Enhancing Capillary Pressure of Porous Aluminum Wicks by Controlling Bi-Porous Structure Using Different-Sized NaCl Space Holders
Abstract
1. Introduction
2. Materials and Methods
2.1. Space Holder Method
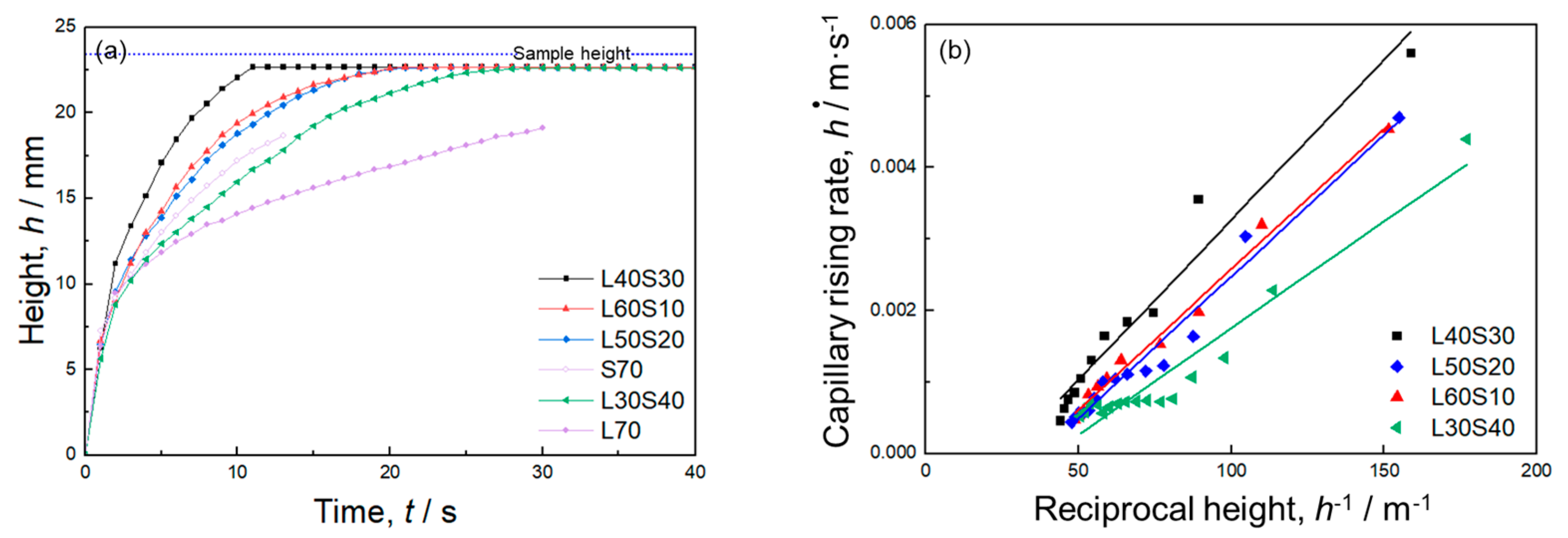
2.2. Capillary Rise Experiment
3. Results
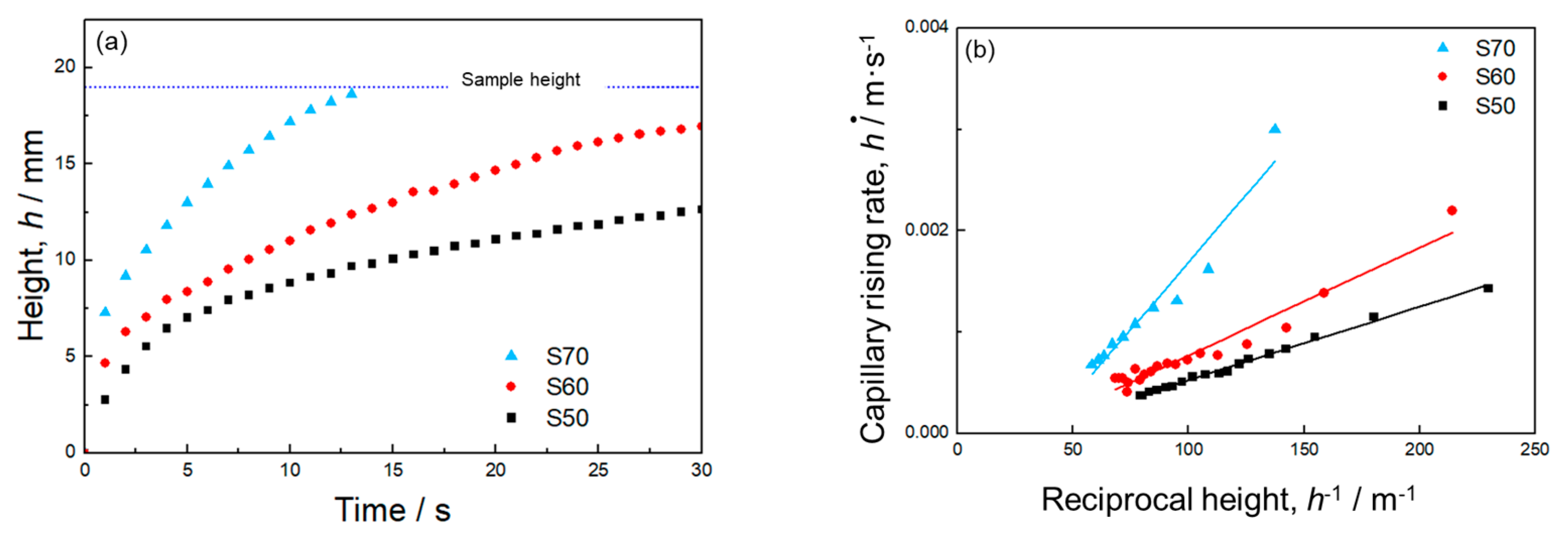
3.1. Mono-Porous Al with Various Porosity
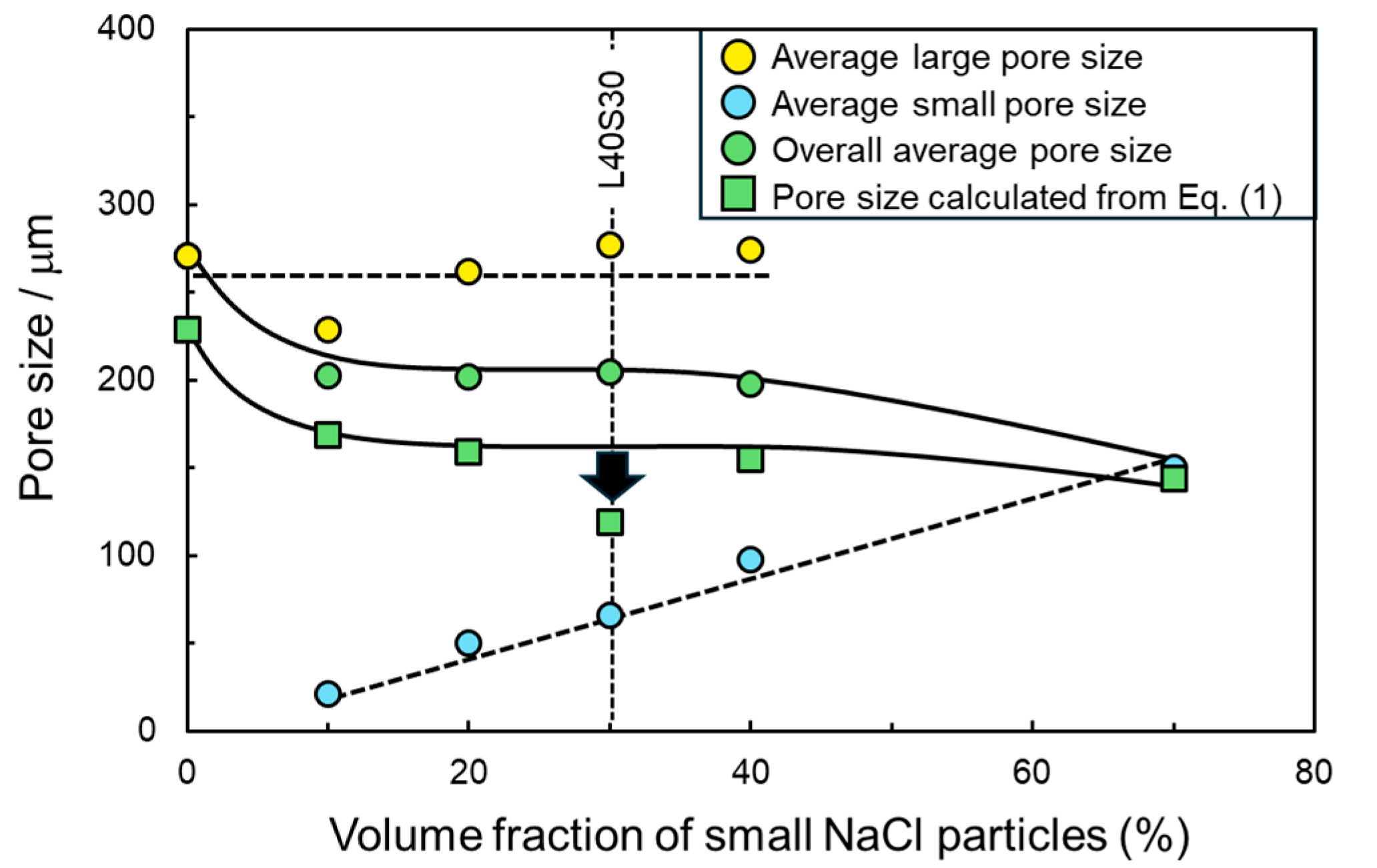
3.2. Bi-Porous Al with Various Volume Fractions of Large and Small Pores under a Constant Total Porosity
4. Discussion
5. Conclusions
- (1)
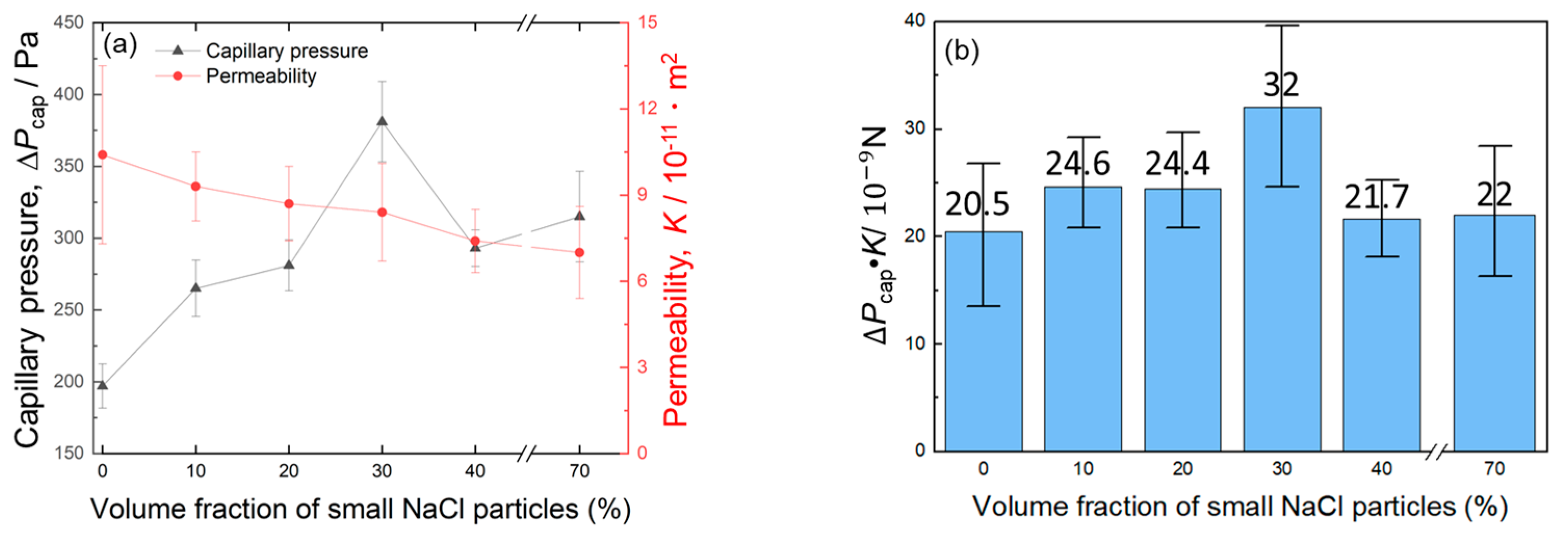
- Mono-porous Al produced using small NaCl particles (30–50 μm) exhibited the trade-off relation between Pcap and K via the porosity. This trend was consistent with the previous results of mono-porous Al fabricated using large NaCl particles (330–430 μm). The pores were well-connected to form large pores as the porosity increased, resulting in the trade-off relation.
- (2)
- The mono-porous Al with a smaller pore size exhibited a higher Pcap and a lower K compared to the mono-porous Al with a larger pore size under a constant porosity. This trend demonstrated the well-known trade-off relation between Pcap and K via the pore size.
- (3)
- The volume fractions of large and small pores in the bi-porous Al were successfully controlled under a constant total porosity of 70% by tailoring the blending volume fractions of large and small NaCl particles. Increasing the volume fraction of small pores from 0% to 30% increased the Pcap from 197 to 381 Pa while slightly decreasing K from 10.4 × 10−11 m2 to 8.4 × 10−11 m2. When the volume fraction of small pores was increased to 40%, Pcap and K degraded to 293 Pa and 7.4 × 10−11 m2.
- (4)
- Almost all the bi-porous samples exhibited intermediate Pcap and K between the mono-porous samples with large and small pores alone. However, the optimized bi-porous structure with 40% large and 30% small pores exhibited a higher Pcap and Pcap·K than the mono-porous samples. The bi-porous structure was not always superior to the mono-porous structure and must be controlled to improve Pcap and Pcap·K.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allioux, F.; Merhebi, S.; Tang, J.; Idrus-Saidi, S.A.; Abbasi, R.; Saborio, M.G.; Ghasemian, M.B.; Han, J.; Namivandi-Zangeneh, R.; O’Mullane, A.P.; et al. Catalytic Metal Foam by Chemical Melting and Sintering of Liquid Metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 1907879. [Google Scholar] [CrossRef]
- Malloy, J.; Quintana, A.; Jensen, C.J.; Liu, K. Efficient and Robust Metallic Nanowire Foams for Deep Submicrometer Particulate Filtration. Nano Lett. 2021, 21, 2968–2974. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, Y.; Ma, B.; Zhu, Y. Experimental Study on Capillary Microflows in High Porosity Open-Cell Metal Foams. Micromachines 2022, 13, 2052. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Qin, Y.; Guo, H.; Wen, P.; Lin, H.; Voshage, M.; Schleifenbaum, J.H.; Cheng, Y.; Zheng, Y. Additively Manufactured Pure Zinc Porous Scaffolds for Critical-Sized Bone Defects of Rabbit Femur. Bioact. Mater. 2023, 19, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Li, B.; Fu, B.; Yang, X.; Lou, T.; Ji, Y. Simulation and Experimental Investigation of Phase Change Materials within Porous Metal Structures with Gradient Porosity. Int. J. Heat Mass Transf. 2024, 234, 126136. [Google Scholar] [CrossRef]
- Li, X.; Yao, D.; Zuo, K.; Xia, Y.; Zeng, Y.-P. Effects of Pore Structures on the Capillary and Thermal Performance of Porous Silicon Nitride as Novel Loop Heat Pipe Wicks. Int. J. Heat Mass Transf. 2021, 169, 120985. [Google Scholar] [CrossRef]
- Nishikawara, M. Pore Network Simulation of Loop Heat Pipe Evaporator with Different Pore Size Distribution. Int. J. Heat Mass Transf. 2024, 218, 124739. [Google Scholar] [CrossRef]
- Kumar, P.; Wangaskar, B.; Khandekar, S.; Balani, K. Thermal-Fluidic Transport Characteristics of Bi-Porous Wicks for Potential Loop Heat Pipe Systems. Exp. Therm. Fluid Sci. 2018, 94, 355–367. [Google Scholar] [CrossRef]
- Feng, C.; Yugeswaran, S.; Chandra, S. Capillary Rise of Liquids in Thermally Sprayed Porous Copper Wicks. Exp. Therm. Fluid Sci. 2018, 98, 206–216. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Feng, C.; Liu, L.; Luan, T. Study of Copper Chemical-Plating Modified Polyacrylonitrile-Based Carbon Fiber Wick Applied to Compact Loop Heat Pipe. Exp. Therm. Fluid Sci. 2019, 100, 104–113. [Google Scholar] [CrossRef]
- Zhang, J.; Lian, L.; Liu, Y.; Wang, R. The Heat Transfer Capability Prediction of Heat Pipes Based on Capillary Rise Test of Wicks. Int. J. Heat Mass Transf. 2021, 164, 120536. [Google Scholar] [CrossRef]
- Lu, L.; Sun, J.; Liu, Q.; Liu, X.; Tang, Y. Influence of Electrochemical Deposition Parameters on Capillary Performance of a Rectangular Grooved Wick with a Porous Layer. Int. J. Heat Mass Transf. 2017, 109, 737–745. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M. Biporous Nanocarbon Foams and the Effect of the Structure on the Capillary Performance. Prog. Nat. Sci. 2020, 30, 360–365. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Chen, G.; Sun, Y.; Tang, Y.; Wang, Z. Capillary Performance Characterization of Porous Sintered Stainless Steel Powder Wicks for Stainless Steel Heat Pipes. Int. Commun. Heat Mass Transf. 2020, 116, 104702. [Google Scholar] [CrossRef]
- Li, X.; Yao, D.; Zuo, K.; Xia, Y.; Yin, J.; Liang, H.; Zeng, Y.-P. Fabrication, Microstructural Characterization and Gas Permeability Behavior of Porous Silicon Nitride Ceramics with Controllable Pore Structures. J. Eur. Ceram. Soc. 2019, 39, 2855–2861. [Google Scholar] [CrossRef]
- Mottet, L.; Prat, M. Numerical Simulation of Heat and Mass Transfer in Bidispersed Capillary Structures: Application to the Evaporator of a Loop Heat Pipe. Appl. Therm. Eng. 2016, 102, 770–784. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Bao, X.; Chen, G.; Chu, H. Experimental Study on Hydraulic and Thermal Characteristics of Composite Porous Wick with Spherical–Dendritic Powders. J. Therm. Anal. Calorim. 2020, 141, 107–117. [Google Scholar] [CrossRef]
- Byon, C.; Kim, S.J. Capillary Performance of Bi-Porous Sintered Metal Wicks. Int. J. Heat Mass Transf. 2012, 55, 4096–4103. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, D.; Guo, C.; Zou, Y. Fabrication and Capillary Performance of Bi-Porous Ti3AlC2 Wicks with Controllable Pore Size Proportion Using Dissolvable Pore Formers. J. Mater. Res. Technol. 2021, 15, 4370–4380. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, C.; Wu, D.; Zou, Y. Effects of Pore Structure Characteristics on Performance of Sintered Bi-Porous Ti3AlC2 Wicks. Mater. Res. Express 2021, 8, 015602. [Google Scholar] [CrossRef]
- Zhu, P.; Wu, Z.; Zhao, Y. Hierarchical Porous Cu with High Surface Area and Fluid Permeability. Scr. Mater. 2019, 172, 119–124. [Google Scholar] [CrossRef]
- Li, H.; Fu, S.; Li, G.; Fu, T.; Zhou, R.; Tang, Y.; Tang, B.; Deng, Y.; Zhou, G. Effect of Fabrication Parameters on Capillary Pumping Performance of Multi-Scale Composite Porous Wicks for Loop Heat Pipe. Appl. Therm. Eng. 2018, 143, 621–629. [Google Scholar] [CrossRef]
- Shu, Y.; Suzuki, A.; Takata, N.; Kobashi, M. Fabrication of Porous NiAl Intermetallic Compounds with a Hierarchical Open-Cell Structure by Combustion Synthesis Reaction and Space Holder Method. J. Mater. Process. Technol. 2019, 264, 182–189. [Google Scholar] [CrossRef]
- Shu, Y.; Suzuki, A.; Takata, N.; Kobashi, M. Effect of Powder Blending Ratio on Porous Structure Formed by Combustion Synthesis Reaction between Ni and Al Together with Space Holder. J. Funct. Graded Mater. 2019, 33, 38–43. [Google Scholar] [CrossRef]
- Kobashi, M.; Inoguchi, N.; Kanetake, N. Effect of Elemental Powder Blending Ratio on Combustion Foaming Behavior of Porous Al–Ti Intermetallics and Al3Ti/Al Composites. Intermetallics 2010, 18, 1039–1045. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Sun, D.X. A Novel Sintering-Dissolution Process for Manufacturing Al Foams. Scr. Mater. 2001, 44, 105–110. [Google Scholar] [CrossRef]
- Shen, H.; Suzuki, A.; Takata, N.; Kobashi, M. Elucidating Dominant Flow Channel Size for Capillary Performance of Open-Cell Porous Wicks. Int. J. Heat Mass Transf. 2024, 223, 125217. [Google Scholar] [CrossRef]
- Suzuki, A.; Arai, Y.; Takata, N.; Kobashi, M. Structural Design and Bonding Strength Evaluation of Al/Epoxy Resin Joint via Interpenetrating Phase Layer. J. Mater. Process. Technol. 2018, 262, 11–18. [Google Scholar] [CrossRef]
- Tang, Y.; Xi, X.; Liang, F.; Zhang, S.; Tang, H.; Wu, C.; Sun, Y. Enhanced Capillary Performance of Ultrathin Nylon Mesh Wick for Flexible Thermal Management Systems. Int. J. Heat Mass Transf. 2023, 200, 123545. [Google Scholar] [CrossRef]
- Kim, S.-G. Development of Direct Joining Process of Metals and Polymers via Additively Fabricated Anchor Layer by Laser Induced In-Situ Reaction. Ph.D. Thesis, Nagoya University, Nagoya, Japan, 2020. [Google Scholar]
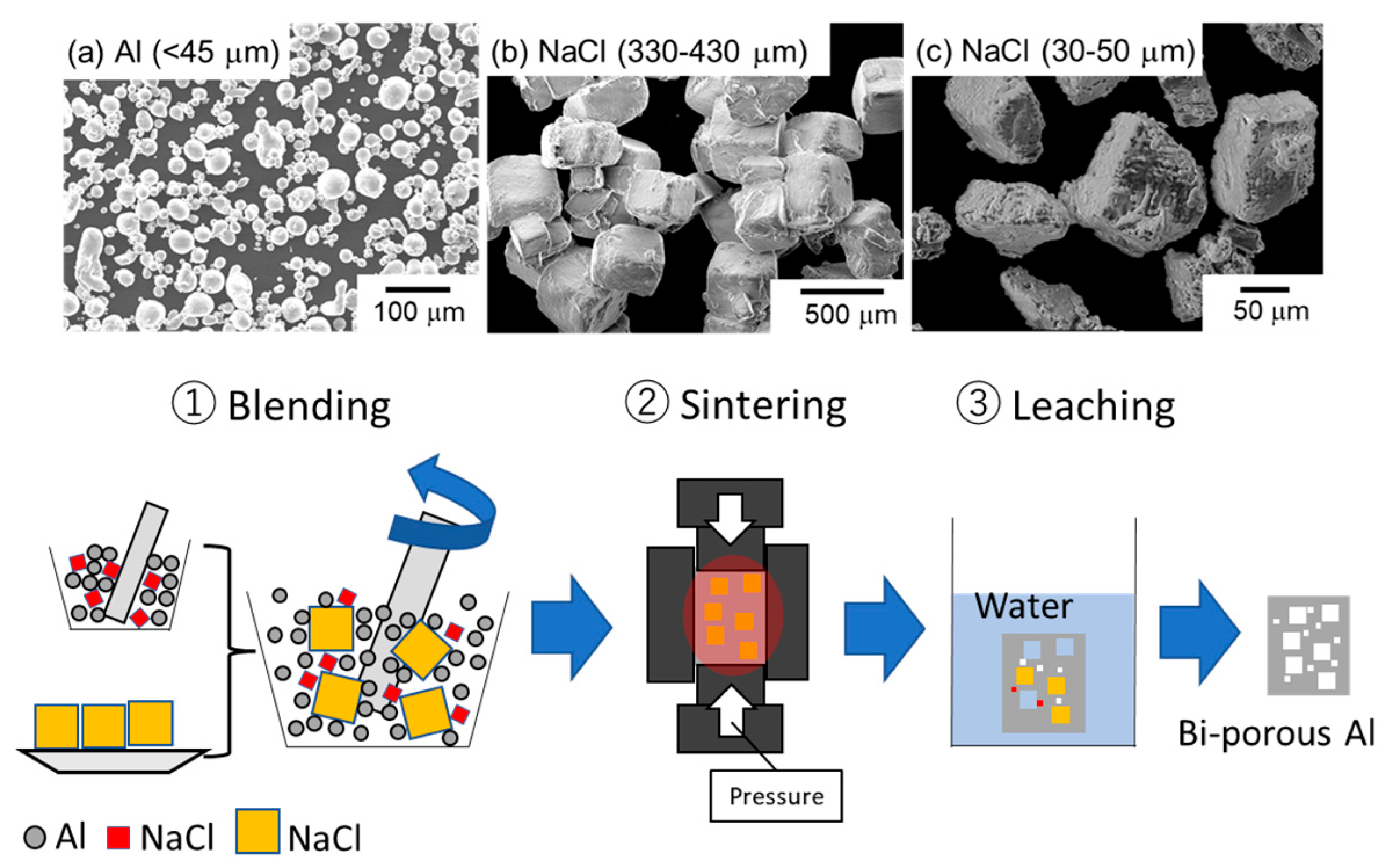
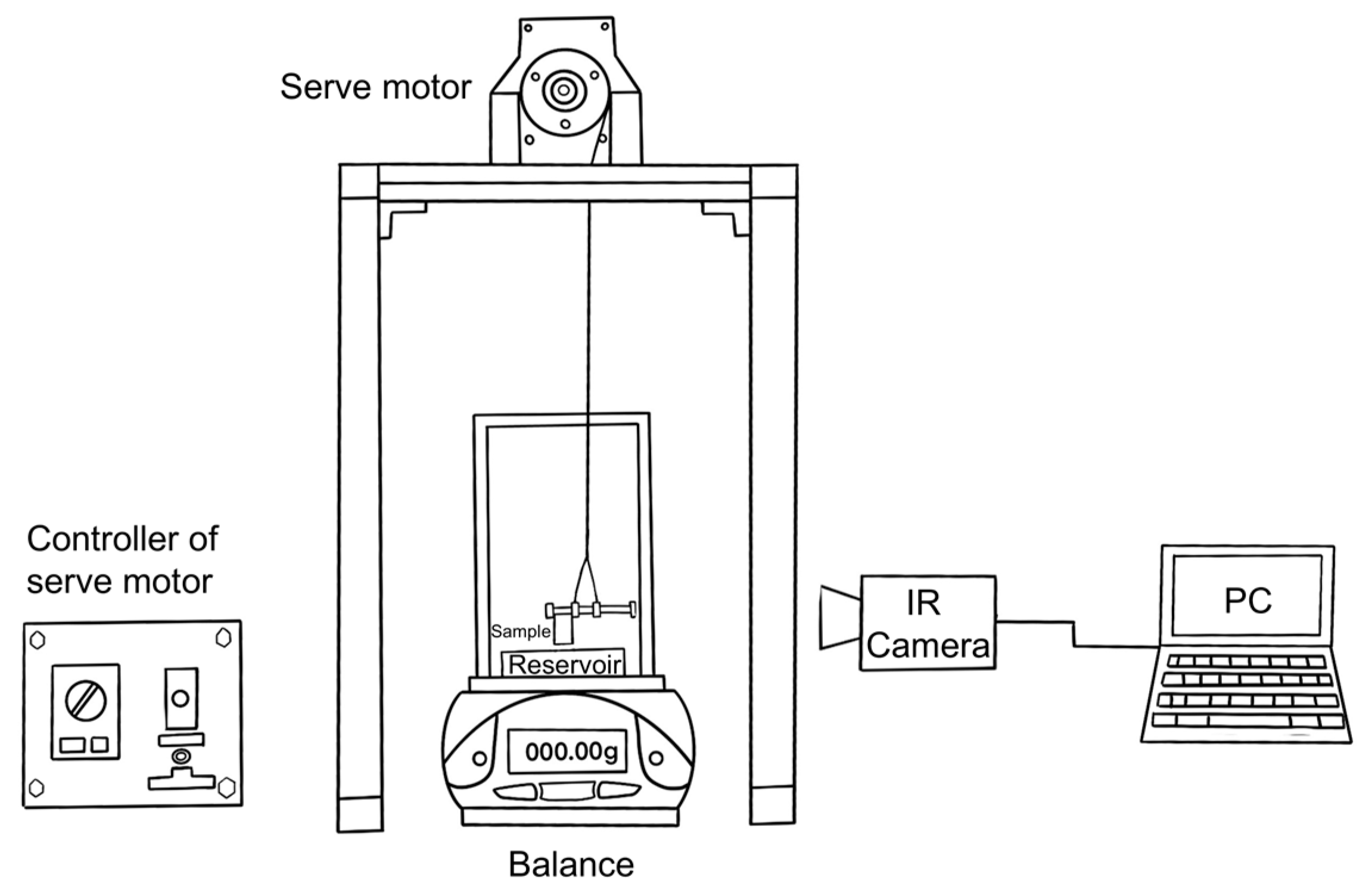
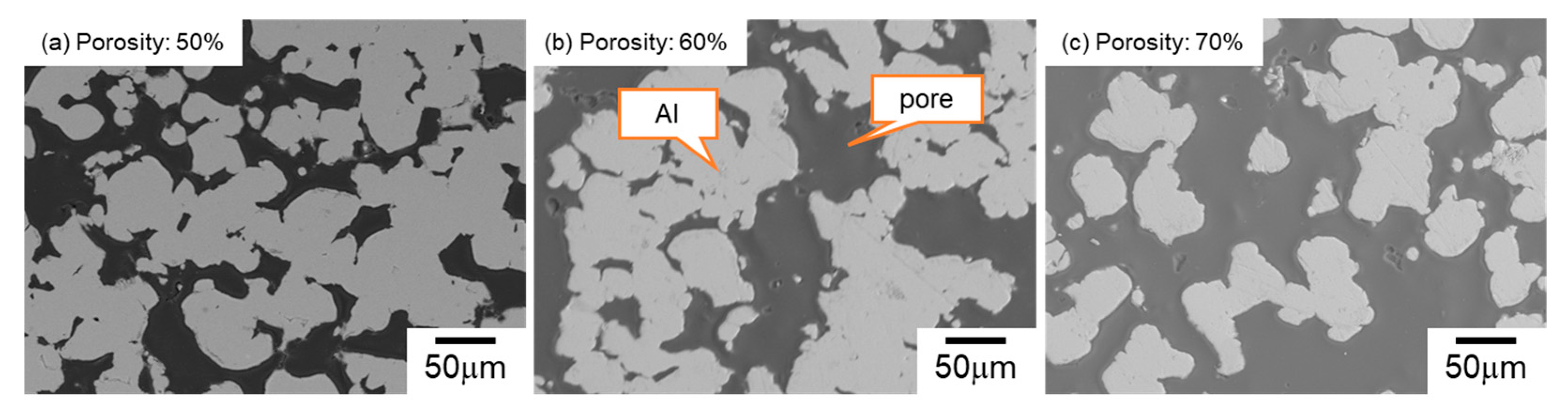
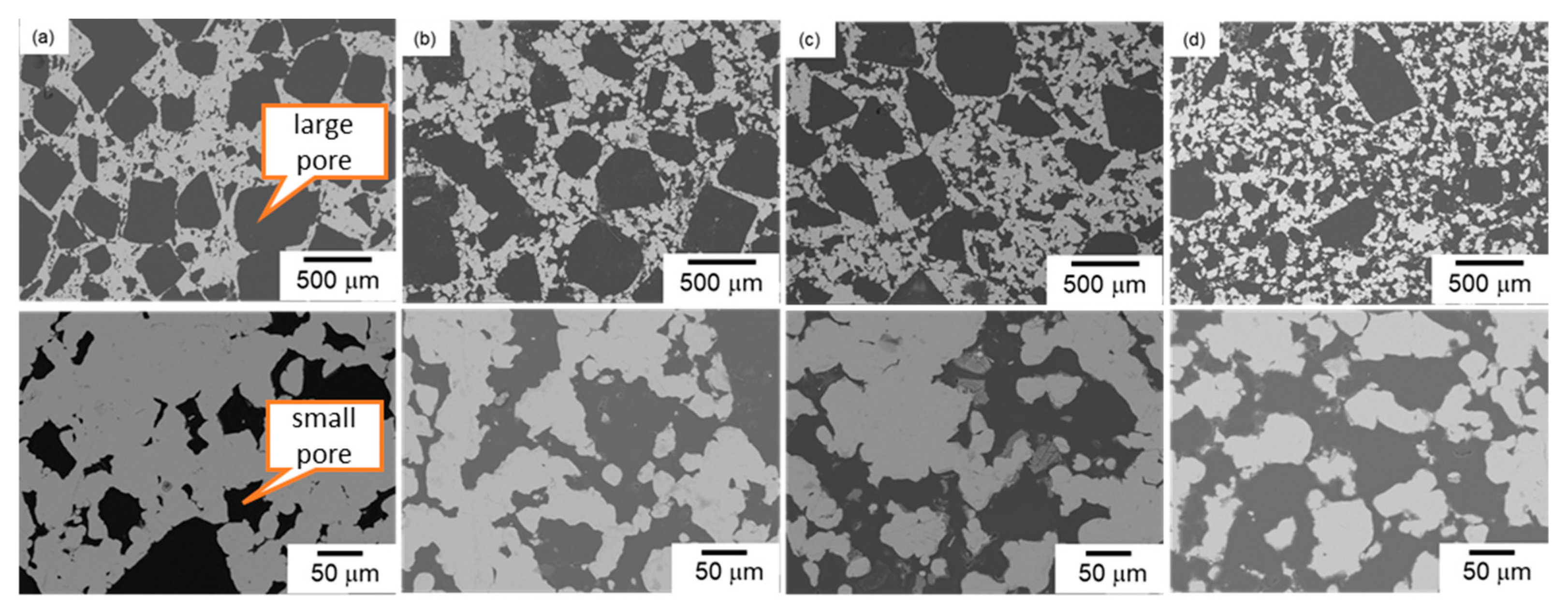


| Sample | 330~430 μm NaCl Volume Fraction (%) | 30~50 μm NaCl Volume Fraction (%) | Designed Total Porosity (%) | Measured Total Porosity (%) | |
|---|---|---|---|---|---|
| Mono-porous | S50 | 0 | 50 | 50 | 48.9 |
| S60 | 0 | 60 | 60 | 59.9 | |
| S70 | 0 | 70 | 70 | 68.7 | |
| Bi-porous | L60S10 | 60 | 10 | 70 | 70.5 |
| L50S20 | 50 | 20 | 70 | 72.3 | |
| L40S30 | 40 | 30 | 70 | 72.6 | |
| L30S40 | 30 | 40 | 70 | 72.0 |
| Sample | Average Large Pore Size (μm) | Average Small Pore Size (μm) | Permeability, | Capillary Pressure, Pcap/Pa | Capillary Factor, N |
|---|---|---|---|---|---|
| S50 | - | 35.2 | 1.1 ± 0.1 | 360 ± 38.0 | 4.0 |
| S60 | - | 53.5 | 2.5 ± 0.6 | 336 ± 13.9 | 8.4 |
| S70 | - | 148.3 | 7 ± 1.6 | 315 ± 31.6 | 22 |
| L70 | 270 | - | 10.4 ± 3.1 | 197 ± 15.4 | 20.5 |
| L60S10 | 228 | 22.1 | 9.3 ± 1.2 | 265 ± 19.6 | 24.6 |
| L50S20 | 260 | 51.1 | 8.7 ± 1.3 | 281 ± 17.8 | 24.4 |
| L40S30 | 276 | 65.7 | 8.4 ± 1.7 | 381 ± 28.0 | 32 |
| L30S40 | 274 | 96.6 | 7.4 ± 1.1 | 293 ± 12.7 | 21.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Suzuki, A.; Takata, N.; Kobashi, M. Enhancing Capillary Pressure of Porous Aluminum Wicks by Controlling Bi-Porous Structure Using Different-Sized NaCl Space Holders. Materials 2024, 17, 4729. https://doi.org/10.3390/ma17194729
Shen H, Suzuki A, Takata N, Kobashi M. Enhancing Capillary Pressure of Porous Aluminum Wicks by Controlling Bi-Porous Structure Using Different-Sized NaCl Space Holders. Materials. 2024; 17(19):4729. https://doi.org/10.3390/ma17194729
Chicago/Turabian StyleShen, Hongfei, Asuka Suzuki, Naoki Takata, and Makoto Kobashi. 2024. "Enhancing Capillary Pressure of Porous Aluminum Wicks by Controlling Bi-Porous Structure Using Different-Sized NaCl Space Holders" Materials 17, no. 19: 4729. https://doi.org/10.3390/ma17194729
APA StyleShen, H., Suzuki, A., Takata, N., & Kobashi, M. (2024). Enhancing Capillary Pressure of Porous Aluminum Wicks by Controlling Bi-Porous Structure Using Different-Sized NaCl Space Holders. Materials, 17(19), 4729. https://doi.org/10.3390/ma17194729










