Thermophysical Properties’ Enhancement of LiNO3-NaNO3-KNO3-NaNO2-KNO2 Mixed with SiO2/MgO Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement Apparatuses and Methods
2.2.1. Testing of Heat Storage Property
2.2.2. Testing of Heat Transfer Property
2.2.3. Microstructure
3. Results and Discussion
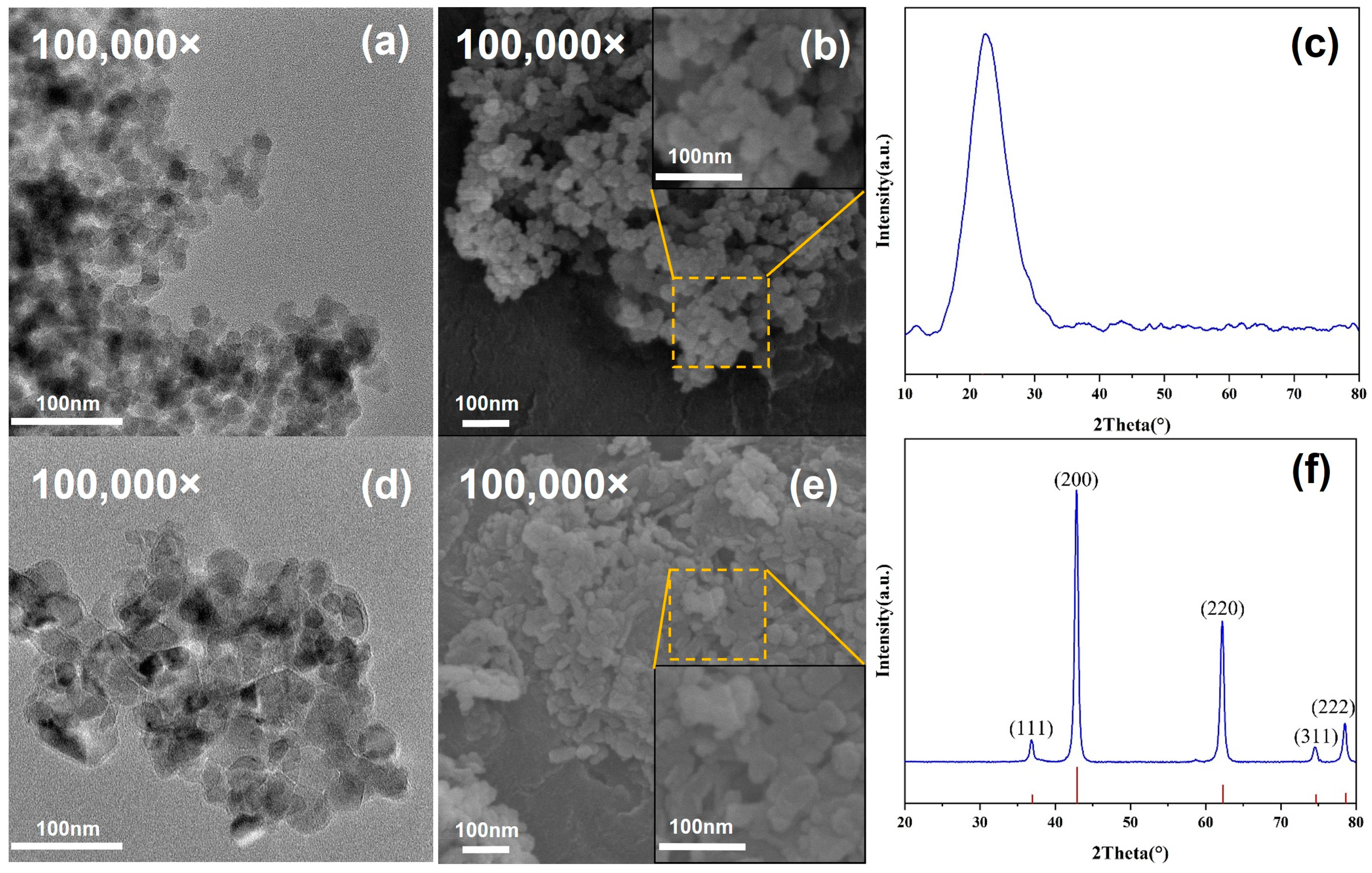
3.1. Characteristics of Nanoparticles
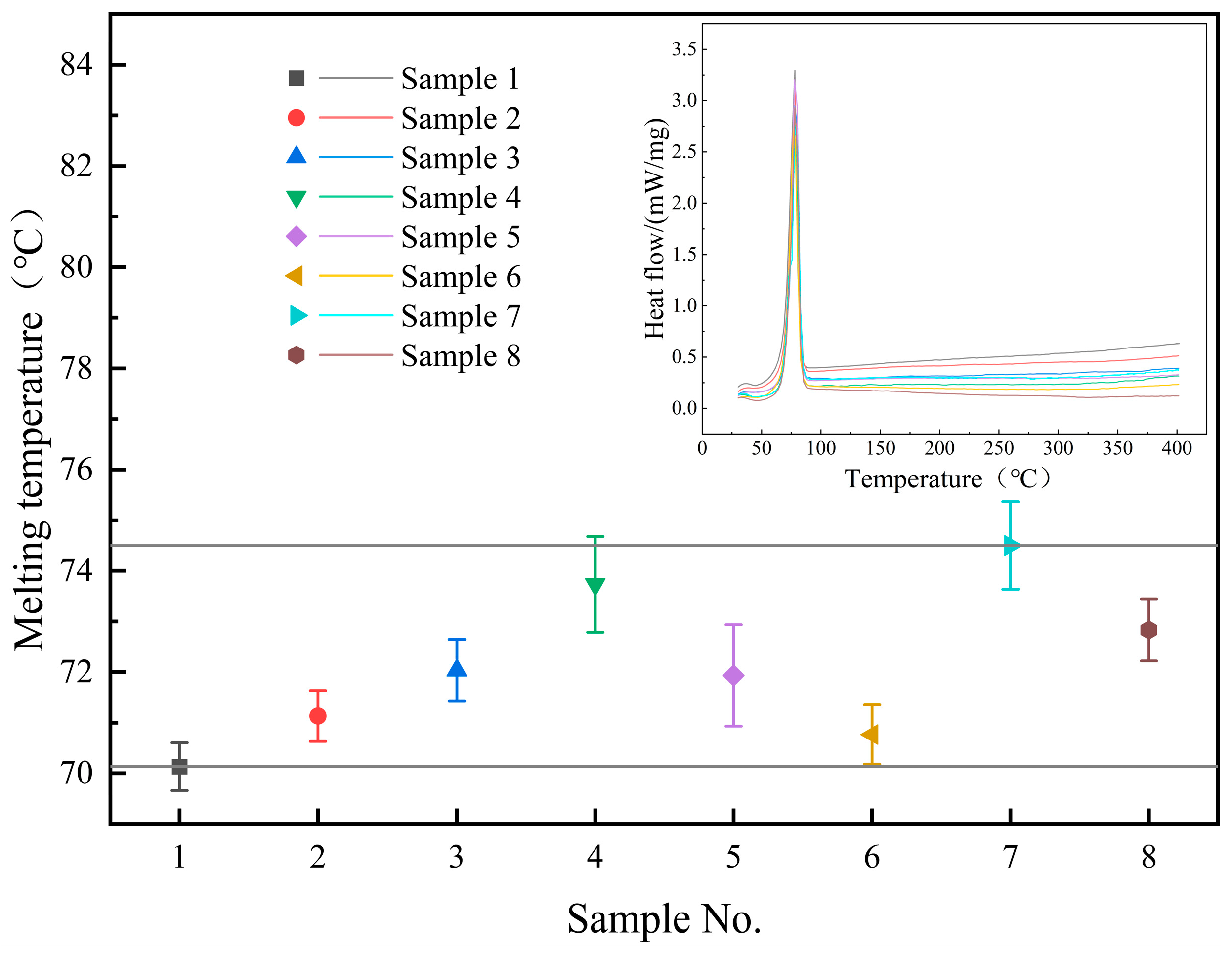
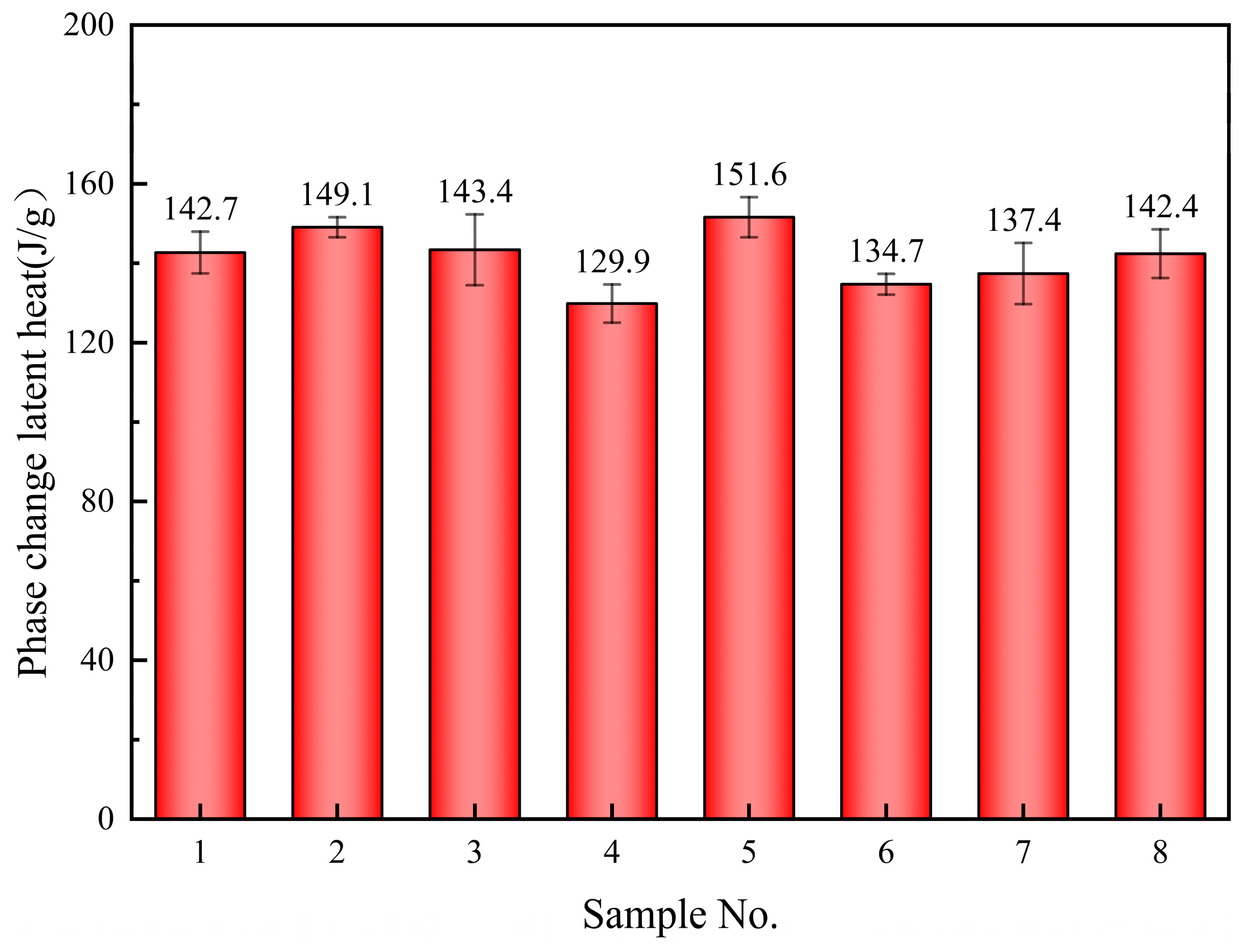
3.2. Melting Point and Latent Heat of Phase Change
3.3. Specific Heat Capacity
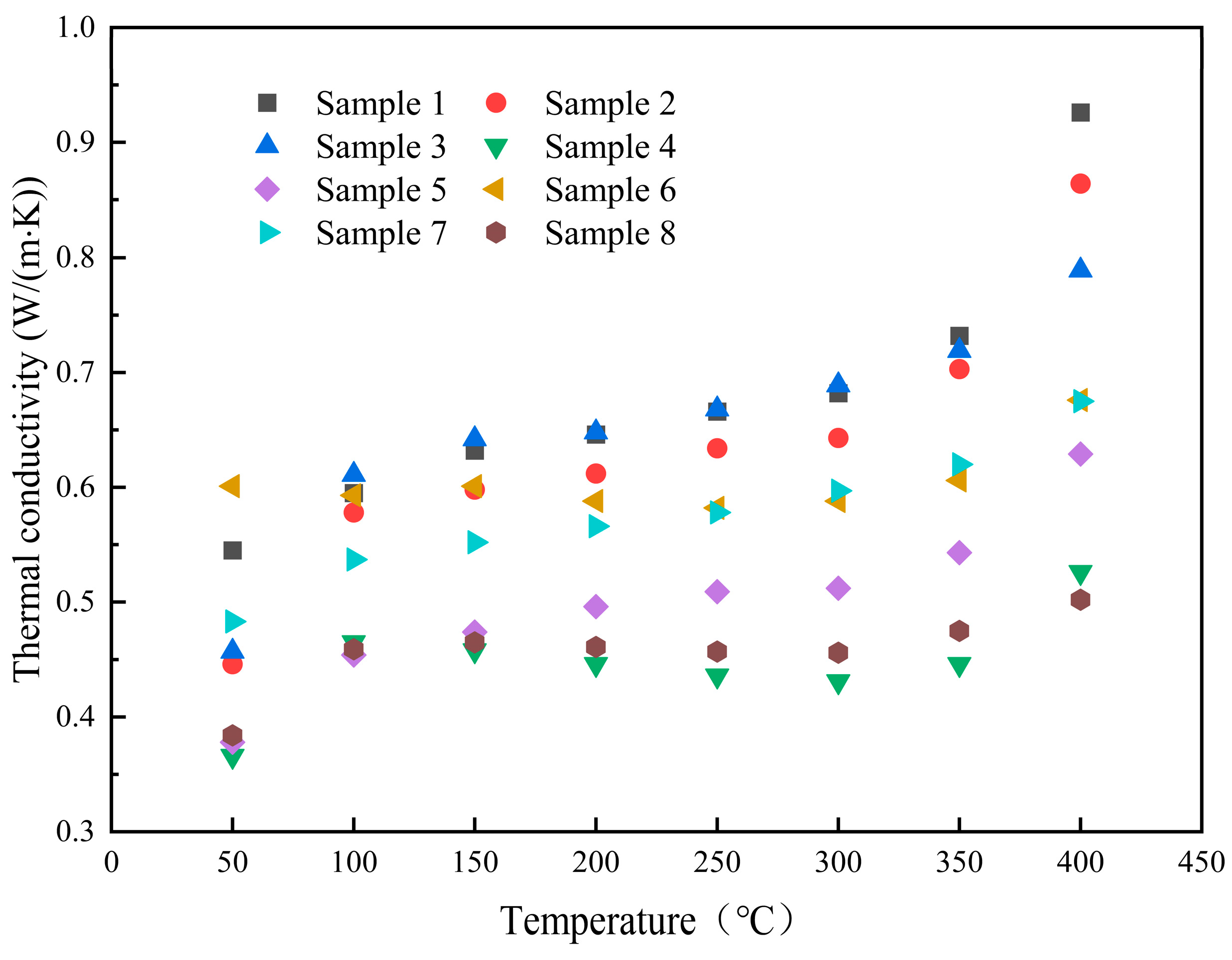
3.4. Heat Transfer Performance
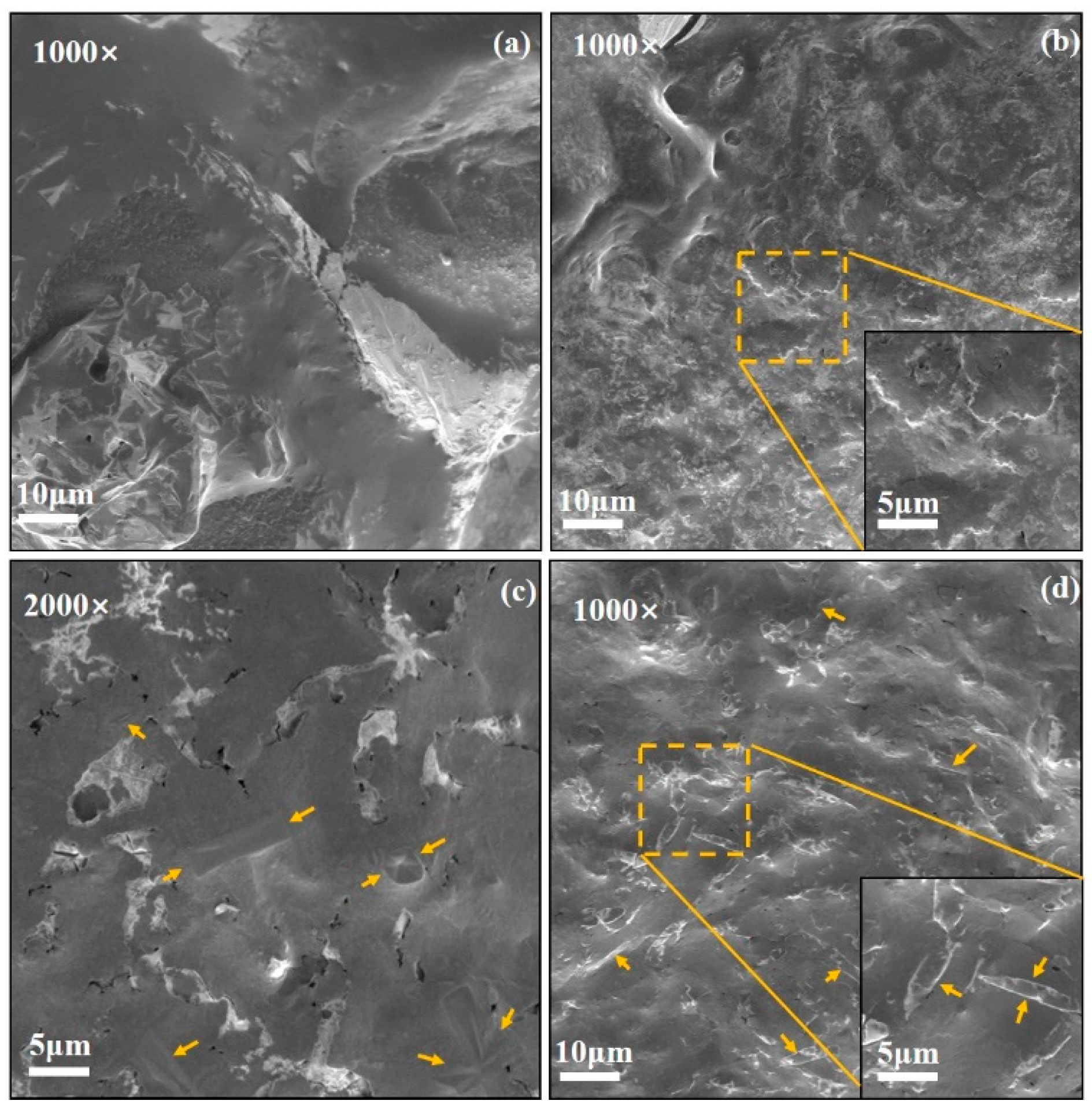
3.5. Microstructures
4. Conclusions
- (1)
- The melting point of the base salt can be influenced by the doping of SiO2 and MgO nanoparticles, albeit with a limited fluctuation range (70–75 °C). The latent heat of the phase change in composite materials can also be affected by doping. Specifically, the doping of 0.5% SiO2 and 0.5% MgO nanoparticles resulted in a significant enhancement in latent heat, reaching 151.6 J/g, which represents a 6.2% increase compared to the base salt;
- (2)
- The doping of 1 wt.% MgO or 0.1% SiO2-0.9% MgO leads to a significant increase in the specific heat capacity. The maximum specific heat capacity of the composite material is 2.36 J/(g·K), exhibiting a 50.4% enhancement over the base salt. Based on current research in the field, it is believed that the phenomenon may be attributed to the interfacial thermal resistance commonly found in composite materials or the electrostatic fields between nanoparticles;
- (3)
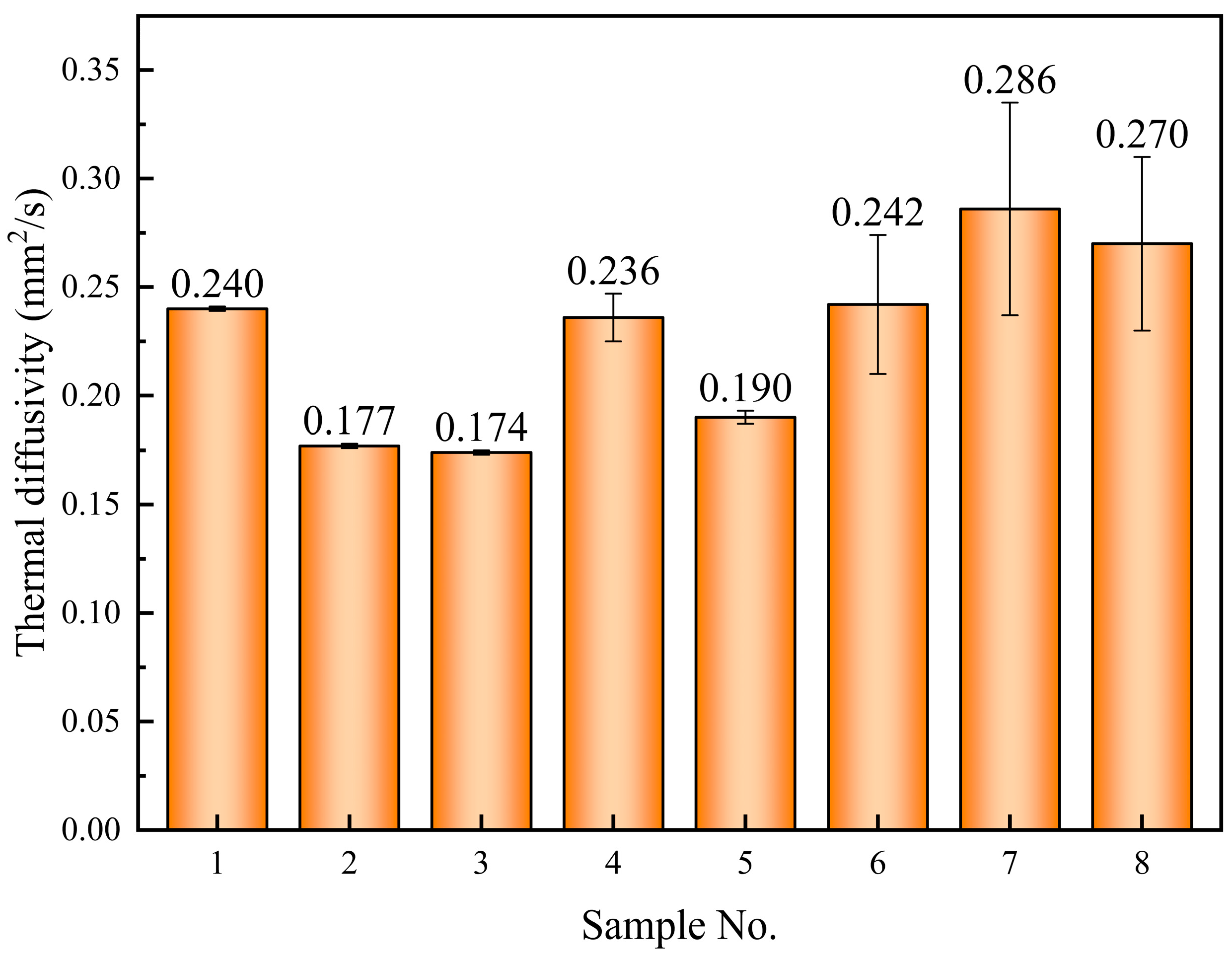
- The doping of 0.9% SiO2-0.1% MgO significantly enhances the thermal diffusion coefficient of the base salt in its solid state, reaching 0.286 mm2/s, representing a 19.2% increase. The SEM images reveal the presence of numerous rod-like structures in the solid state of this sample, which can demonstrate favorable efficiency in phonon heat transfer;
- (4)
- Although interface thermal resistance can enhance specific heat, it can also result in the reduction of heat transfer efficiency in materials. The specific heat capacities of samples 2 and 3 in this study are higher, while their thermal diffusivities are lower. This phenomenon is consistent with the characteristics of interfacial thermal resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, G.; Ji, Y.; Fu, J.; Gao, M.; Li, B. Research Status, Hot Trends and Application Progress of Thermal Energy Storage Technology. Proc. CSEE 2023, 43, 127–142. [Google Scholar] [CrossRef]
- Benato, A.; Stoppato, A. Pumped Thermal Electricity Storage: A technology overview. Therm. Sci. Eng. Prog. 2018, 6, 301–315. [Google Scholar] [CrossRef]
- Palacios, A.; Navarro, M.E.; Barreneche, C.; Ding, Y.L. Hybrid 3 in 1 thermal energy storage system—Outlook for a novel storage strategy. Appl. Energy 2020, 274, 115024. [Google Scholar] [CrossRef]
- Funayama, S.; Kato, T.; Tamano, S.; Mochizuki, K.; Zamengo, M.; Harada, T.; Takasu, H.; Kato, Y. Thermal energy storage with flexible discharge performance based on molten-salt thermocline and thermochemical energy storage. Appl. Therm. Eng. 2024, 238, 121947. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Chen, L.; Cheng, S.; Wang, S.; Wang, J. Research status of solar thermal power generation system. Energy Conserv. 2024, 43, 118–122. [Google Scholar] [CrossRef]
- Duan, S.; Ma, N.; Chen, X.; Duan, Z.; Chen, B.; Wang, Z. Status and Development Trend of Molten Salt Thermal Storage Technology Based on Photothermal Technology. Xinjiang Oil Gas 2024, 20, 87–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Li, J.; Zhao, Y.; Wang, H. Study on thermophysical properties of solar salt composites doped with nanoparticles. Inorg. Chem. Ind. 2022, 54, 54–60. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Cui, L.; Du, X. Stability of the thermal performances of molten salt-based nanofluid. Energy Storage Sci. Technol. 2020, 9, 1775–1783. [Google Scholar] [CrossRef]
- Jeong, S.; Jo, B. Understanding mechanism of enhanced specific heat of single molten salt-based nanofluids: Comparison with acid-modified salt. J. Mol. Liq. 2021, 336, 116561. [Google Scholar] [CrossRef]
- Dudda, B.; Shin, D. Effect of nanoparticle dispersion on specific heat capacity of a binary nitrate salt eutectic for concentrated solar power applications. Int. J. Therm. Sci. 2013, 69, 37–42. [Google Scholar] [CrossRef]
- Meng, Q.; Yang, Y.; Xiong, Y. Study on thermal stability of molten salt composites added with SiO2 nanoparticles. Integr. Intell. Energy 2023, 45, 32–39. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, C.C.; Lu, Y.W.; Kong, Q.L.; Wei, H.J.; Yang, Y.C.; Gao, Q.; Wu, Y.T.; Sciacovelli, A. Comprehensive performance of composite phase change materials based on eutectic chloride with SiO2 nanoparticles and expanded graphite for thermal energy storage system. Renew. Energy 2021, 172, 1120–1132. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.T.; Zhang, L.D.; Wang, X.; Ma, C.F. Experimental study on thermophysical properties of molten salt nanofluids prepared by high-temperature melting. Sol. Energy Mater. Sol. Cells 2019, 191, 209–217. [Google Scholar] [CrossRef]
- Abir, F.M.; Shin, D. Specific Heat Capacity of Solar Salt-Based Nanofluids: Molecular Dynamics Simulation and Experiment. Materials 2024, 17, 506. [Google Scholar] [CrossRef]
- Li, Z.; Cui, L.; Li, B.R.; Du, X.Z. Mechanism exploration of the enhancement of thermal energy storage in molten salt nanofluid. Phys. Chem. Chem. Phys. 2021, 23, 13181–13189. [Google Scholar] [CrossRef]
- Yang, X.M.; Ji, C.; Liu, J.T.; Ma, Y.F.; Cao, B.Y. New insights into the heat capacity enhancement of nano-SiO2 doped alkali metal chloride molten salt for thermal energy storage: A molecular dynamics study. J. Energy Storage 2023, 63, 107015. [Google Scholar] [CrossRef]
- Rong, Z.Z.; Ding, J.; Lu, J.F.; Wang, W.L.; Yan, J.Y. Experimental and theoretical investigation of an innovative composite nanofluid for solar energy photothermal conversion and storage. J. Energy Storage 2022, 52, 104800. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y.; Yu, Q.; Wu, Y. Effect of microwave method on thermal properties of nano-quaternary mixed nitrate. Acta Energiae Solaris Sin. 2021, 42, 153–159. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wu, S.; Xie, L. Preparation and investigation of multicomponent alkali nitrate/nitrite salts for low temperature thermal energy storage. Energy 2018, 160, 1021–1029. [Google Scholar] [CrossRef]
- Zhu, C.; Gong, L. Influence of electric field on structure and properties of ternary carbonate molten salt. IOP Conf. Ser. Earth Environ. Sci. 2020, 565, 012043. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Molecular dynamics study on interfacial thermal resistance between organic nanoparticles and alkali molten salt mixtures. Int. J. Multiscale Comput. Eng. 2017, 15, 199–217. [Google Scholar] [CrossRef]
- Lee, D.; Jo, B. Thermal energy storage characteristics of binary molten salt nanofluids: Specific heat and latent heat. Int. J. Energy Res. 2021, 45, 3231–3241. [Google Scholar] [CrossRef]
- Seo, J.; Shin, D. Size effect of nanoparticle on specific heat in a ternary nitrate (LiNO3-NaNO3-KNO3) salt eutectic for thermal energy storage. Appl. Therm. Eng. 2016, 102, 144–148. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhancement of specific heat capacity of high-temperature silica-nanofluids synthesized in alkali chloride salt eutectics for solar thermal-energy storage applications. Int. J. Heat Mass Transf. 2011, 54, 1064–1070. [Google Scholar] [CrossRef]
- Tiznobaik, H.; Shin, D. Enhanced specific heat capacity of high-temperature molten salt-based nanofluids. Int. J. Heat Mass Transf. 2013, 57, 542–548. [Google Scholar] [CrossRef]
- Carrillo-Berdugo, I.; Grau-Crespo, R.; Zorrilla, D.; Navas, J. Interfacial molecular layering enhances specific heat of nanofluids: Evidence from molecular dynamics. J. Mol. Liq. 2021, 325, 115217. [Google Scholar] [CrossRef]
- Yu, Q.; Lu, Y.W.; Zhang, X.P.; Yang, Y.C.; Zhang, C.C.; Wu, Y.T. Comprehensive thermal properties of molten salt nanocomposite materials base on mixed nitrate salts with SiO2/TiO2 nanoparticles for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 230, 111215. [Google Scholar] [CrossRef]
- Rao, Z.H.; Ye, K.; Wang, H.; Liao, S.M. Effects of interface layer on the thermophysical properties of solar salt-SiO2 nanofluids: A molecular dynamics simulation. Int. J. Energy Res. 2021, 45, 13323–13337. [Google Scholar] [CrossRef]
- Abir, F.M.; Altwarah, Q.; Rana, M.T.; Shin, D. Recent Advances in Molten Salt-Based Nanofluids as Thermal Energy Storage in Concentrated Solar Power: A Comprehensive Review. Materials 2024, 17, 955. [Google Scholar] [CrossRef]
- Cui, X.D.; Cheng, X.M.; Xu, H.; Li, B.; Zhu, J.Q. Enhancement of thermophysical coefficients in nanofluids: A simulation study. Int. J. Mod. Phys. B 2020, 34, 2050222. [Google Scholar] [CrossRef]
- Wu, Y.; Min, W.; Li, J.; Zhao, Y.; Wang, H.; Wei, M. Research progress in improving heat transfer and heat storage performance of molten nitrate by nanomaterials. J. Mater. Eng. 2020, 48, 10–18. [Google Scholar] [CrossRef]
- Ishibe, T.; Kaneko, T.; Uematsu, Y.; Sato-Akaba, H.; Komura, M.; Iyoda, T.; Nakamura, Y. Tunable Thermal Switch via Order-Order Transition in Liquid Crystalline Block Copolymer. Nano Lett. 2022, 22, 6105–6111. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cui, L.; Li, B.R.; Du, X.Z. Effects of SiO2 Nanoparticle Dispersion on the Heat Storage Property of the Solar Salt for Solar Power Applications. Energies 2021, 14, 703. [Google Scholar] [CrossRef]
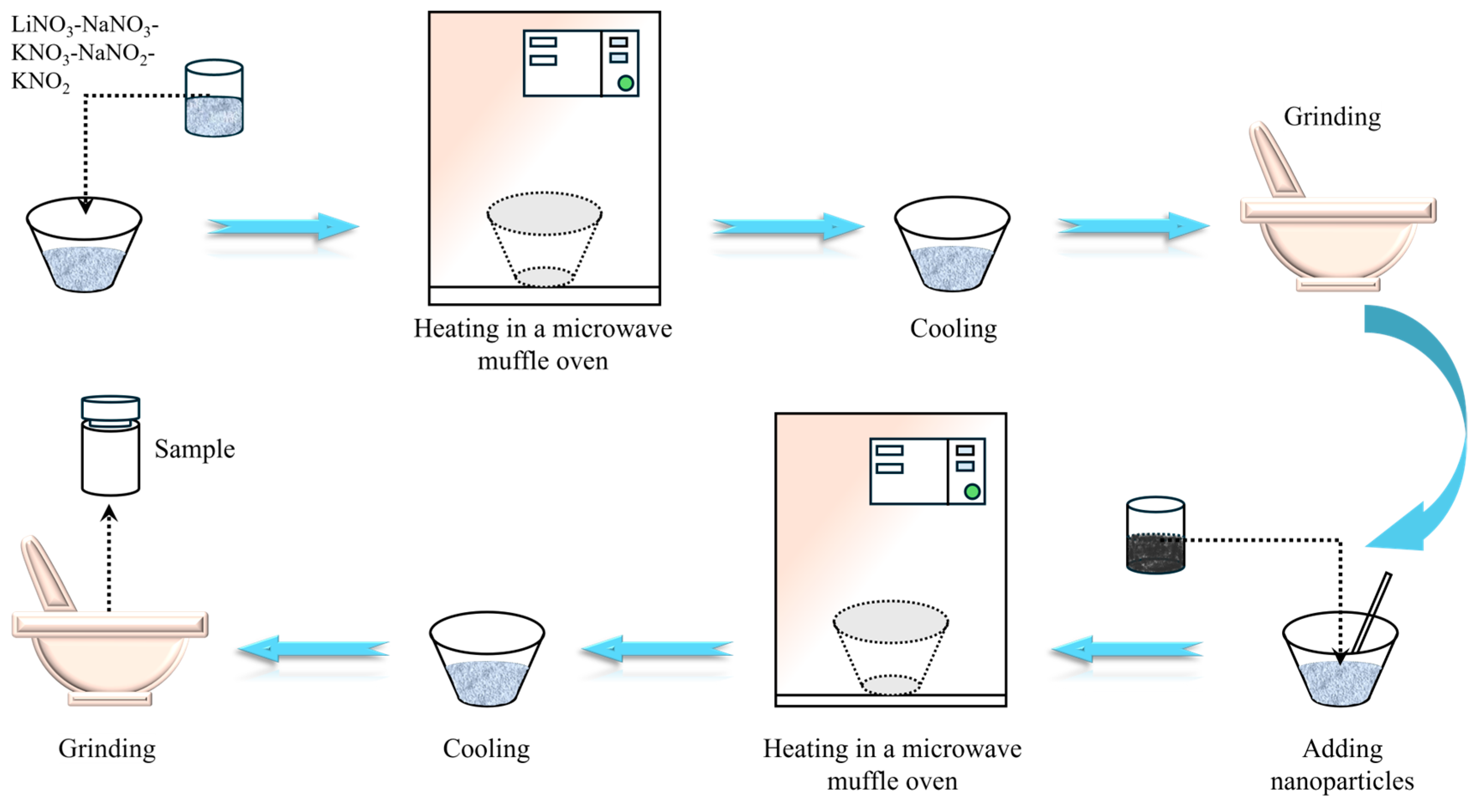


| Raw Material | Melting Point (°C) | Density (g/cm3) | Purity (%) |
|---|---|---|---|
| LiNO3 | 264 | 2.38 | ≥99.0 |
| NaNO3 | 307 | 2.26 | ≥99.0 |
| KNO3 | 334 | 2.11 | ≥99.0 |
| NaNO2 | 271 | 2.17 | ≥99.0 |
| KNO2 | 350 (decompose) | 1.92 | ≥99.0 |
| Characteristics | Parameter | |
|---|---|---|
| Types of Nanoparticles | SiO2 | MgO |
| Purity (%) | 99.9 | 99 |
| Average size (nm) | About 30 | About 50 |
| Specific surface area (m2/g) | 2.65 | 3.58 |
| Density (g/cm3) | 143 | 50 |
| Thermal conductivity (W/(m·K)) | 8.3 | 36 |
| Specific heat (J/(g·K)) | 0.99 | 0.92 |
| Sample No. | LiNO3-NaNO3-KNO3-NaNO2-KNO2 (wt.%) | SiO2 Nanoparticles (wt.%) | MgO Nanoparticles (wt.%) |
|---|---|---|---|
| 1 | 100 | 0 | 0 |
| 2 | 99 | 0 | 1.0 |
| 3 | 99 | 0.1 | 0.9 |
| 4 | 99 | 0.3 | 0.7 |
| 5 | 99 | 0.5 | 0.5 |
| 6 | 99 | 0.7 | 0.3 |
| 7 | 99 | 0.9 | 0.1 |
| 8 | 99 | 1.0 | 0 |
| Sample No. | Specific Heat (J/(g·K)) | |||||||
|---|---|---|---|---|---|---|---|---|
| 50 °C | 100 °C | 150 °C | 200 °C | 250 °C | 300 °C | 350 °C | 400 °C | |
| 1 | 1.05 (±0.10) * | 1.54 (±0.03) * | 1.51 (±0.07) * | 1.52 (±0.08) * | 1.54 (±0.09) * | 1.56 (±0.11) * | 1.65 (±0.14) * | 1.77 (±0.19) * |
| 2 | 1.16 (±0.04) * | 1.92 (±0.02) * | 1.90 (±0.03) * | 1.88 (±0.04) * | 1.92 (±0.04) * | 1.86 (±0.09) * | 1.88 (±0.13) * | 2.01 (±0.16) * |
| 3 | 1.21 (±0.21) * | 2.13 (±0.03) * | 2.27 (±0.02) * | 2.30 (±0.06) * | 2.37 (±0.08) * | 2.39 (±0.11) * | 2.44 (±0.14) * | 2.55 (±0.14) * |
| 4 | 0.72 (±0.31) * | 1.31 (±0.10) * | 1.17 (±0.10) * | 1.11 (±0.12) * | 1.02 (±0.15) * | 0.98 (±0.23) * | 1.03 (±0.19) * | 1.23 (±0.18) * |
| 5 | 0.92 (±0.20) * | 1.42 (±0.05) * | 1.44 (±0.04) * | 1.45 (±0.01) * | 1.45 (±0.04) * | 1.42 (±0.06) * | 1.43 (±0.06) * | 1.55 (±0.09) * |
| 6 | 1.15 (±0.28) * | 2.00 (±0.04) * | 1.87 (±0.06) * | 1.69 (±0.13) * | 1.59 (±0.19) * | 1.55 (±0.24) * | 1.57 (±0.27) * | 1.77 (±0.37) * |
| 7 | 0.78 (±0.15) * | 1.68 (±0.11) * | 1.61 (±0.10) * | 1.58 (±0.08) * | 1.57 (±0.06) * | 1.60 (±0.04) * | 1.71 (±0.02) * | 1.99 (±0.17) * |
| 8 | 0.66 (±0.20) * | 1.30 (±0.02) * | 1.18 (±0.01) * | 1.05 (±0.02) * | 0.94 (±0.03) * | 0.89 (±0.04) * | 0.88 (±0.05) * | 0.91 (±0.05) * |
| Sample No. | 100 °C | 200 °C | 300 °C | 400 °C | Average Value | Change Rate |
|---|---|---|---|---|---|---|
| 1 | 0.208 (±0.008) * | 0.228 (±0.001) * | 0.235 (±0.000) * | 0.280 (±0.001) * | 0.235 | - |
| 2 | 0.162 (±0.004) * | 0.174 (±0.001) * | 0.185 (±0.000) * | 0.231 (±0.001) * | 0.186 | −20.9% |
| 3 | 0.154 (±0.001) * | 0.151 (±0.000) * | 0.155 (±0.000) * | 0.166 (±0.002) * | 0.155 | −34.0% |
| 4 | 0.190 (±0.003) * | 0.217 (±0.000) * | 0.236 (±0.001) * | 0.229 (±0.003) * | 0.221 | −6.0% |
| 5 | 0.171 (±0.003) * | 0.184 (±0.001) * | 0.194 (±0.000) * | 0.218 (±0.001) * | 0.191 | −18.7% |
| 6 | 0.159 (±0.002) * | 0.187 (±0.000) * | 0.203 (±0.000) * | 0.205 (±0.003) * | 0.190 | −19.1% |
| 7 | 0.172 (±0.002) * | 0.192 (±0.000) * | 0.200 (±0.001) * | 0.182 (±0.003) * | 0.189 | −19.6% |
| 8 | 0.189 (±0.004) * | 0.236 (±0.000) * | 0.274 (±0.000) * | 0.297 (±0.004) * | 0.251 | 6.8% |
| Sample No. | Thermal Conductivity | Change Rate |
|---|---|---|
| 1 | 0.545 (±0.001) * | - |
| 2 | 0.446 (±0.002) * | −18.2 |
| 3 | 0.457 (±0.001) * | −16.1% |
| 4 | 0.366 (±0.009) * | −32.8% |
| 5 | 0.378 (±0.004) * | −30.6% |
| 6 | 0.601 (±0.040) * | 10.3% |
| 7 | 0.483 (±0.042) * | −11.4% |
| 8 | 0.384 (±0.029) * | −29.5% |
| Sample No. | 100 °C | 200 °C | 300 °C | 400 °C | Average Value | Change Rate |
|---|---|---|---|---|---|---|
| 1 | 0.595 (±0.022) * | 0.646 (±0.001) * | 0.682 (±0.000) * | 0.926 (±0.003) * | 0.697 | - |
| 2 | 0.578 (±0.012) * | 0.612 (±0.001) * | 0.643 (±0.001) * | 0.864 (±0.003) * | 0.662 | −5.0% |
| 3 | 0.611 (±0.002) * | 0.648 (±0.001) * | 0.689 (±0.001) * | 0.789 (±0.007) * | 0.681 | −2.3% |
| 4 | 0.465 (±0.008) * | 0.446 (±0.001) * | 0.431 (±0.003) * | 0.526 (±0.007) * | 0.458 | −34.3% |
| 5 | 0.454 (±0.007) * | 0.496 (±0.001) * | 0.512 (±0.001) * | 0.629 (±0.001) * | 0.517 | −25.8% |
| 6 | 0.593 (±0.009) * | 0.588 (±0.001) * | 0.588 (±0.001) * | 0.676 (±0.009) * | 0.605 | −13.2% |
| 7 | 0.537 (±0.005) * | 0.566 (±0.001) * | 0.597 (±0.001) * | 0.675 (±0.011) * | 0.589 | −15.5% |
| 8 | 0.459 (±0.010) * | 0.461 (±0.001) * | 0.456 (±0.001) * | 0.502 (±0.006) * | 0.468 | −32.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Xu, M.; Tian, B.; Gu, M.; Gong, L. Thermophysical Properties’ Enhancement of LiNO3-NaNO3-KNO3-NaNO2-KNO2 Mixed with SiO2/MgO Nanoparticles. Materials 2024, 17, 4611. https://doi.org/10.3390/ma17184611
Zhu C, Xu M, Tian B, Gu M, Gong L. Thermophysical Properties’ Enhancement of LiNO3-NaNO3-KNO3-NaNO2-KNO2 Mixed with SiO2/MgO Nanoparticles. Materials. 2024; 17(18):4611. https://doi.org/10.3390/ma17184611
Chicago/Turabian StyleZhu, Chuang, Minhao Xu, Baiyuan Tian, Manting Gu, and Li Gong. 2024. "Thermophysical Properties’ Enhancement of LiNO3-NaNO3-KNO3-NaNO2-KNO2 Mixed with SiO2/MgO Nanoparticles" Materials 17, no. 18: 4611. https://doi.org/10.3390/ma17184611
APA StyleZhu, C., Xu, M., Tian, B., Gu, M., & Gong, L. (2024). Thermophysical Properties’ Enhancement of LiNO3-NaNO3-KNO3-NaNO2-KNO2 Mixed with SiO2/MgO Nanoparticles. Materials, 17(18), 4611. https://doi.org/10.3390/ma17184611






