. 2022, 2, 100175. [Google Scholar] [CrossRef]
- Liu, Z.C.; Will, H. Effect of hydrophobic surface treatment on freeze-thaw durability of concrete. Cem. Concr. Compos. 2016, 69, 49–60. [Google Scholar] [CrossRef]
- Yu, H.; He, Z.; Qian, G.P.; Gong, X.B.; Qu, X. Research on the anti-icing properties of silicone modified polyurea coatings (SMPC) for asphalt pavement. Constr. Build. Mater. 2020, 242, 117793. [Google Scholar] [CrossRef]
- Chen, L.L.; Wang, Y.Q.; Wang, Z.F.; Chang, H.T.; Fan, F.F. Diffusion resisting performance of concrete modified with sodium methyl silicate in saline soil area. Constr. Build. Mater. 2022, 350, 128767. [Google Scholar] [CrossRef]
- Zhou, H.Q.; Han, C.P.; Li, X.L.; Xue, H.; Zhao, J.; Yin, G.H.; Zong, Y.C.; Li, Y.L.; Liu, Z.Q.; Song, X. Research on waterproof performance and mechanism of organic silicon hydrophobic material for roadbed soil. Road. Mater. Pavement. 2023, 24, 2113–2132. [Google Scholar] [CrossRef]
- Ma, Q.W.; Liu, Q.; Cheng, K.D.; Liu, S.H. Study on the Durability of Hydraulic Lime Soil Mixed with Sodium Methyl Silicate. Coatings 2022, 12, 903. [Google Scholar] [CrossRef]
- He, T.S.; Kang, Z.Q.; Chen, C. The effect of sodium methyl silicate on the water resistance of desulfurized gypsum blocks. J. Build. Mater. 2021, 24, 247–253+259. (In Chinese) [Google Scholar]
- Cao, P.K.; Ma, Q.W.; Zha, M.M.; Zhang, J.; Huo, Z.J. Study on the Modification of Silty Soil Sites Using Nanosilica and Methylsilicate. Materials 2023, 16, 5646. [Google Scholar] [CrossRef]
- Lv, X.S.; Qin, Y.; Liang, H.; Han, Y.C.; Li, J.; He, Y.; Cui, X. Potassium methyl silicate (CH5SiO3Na) assisted activation and modification of alkali-activated-slag-based drying powder coating for protecting cement concrete. Constr. Build. Mater. 2022, 326, 126858. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, X.; Gu, X.Y.; Liu, J.Q. Investigation into the degradation of air and runoff pollutants using nano g-C3N4 photocatalytic road surfaces. Constr. Build. Mater. 2024, 411, 134553. [Google Scholar] [CrossRef]
- Jagadesh, P.; Nagarajan, V.; Prabhu, K.T.; Arunachalam, K.K. Effect of nano titanium di oxide on mechanical properties of fly ash and ground granulated blast furnace slag based geopolymer concrete. J. Build. Eng. 2022, 61, 105235. [Google Scholar]
- Zhang, R.; Cheng, X.; Hou, P.K.; Ye, Z.M. Influences of Nano-TiO2 on the Properties of Cement-Based Materials: Hydration and Drying Shrinkage. Constr. Build. Mater. 2015, 81, 35–41. [Google Scholar] [CrossRef]
- Senff, L.; Hotza, D.; Lucas, S.; Ferreira, V.M.; Labrincha, J.A. Effect of Nano-SiO2 and Nano-TiO2 Addition on the Rheological Behaviour and the Hardened Properties of Cement Mortars. Mat. Sci. Eng. A—Struct. 2012, 532, 354–361. [Google Scholar] [CrossRef]
- Ma, B.G.; Li, H.N.; Mei, J.P.; Li, X.G.; Chen, F.J. Effects of Nano-TiO2 on the Toughness and Durability of Cement-Based Material. Adv. Mater. Sci. Eng. 2015, 2015, 583106. [Google Scholar] [CrossRef]
- Abbas, B.; Mahmoud, G.; Navid, G. Shear Strength Parameters of Clayey Sand Treated with Cement and Nano Titanium Dioxide. Geotech. Geol. Eng. 2021, 4, 133–151. [Google Scholar]
- Anuj, T.; Tarun, S.; Sandeep, S. Strength properties and durability of clay soil treated with mixture of nano silica and Polypropylene fiber. Mater. Today Proceed. 2020, 26, 3449–3457. [Google Scholar]
- Du, M.R.; Zhang, B.Y.; Li, P.B.; Zhao, P.; Su, H.J.; Du, X.M. Study on the nanoscale mechanical properties of graphene oxide–enhanced shear resisting cement. Rev. Adv. Mater. Sci. 2022, 61, 552–562. [Google Scholar] [CrossRef]
- Mohammed, A.; Sanjayan, J.; Duan, W.; Nazari, A. Incorporating graphene oxide in cement composites: A study of transport properties. Constr. Build. Mater. 2015, 84, 341–347. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxid. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Seyed, S.M.; Masoud, F.; Yahya, Z.Y.; Mohammad, R.B.; Anirudh, N.J.; Imran, K. Finite element based post-buckling analysis of refined graphene oxide reinforced concrete beams with geometrica limperfectio. Comput. Concr. 2020, 25, 283–291. [Google Scholar]
- Xu, Z.F.; Dai, T.; Gui, C.; Zhang, X.; Ye, D.D. Study on Properties of Graphene Oxide Modified Recycled Cement-Based Composites. Integr. Ferroelectr. 2022, 227, 132–144. [Google Scholar]
- Li, W.; He, Y.; Bao, W.B.; Bao, H.L.; Li, D.Y.; Zhang, C.L.; Wang, M. Novel TiO2/GO/M-MMT nano-heterostructured composites exhibiting high photocatalytic activity. Front. Chem. 2023, 6, 1113186. [Google Scholar] [CrossRef]
- Tong, T.; Fan, Z.; Liu, Q.; Wang, S.; Tan, S.S.; Yu, Q. Investigation of the effects of graphene and graphene oxide nanoplatelets on the micro-and macro-properties of cementitious materials. Constr. Build. Mater. 2016, 106, 102–114. [Google Scholar] [CrossRef]
- Saleem, A.; Rehman, Z.U.; Qamar, S.; Mohammedsaleh, K.K.; Khan, A.H.; Akhtar, M.N.; Qamar, N.; Alrowaili, Z.A.; Saeed, U.; Ullah, S.; et al. Investigations of graphene oxides and cement on strength performance of soil. J. Build. Eng. 2023, 73, 106857. [Google Scholar] [CrossRef]
- Aziz, M.; Hamza, M.; Rasool, A.M.; Ali, U.; Ahmed, T.; Kharal, Z.N.; Khan, A.H.; Rehman, Z.U. Use of Graphene Oxide Nanomaterial to Improve Mechanical Properties of Cement-Treated Silty Soil. Arab. Sci. Eng. 2022, 48, 5603–5618. [Google Scholar] [CrossRef]
- Gao, Y.; Jing, H.W.; Fu, G.P.; Zhao, Z.L.; Shi, X.S. Studies on combined effects of graphene oxide-fly ash hybrid on the work ability, mechanical performance and pore structures of cementitious grouting under high W/C ratio. Constr. Build. Mater. 2021, 281, 122578. [Google Scholar] [CrossRef]
- Zhou, A.C.; Yu, T.Q.; Liang, X.C.; Yin, S.B. H2O2-free strategy derived from Hummers method for preparing graphene oxide with high oxidation degree. Flatchem 2023, 38, 100487. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Geotechnical Test Method Standard. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2019.
- Driss, A.A.E.; Khelifa, H.; Mohamed, G.; Sedat, S.; Ertan, B. Effect of Natural Pozzolana on the Unconsolidated Undrained Shear Strength of a Lime-Stabilized Clay Soil. Int. J. Civ. Eng. 2023, 21, 1007–1026. [Google Scholar] [CrossRef]
- WW/T 0039-2012; Technical Specification for the Protection Experiment of Earthen Ruins. State Administration of Cultural Relics of the People’s Republic of China: Beijing, China, 2012.
- Goffredo, B.G.; Placido, M. Preservation of Historical Stone Surfaces by TiO2 Nanocoatings. Coatings 2015, 5, 222–231. [Google Scholar] [CrossRef]
- Li, J.J.; Ma, B.G.; Zhang, X.J.; Lu, X.M. Enhancement and mechanism of macro-defect free (MDF) gypsum water resistance achieved by hydrophobic modification. Case. Stud. Constr. Mat. 2024, 20, e02791. [Google Scholar] [CrossRef]
- Materak, K.; Wieczorek, A.; Bednarska, D.; Chałupka-Śpiewak, K.; Małecka, M.; Buczkowski, A.; Koniorczyk, M. Application of potassium methylsiliconate in cement-based materials: Retarding of hydration process and improvement of compressive strength. Constr. Build. Mater. 2023, 406, 133400. [Google Scholar] [CrossRef]
- Foad, C.; Abdolhosein, H. Strength properties of soft clay treated with mixture of nano-SiO2 and recycled polyester fiber. J. Rock. Mech. Geotech. 2015, 7, 367–378. [Google Scholar]
- Gao, L.; Ren, K.Y.; Ren, Z.; Yu, X.J. Study on the shear property of nano-MgO-modified soil. Mar. Georesour. Geotec. 2018, 36, 465–470. [Google Scholar] [CrossRef]
- Lu, S.Y.; Wang, M.R.; He, P.G.; Sun, X.H.; Wang, X.Y.; Tang, D.Y.; Jia, D.C. Effect of sodium methyl-silicate on the performance and structure of geopolymer. Mater. Lett. 2023, 350, 134893. [Google Scholar] [CrossRef]
- Li, Z.; Han, B.G.; Xun, Y.; Dong, S.F.; Zhang, L.Q.; Dong, X.F.; Ou, J.P. Effect of nano-titanium dioxide on mechanical and electrical properties and microstructure of reactive powder concrete. Mater. Res. Express 2017, 4, 095008. [Google Scholar] [CrossRef]
- Feng, X.M.; Teng, J.D.; Wang, H.W. Influence Mechanism of Water Content and Compaction Degree on Shear Strength of Red Clay with High Liquid Limit. Materials 2023, 17, 162. [Google Scholar] [CrossRef]
- Bonagiri, V.; Arif, A.B.M.; Ateekh, U.R.; Bhaskar, C.S.C. Shear, Consolidation Characteristics and Carbon Footprint Analysis of Clayey Soil Blended with Calcium Lignosulphonate and Granite Sand for Earthen Dam Application. Sustainability 2023, 15, 6117. [Google Scholar] [CrossRef]
- Gou, M.F.; Zhao, M.K.; Zhou, L.F.; Zhao, J.H.; Hou, W.L.; Ma, W.J.; Hou, Z.B. Hydration and mechanical properties of FGD gypsum-cement-mineral powder composites. J. Build. Eng. 2023, 69, 106288. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Dusan, K.; Martina, Z.; Mohamed, H.E. Mechanical properties stabilization of low plasticity Kaolin soil using fly ash and hydrated lime. Case. Stud. Constr. Mat. 2024, 21, e03662. [Google Scholar]
- Zheng, D.; Qian, Z.D.; Li, P.; Wang, L.B. Performance evaluation of high-elasticity asphalt mixture containing inorganic nano-titanium dioxide for applications in high altitude regions. Constr. Build. Mater. 2019, 199, 594–600. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, G.D.; Wang, Y.; Zheng, H.Y.; Shan, S.H.; Lu, C.X. Research progress on the effect of graphene oxide on theproperties of cement-based composites. New Carbon Mater. 2021, 36, 729–750. [Google Scholar] [CrossRef]
- Lv, J.; Zhou, T.H.; Du, Q.; Wu, H.H. Experimental investigation on properties of gypsum-quicklime-soil grout material in the reparation of earthen site cracks. Constr. Build. Mater. 2017, 157, 253–262. [Google Scholar] [CrossRef]
- Lu, S.C.; Wang, X.Y.; Meng, Z.R.; Deng, Q.C.; Peng, F.F.; Yu, C.C.; Hu, X.; Zhao, Y.; Ke, Y.H.; Qi, F.Z. The mechanical properties, microstructures and mechanism of carbon nanotube-reinforced oil well cement-based nanocomposites. Rsc. Adv. 2019, 9, 26691–26702. [Google Scholar] [CrossRef]

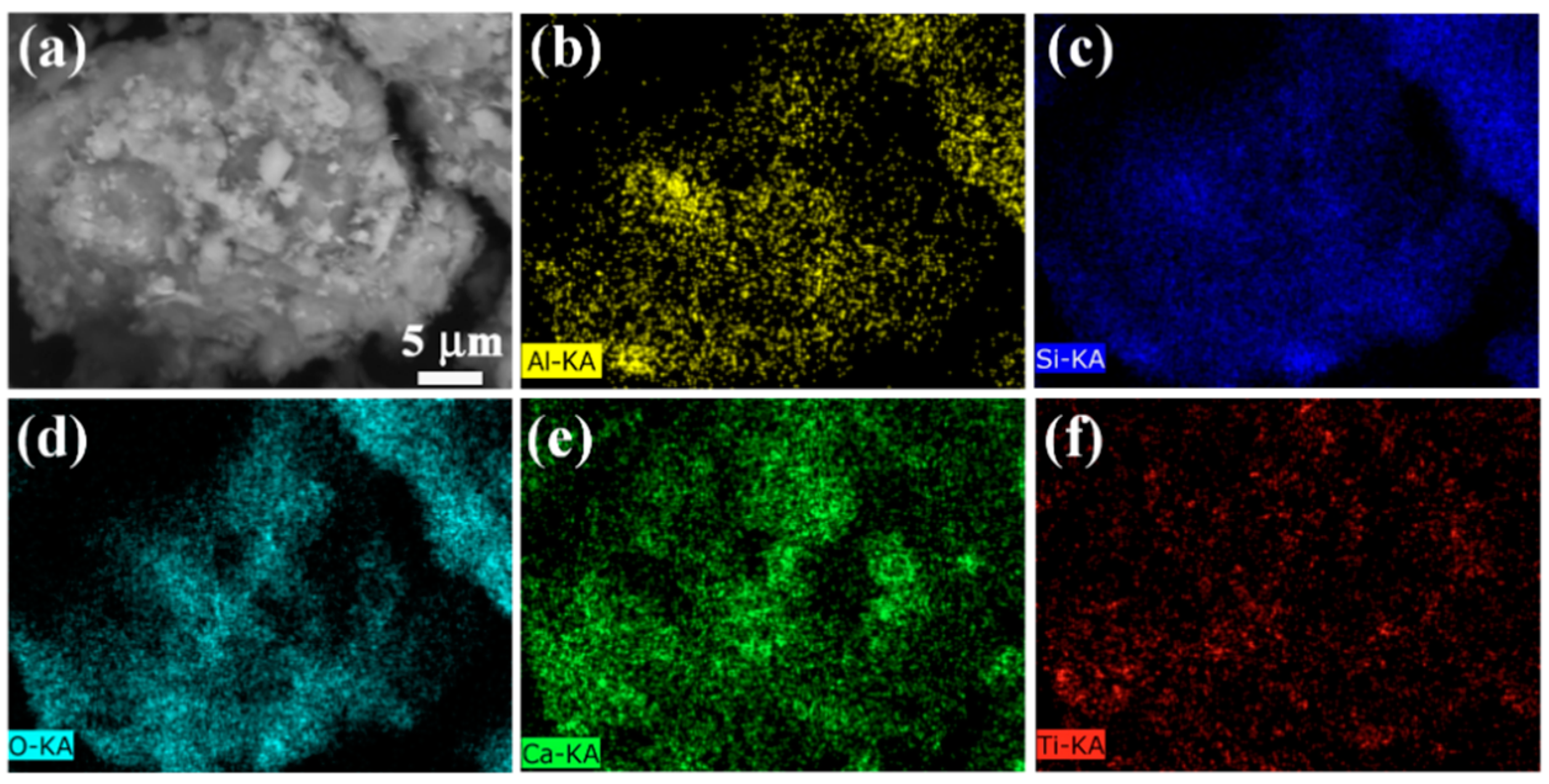


| Element | Atomic Percent |
|---|---|
| O | 55.63 |
| Si | 26.11 |
| Ca | 4.71 |
| Al | 11.85 |
| Ti | 1.70 |
| Weight | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Bao, W.; Huang, Z.; Li, Y.; Guo, Y.; Wang, M. Enhanced Water Resistance of TiO2–GO–SMS-Modified Soil Composite for Use as a Repair Material in Earthen Sites. Materials 2024, 17, 4610. https://doi.org/10.3390/ma17184610
Li W, Bao W, Huang Z, Li Y, Guo Y, Wang M. Enhanced Water Resistance of TiO2–GO–SMS-Modified Soil Composite for Use as a Repair Material in Earthen Sites. Materials. 2024; 17(18):4610. https://doi.org/10.3390/ma17184610
Chicago/Turabian StyleLi, Wei, Wenbo Bao, Zhiqiang Huang, Yike Li, Yuxuan Guo, and Ming Wang. 2024. "Enhanced Water Resistance of TiO2–GO–SMS-Modified Soil Composite for Use as a Repair Material in Earthen Sites" Materials 17, no. 18: 4610. https://doi.org/10.3390/ma17184610





