Investigation of MO Adsorption Kinetics and Photocatalytic Degradation Utilizing Hollow Fibers of Cu-CuO/TiO2 Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Hollow Fibers Preparation
2.2. Characterization Techniques
2.3. Photocatalytic Batch Procedure of the Developed Nanocomposite Material
3. Results
3.1. Characterization Results
3.2. Evaluation of MO Adsorption Capacity
3.3. Study of MO Adsorption Kinetics
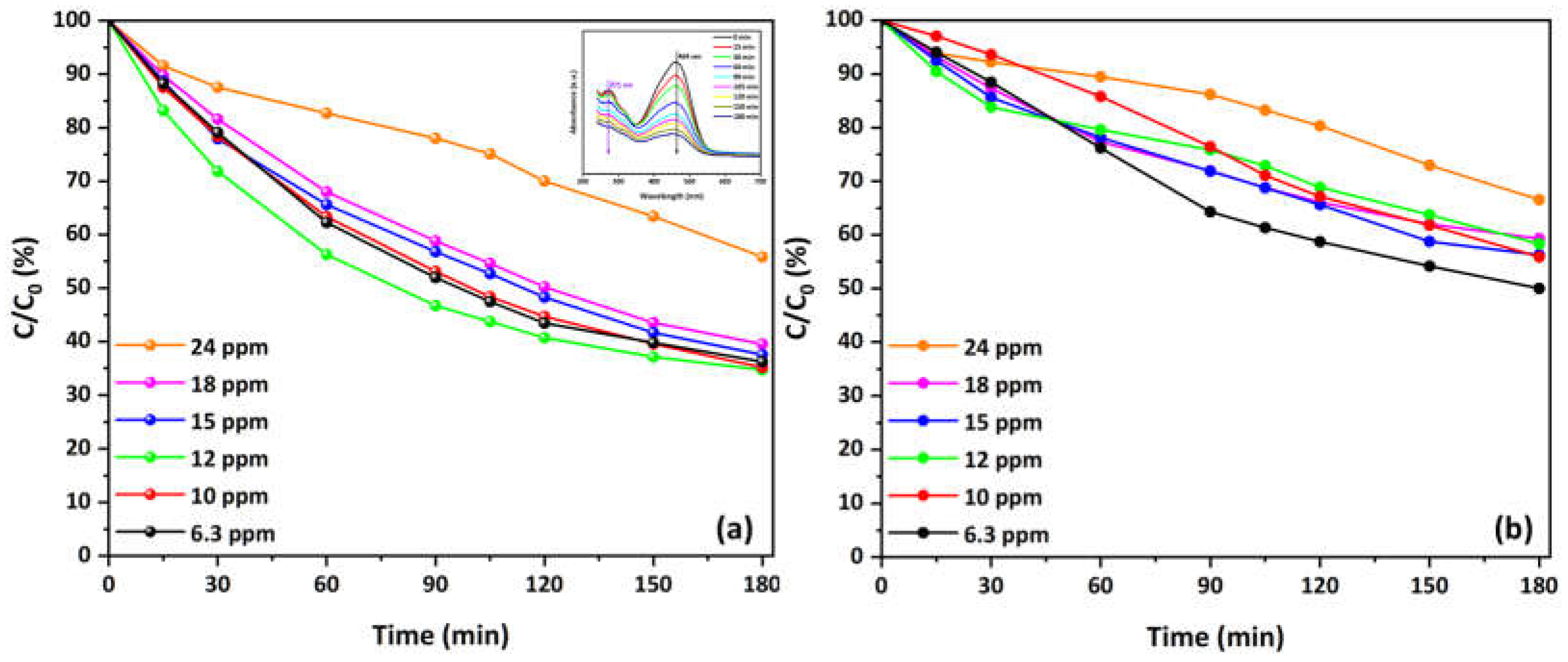
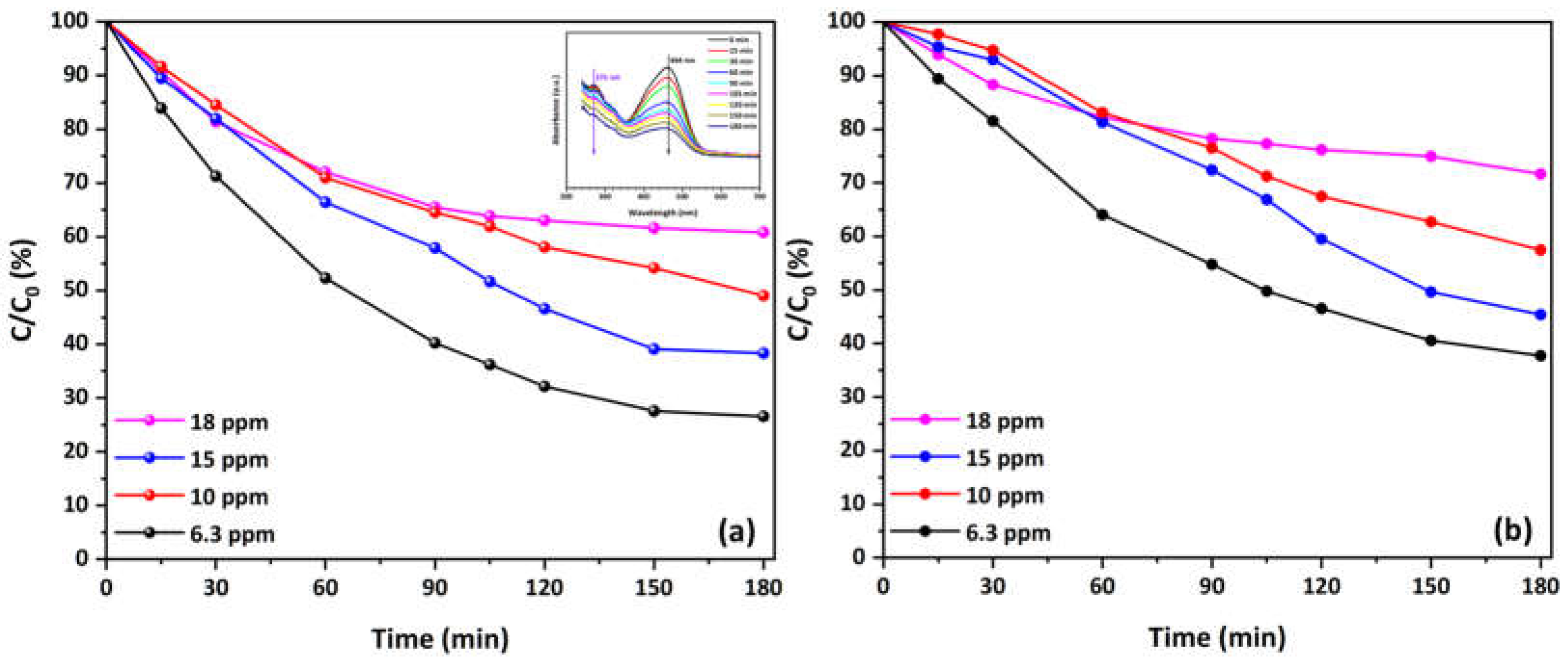
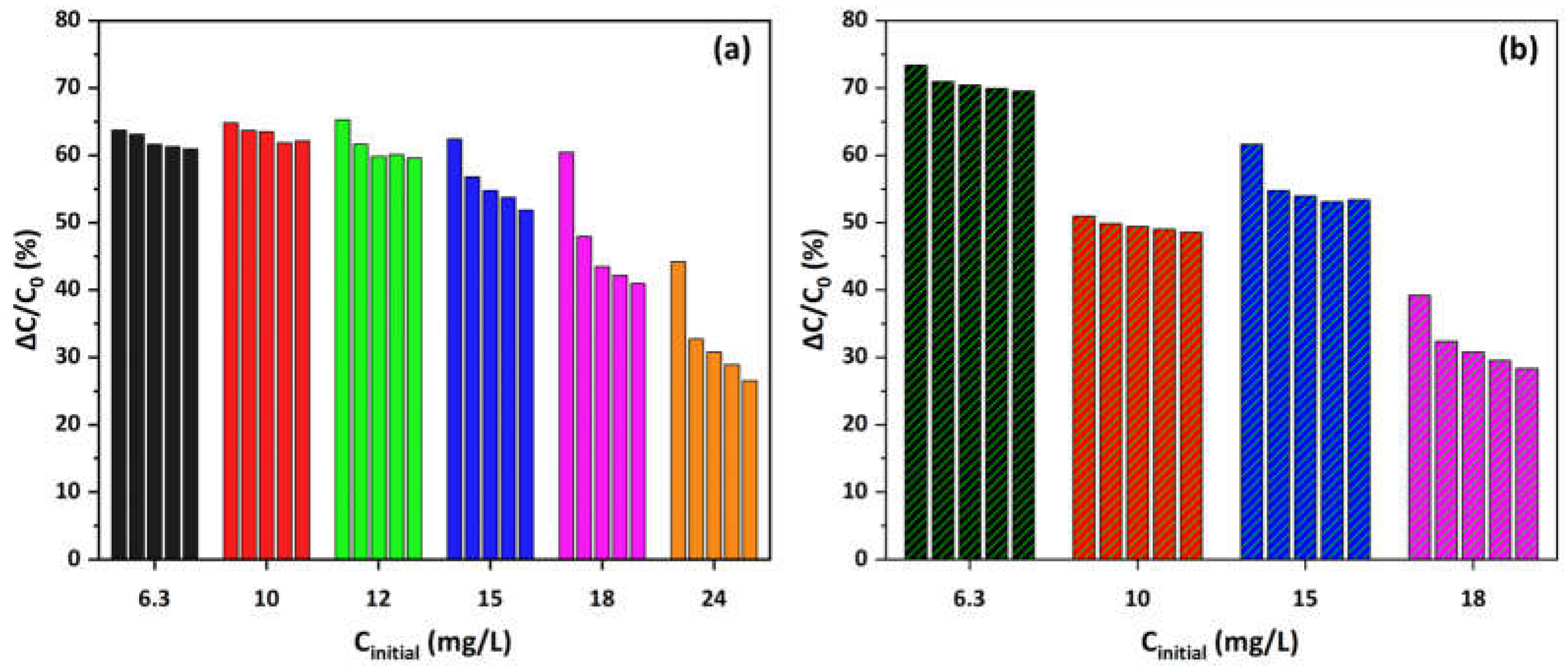
3.4. Photocatalytic Evaluation and Reaction Kinetics of Cu-CuO/TiO2 Nanocomposite—First Experimental Cycles
3.5. Half-Time of Reaction
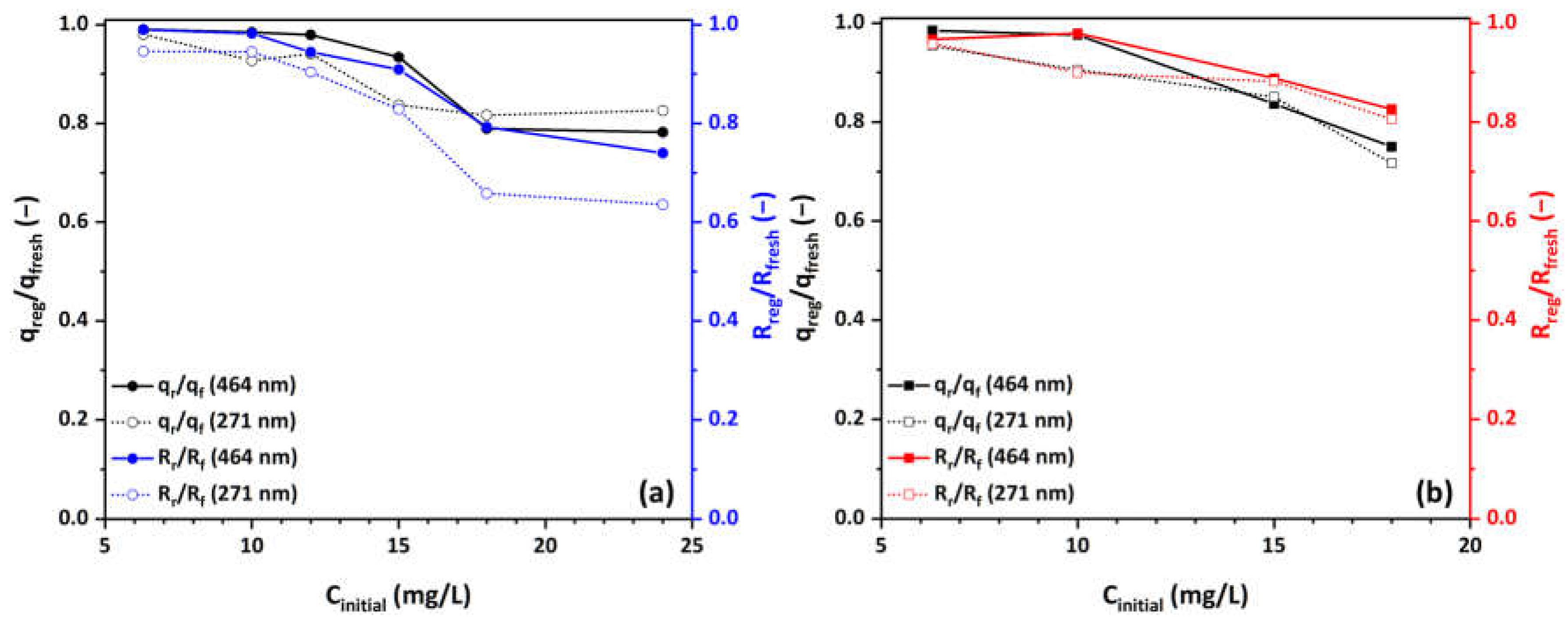
3.6. Regeneration and Reusability of Cu-CuO/TiO2 Nanocomposite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdi, Z.G.; Lai, J.-Y.; Chung, T.-S. Green modification of P84 co-polyimide with β-cyclodextrin for separation of dye/salt mixtures. Desalination 2023, 549, 116365. [Google Scholar] [CrossRef]
- Mahmoodian, H.; Moradi, O.; Shariatzadeha, B.; Salehf, T.A.; Tyagi, I.; Maity, A.; Asif, M.; Gupta, V.K. Enhanced removal of methyl orange from aqueous solutions by polyHEMA–chitosan-MWCNT nano-composite. J. Mol. Liq. 2015, 202, 189–198. [Google Scholar] [CrossRef]
- Bahrudin, N.N.; Nawi, M.A.; Zainal, Z. Insight into the synergistic photocatalytic-adsorptive removal of methyl orange dye using TiO2/chitosan based photocatalyst. Int. J. Biol. Macromol. 2020, 165, 2462–2474. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.A.A.; Rashada, M.; AL-Aoh, H.A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dye. Pigment. 2019, 160, 563–571. [Google Scholar] [CrossRef]
- Yu, L.; Bi, J.; Song, Y.; Wang, M. Isotherm, thermodynamics, and kinetics of methyl orange adsorption onto magnetic resin of chitosan microspheres. Int. J. Mol. Sci. 2022, 23, 13839. [Google Scholar] [CrossRef] [PubMed]
- Piaskowski, K.; Świderska -Dąbrowska, R.; Zarzycki, P.K. Dye removal from water and wastewater using various physical, chemical, and biological processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 2021, 265, 129087. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Shah, M.U.H.; Reddy, A.V.B.; Aminabhavi, T.M. Recent advances in the removal of dyes from wastewater using low-cost adsorbents. J. Environ. Manag. 2022, 321, 115981. [Google Scholar] [CrossRef]
- Sarojini, G.; Kannan, P.; Rajamohan, N.; Rajasimman, M.; Vo, D.-V.N. Dyes removal from water using polymeric nanocomposites: A review. Environ. Chem. Lett. 2023, 21, 1029–1058. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dutta, S.; Ghosh, S.; Vedajnananda, S.; Bandyopadhyay, S. Crossflow microfiltration using ceramic membrane for treatment of sulphur black effluent from garment processing industry. Desalination 2010, 261, 67–72. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2020, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Moradihamedani, P. Recent advances in dye removal from wastewater by membrane technology: A review. Polym. Bull. 2022, 79, 2603–2631. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, M.; Nehra, S.; Sharma, R.; Kumar, D. Dye removal from industrial water using nanofiltration membrane. In Nanofiltration Membrane for Water Purification, 1st ed.; Ahmad, A., Alshammari, M.B., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2023; pp. 83–117. [Google Scholar]
- Ma, H.; Wang, B.; Luo, X. Studies on degradation of methyl orange wastewater by combined electrochemical process. J. Hazard. Mater. 2007, 149, 492–498. [Google Scholar] [CrossRef]
- Droguett, T.; Mora-Gómez, J.; García-Gabaldón, M.; Ortega, E.; Mestre, S.; Cifuentes, G.; Pérez-Herranz, V. Electrochemical degradation of reactive black 5 using two-different reactor configuration. Sci. Rep. 2020, 10, 4482. [Google Scholar] [CrossRef]
- Łuba, M.; Mikołajczyk, T.; Pierożyński, B.; Smoczyński, L.; Wojtacha, P.; Kuczyński, M. Electrochemical degradation of industrial dyes in wastewater through the dissolution of aluminum sacrificial anode of Cu/Al macro-corrosion galvanic cell. Molecules 2020, 25, 4108. [Google Scholar] [CrossRef]
- Goren, A.Y.; Recepoğlu, Y.K.; Edebali, Ö.; Sahin, C.; Genisoglu, M.; Okten, H.E. Electrochemical degradation of methylene blue by a flexible graphite electrode: Techno-economic evaluation. ACS Omega 2022, 7, 32640–32652. [Google Scholar] [CrossRef]
- Abilaji, S.; Narenkumar, J.; Das, B.; Suresh, S.; Rajakrishnan, R.; Sathishkumar, K.; Rajamohan, R.; Rajasekar, A. Electrochemical oxidation of azo dyes degradation by RuO2–IrO2–TiO2 electrode with biodegradation aeromonas hydrophila AR1 and its degradation pathway: An integrated approach. Chemosphere 2023, 345, 140516. [Google Scholar] [CrossRef]
- Sarfo, D.K.; Kaur, A.; Marshall, D.L.; O’Mullane, A.P. Electrochemical degradation and mineralisation of organic dyes in aqueous nitrate solutions. Chemosphere 2023, 316, 137821. [Google Scholar] [CrossRef]
- Pandit, P.; Basu, S. Removal of organic dyes from water by liquid–liquid extraction using reverse micelles. J. Colloid Interface Sci. 2002, 245, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Sun, S.; Dai, F. Removal, separation, and recovery of mixed ionic dyes by solvent extraction using reverse micellar systems. Chem. Lett. 2015, 44, 1173–1175. [Google Scholar] [CrossRef]
- Shang, X.; Li, B.; Zhang, T.; Li, C.; Wang, X. Photocatalytic degradation of methyl orange with commercial organic pigment sensitized TiO2. Procedia Environ. Sci. 2013, 18, 478–485. [Google Scholar] [CrossRef]
- Petrella, A.; Boghetich, G.; Petrella, M.; Mastrorilli, P.; Petruzzelli, V.; Petruzzelli, D. Photocatalytic degradation of azo dyes. Pilot plant investigation. Ind. Eng. Chem. Res. 2014, 53, 2566–2571. [Google Scholar] [CrossRef]
- Raliya, R.; Avery, C.; Chakrabarti, S.; Biswas, P. Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl. Nanosci. 2017, 7, 253–259. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, C.; Chen, Y. Experimental study on photocatalytic degradation efficiency of mixed crystal nano-TiO2 concrete. Nanotechnol. Rev. 2020, 9, 219–229. [Google Scholar] [CrossRef]
- Huang, L.; Peng, F.; Yu, H.; Wang, H. Preparation of cuprous oxides with different sizes and their behaviors of adsorption, visible-light driven photocatalysis and photocorrosion. Solid State Sci. 2009, 11, 129–138. [Google Scholar] [CrossRef]
- Dong, C.; Zhong, M.; Huang, T.; Ma, M.; Wortmann, D.; Brajdic, M.; Kelbassa, I. Photodegradation of methyl orange under visible light by micro-nano hierarchical Cu2O structure fabricated by hybrid laser processing and chemical dealloying. ACS Appl. Mater. Interfaces 2011, 3, 4332–4338. [Google Scholar] [CrossRef]
- Athanasekou, C.; Romanos, G.E.; Papageorgiou, S.K.; Manolis, G.K.; Katsaros, F.; Falaras, P. Photocatalytic degradation of hexavalent chromium emerging contaminant via advanced titanium dioxide nanostructures. Chem. Eng. J. 2017, 318, 171–180. [Google Scholar] [CrossRef]
- Teng, F.; Li, M.; Gao, C.; Zhang, G.; Zhang, P.; Wang, Y.; Chen, L.; Xie, E. Preparation of black TiO2 by hydrogen plasma assisted chemical vapor deposition and its photocatalytic activity. Appl. Catal. B Environ. 2014, 148–149, 339–343. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, Y.; Wang, Y.; Hu, Z.; Zhao, H.; Xie, W. A facile approach to prepare black TiO2 with oxygen vacancy for enhancing photocatalytic activity. Nanomaterials 2018, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- An, H.-R.; Park, S.Y.; Kim, H.; Lee, C.Y.; Choi, S.; Lee, S.C.; Seo, S.; Park, E.C.; Oh, Y.-K.; Song, C.-G.; et al. Advanced nanoporous TiO2 photocatalysts by hydrogen plasma for efficient solar-light photocatalytic application. Sci. Rep. 2016, 6, 29683. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Jagminas, A. Synthesis, characterisation, and applications of TiO and other black titania nanostructures species (review). Crystals 2024, 14, 647. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ahmed, E.; Hong, Z.; Ahmad, M.; Zhang, Y.; Khalid, S. Cu-doped TiO2 nanoparticles/graphene composites for efficient visible-light photocatalysis. Ceram. Int. 2013, 39, 7107–7113. [Google Scholar] [CrossRef]
- Khairy, M.; Zakaria, W. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef]
- Reda, S.M.; Khairy, M.; Mousa, M.A. Photocatalytic activity of nitrogen and copper doped TiO2 nanoparticles prepared by microwave-assisted sol-gel process. Arab. J. Chem. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Hampel, B.; Pap, Z.; Sapi, A.; Szamosvolgyi, A.; Baia, L.; Hernadi, K. Application of TiO2-Cu Composites in photocatalytic degradation different pollutants and hydrogen production. Catalysts 2020, 10, 85. [Google Scholar] [CrossRef]
- A’srai, A.I.M.; Razali, M.H.; Amin, K.A.M.; Osman, U.M. CuO/TiO2 nanocomposite photocatalyst for efficient MO degradation. Dig. J. Nanomater. Biostruct. 2023, 18, 1005–1124. [Google Scholar] [CrossRef]
- Vaez, M.; Moghaddam, A.Z.; Mahmoodi, N.M.; Alijani, S. Decolorization and degradation of acid dye with immobilized titania nanoparticles. Process Saf. Environ. Prot. 2012, 90, 56–64. [Google Scholar] [CrossRef]
- Ahuja, T.; Brighu, U.; Saxena, K. Recent advances in photocatalytic materials and their applications for treatment of wastewater: A review. J. Water Process Eng. 2023, 53, 103759. [Google Scholar] [CrossRef]
- Shokri, A.; Fard, M.S. A critical review in the features and application of photocatalysts in wastewater treatment. Chem. Pap. 2022, 76, 5309–5339. [Google Scholar] [CrossRef]
- Lai, C.W.; Lee, K.M.; Juan, J.C. Polymeric nanocomposites for visible-light-induced photocatalysis. In Nanocomposites for Visible Light-induced Photocatalysis, 1st ed.; Khan, M.M., Pradhan, D., Sohn, Y., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 175–201. [Google Scholar]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic materials: Recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Fang, Y.; Zheng, Y.; Fang, T.; Chen, Y.; Zhu, Y.; Liang, Q.; Sheng, H.; Li, Z.; Chen, C.; Wang, X. Photocatalysis: An overview of recent developments and technological advancements. Sci. China Chem. 2020, 63, 149–181. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Amani, A.M. Photocatalytic systems: Reactions, mechanism, and applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef]
- McEvoy, J.G.; Zhang, Z. Synthesis and characterization of Ag/AgBr–activated carbon composites for visible light induced photocatalytic detoxification and disinfection. J. Photochem. Photobiol. A Chem. 2016, 321, 161–170. [Google Scholar] [CrossRef]
- Bouarioua, A.; Zerdaoui, M. Photocatalytic activities of TiO2 layers immobilized on glass substrates by dip-coating technique toward the decolorization of methyl orange as a model organic pollutant. J. Environ. Chem. Eng. 2017, 5, 1565–1574. [Google Scholar] [CrossRef]
- Shifu, C.; Xuqiang, L.; Yunzhang, L.; Gengyu, C. The preparation of nitrogen-doped TiO2−xNx photocatalyst coated on hollow glass microbeads. Appl. Surf. Sci. 2007, 253, 3077–3082. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, W.; Cao, Y.; Han, Z.; Ding, Z. Preparation and catalytic activity of Fe alginate gel beads for oxidative degradation of azo dyes under visible light irradiation. Catal. Today 2011, 175, 346–355. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Appunni, S.; Gopalram, K. Immobilized TiO2/chitosan beads for photocatalytic degradation of 2,4-dichlorophenoxyacetic acid. Int. J. Biol. Macromol. 2020, 161, 282–291. [Google Scholar] [CrossRef]
- Mehmood, C.T.; Zhong, Z.; Zhou, H.; Zhang, C.; Xiao, Y. Immobilizing a visible light-responsive photocatalyst on a recyclable polymeric composite for floating and suspended applications in water treatment. RSC Adv. 2020, 10, 36349. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.-B.; Xie, R.; Qin, J.-W.; Deng, X.-B.; Ju, X.-J.; Wang, W.; Liu, Z.; Chu, L.-Y. Facile fabrication of photocatalyst-immobilized gel beads with interconnected macropores for the efficient removal of pollutants in water. Ind. Eng. Chem. Res. 2021, 60, 8762–8775. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.V.; Katsaros, F.K.; Papageorgiou, S.K.; Beazi-Katsioti, M.; Romanos, G.E. Engineering commercial TiO2 powder into tailored beads for efficient water purification. Materials 2022, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Zhao, W.; Xiong, D.; Li, S.; Ye, Y.; Du, L. Novel alginate immobilized TiO2 reusable functional hydrogel beads with high photocatalytic removal of dye pollutions. J. Polym. Eng. 2022, 42, 978–985. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Favvas, E.P.; Romanos, G.E.; Athanasekou, C.P.; Beltsios, K.G.; Tzialla, O.I.; Falaras, P. Alginate fibers as photocatalyst immobilizing agents applied in hybrid photocatalytic/ultrafiltration water treatment processes. Water Res. 2012, 46, 1858–1872. [Google Scholar] [CrossRef]
- Shi, Z.; Zhou, M.; Zheng, D.; Liu, H.; Yao, S. Preparation of Ce-doped TiO2 hollow fibers and their photocatalytic degradation properties for dye compound. J. Chin. Chem. Soc. 2013, 60, 1156–1162. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Photocatalytic degradation of nonylphenol by immobilized TiO2 in dual layer hollow fibre membranes. Chem. Eng. J. 2015, 269, 255–261. [Google Scholar] [CrossRef]
- Chakraborty, S.; Loutatidou, S.; Palmisano, G.; Kujawa, J.; Mavukkandy, M.O.; Al-Gharabli, S.; Curcio, E.; Arafat, H.A. Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J. Environ. Chem. Eng. 2017, 5, 5014–5024. [Google Scholar] [CrossRef]
- Galiano, F.; Song, X.; Marino, T.; Boerrigter, M.; Saoncella, O.; Simone, S.; Faccini, M.; Chaumette, C.; Drioli, E.; Figoli, A. Novel photocatalytic PVDF/Nano-TiO2 hollow fibers for environmental remediation. Polymers 2018, 10, 1134. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.V.; Romanos, G.E.; Katsaros, F.K.; Papageorgiou, S.K.; Kontos, A.G.; Spyrou, K.; Beazi-Katsioti, M.; Falaras, P. Structuring efficient photocatalysts into bespoke fiber shaped systems for applied water treatment. Chemosphere 2021, 277, 130253. [Google Scholar] [CrossRef]
- Umadevi, D.; Panigrahi, S.; Sastry, G.N. Noncovalent interaction of carbon nanostructures. Acc. Chem. Res. 2014, 47, 2574–2581. [Google Scholar] [CrossRef] [PubMed]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of pharmaceutical aromatic pollutants on heat-treated activated carbons: Effect of carbonaceous structure and the adsorbent−adsorbate interactions. ACS Omega 2020, 5, 15247–15256. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Duan, L.; Zhu, D. Adsorption of polar and nonpolar organic chemicals to carbon nanotubes. Environ. Sci. Technol. 2007, 41, 8295–8300. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, I.; Khamo, J.S.; Pandit, S.; Fathi, P.; Huang, X.; Cao, A.; Haasch, R.T.; Nie, S.; Zhang, K.; Pan, D. Influence of electron acceptor and electron donor on the photophysical properties of carbon dots: A comparative investigation at the bulk-state and single-particle level. Adv. Funct. Mater. 2019, 29, 1902466. [Google Scholar] [CrossRef]
- Zhao, F.; Shan, R.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y. Magnetically recyclable loofah biochar by KMnO4 modification for adsorption of Cu(II) from aqueous solutions. ACS Omega 2022, 7, 8844–8853. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Guettaï, N.; Amar, H.A. Photocatalytic oxidation of methyl orange in presence of titanium dioxide in aqueous suspension. Part II: Kinetics study. Desalination 2005, 185, 439–448. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and solid-diftusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Temkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta USSR 1940, 12, 327–356. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Proc. Acad. Sci. Phys. Chem. USSR 1947, 55, 331–333. [Google Scholar]
- Porter, J.F.; McKay, G.; Choy, K.H. The prediction of sorption from a binary mixture of acidic dyes using single- and mixed-isotherm variants of the ideal adsorbed solute theory. Chem. Eng. Sci. 1999, 54, 58635885. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorptionof lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- López-Luna, J.; ·Ramírez-Montes, L.E.; Martinez-Vargas, S.; Martínez, A.I.; Mijangos-Ricardez, O.F.; González-Chávez, M.d.C.A.; Carrillo-González, R.; Solís-Domínguez, F.A.; Cuevas-Díaz, M.d.C.; Vázquez-Hipólito, V. Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl. Sci. 2019, 1, 950. [Google Scholar] [CrossRef]
- Subramanyam, B.; Das, A. Linearised and non-linearised isotherm models optimization analysis by error functions and statistical means. J. Environ. Health Sci. Eng. 2014, 12, 92. [Google Scholar] [CrossRef]
- Tekin, N.; Bayrak, M.A.; Can, E. Adsorption of brilliant yellow onto sepiolite: Evaluation of thermodynamics and kinetics and the application of nonlinear isotherm models. J. Disper. Sci. Technol. 2016, 37, 1783–1792. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Adsorption Mechanism. In Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density, 1st ed.; Lowell, S., Shields, J.E., Thomas, M.A., Thommes, M., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 16, pp. 15–57. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Trivedi, H.C.; Patel, V.M.; Patel, R.D. Adsorption of cellulose triacetate on calcium silicate. Eur. Polym. J. 1973, 9, 525–531. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Allen, J.A.; Scaife, P.H. The Elovich equation and chemisorption kinetics. Aust. J. Chem. 1966, 19, 2015–2023. [Google Scholar] [CrossRef]
- Gillis-D’Hamers, I.; Van Der Voort, P.; Vrancken, K.C.; De Roy, G.; Vansant, E.F. Kinetic study of the chemisorption of diborane on silica gel: Application of the Elovich equation. J. Chem. Soc. Faraday Trans. 1992, 88, 65–69. [Google Scholar] [CrossRef]
- Juang, R.-S.; Chen, M.-L. Application of the Elovich equation to the kinetics of metal sorption with solvent-impregnated resins. Ind. Eng. Chem. Res. 1997, 36, 813–820. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Taylor, H.A.; Thon, N. Kinetics of chemisorption. J. Am. Chem. Soc. 1952, 74, 4169–4173. [Google Scholar] [CrossRef]
- Kavitha, D.; Namasivayam, C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour. Technol. 2007, 98, 14–21. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of rhodamine B dye onto raphia hookerie fruit epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef]
- Malekbala, M.R.; Hosseini, S.; Yazdi, S.K.; Soltani, S.M.; Malekbala, M.R. The study of the potential capability of sugar beet pulp on the removal efficiency of two cationic dyes. Chem. Eng. Res. Des. 2012, 90, 704–712. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Tütem, E.; Apak, R.; Ünal, Ҫ.F. Adsorptive removal of chlorophenols from water by bituminous shale. Wat. Res. 1998, 32, 2315–2324. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Swamy, M.M.; Mall, I.D.; Prasad, B.; Mishra, I.M. Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 89–104. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Gryglewicz, G. Adsorption characteristics of congo red on coal-based mesoporous activated carbon. Dye. Pigment. 2007, 74, 34–40. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, Y.; Lin, J.-M.; Chen, G. Study on the photocatalytic degradation of methyl orange in water using Ag/ZnO as catalyst by liquid chromatography electrospray ionization ion-trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2008, 19, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; Zhang, Y.; Qiu, L.; Xing, Y. A 0D/2D Bi4V2O11/g-C3N4 S-scheme heterojunction with rapid interfacial charges migration for photocatalytic antibiotic degradation. Acta Phys. -Chim. Sin. 2022, 38, 2112027. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Zhang, J.; Dai, K. Overall utilization of photoexcited charges for simultaneous photocatalytic redox reactions. Acta Phys.-Chim. Sin. 2023, 39, 2209037. [Google Scholar] [CrossRef]
- Arfanis, M.K.; Theodorakopoulos, G.V.; Anagnostopoulos, C.; Georgaki, I.; Karanasios, E.; Romanos, G.E.; Markellou, E.; Falaras, P. Photocatalytic removal of thiamethoxam and flonicamid pesticides present in agro-industrial water Effluents. Catalysts 2023, 13, 516. [Google Scholar] [CrossRef]
- Cao, J.; Wei, L.; Huang, Q.; Wang, L.; Han, S. Reducing degradation of azo dye by zero-valent iron in aqueous solution. Chemosphere 1999, 38, 565–571. [Google Scholar] [CrossRef]
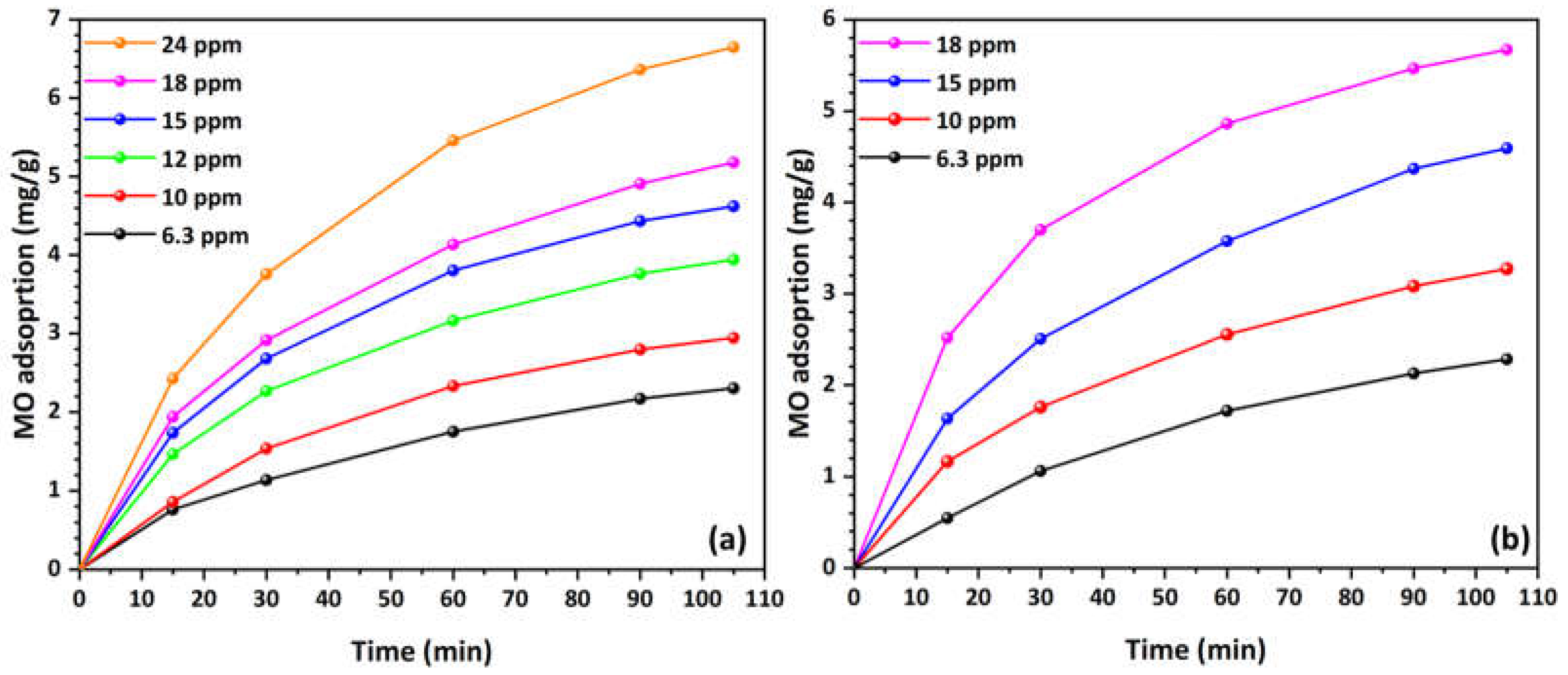

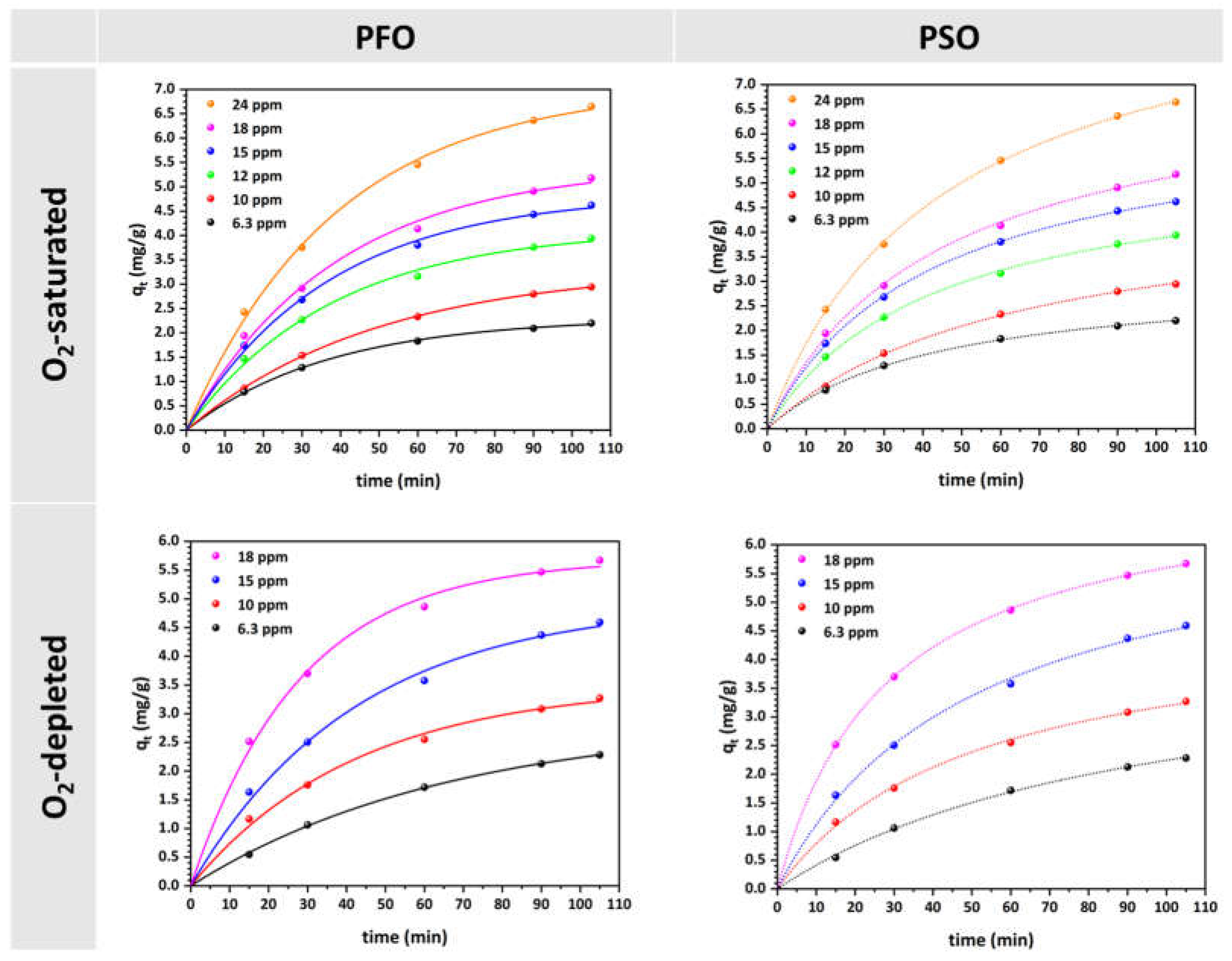
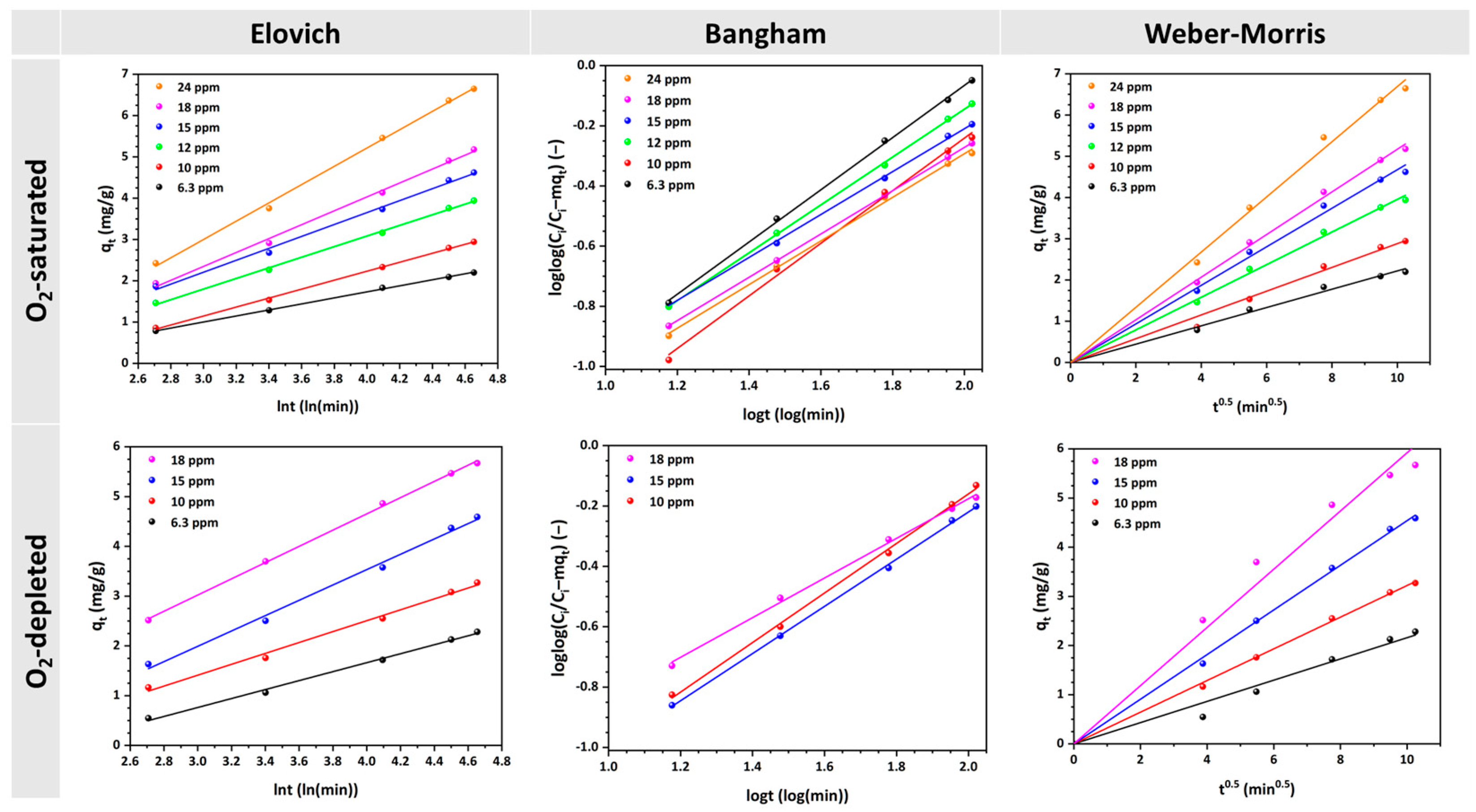

| Kinetic Model | Parameters | Solution | Concentration (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| 6.3 | 10 | 12 | 15 | 18 | 24 | |||
| Experimental | qe (mg/g) | O2-saturated | 2.30 | 2.94 | 3.94 | 4.62 | 5.18 | 6.65 |
| O2-depleted | 2.28 | 3.27 | - | 4.59 | 5.67 | - | ||
| PFO | qe (mg/g) | O2-saturated | 2.31 | 3.34 | 4.13 | 4.85 | 5.44 | 7.05 |
| k1 (min–1) | 0.0270 | 0.0202 | 0.0266 | 0.0273 | 0.0260 | 0.0259 | ||
| R2 (−) | 0.996 | 0.9999 | 0.9972 | 0.9984 | 0.9964 | 0.9988 | ||
| qe (mg/g) | O2-depleted | 2.88 | 3.51 | - | 4.93 | 5.69 | - | |
| k1 (min–1) | 0.0150 | 0.0235 | - | 0.0237 | 0.0357 | - | ||
| R2 (−) | 0.9996 | 0.9963 | - | 0.9963 | 0.9972 | - | ||
| PSO | qe (mg/g) | O2-saturated | 3.10 | 4.79 | 5.53 | 6.46 | 7.30 | 9.51 |
| k2 (g/mg·min) | 0.00756 | 0.00324 | 0.00420 | 0.00371 | 0.00311 | 0.00234 | ||
| h (mg/g·min) | 0.07 | 0.07 | 0.13 | 0.15 | 0.17 | 0.21 | ||
| R2 (−) | 0.9998 | 0.9995 | 0.9995 | 0.9999 | 0.9992 | 0.9998 | ||
| qe (mg/g) | O2-depleted | 4.40 | 4.81 | - | 6.75 | 7.17 | - | |
| k2 (g/mg·min) | 0.00236 | 0.00410 | - | 0.00295 | 0.00497 | - | ||
| h (mg/g·min) | 0.05 | 0.09 | - | 0.13 | 0.26 | - | ||
| R2 (−) | 0.9992 | 0.9988 | - | 0.9988 | 0.9999 | - | ||
| Elovich | α (mg/g·min) | O2-saturated | 0.14 | 0.16 | 0.26 | 0.31 | 0.34 | 0.43 |
| β (g/mg) | 1.37 | 0.92 | 0.78 | 0.66 | 0.59 | 0.45 | ||
| R2 (−) | 0.9992 | 0.9989 | 0.9984 | 0.9988 | 0.9966 | 0.9980 | ||
| α (mg/g·min) | O2-depleted | 0.10 | 0.20 | - | 0.28 | 0.52 | - | |
| β (g/mg) | 1.11 | 0.91 | - | 0.65 | 0.61 | - | ||
| R2 (−) | 0.9962 | 0.9936 | - | 0.9941 | 0.9990 | - | ||
| Bangham | α (−) | O2-saturated | 0.868 | 0.874 | 0.795 | 0.754 | 0.719 | 0.725 |
| k0 (mL/g/L) | 0.437 | 0.284 | 0.508 | 0.540 | 0.538 | 0.500 | ||
| R2 (−) | 0.9991 | 0.9968 | 0.9996 | 0.9992 | 1.0000 | 0.9980 | ||
| α (−) | O2-depleted | 1.145 | 0.821 | - | 0.782 | 0.657 | - | |
| k0 (mL/g/L) | 0.131 | 0.437 | - | 0.456 | 0.896 | - | ||
| R2 (−) | 0.9988 | 0.9984 | - | 0.9993 | 0.9973 | - | ||
| Weber–Morris | kid (mg·g−1·min−0.5) | O2-saturated | 0.2221 | 0.2883 | 0.3956 | 0.4672 | 0.5172 | 0.6692 |
| R2 (−) | 0.9981 | 0.9968 | 0.9992 | 0.9988 | 0.9995 | 0.9988 | ||
| kid (mg·g−1·min−0.5) | O2-depleted | 0.2160 | 0.3222 | - | 0.4541 | 0.5922 | - | |
| R2 (−) | 0.9921 | 0.9996 | - | 0.9996 | 0.9952 | - | ||
| Type | Catalyst Amount (g/L) | Light Intensity (mW/cm2) | Results | Reference |
|---|---|---|---|---|
| doped | 1 | n/a * (vis) | R ≈ 45% (10 mg/L, 3 h) | [36] |
| doped | n/a | n/a | R = 73% (50 mg/L, 0.5 h) | [37] |
| doped | n/a | n/a | R = 82% (9.8 mg/L, 2 h) | [38] |
| nanocomposite | 1 | n/a | R = 39.1% (40.9 mg/L, 2 h) | [39] |
| nanocomposite | 1 | n/a | R = 80% (20 mg/L, 3 h) | [40] |
| nanocomposite | 2.5 | 0.5 | R = 65.3% (O2, 12 mg/L, 3 h) | This work |
| R = 73.5% (Inert, 6.3 mg/L, 3 h) |
| Cinitial (mg/L) | kapp_o × 102 (min–1) | r′_o (mg/L/min) | kapp_i × 102 (min–1) | r′_i (mg/L/min) |
|---|---|---|---|---|
| 6.3 | 0.79 | 0.017 | 1.09 | 0.020 |
| 10 | 0.78 | 0.021 | 0.57 | 0.013 |
| 12 | 0.81 | 0.017 | - | - |
| 15 | 0.73 | 0.033 | 0.68 | 0.027 |
| 18 | 0.65 | 0.033 | 0.68 | 0.026 |
| 24 | 0.38 | 0.028 | - | - |
| Cinitial (mg/L) | t1/2_o (min) | t1/2_i (min) | t′1/2_o (min) | t′1/2_i (min) | Δt1/2_o (min) | Δt1/2_i (min) |
|---|---|---|---|---|---|---|
| 6.3 | 83.29 | 70.93 | 87.67 | 63.36 | 4.38 | n/a |
| 10 | 85.10 | 76.87 | 89.31 | 121.58 | 4.22 | 44.71 |
| 12 | 83.17 | - | 85.42 | - | 2.25 | - |
| 15 | 92.13 | 96.46 | 94.72 | 101.74 | 2.59 | 5.27 |
| 18 | 94.48 | 94.62 | 106.08 | 101.91 | 11.60 | 7.29 |
| 24 | 103.56 | - | 181.47 | - | 77.91 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorakopoulos, G.V.; Papageorgiou, S.K.; Katsaros, F.K.; Romanos, G.E.; Beazi-Katsioti, M. Investigation of MO Adsorption Kinetics and Photocatalytic Degradation Utilizing Hollow Fibers of Cu-CuO/TiO2 Nanocomposite. Materials 2024, 17, 4663. https://doi.org/10.3390/ma17184663
Theodorakopoulos GV, Papageorgiou SK, Katsaros FK, Romanos GE, Beazi-Katsioti M. Investigation of MO Adsorption Kinetics and Photocatalytic Degradation Utilizing Hollow Fibers of Cu-CuO/TiO2 Nanocomposite. Materials. 2024; 17(18):4663. https://doi.org/10.3390/ma17184663
Chicago/Turabian StyleTheodorakopoulos, George V., Sergios K. Papageorgiou, Fotios K. Katsaros, George Em. Romanos, and Margarita Beazi-Katsioti. 2024. "Investigation of MO Adsorption Kinetics and Photocatalytic Degradation Utilizing Hollow Fibers of Cu-CuO/TiO2 Nanocomposite" Materials 17, no. 18: 4663. https://doi.org/10.3390/ma17184663
APA StyleTheodorakopoulos, G. V., Papageorgiou, S. K., Katsaros, F. K., Romanos, G. E., & Beazi-Katsioti, M. (2024). Investigation of MO Adsorption Kinetics and Photocatalytic Degradation Utilizing Hollow Fibers of Cu-CuO/TiO2 Nanocomposite. Materials, 17(18), 4663. https://doi.org/10.3390/ma17184663








