Metal–Organic Skeleton-Derived W-Doped Ga2O3-NC Catalysts for Aerobic Oxidative Dehydrogenation of N-Heterocycles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalytic Reaction
2.4. Characterization of Catalysts
3. Results and Discussion
3.1. Characterization
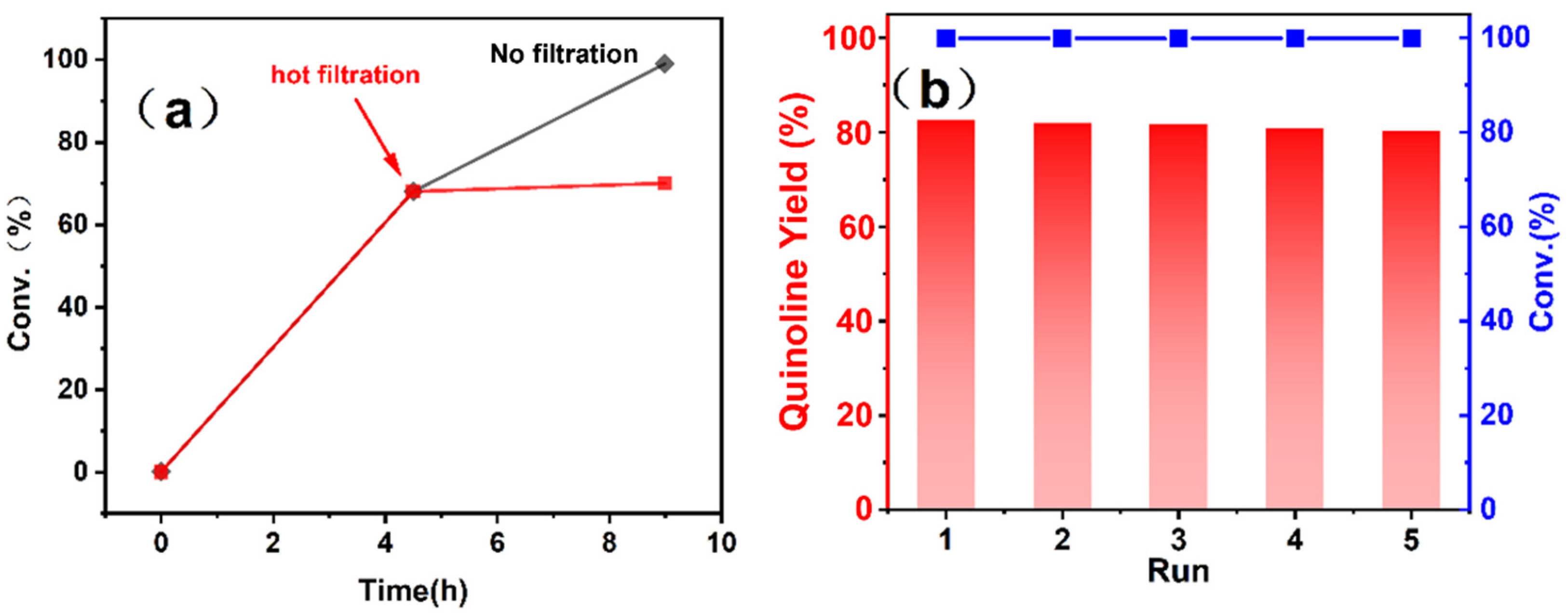
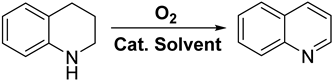
3.2. Catalytic Performance
3.3. Substrate Expansion
3.4. Catalytic Mechanism of THQ Dehydrogenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.-H.; Sun, Y.-C.; Wang, K.; Wang, Z.-Y.; Guo, P.-P.; Jiang, C.-S.; Yao, M.-Q.; Li, Z.-H.; Liu, Z.-T. Reversible aerobic oxidative dehydrogenation/hydrogenation of N-heterocycles over AlN supported redox cobalt catalysts. Mol. Catal. 2020, 496, 111192. [Google Scholar] [CrossRef]
- Jaiswal, G.; Subaramanian, M.; Sahoo, M.K.; Balaraman, E. A reusable cobalt catalyst for reversible acceptorless dehydrogenation and hydrogenation of N-heterocycles. ChemCatChem 2019, 11, 2449–2457. [Google Scholar] [CrossRef]
- Li, J.; Liu, G.; Long, X.; Gao, G.; Wu, J.; Li, F. Different active sites in a bifunctional Co@ N-doped graphene shells based catalyst for the oxidative dehydrogenation and hydrogenation reactions. J. Catal. 2017, 355, 53–62. [Google Scholar] [CrossRef]
- Cui, X.; Huang, Z.; Muyden, A.P.V.; Fei, Z.; Wang, T.; Dyson, P.J. Acceptorless dehydrogenation and hydrogenation of N- and O-containing compounds on Pd3Au1(111) facets. Sci. Adv. 2020, 6, eabb3831. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Hu, L.; Wang, J.; Li, X.; Qi, F.; Lu, J.; Cao, X.; Gu, H. Reversible hydrogenation-oxidative dehydrogenation of quinolines over a highly active Pt nanowire catalyst under mild conditions. ChemCatChem 2013, 5, 2183–2186. [Google Scholar] [CrossRef]
- Moromi, S.K.; Siddiki, S.M.A.H.; Kon, K.; Toyao, T.; Shimizu, K.-I. Acceptorless dehydrogenation of N-heterocycles by supported Pt catalysts. Catal. Today 2017, 281, 507–511. [Google Scholar] [CrossRef]
- Wang, Q.; Chai, H.; Yu, Z. Acceptorless dehydrogenation of N-heterocycles and secondary alcohols by Ru(II)-NNC complexes bearing a pyrazoyl-indolyl-pyridine ligand. Organometallics 2018, 37, 584–591. [Google Scholar] [CrossRef]
- Kim, S.; Loose, F.; Bezdek, M.J.; Wang, X.; Chirik, P.J. Hydrogenation of N-heteroarenes using rhodium precatalysts: Reductive elimination leads to formation of multimetallic clusters. J. Am. Chem. Soc. 2019, 141, 17900–17908. [Google Scholar] [CrossRef]
- Wu, J.; Barnard, J.H.; Zhang, Y.; Talwar, D.; Robertson, C.M.; Xiao, J. Robust cyclometallated Ir(III) catalysts for the homogeneous hydrogenation of N-heterocycles under mild conditions. Chem. Commun. 2013, 49, 7052–7054. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Ahn, J.; Shi, S.; Wang, P.; Gao, R.; Qin, D. Noble-metal nanoframes and their catalytic applications. Chem. Rev. 2021, 121, 796–833. [Google Scholar] [CrossRef]
- Ullah, S.; Shaban, M.; Siddique, A.B.; Zulfiqar, A.; Lali, N.S.; Naeem-ul-Hassan, M.; Irfan, M.I.; Sher, M.; Rehman, M.F.U.; Hanbashi, A.; et al. Greenly synthesized zinc oxide nanoparticles: An efficient, cost-effective catalyst for dehydrogenation of formic acid and with improved antioxidant and phyto-toxic properties. J. Environ. Chem. Eng. 2024, 12, 113350. [Google Scholar] [CrossRef]
- Cui, X.; Li, Y.; Bachmann, S.; Scalone, M.; Surkus, A.-E.; Junge, K.; Topf, C.; Beller, M. Synthesis and characterization of iron–nitrogen-doped graphene/core–shell catalysts: Efficient oxidative dehydrogenation of N-heterocycles. J. Am. Chem. Soc. 2015, 137, 10652–10658. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, X.; Yao, K.; Yuan, Z.; Chi, Q.; Zhang, Z. Efficient oxidative dehydrogenation of N-heterocycles over Nitrogen-doped carbon-supported cobalt nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 13646–13654. [Google Scholar] [CrossRef]
- Tan, K.C.; He, T.; Chua, Y.S.; Chen, P. Recent advances of catalysis in the hydrogenation and dehydrogenation of N-heterocycles for hydrogen storage. J. Phy. Chem. C 2021, 125, 18553–18566. [Google Scholar] [CrossRef]
- Iosub, A.V.; Stahl, S.S. Catalytic aerobic dehydrogenation of nitrogen heterocycles using heterogeneous cobalt oxide supported on nitrogen-doped carbon. Org. Let. 2015, 17, 4404–4407. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Li, Y.; Pan, Y.; Behera, N.; Jin, W. Metal-organic framework nanosheets: An emerging family of multifunctional 2D materials. Coordin. Chem. Rev. 2019, 395, 25–45. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging multifunctional metal–organic framework materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Searles, K.; Chan, K.W.; Mendes Burak, J.A.; Zemlyanov, D.; Safonova, O.; Copéret, C. Highly productive propane dehydrogenation catalyst using silica-supported Ga–Pt nanoparticles generated from single-sites. J. Am. Chem. Soc. 2018, 140, 11674–11679. [Google Scholar] [CrossRef] [PubMed]
- Sattler, J.J.; Gonzalez-Jimenez, I.D.; Luo, L.; Stears, B.A.; Malek, A.; Barton, D.G.; Kilos, B.A.; Kaminsky, M.P.; Verhoeven, T.W.; Koers, E.J. Platinum-promoted Ga/Al2O3 as highly active, selective, and stable catalyst for the dehydrogenation of propane. Angew. Chem. Int. Ed. 2014, 126, 9405–9410. [Google Scholar] [CrossRef]
- Dizaji, A.K.; Mokhtarani, B.; Mortaheb, H.R. Deep and fast oxidative desulfurization of fuels using graphene oxide-based phosphotungstic acid catalysts. Fuel 2019, 236, 717–729. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Zhou, Y.; Wang, Z.; Du, M.; Wen, Z.; Yan, B.; Ma, Q.; Liu, N.; Xue, B. Phosphotungstic acid supported on Zr-SBA-15 as an efficient catalyst for one-pot conversion of furfural to γ-valerolactone. Fuel 2024, 356, 129631. [Google Scholar] [CrossRef]
- Zhai, S.; Lu, Z.; Ai, Y.; Jia, X.; Yang, Y.; Liu, X.; Tian, M.; Bian, X.; Lin, J.; He, S. High performance nanocomposite proton exchange membranes based on the nanohybrids formed by chemically bonding phosphotungstic acid with covalent organic frameworks. J. Power Sources 2023, 554, 232332. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L. Mesoporous silica supported phosphotungstic acid catalyst for glycerol dehydration to acrolein. Catal. Today 2021, 376, 55–64. [Google Scholar] [CrossRef]
- Yan, X.-M.; Mei, P.; Lei, J.; Mi, Y.; Xiong, L.; Guo, L. Synthesis and characterization of mesoporous phosphotungstic acid/TiO2 nanocomposite as a novel oxidative desulfurization catalyst. J. Mol. Catal. A Chem. 2009, 304, 52–57. [Google Scholar] [CrossRef]
- Shahzad, R.; Muneer, M.; Khalid, R.; Amin, H.M.A. ZnO-Bi2O3 heterostructured composite for the photocatalytic degradation of orange 16 reactive dye: Synergistic effect of UV irradiation and hydrogen peroxide. Catalysts 2023, 13, 1328. [Google Scholar] [CrossRef]
- Zan, L.; Amin, H.M.A.; Mostafa, E.; Abd-El-Latif, A.A.; Iqbal, S.; Baltruschat, H. Electrodeposited cobalt nanosheets on smooth silver as a bifunctional catalyst for OER and ORR: In situ structural and catalytic characterization. ACS Appl. Mater. Interfaces 2022, 14, 55458–55470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, P. Role of graphitic carbon in g-C3N4 nanoarchitectonics towards efficient photocatalytic reaction kinetics: A review. Carbon 2023, 216, 118584. [Google Scholar] [CrossRef]
- Pustovarenko, A.; Goesten, M.G.; Sachdeva, S.; Shan, M.; Amghouz, Z.; Belmabkhout, Y.; Dikhtiarenko, A.; Rodenas, T.; Keskin, D.; Voets, I.K.; et al. Nanosheets of nonlayered aluminum metal–organic frameworks through a surfactant-assisted method. Adv. Mater. 2018, 30, 1707234. [Google Scholar] [CrossRef]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, Y.; Lian, H.; Wang, Y.; Xue, X.; Zhang, G.; Zhang, Y. Enhancement of CH4/N2 separation capacity of coal-based porous carbons via hydrothermal coupled KOH activation. J. Environ. Chem. Eng. 2024, 12, 112477. [Google Scholar] [CrossRef]
- Baldovino-Medrano, V.G.; Niño-Celis, V.; Isaacs Giraldo, R. Systematic analysis of the nitrogen adsorption–desorption isotherms recorded for a series of materials based on microporous–mesoporous amorphous aluminosilicates using classical methods. J. Chem. Eng. Data 2023, 68, 2512–2528. [Google Scholar] [CrossRef]
- Liang, K.; Wu, T.; Misra, S.; Dun, C.; Husmann, S.; Prenger, K.; Urban, J.J.; Presser, V.; Unocic, R.R.; Jiang, D.E. Nitrogen-doped graphene-like carbon intercalated MXene heterostructure electrodes for enhanced sodium-and lithium-ion storage. Adv. Sci. 2024, 11, 2402708. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.; Li, N.; Jiang, B.; Tu, R.; Wu, T.; Guan, P.; Ye, Y.; Cheong, W.-C.; Sun, K.; Liu, S.; et al. Rationally engineered Co and N co-doped WS2 as bifunctional catalysts for pH-universal hydrogen evolution and oxidative dehydrogenation reactions. Nano Res. 2022, 15, 1993–2002. [Google Scholar] [CrossRef]
- Huang, K.; Wang, J.; Zhang, Q.; Yuan, K.; Yang, Q.; Li, F.; Sun, X.; Chang, H.; Liang, Y.; Zhao, J.; et al. Sub 150 nm nanoscale Gallium based metal-organic frameworks armored antibiotics as super penetrating bombs for eradicating persistent bacteria. Adv. Fun. Mater. 2022, 32, 2204906. [Google Scholar] [CrossRef]
- Wang, K.; Ye, W.; Yin, W.; Chai, W.; Tang, B.; Rui, Y. One-step synthesis of MOF-derived Ga/Ga2O3@C dodecahedra as an anode material for high-performance lithium-ion batteries. Dalton Tran. 2019, 48, 12386–12390. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Meng, J.; Chen, J.; Wu, R.; Zhang, L.; Jiang, J.; Deng, J.; Yin, Z.; Zhang, X. Enhanced electrical conductivity and reduced work function of β-Ga2O3 thin films by hydrogen plasma treatment. J. Alloy. Compound. 2024, 974, 172946. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Hou, Q.; Xu, E.; Wang, L.; Li, F.; Wei, M. Metal-acid bifunctional catalysts toward tandem reaction: One-step hydroalkylation of benzene to cyclohexylbenzene. ACS Appl. Mater. Inter. 2022, 14, 31998–32008. [Google Scholar] [CrossRef] [PubMed]
- Naveen, K.; Mahvelati-Shamsabadi, T.; Sharma, P.; Lee, S.; Hur, S.H.; Choi, W.M.; Shin, T.J.; Chung, J.S. MOF-derived Co/Zn single-atom catalysts for reversible hydrogenation and dehydrogenation of quinoline hydrogen carrier. Appl. Catal. B Environ. 2023, 328, 122482. [Google Scholar] [CrossRef]
- Su, H.; Sun, L.-H.; Xue, Z.-H.; Gao, P.; Zhang, S.-N.; Zhai, G.-Y.; Zhang, Y.-M.; Lin, Y.-X.; Li, X.-H.; Chen, J.-S. Nitrogen-thermal modification of the bifunctional interfaces of transition metal/carbon dyads for the reversible hydrogenation and dehydrogenation of heteroarenes. Chem. Commun. 2019, 55, 11394–11397. [Google Scholar] [CrossRef]
- Xu, D.; Liu, R.; Li, J.; Zhao, H.; Ma, J.; Dong, Z. Atomically dispersed Co-N4 sites anchored on N-doped carbon for aqueous phase transfer hydrogenation between nitroarenes and saturated N-heterocycles. Appl. Catal. B Environ. 2021, 299, 120681. [Google Scholar] [CrossRef]
- Tang, F.; Zhang, G.; Wangd, L.; Huang, J.; Liu, Y.-N. Unsymmetrically N, S-coordinated single-atom cobalt with electron redistribution for catalytic hydrogenation of quinolines. J. Catal. 2022, 414, 101–108. [Google Scholar] [CrossRef]
- Sun, X.; Olivos-Suarez, A.I.; Osadchii, D.; Romero, M.J.V.; Kapteijn, F.; Gascon, J. Single cobalt sites in mesoporous N-doped carbon matrix for selective catalytic hydrogenation of nitroarenes. J. Catal. 2018, 357, 20–28. [Google Scholar] [CrossRef]





 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Conv. b (%) | Sel. b (%) | Yield (%) |
| 1 | H3O40PW12-xH2O | 54 | 91 | 49 |
| 2 | Ga2O3-NC | 48 | 85 | 41 |
| 3 | W/Ga2O3-NC (130-600) | 98 | 80 | 78 |
| 4 | W/Ga2O3-NC c | 99 | 89 | 88 |
| 5 | W/Ga2O3-NC (170-600) | 94 | 71 | 67 |
| 6 | W/Ga2O3-NC (150-500) | 97 | 60 | 58 |
| 7 | W/Ga2O3-NC (150-700) | 98 | 79 | 77 |
| 8 | W/Ga2O3-NC (150-800) | 70 | 70 | 49 |
| 9 c | W/Ga2O3-NC-1 | 83 | 82 | 68 |
| 10 c | W/Ga2O3-NC-2 | 88 | 80 | 70 |
| 11 c | W/Ga2O3-NC-3 | 97 | 79 | 77 |
| 12 c | W/Ga2O3-NC-4 | 93 | 73 | 68 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Substrates | Products | T (h) | Conv. b (%) | Sel. b (%) | Yield b (%) |
| 1 |  |  | 12 | >99 | 91 | 91 |
| 2 | 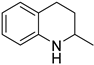 |  | 12 | >99 | 58 | 58 |
| 3 | 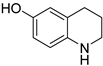 |  | 15 | >99 | 65 | 65 |
| 4 | 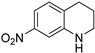 | 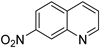 | 15 | 87 | >99 | 87 |
| 5 | 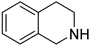 |  | 12 | 85 | >99 | 85 |
| 6 | 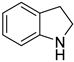 | 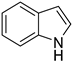 | 12 | >99 | 100 | >99 |
| 7 | 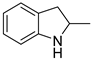 |  | 12 | 51 | 100 | 51 |
| 8 |  | 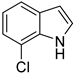 | 12 | 89 | 100 | 89 |
| 9 | 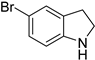 |  | 12 | 23 | 100 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Zhang, Q.; Zhang, F.; Luo, X.; Wang, W. Metal–Organic Skeleton-Derived W-Doped Ga2O3-NC Catalysts for Aerobic Oxidative Dehydrogenation of N-Heterocycles. Materials 2024, 17, 4804. https://doi.org/10.3390/ma17194804
Zhang F, Zhang Q, Zhang F, Luo X, Wang W. Metal–Organic Skeleton-Derived W-Doped Ga2O3-NC Catalysts for Aerobic Oxidative Dehydrogenation of N-Heterocycles. Materials. 2024; 17(19):4804. https://doi.org/10.3390/ma17194804
Chicago/Turabian StyleZhang, Fan, Qiwen Zhang, Feng Zhang, Xiaolin Luo, and Wei Wang. 2024. "Metal–Organic Skeleton-Derived W-Doped Ga2O3-NC Catalysts for Aerobic Oxidative Dehydrogenation of N-Heterocycles" Materials 17, no. 19: 4804. https://doi.org/10.3390/ma17194804
APA StyleZhang, F., Zhang, Q., Zhang, F., Luo, X., & Wang, W. (2024). Metal–Organic Skeleton-Derived W-Doped Ga2O3-NC Catalysts for Aerobic Oxidative Dehydrogenation of N-Heterocycles. Materials, 17(19), 4804. https://doi.org/10.3390/ma17194804





