A Third Generation Calphad Description of Pure Lithium
Abstract
1. Introduction
2. Literature Review
2.1. Low-Temperature Phase Transition of Li
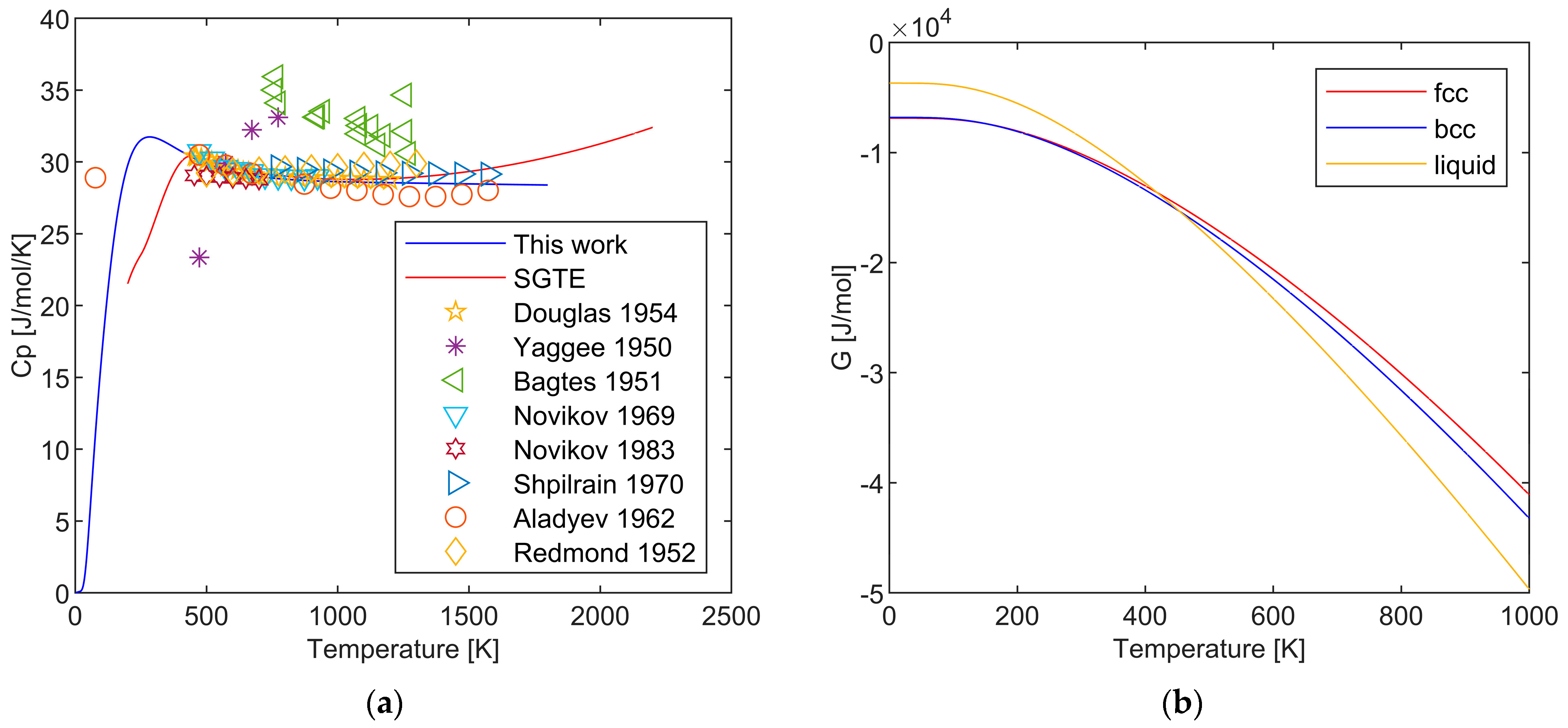
2.2. Heat Capacity of the Crystalline and Liquid Phases of Li
2.2.1. Low Temperature Data
2.2.2. Bcc and Liquid Phase
2.3. Enthalpy Data
3. Thermodynamic Model
3.1. Extended Debye Model and Extended Einstein Model
3.2. Two-State Model
4. Results
4.1. Fitting by the Extended Debye Model
4.2. Overall Description by Extended Einstein Model and Two-State Model
4.3. Extension of the SGTE Database
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinsdale, A. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Chase, M.; Ansara, I.; Dinsdale, A.; Eriksson, G.; Grimvall, G.; Hoglund, H.; Yokokawa, H. Group1: Heat capacity models for crystalline phases from 0 K to 6000 K. Calphad 1995, 19, 437–447. [Google Scholar]
- Chen, Q.; Sundman, B. Modeling of thermodynamic properties for Bcc, Fcc, liquid, and amorphous iron. J. Phase Equilibria 2001, 22, 631–644. [Google Scholar] [CrossRef]
- Roslyakova, I.; Sundman, B.; Dette, H.; Zhang, L.; Steinbach, I. Modeling of Gibbsenergies of pure elements down to 0 K using segmented regression. Calphad 2016, 55, 165–180. [Google Scholar] [CrossRef]
- Jiang, Y.; Zomorodpoosh, S.; Roslyakova, I.; Zhang, L. Thermodynamic re-assessment of binary Cr-Nb system down to 0 K. Calphad 2018, 62, 109–118. [Google Scholar] [CrossRef]
- Jiang, Y.; Zomorodpoosh, S.; Roslyakova, I.; Zhang, L. Thermodynamic re-assessment of the binary Cr–Ta system down to 0 K. Int. J. Mater. Res. 2019, 110, 797–807. [Google Scholar]
- Zhang, E.; Tang, Y.; Wen, M.; Obaied, A.; Roslyakova, I.; Zhang, L. On phase stability of Mo-Nb-Ta-W refractory high entropy alloys. Int. J. Refract. Met. Hard Mater. 2022, 103, 105780. [Google Scholar] [CrossRef]
- Obaied, A.; Bocklund, B.; Zomorodpoosh, S.; Zhang, L.; Otis, R.; Liu, Z.-K.; Roslyakova, I. Thermodynamic re-assessment of pure chromium using modified segmented regression model. Calphad 2020, 69, 101762. [Google Scholar] [CrossRef]
- Voronin, G.F.; Kutsenok, I.B. Universal Method for Approximating the Standard Thermodynamic Functions of Solids. J. Chem. Eng. Data 2013, 58, 2083–2094. [Google Scholar] [CrossRef]
- Khvan, A.; Uspenskaya, I.; Aristova, N.; Chen, Q.; Trimarchi, G.; Konstantinova, N.; Dinsdale, A. Description of the thermodynamic properties of pure gold in the solid and liquid states from 0 K. Calphad 2019, 68, 101724. [Google Scholar] [CrossRef]
- Khvan, A.; Dinsdale, A.; Uspenskaya, I.; Zhilin, M.; Babkina, T.; Phiri, A. A thermodynamic description of data for pure Pb from 0 K using the expanded Einstein model for the solid and the two state model for the liquid phase. Calphad 2018, 60, 144–155. [Google Scholar] [CrossRef]
- Khvan, A.V.; Uspenskaya, I.A.; Aristova, N.M. Critical assessment of the data for Pure Cu from 0 K, using two-state model for the description of the liquid phase. Calphad 2024, 84, 102637. [Google Scholar] [CrossRef]
- He, Z.; Selleby, M. A third generation Calphad description of pure W. Mater. Chem. Phys. 2022, 276, 125445. [Google Scholar] [CrossRef]
- He, Z.; Selleby, M. A third generation Calphad description of W–C including a revision of liquid C. Calphad 2022, 78, 102499. [Google Scholar] [CrossRef]
- He, Z.; Kaplan, B.; Mao, H.; Selleby, M. The third generation Calphad description of Al–C including revisions of pure Al and C. Calphad 2021, 72, 102250. [Google Scholar] [CrossRef]
- Vřešt’áL, J.; Štrof, J.; Pavlů, J. Extension of SGTE data for pure elements to zero Kelvin temperature—A case study. Calphad 2012, 37, 37–48. [Google Scholar] [CrossRef]
- Barrett, C.S. A Low Temperature Transformation in Lithium. Phys. Rev. B 1947, 72, 245. [Google Scholar] [CrossRef]
- Barrett, C.S. X-ray study of the alkali metals at low temperatures. Acta Crystallogr. 1956, 9, 671–677. [Google Scholar] [CrossRef]
- Overhauser, A.W. Crystal structure of lithium at 4.2 K. Phys. Rev. Lett. 1984, 53, 64. [Google Scholar] [CrossRef]
- Harris, S.W.; Hartmann, O.; Hempelmann, R. Muon spin relaxation investigation of the 9R-related phase change in lithium and sodium. J. Phys. Condens. Matter 1991, 3, 5665–5670. [Google Scholar] [CrossRef]
- Staikov, P.; Kara, A.; Rahman, T.S. First-principles studies of the thermodynamic properties of bulk Li. J. Phys. Condens. Matter 1997, 9, 2135–2148. [Google Scholar] [CrossRef]
- Schwarz, W.; Blaschko, O.; Gorgas, I. bcc instability of lithium at low temperatures. Phys. Rev. B 1991, 44, 6785–6790. [Google Scholar] [CrossRef]
- Pichl, W.; Krystian, M.; Prem, M.; Krexner, G. The martensite phase of high-purity lithium. J. Phys. IV 2003, 112, 1095–1098. [Google Scholar] [CrossRef]
- Ackland, G.J.; Dunuwille, M.; Martinez-Canales, M.; Loa, I.; Zhang, R.; Sinogeikin, S.; Cai, W.; Deemyad, S. Quantum and isotope effects in lithium metal. Science 2017, 356, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.H.; Jacobson, R.; Munson, T.R.; Wagman, D.D. Thermodynamic properties of the alkali metals. J. Res. Natl. Bur. Stand. 1955, 55, 83. [Google Scholar] [CrossRef]
- Shpil, E.E.; Totskyi, K.A.Y.E.E.; Timrot, D.L.; Fomin, V.A. Thermophysical Properties of Alkali Metals; Izd-vo Standartov: Moscow, Russia, 1970. [Google Scholar]
- Glushko, V.P.; Medvedev, V.A. Thermal Constants of Substances; VINITI: Moscow, Russia, 1981. [Google Scholar]
- Hultgren, R.; Desai, P.D.; GJeiser, D.T.H.M.; Kelley, K.K.; Wagman, D.D. Selected Values of the Thermodynamic Properties of the Elements; American Society for Metals: Metals Park, OH, USA, 1973. [Google Scholar]
- Chase, M.W. NIST-JANAF Thermochemical Tables for Oxygen Fluorides. J. Phys. Chem. Ref. Data 1996, 25, 551–603. [Google Scholar] [CrossRef]
- Fink, J.K.; Leibowitz, L. Handbook of Thermodynamic and Transport Properties of Alkali Metals; Ohse, R.W., Ed.; Blackwell Scientific Publications: Oxford, UK, 1985; pp. 411–434. [Google Scholar]
- Cox, J.D.; Wagman, D.D.; Medvedev, V.A. CODATA Key Values for Thermodynamics; Hemisphere Publishing Corporation: New York, NY, USA, 1998; p. 251. [Google Scholar]
- Gurvich, L.V.; Veits, I.V.; Medvedev, V.A.; Glushko, V.P. (Eds.) Thermodynamic Properties of Individual Substances; Nauka: Moscow, Russia, 1982; Volume 4, pp. 244–247. [Google Scholar]
- Alcock, C.B.; Chase, M.W.; Itkin, V.P. Thermodynamic Properties of the Group IA Elements. J. Phys. Chem. Ref. Data 1994, 23, 385–497. [Google Scholar] [CrossRef][Green Version]
- Martin, D.L. The Specific Heat of Litium from 20 to 300 K: The martensitic transformation. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1960, 254, 444–454. [Google Scholar] [CrossRef]
- Douglas, T.B.; Dever, J.L.; Epstein, L.F.; Howland, W.H. Lithium: Heat Content from 0 to 900°, Triple Point and Heat of Fusion, and Thermodynamic Properties of the Solid and Liquid1. J. Am. Chem. Soc. 1955, 77, 2144–2150. [Google Scholar] [CrossRef]
- Martin, D.L. Specific heats of lithium isotopes from 20° to 300 °K. Physica 1959, 25, 1193–1199. [Google Scholar] [CrossRef]
- Martin, D.L. A modified continuous-heating calorimeter for the temperature range 15 to 300 K. Can. J. Phys. 1962, 40, 1166–1173. [Google Scholar] [CrossRef]
- Simon, F.; Swain, R.C. Untersuchungen über die spezifischen Wärmen bei tiefen Temperaturen. Z. Phys. Chem. 1935, 28B, 189–198. [Google Scholar] [CrossRef]
- Martin, D.L. The electronic specific heat of lithium isotopes. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1961, 263, 378–386. [Google Scholar] [CrossRef]
- Martin, B.D.; Zych, D.A.; Heer, C.V. Atomic Heats of Cesium, Rubidium, and Lithium Between 0.35 and 2 °K. Phys. Rev. B 1964, 135, A671–A679. [Google Scholar] [CrossRef]
- Roberts, L.M. The atomic heats of lithium, sodium and potassium between 1.5 and 20 K. Proc. Roy. Soc. B 1957, 70, 744. [Google Scholar] [CrossRef]
- Filby, J.D.; Martin, D.L. The specific heats below 30 °K of lithium metal of various isotopic compositions and of sodium metal. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1963, 276, 187–203. [Google Scholar] [CrossRef]
- Bates, A.G.; Smith, D.J. Specific Heat and Enthalpy of Liquid Lithium in the Range of 500 °C to 1000 °C; Carbide and Carbon Chemicals Division, Union Carbine and Carbon Corporation: Houston, TX, USA, 1951. [Google Scholar] [CrossRef]
- Yaggee, F.L.; Untermyer, S. The Relative Thermal Conductivities of Liquid Lithium, Sodium, and Eutectic NaK, and the Specific Heat of Liquid Lithium; University of Chicago: Chicago, IL, USA, 1950. [Google Scholar] [CrossRef]
- Cabbage, A.M. Enthalpy, Mean Heat Capacity, and Absolute Heat Capacity of Sold and Liquid Lithium; USAEC Report AECD-3240; NEPA Division: Lackland, TX, USA, 1950. [Google Scholar]
- Novikov, I.I.; Gruzdev, V.A.; Odintsov, O.A.K.A.A.; Roschupkin, V.V. Thermophysical properties of liquid alkali metals at high temperatures. High Temp. 1969, 7, 65–68. [Google Scholar]
- Novikov, I.I.; Roschupkin, V.V.; Fordeyeva, L.C. Solid and liquid lithium enthalpy: Experimental investigation near the melting point. Int. J. Thermophys. 1983, 4, 227–233. [Google Scholar] [CrossRef]
- Pchelkin, I.M. I. T. Aladyev, Supervisor. Ph.D. Thesis, ENIN, Moscow, Russia, 1962. [Google Scholar]
- Redmond, R.F.; Lones, J. Enthalpies and Heat Capacities of Stainless Steel (316), Zirconium, and Lithium at Elevated Temperatures; USAEC Rept., ORNL-1342; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 1952. [Google Scholar] [CrossRef]
- Cristescu, S.; Simon, F. Die spezifischen Wärmen von Beryllium, Germanium und Hafnium bei tiefen Temperaturen. Z. Phys. Chem. Int. J. Res. Phys. Chem. Chem. Phys. 1934, 25B, 273–282. [Google Scholar] [CrossRef]
- Dauphinee, T.M.; Macdonald, D.K.C.; Preston-Thomas, H. A new semi-automatic apparatus for measurement of specific heats and the specific heat of sodium between 55 and 315 K. Proc. R. Soc. Lond. A 1954, 221, 267–276. [Google Scholar] [CrossRef]
- Schneider, A.; Hilmer, O. Wärmeinhalte und Schmelzentropien von NaTl-Phasen. Anorg. Allgem. Chem. 1956, 286, 97–117. [Google Scholar] [CrossRef]
- Achener, P.Y.; Fisher, D.L. ALKALI METALS EVALUATION PROGRAM. THE SPECIFIC HEAT OF LIQUID SODIUM AND LITHIUM. Report AGN-8191; U.S. Aerojet General Corp: San Ramon, CA, USA, 1967; Volume 6. [Google Scholar]
- Barron, T.H.K.; Morrison, J.A. On the specific heat of solids at low temperatures. Can. J. Phys. 1957, 35, 799–810. [Google Scholar] [CrossRef]
- Becker, C.A.; Ågren, J.; Baricco, M.; Chen, Q.; Decterov, S.A.; Kattner, U.R.; Perepezko, J.H.; Pottlacher, G.R.; Selleby, M. Thermodynamic modelling of liquids: CALPHAD approaches and contributions from statistical physics. Phys. Status Solidi (b) 2013, 251, 33–52. [Google Scholar] [CrossRef]
- Agren, J. Thermodynamics of Supercooled Liquids and their Glass Transition. Phys. Chem. Liq. 1988, 18, 123–139. [Google Scholar] [CrossRef]

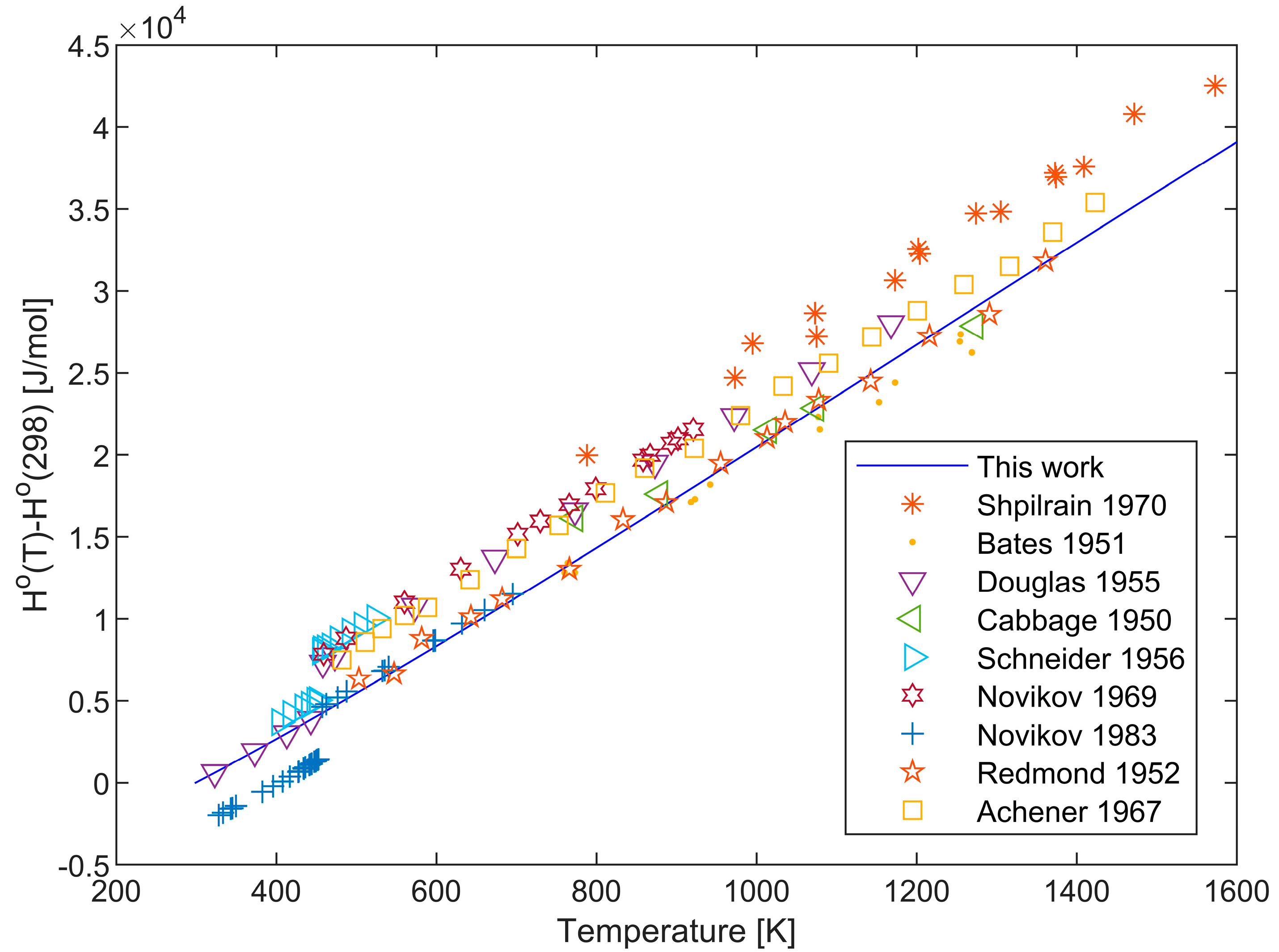



| Temperature Range, K | Relative Uncertainty, % | Purity as Reported in the Original Publications, % | Method | Ref. |
|---|---|---|---|---|
| 20–300 | 0.2 above 80 K, 2% below 20 K | 99.93 6Li, 99.99 7Li | Adiabatic calorimetry | D. L. Martin [36] 1959 |
| 20–300 | 0.2 above 80 K, 2% below 20 K | b Natural Li (99.95%) | Adiabatic calorimetry | D. L. Martin [34] 1960 |
| 100–300 | Reported to be 0.1 | b Natural Li (99.95%) | Continuous-heating method of calorimetry | D. L. Martin [37] 1962 |
| 15–300 | a 4 | n/a | Calorimetry method | Simon [38] |
| 0.4–1.5 | Reported to be 5 | b Natural Li (99.95% and 99.3%) | Adiabatic calorimetry | D. L. Martin [39] 1961 |
| 0.35–2 | n/a | 99.999 | Low-temperature calorimetry | B. D. Martin [40] |
| 1.5–20 | Reported to be 2 | 99. 5 | Calorimetry method | Roberts [41] |
| 3–30 | Reported to be 0. 5 | Natural Li b (99.95%) | Adiabatic calorimetry | Filby [42] |
| 298.16–1200 | Reported to be 5 | 99.9 0. 028%O, 0.003%N, 0.0036%Fe, 0.0006%Ni, 0. 029%Ca, 0.016%Na | Bunsen ice calorimetry and a drop method | Douglas [35] |
| 760–1269 | Reported to be 5 | 98.5 | Bunsen ice calorimetry | Bates [43] |
| 473–733 | Reported to be 10 | A commercial grade manufactured by the Maywood Chemical Company, Maywood, NJ, USA | Comparing the cooling rates in air of thin-wall stainless steel capsules | Yaggee [44] |
| 378–1276 773–1273 | n/a | 99 0.37% Li2O, 0.92% LiN | The drop method | Cabbage [45] |
| 473–923 | Reported to be 0.3 | 99.33 0.38% Na, 0.14% Mg, 0.01% K and Al, 0.001% Fe, 0.003% Ca, 0.005% heavy metals, 0.012%N | Ice drop calorimetry | Novikov [46] 1969 |
| 350–750 | 0.6 | 99.5 0.06%Na, 0.005%K, 0.02%Mg, 0.03%Ca, 0.001%Mn, 0.005%Fe, 0.003%Al, 0.01%SiO2, 0.05%N (nitrides) | The drop method | Novikov [47] 1983 |
| 473–1573 | Reported to be 8 | 99.5 0.5% Na and 0.02% K | Isothermal calorimetry | Aladyev [48] |
| 500–1300 | Reported to be 5 | n/a | Bunsen ice calorimetry | Redmond [49] |
| 773–1573 | Reported to be 2 | 99 0.26%Na, 0.001%K, 0.003%Ca, 0.0072 N, other impurities < 0.015% | Boiling-point calorimetry | Shpil’rain [26] |
| Ref. | Cp(298.15) [J/mol/K] | So(298.15) [J/mol/K] | Ho(298.15)-Ho(0) [J/mol] | ∆fusH [J/mol] | Annotation |
|---|---|---|---|---|---|
| This work | 25.44 | 27.46 | 4518.8 | 3003 | Extended Debye model |
| 24.44 | 28.81 | 4568.9 | - | Extended Einstein model | |
| SGTE [1] | 24.79 | 29.12 | 4632 | 2999.93 | |
| Hultgren [28] | 26.148 | 29.275 | 1106 (cal/mol) | 710 ± 10 (cal/mol) | |
| Chase [29] | 24.623 | 29.085 | - | 3000 ± 15 | |
| Alcock [33] | 24.78 ± 0.1 | 28.99 ± 0.3 | 4671 ± 30 | 3000 ± 30 |
| Phase | Expression of Cp | a | b | c | ΘD |
|---|---|---|---|---|---|
| fcc | 0.0016 | −9.9996 × 10−7 | 2.2225 × 10−14 | 380 | |
| bcc | 9.4405 × 10−6 | 6.9752 × 10−6 | 9.3676 × 10−11 | 375 |
| Phase | |
|---|---|
| fcc | ΘE = 320 K |
| bcc | ΘE = 330 K |
| Liquid | ΘE = 259.9 K |
| Phase | |
|---|---|
| fcc | ΘE = 275 K |
| bcc | ΘE = 291.1 K |
| Liquid | ΘE = 204 K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Li, X.; Ou, M.; Ma, J. A Third Generation Calphad Description of Pure Lithium. Materials 2024, 17, 4750. https://doi.org/10.3390/ma17194750
Xu W, Li X, Ou M, Ma J. A Third Generation Calphad Description of Pure Lithium. Materials. 2024; 17(19):4750. https://doi.org/10.3390/ma17194750
Chicago/Turabian StyleXu, Wenjun, Xiaobo Li, Mingyu Ou, and Jinning Ma. 2024. "A Third Generation Calphad Description of Pure Lithium" Materials 17, no. 19: 4750. https://doi.org/10.3390/ma17194750
APA StyleXu, W., Li, X., Ou, M., & Ma, J. (2024). A Third Generation Calphad Description of Pure Lithium. Materials, 17(19), 4750. https://doi.org/10.3390/ma17194750





