Impact of a Bio-Cross-Linking Agent Obtained from Spent Coffee Grounds on the Physicochemical and Thermal Properties of Gelatin/Κ-Carrageenan Hydrogels
Abstract
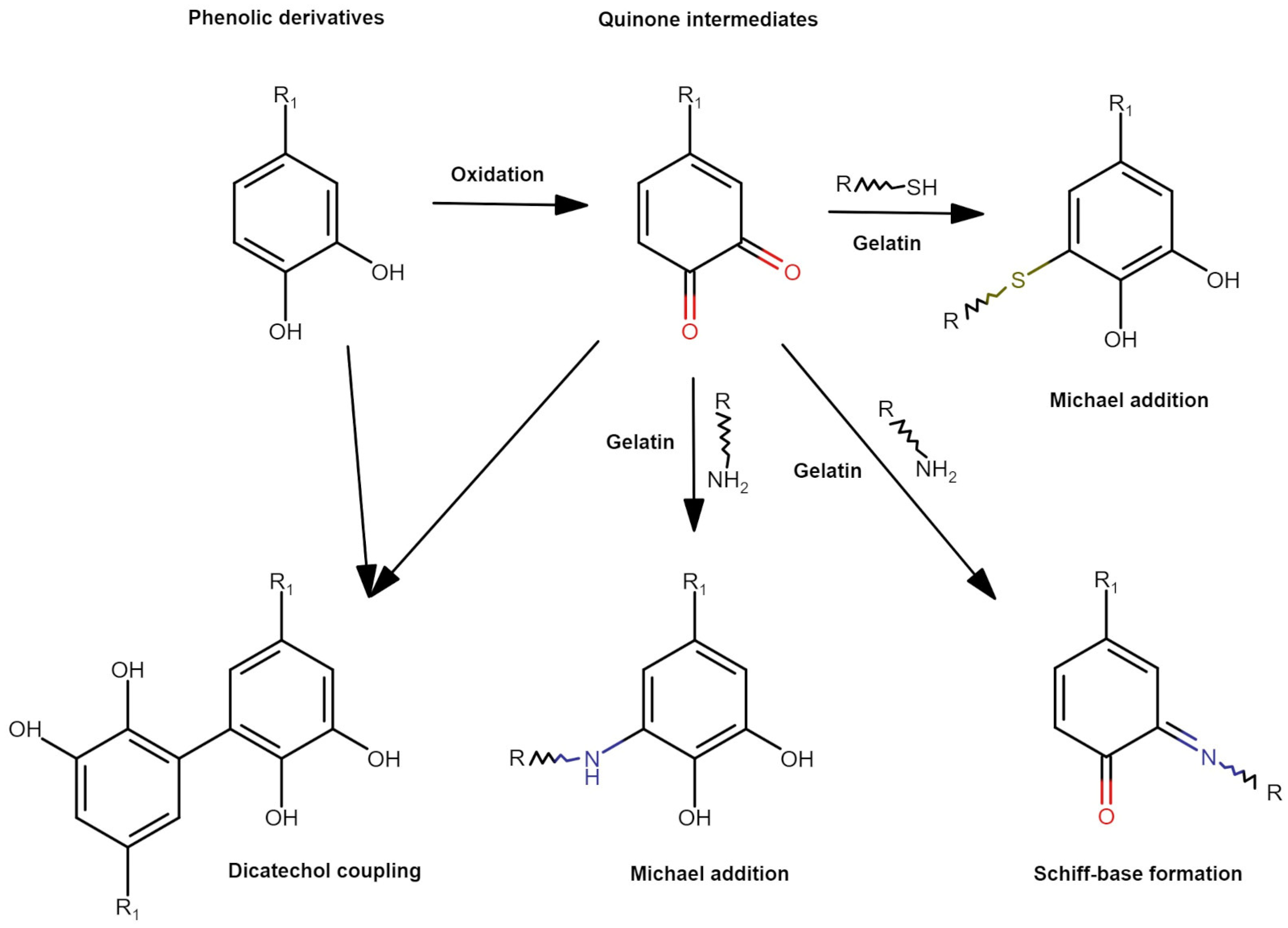
:1. Introduction
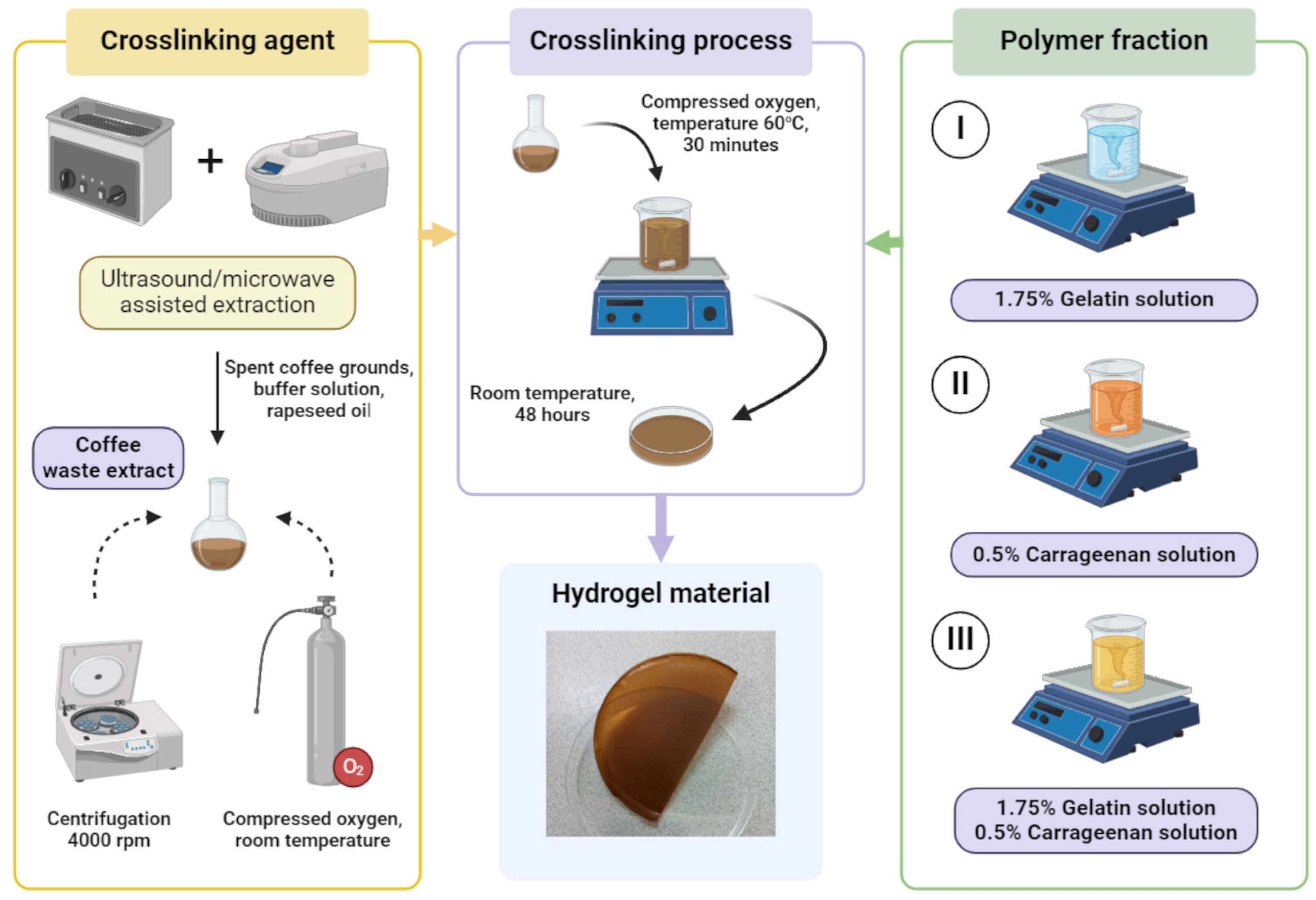
2. Materials and Methods
3. Results and Discussion
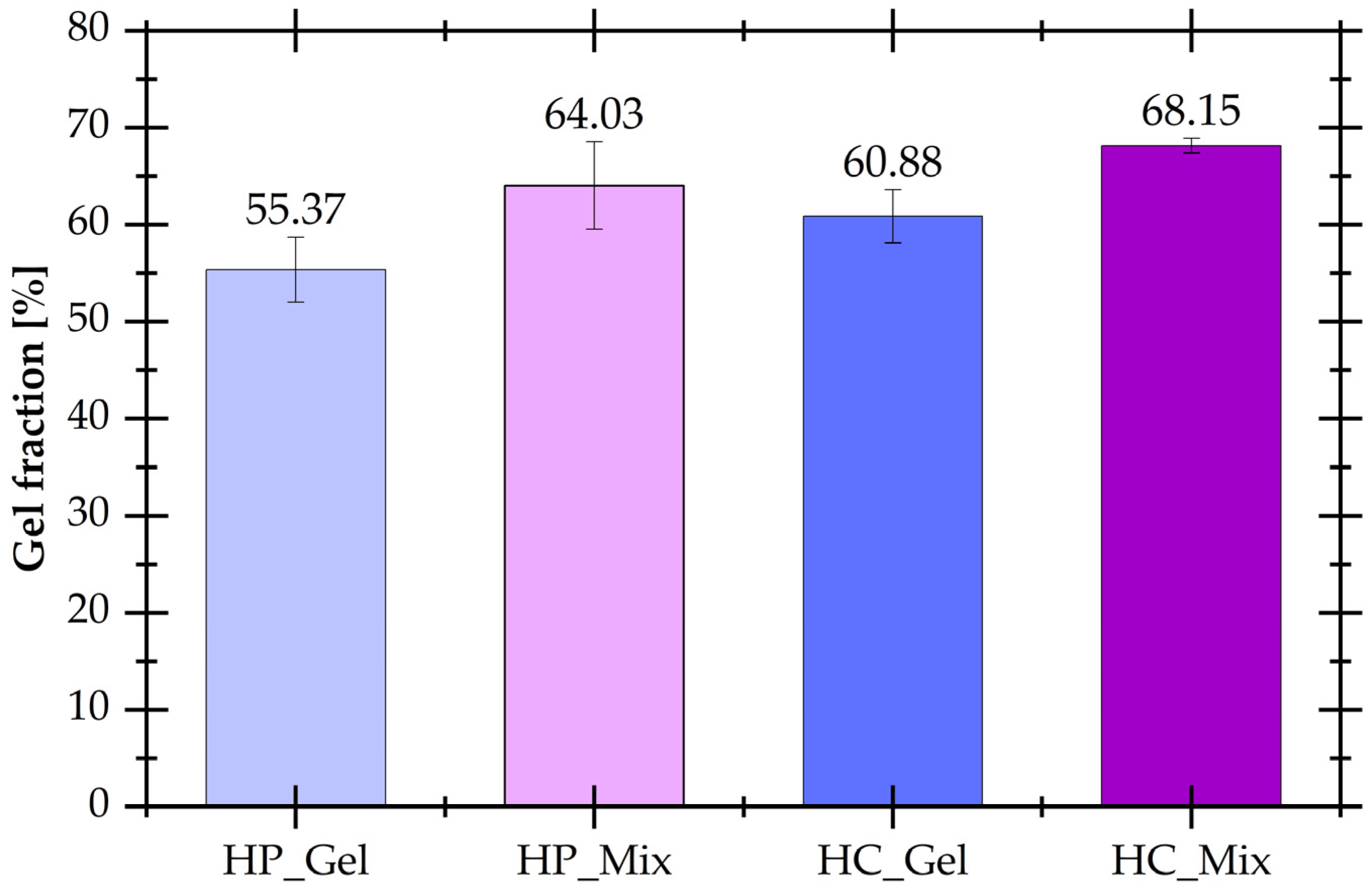
3.1. Gel Fraction
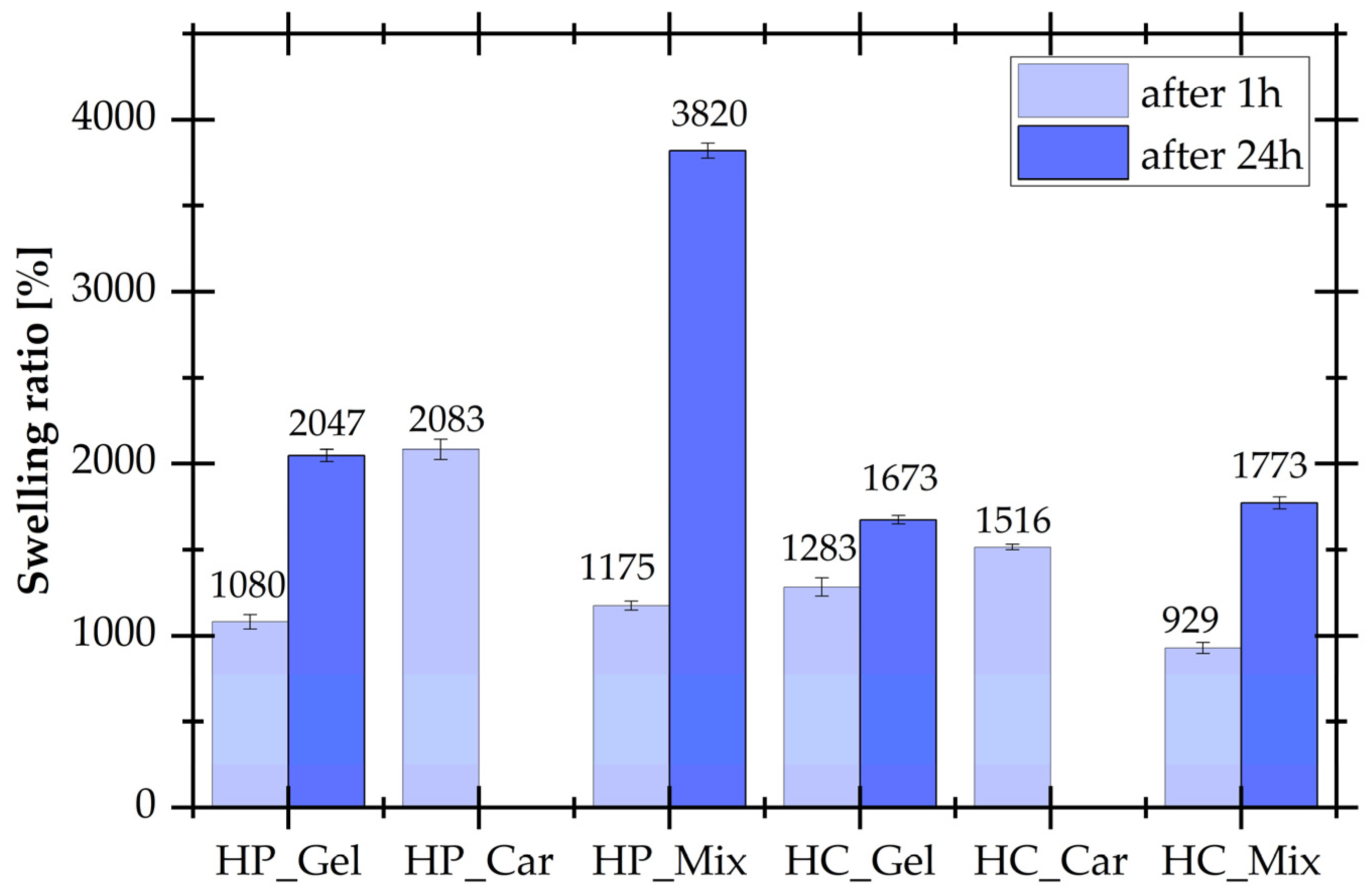
3.2. Determination of Swelling Ratios
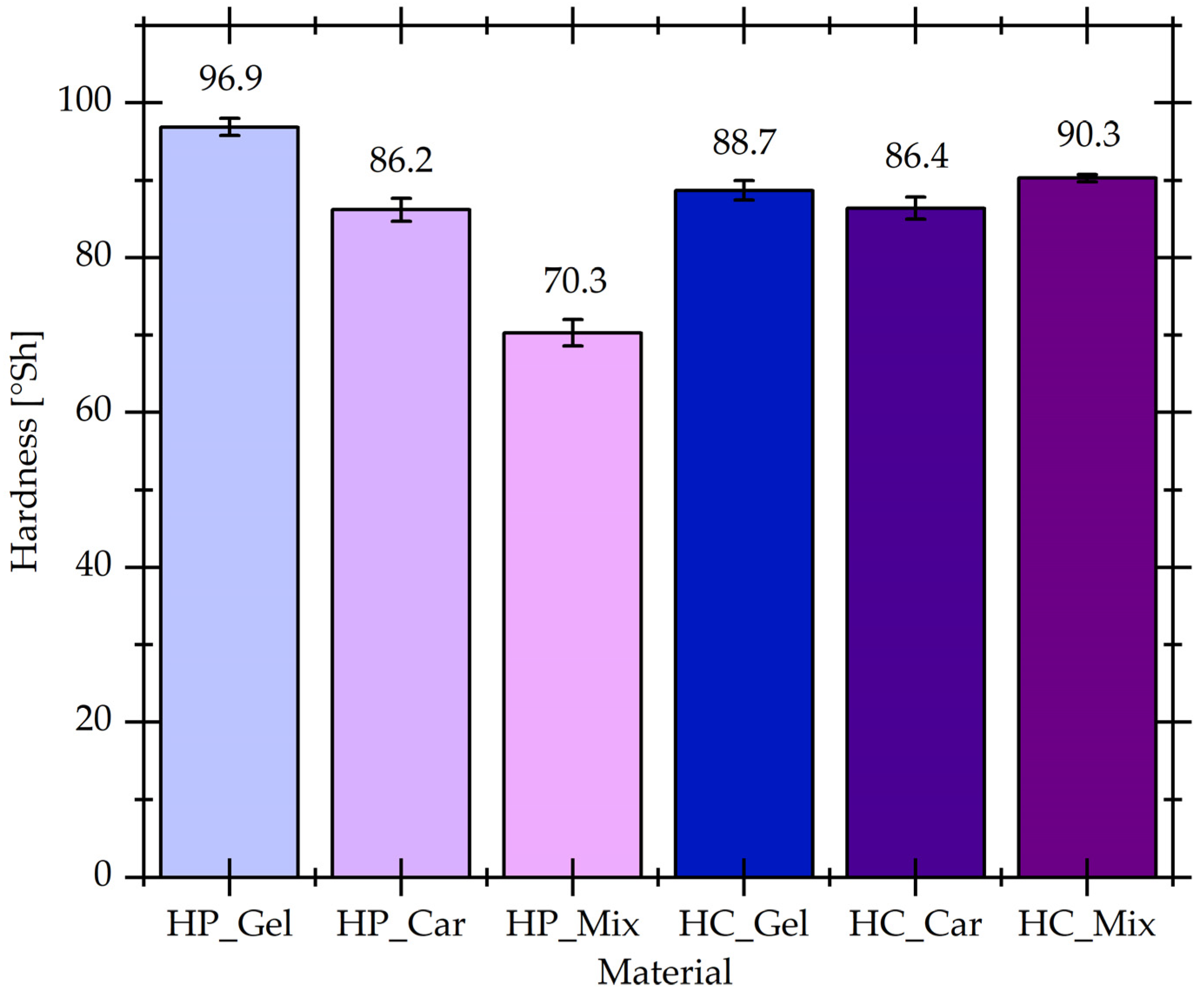
3.3. Hardness
3.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
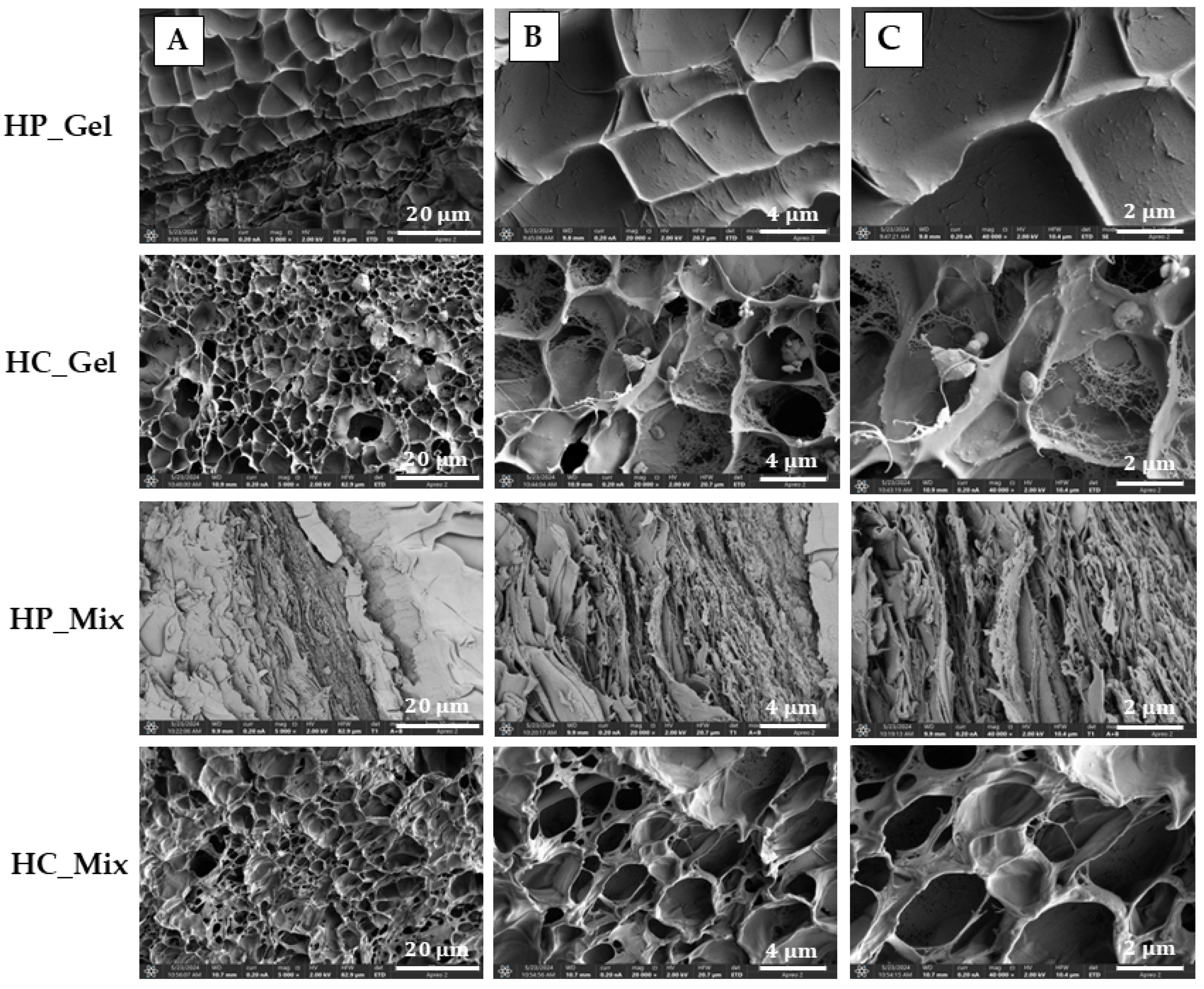
3.5. SEM Analysis
3.6. Thermogravimetric Analysis (TGA)
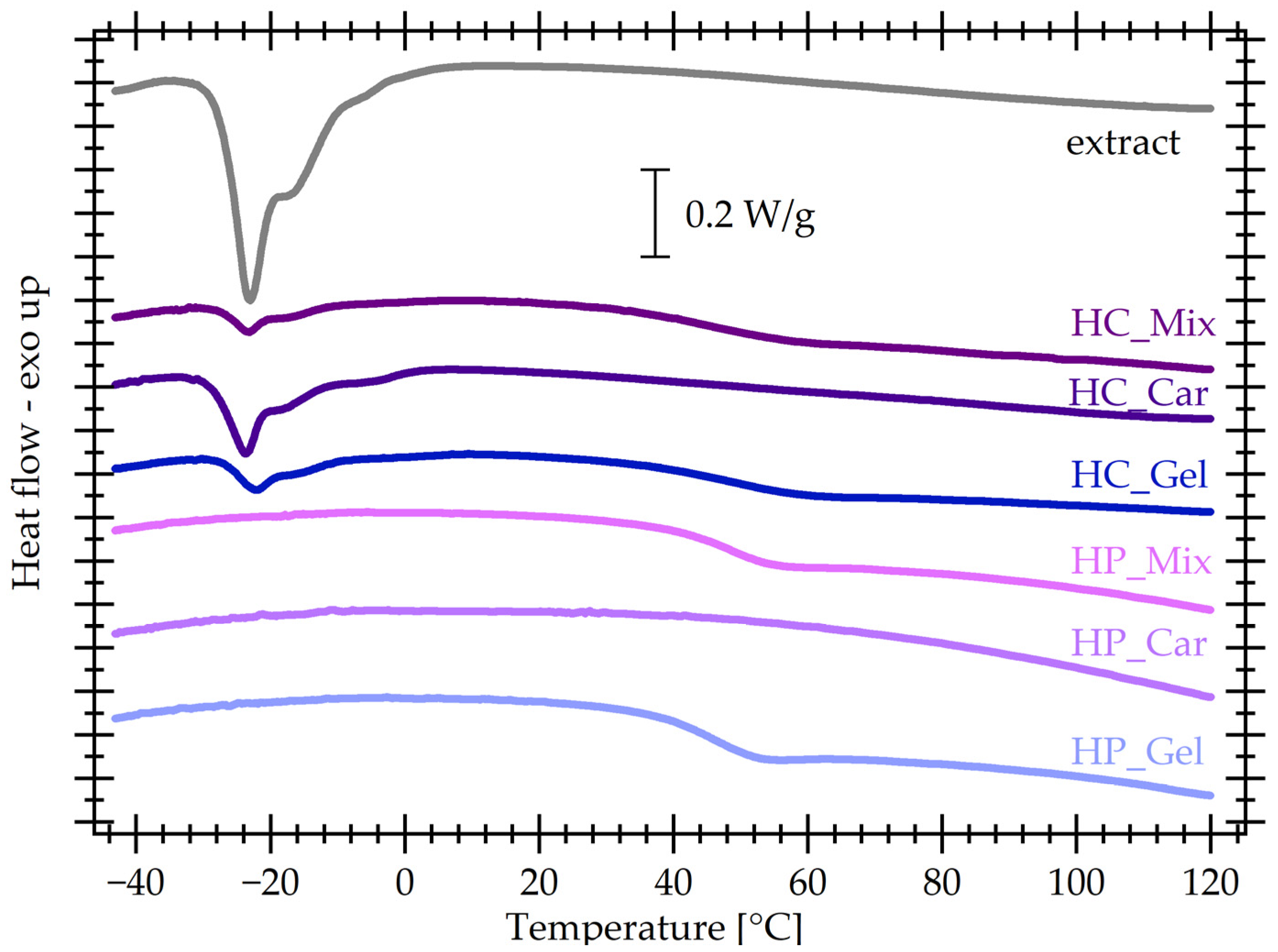
3.7. Differential Scanning Calorimetry (DSC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmadi, S.; Pourebrahimi, S.; Malloum, A.; Pirooz, M.; Osagie, C.; Ghosh, S.; Zafar, M.N.; Dehghani, M.H. Hydrogel-based materials as antibacterial agents and super adsorbents for the remediation of emerging pollutants: A comprehensive review. Emerg. Contam. 2024, 10, 100336. [Google Scholar] [CrossRef]
- Liaw, C.Y.; Ji, S.; Guvendiren, M. Engineering 3D Hydrogels for Personalized In Vitro Human Tissue Models. Adv. Healthc. Mater. 2018, 7, 1701165. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, G.; Zhu, Y.; Cheng, W.; Cao, K.; Xu, G.; Zhao, D.; Yu, H. A Scalable Bacterial Cellulose Ionogel for Multisensory Electronic Skin. Research 2022, 2022, 9814767. [Google Scholar] [CrossRef]
- Filip, D.; Macocinschi, D.; Zaltariov, M.-F.; Ciubotaru, B.-I.; Bargan, A.; Varganici, C.-D.; Vasiliu, A.-L.; Peptanariu, D.; Balan-Porcarasu, M.; Timofte-Zorila, M.-M. Hydroxypropyl Cellulose/Pluronic-Based Composite Hydrogels as Bio-degradable Mucoadhesive Scaffolds for Tissue Engineering. Gels 2022, 8, 519. [Google Scholar] [CrossRef]
- Hasan, N.; Lee, J.; Ahn, H.-J.; Hwang, W.R.; Bahar, M.A.; Habibie, H.; Amir, M.N.; Lallo, S.; Son, H.-J.; Yoo, J.-W. Nitric Oxide-Releasing Bacterial Cellulose/Chitosan Crosslinked Hydrogels for the Treatment of Polymicrobial Wound Infections. Pharmaceutics 2022, 14, 22. [Google Scholar] [CrossRef]
- Chen, H.; Wu, D.; Ma, W.; Wu, C.; Liu, J.; Du, M. Strong fish gelatin hydrogels double crosslinked by transglutaminase and carrageenan. Food Chem. 2022, 376, 131873. [Google Scholar] [CrossRef]
- De Alcântara, M.G.; de Freitas Ortega, N.; Souza, C.J.F.; Garcia-Rojas, E.E. Electrostatic hydrogels formed by gelatin and carrageenan induced by acidification: Rheological and structural characterization. Food Struct. 2020, 24, 100137. [Google Scholar] [CrossRef]
- Padhi, J.R.; Nayak, D.; Nanda, A.; Rauta, P.R.; Ashe, S.; Nayak, B. Development of highly biocompatible Gelatin & i-Carrageenan based composite hydrogels: In depth physiochemical analysis for biomedical applications. Carbohydr. Polym. 2016, 153, 292–301. [Google Scholar] [CrossRef]
- Tytgat, L.; Van Damme, L.; Arevalo, M.d.P.O.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-based 3D printing of photo-crosslinkable gelatin and κ-carrageenan hydrogel blends for adipose tissue regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z.; Chen, Q.; Ye, X.; Zeng, Q.; Yuan, Y.; Dong, L.; Huang, F.; Su, D. Effects of gelatin-polyphenol and gelatin—Genipin crosslinking on the structure of gelatin hydrogels. Int. J. Food Prop. 2017, 20, S2822–S2832. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X. Hydrogel with the network structure fabricated by anthocyanin–gelatin cross-linking and improved mineral encapsulation ability. Int. J. Food Sci. Technol. 2022, 57, 7143–7155. [Google Scholar] [CrossRef]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Hezaveh, H.; Muhamad, I.I. Modification and swelling kinetic study of kappa-carrageenan-based hydrogel for controlled release study. J. Taiwan Inst. Chem. Eng. 2013, 44, 182–191. [Google Scholar] [CrossRef]
- Azizi, S.; Mohamad, R.; Rahim, R.A.; Mohammadinejad, R.; Bin Ariff, A. Hydrogel beads bio-nanocomposite based on Kappa-Carrageenan and green synthesized silver nanoparticles for biomedical applications. Int. J. Biol. Macromol. 2017, 104, 423–431. [Google Scholar] [CrossRef]
- Selvakumaran, S.; Muhamad, I.I. Evaluation of kappa carrageenan as potential carrier for floating drug delivery system: Effect of cross linker. Int. J. Pharm. 2015, 496, 323–331. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Ye, J.; Xiao, Z.; Gao, L.; Zhang, J.; He, L.; Zhang, H.; Liu, Q.; Yang, G. Assessment of the effects of four crosslinking agents on gelatin hydrogel for myocardial tissue engineering applications. Biomed. Mater. 2021, 16, 045026. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Venezia, V.; Verrillo, M.; Avallone, P.R.; Silvestri, B.; Cangemi, S.; Pasquino, R.; Grizzuti, N.; Spaccini, R.; Luciani, G. Waste to Wealth Approach: Improved Antimicrobial Properties in Bioactive Hydrogels through Humic Substance—Gelatin Chemical Conjugation. Biomacromolecules 2023, 24, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Maji, B.; Yadav, H. Progress on green crosslinking of polysaccharide hydrogels for drug delivery and tissue engineering applications. Carbohydr. Polym. 2024, 326, 121584. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Chen, H.; Chen, K.; Tao, W.; Ouyang, X.-K.; Mei, L.; Zeng, X. Polyphenol-based hydrogels: Pyramid evolution from crosslinked structures to biomedical applications and the reverse design. Bioact. Mater. 2022, 17, 49–70. [Google Scholar] [CrossRef]
- Wu, L.; Shao, H.; Fang, Z.; Zhao, Y.; Cao, C.Y.; Li, Q. Mechanism and Effects of Polyphenol Derivatives for Modifying Collagen. ACS Biomater. Sci. Eng. 2019, 5, 4272–4284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lei, Y.; Qi, S.; Fan, M.; Zheng, S.; Huang, Q.; Lu, X. Ultrasonic-microwave-assisted extraction for enhancing antioxidant activity of Dictyophora indusiata polysaccharides: The difference mechanisms between single and combined assisted extraction. Ultrason. Sonochem. 2023, 95, 106356. [Google Scholar] [CrossRef] [PubMed]
- Sapuła, P.; Bialik-Wąs, K. Naturalny Czynnik Sieciujący i Sposób Otrzymywania Naturalnego Czynnika Sieciującego. Polish Patent Application P.446027, 6 September 2023. [Google Scholar]
- Sapuła, P.; Bialik-Wąs, K. Sposób Otrzymywania Materiału Hydrożelowego Przy Zastosowaniu Naturalnego Czynnika Sieciującego. Polish Patent Application P.449047, 28 June 2024. [Google Scholar]
- PN-ISO 868; Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). PKN: Płock, Poland, 2003.
- Hellio, D.; Djabourov, M. Physically and chemically crosslinked gelatin gels. Macromol. Symp. 2006, 241, 23–27. [Google Scholar] [CrossRef]
- Bukhari, S.M.H.; Khan, S.; Rehanullah, M.; Ranjha, N.M. Synthesis and Characterization of Chemically Cross-Linked Acrylic Acid/Gelatin Hydrogels: Effect of pH and Composition on Swelling and Drug Release. Int. J. Polym. Sci. 2015, 2015, 187961. [Google Scholar] [CrossRef]
- Zou, J.; Wang, L.; Sun, G. Mechanisms and Performances of Physically and Chemically Crosslinked Gelatin-Based Hydrogels as Advanced Sustainable and Reusable “Jelly Ice Cube” Coolants. ACS Appl. Mater. Interfaces 2023, 15, 34087–34096. [Google Scholar] [CrossRef]
- Miyawaki, O.; Omote, C.; Matsuhira, K. Thermodynamic Analysis of Sol—Gel Transition of Gelatin in Terms of Water Activity in Various Solutions. Biopolymers 2015, 103, 685–691. [Google Scholar] [CrossRef]
- Derkach, S.R.; Ilyin, S.O.; Maklakova, A.A.; Kulichikhin, V.G.; Malkin, A.Y. The rheology of gelatin hydrogels modified by κ-carrageenan. LWT-Food Sci. Technol. 2015, 63, 612–619. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Xu, M.; Wei, L.; Xiao, Y.; Bi, H.; Yang, H.; Du, Y. Physicochemical and functional properties of gelatin extracted from Yak skin. Int. J. Biol. Macromol. 2017, 95, 1246–1253. [Google Scholar] [CrossRef]
- Hung, L.D.; Nguyen, H.T.T.; Trang, V.T.D. Kappa carrageenan from the red alga Kappaphycus striatus cultivated at Vanphong Bay, Vietnam: Physicochemical properties and structure. J. Appl. Phycol. 2021, 33, 1819–1824. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Tang, P.L.; Chew, S.Y.; Hou, X.; Deng, J.; Badri, K. Novel use of sugarcane leaf polysaccharide in κ-carrageenan blend hydrogel. Biomass Convers. Biorefin. 2024, 14, 5489–5503. [Google Scholar] [CrossRef]
- Welna, M.; Szymczycha-Madeja, A.; Zyrnicki, W. Applicability of ICP-OES, UV-VIS, and FT-IR Methods for the Analysis of Coffee Products. Anal. Lett. 2013, 46, 2927–2940. [Google Scholar] [CrossRef]
- Garrigues, J.M.; Bouhsain, Z.; Garrigues, S.; de la Guardia, M. Fourier transform infrared determination of caffeine in roasted coffee samples. Fres. J. Anal. Chem. 2000, 366, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Llavata-Cabrero, B.; Martínez-Sanz, M.; Fabra, M.J.; López-Rubio, A. Self-assembled gelatin-ι-carrageenan encapsulation structures for intestinal-targeted release applications. J. Colloid Interface Sci. 2018, 517, 113–123. [Google Scholar] [CrossRef]
- Carvalho, I.C.; Mansur, H.S. Engineered 3D-scaffolds of photocrosslinked chitosan-gelatin hydrogel hybrids for chronic wound dressings and regeneration. Mater. Sci. Eng. C 2017, 78, 690–705. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, R.; Wang, W.; Wei, X.; Li, J.; Chen, X.; Liu, Y.; Lu, F.; Li, Y. Tunable physical and mechanical properties of gelatin hydrogel after transglutaminase crosslinking on two gelatin types. Int. J. Biol. Macromol. 2020, 162, 405–413. [Google Scholar] [CrossRef]
- Stutz, H.; Illers, K.-H.; Mertes, J. A generalized theory for the glass transition temperature of crosslinked and uncrosslinked polymers. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 1483–1498. [Google Scholar] [CrossRef]


| Sample | Gelatin [% v/v] | Κ-Carrageenan [% v/v] | Cross-Linking Agent [% v/v] |
|---|---|---|---|
| HP_Gel | 100 | - | - |
| HP_Car | - | 100 | - |
| HP_Mix | 67 | 33 | - |
| HC_Gel | 67 | - | 33 |
| HC_Car | - | 50 | 50 |
| HC_Mix | 50 | 25 | 25 |
| Sample | Tmax (°C) | Tg (°C) | ΔcP (J/gK) | |
|---|---|---|---|---|
| Polymer | Extract | |||
| HP_Gel | 317 | - | 44.0 | 0.68 |
| HP_Car | 278 | - | - | - |
| HP_Mix | 275, 314 | - | 46.7 | 0.61 |
| HC_Gel | 320 | 366, 450 | 47.4 | 0.42 |
| HC_Car | 288 | 371, 452 | - | - |
| HC_Mix | 288, 329 | 378, 454 | 43.7 | 0.47 |
| extract | - | 385, 453 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapuła, P.; Zając, P.; Pielichowski, K.; Raftopoulos, K.N.; Bialik-Wąs, K. Impact of a Bio-Cross-Linking Agent Obtained from Spent Coffee Grounds on the Physicochemical and Thermal Properties of Gelatin/Κ-Carrageenan Hydrogels. Materials 2024, 17, 4724. https://doi.org/10.3390/ma17194724
Sapuła P, Zając P, Pielichowski K, Raftopoulos KN, Bialik-Wąs K. Impact of a Bio-Cross-Linking Agent Obtained from Spent Coffee Grounds on the Physicochemical and Thermal Properties of Gelatin/Κ-Carrageenan Hydrogels. Materials. 2024; 17(19):4724. https://doi.org/10.3390/ma17194724
Chicago/Turabian StyleSapuła, Paulina, Paulina Zając, Krzysztof Pielichowski, Konstantinos N. Raftopoulos, and Katarzyna Bialik-Wąs. 2024. "Impact of a Bio-Cross-Linking Agent Obtained from Spent Coffee Grounds on the Physicochemical and Thermal Properties of Gelatin/Κ-Carrageenan Hydrogels" Materials 17, no. 19: 4724. https://doi.org/10.3390/ma17194724








