Preparation and Electrothermal Transport Behavior of Sn8[(Ga2Te3)34(SnTe)66]92 Bulk Glass
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussions
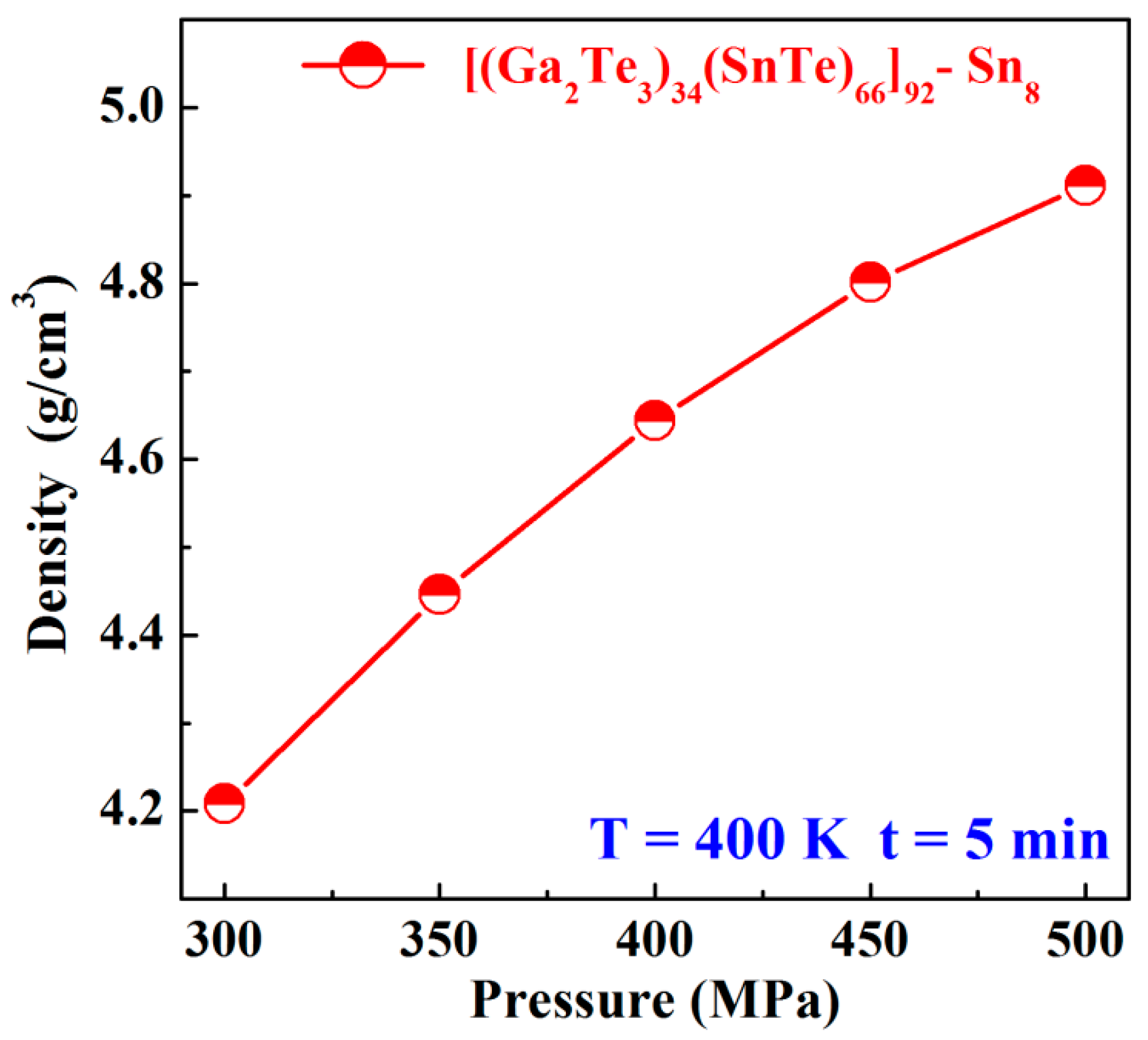
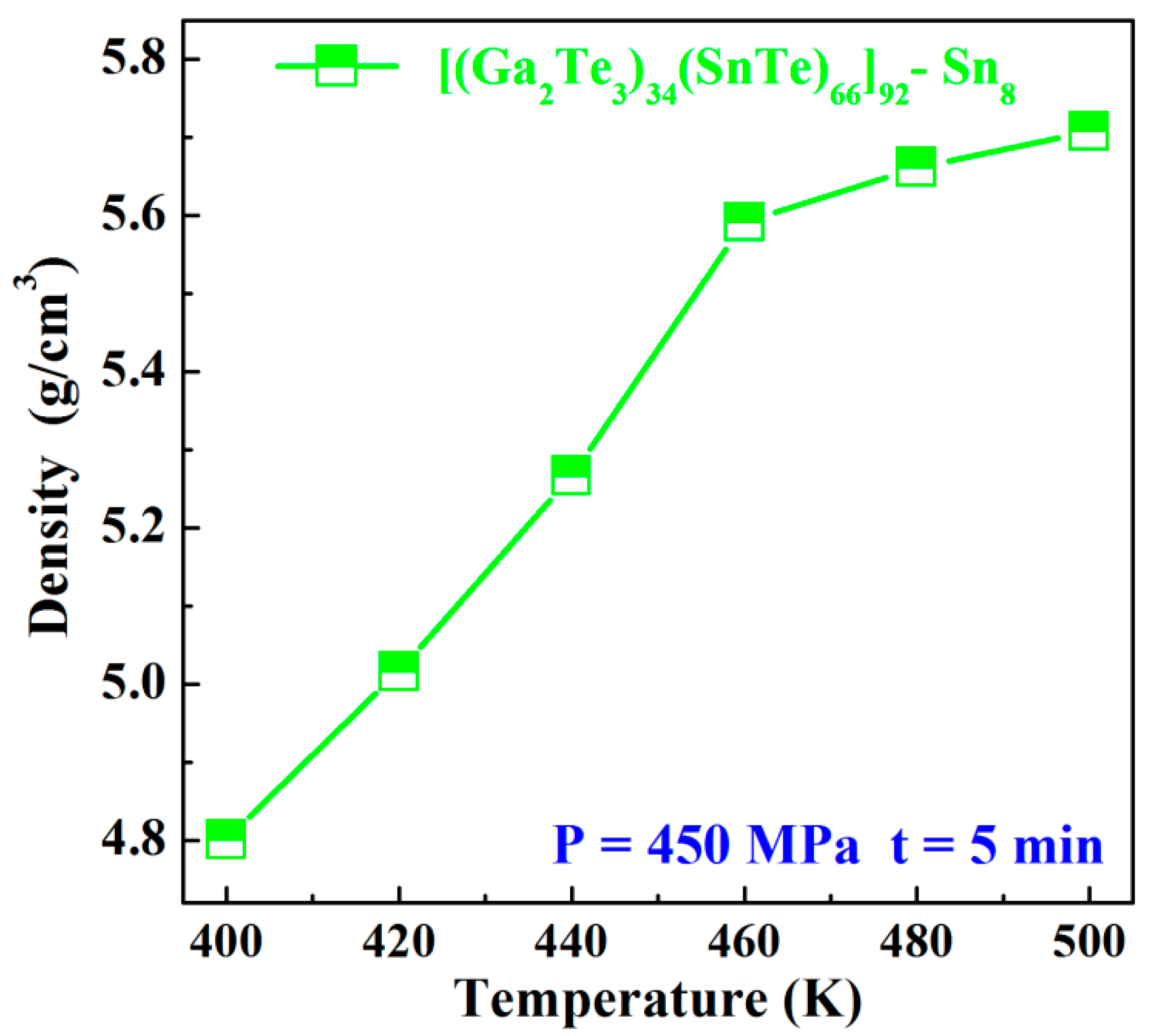
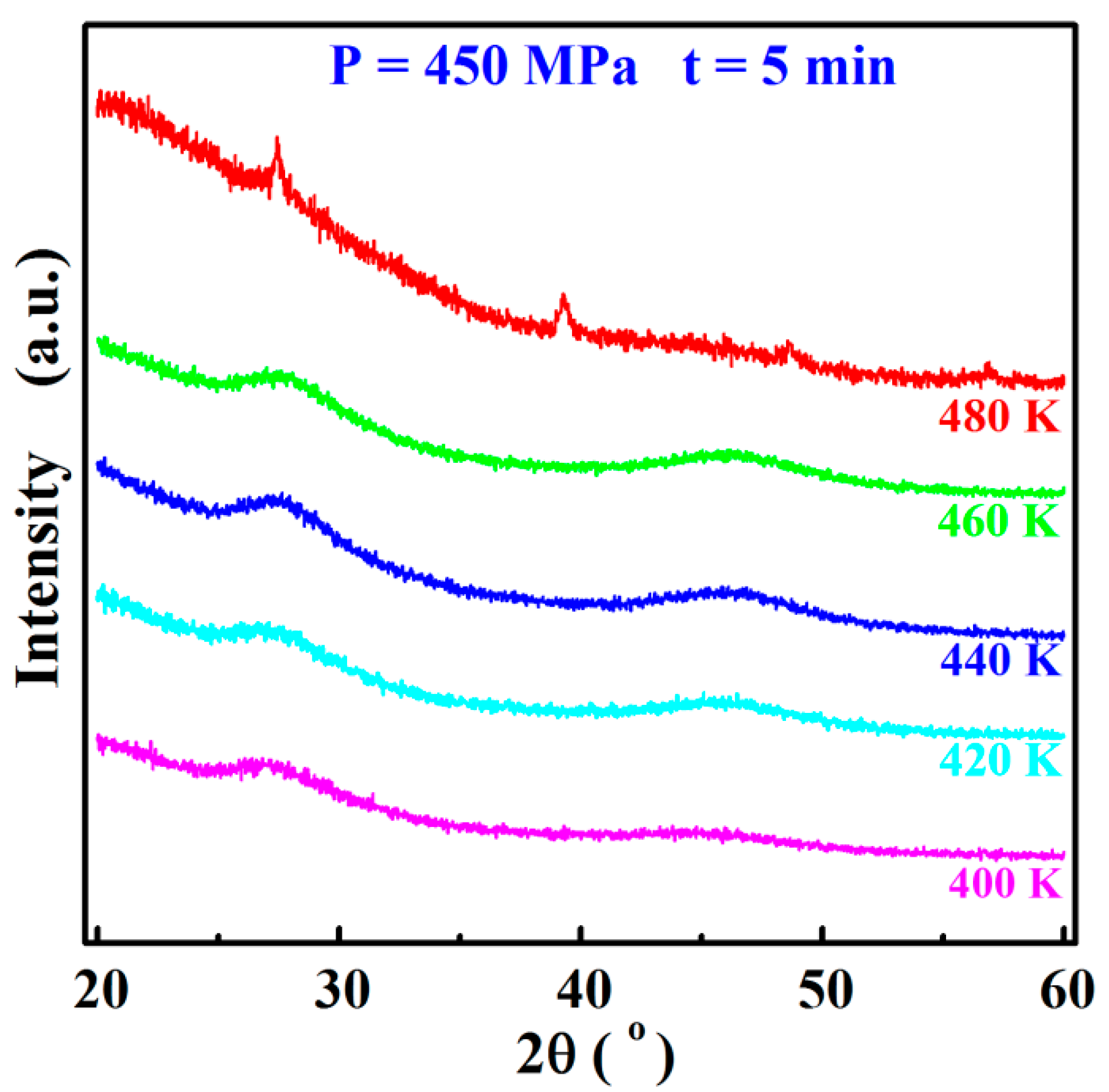
3.1. Sn8 Bulk Glass Preparation
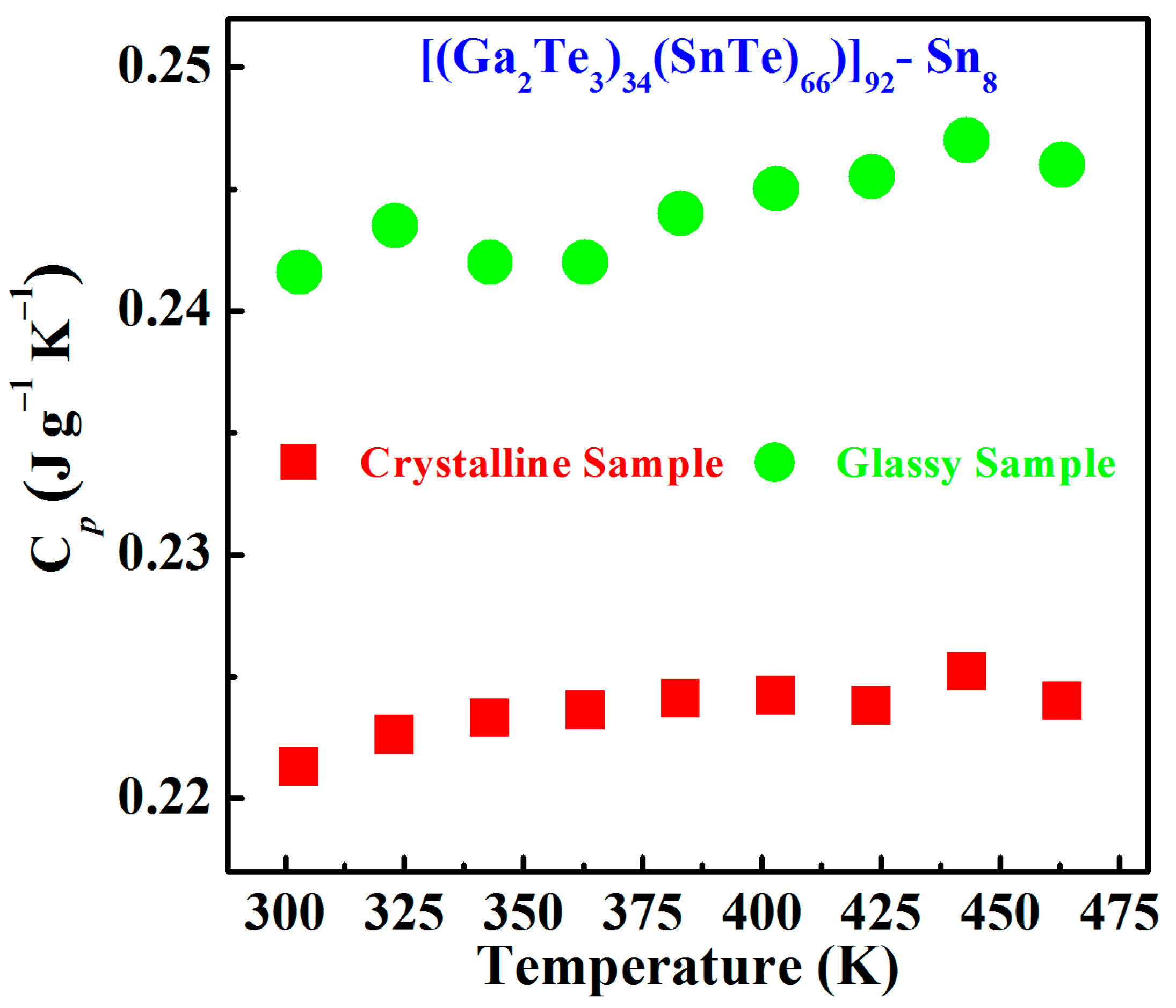
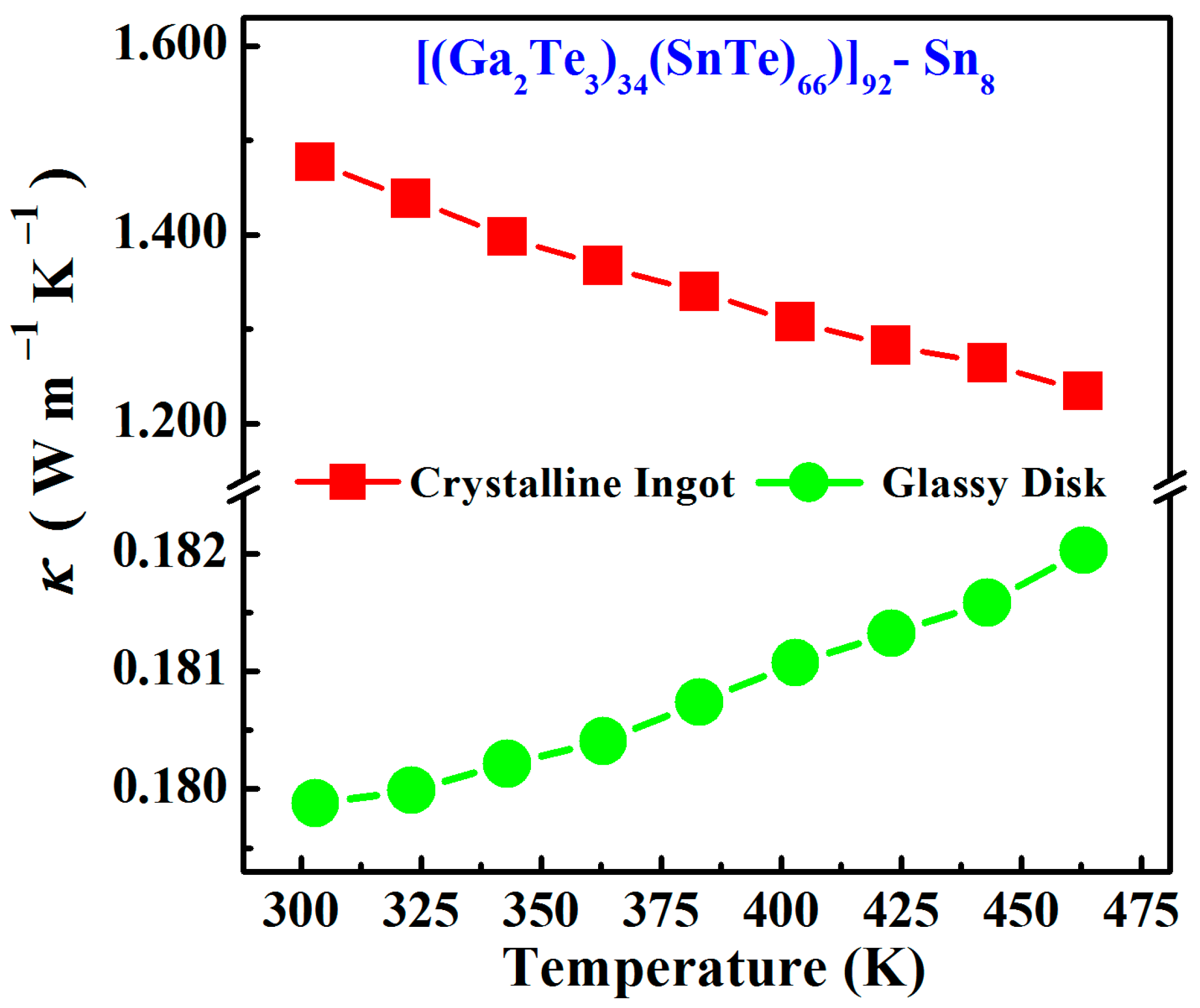
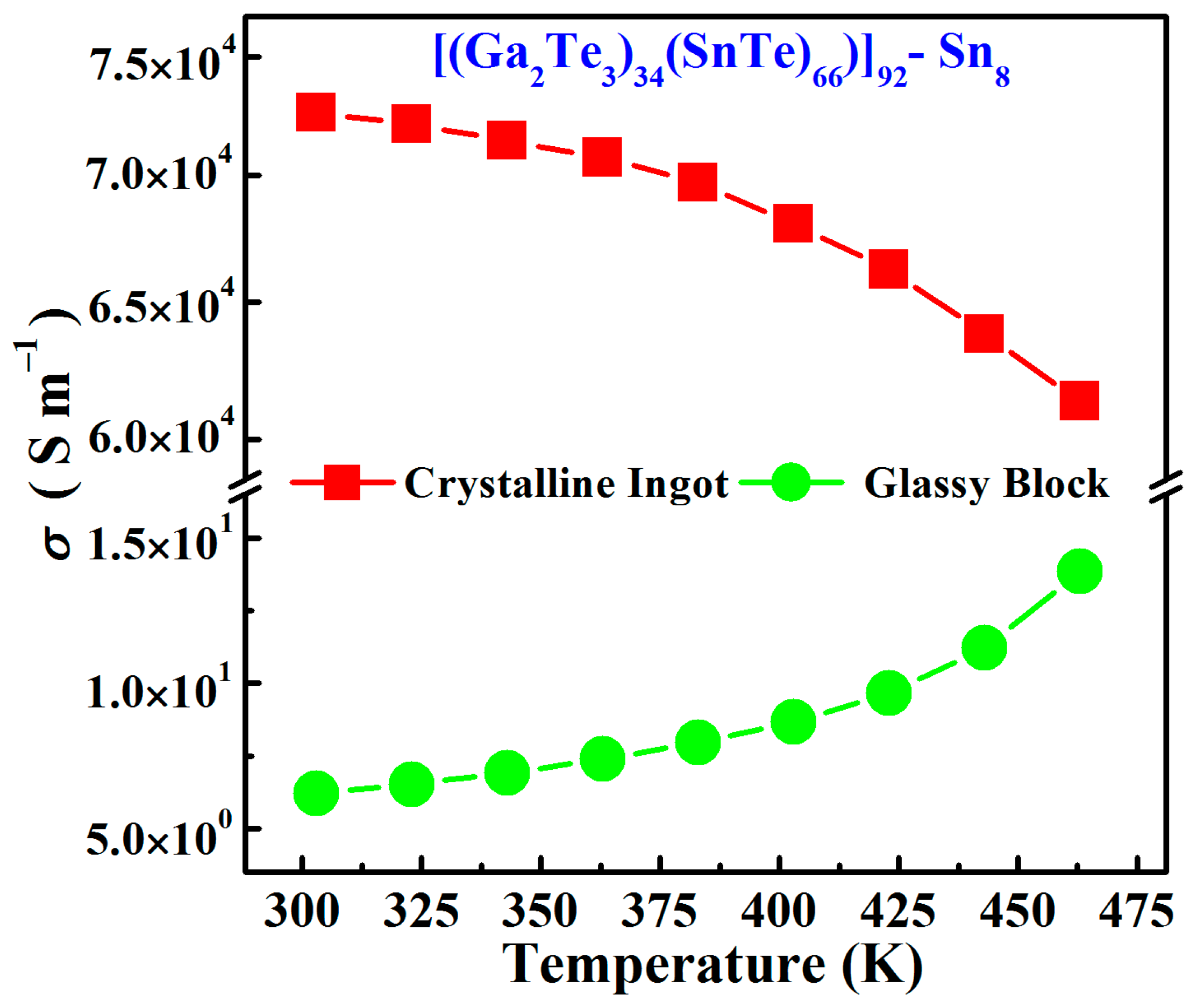
3.2. Electrical and Thermal Transport Properties
4. Conclusions
- (1)
- The high-density (>96%) Sn8 bulk glass with the density of 5.5917 g/cm3 was successfully prepared by the SPS technology at 460 K, using a 5 min dwell time and 450 MPa pressure.
- (2)
- For the Sn8 bulk materials, the room-temperature thermal conductivity significantly decreased from 1.476 W m−1∙K−1 in the crystalline sample to 0.179 W m−1∙K−1 in the glass, and the Seebeck coefficient obviously increased from 35 μV∙K−1 in to 286 μV∙K−1. Thus, the glass transition of tellurium-based semiconductors could significantly degrade the phonon energy and transmission rate, presenting lower thermal conductivities and higher Seebeck coefficients, which render tellurium-based glasses appear as newly emerging thermoelectric materials.
- (3)
- Compared to the conventional tellurium-based glassy systems, the newly fabricated Sn8 bulk glass presented a high room-temperature conductivity (σ = 6.2 S∙m−1) and a large glass transition temperature (Tg = 488 K), which was expected to be a promising thermoelectric material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, W.; Sun, X.; Liu, H.; Yu, C.; Li, W.; Inoue, A.; Şopu, D.; Eckert, J.; Tang, C. Structural Homology of the Strength for Metallic Glasses. J. Mater. Sci. Technol. 2021, 81, 123–130. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Ge, J.; Wu, Z.; Ke, Y.; Li, Q.; Sun, B.; Feng, T.; Wu, Y.; Wang, J.T.; et al. Deformation-Enhanced Hierarchical Multiscale Structure Heterogeneity in a Pd-Si Bulk Metallic Glass. Acta Mater. 2020, 200, 42–55. [Google Scholar] [CrossRef]
- Sohrabi, S.; Fu, J.; Li, L.; Zhang, Y.; Li, X.; Sun, F.; Ma, J.; Wang, W.H. Manufacturing of Metallic Glass Components: Processes, Structures and Properties. Prog. Mater. Sci. 2024, 144, 101283. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, Y.; Wang, J.-Q.; Huo, J.; Wang, L.-M. Quantifying Concentration Fluctuations in Binary Glass-Forming Systems by Small- and Wide-Angle X-Ray Scattering. J. Phys. Chem. Lett. 2022, 13, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-Y.; Yang, Q.; Yu, H.-B. Toward Atomic-Scale Understanding of Structure-Dynamics-Properties Relations for Metallic Glasses. Prog. Mater. Sci. 2024, 145, 101311. [Google Scholar] [CrossRef]
- Ramachandran, T.; Natarajan, S.; Hamed, F. The Role of Dysprosium Levels in the Formation of Mixed Oxidation States within Spinel MnCo2−xDyxO4 Nanocrystalline Powders. J. Electron Spectrosc. Relat. Phenom. 2020, 242, 146952. [Google Scholar] [CrossRef]
- Ramachandran, T.; Thiemann, T.; Hamed, F. Phase Evolution and Magnetic Properties of Dy3Fe5+xO12−x Nanocrystalline Powders: A Choice of Fuel Approach. Mater. Chem. Phys. 2020, 240, 122138. [Google Scholar] [CrossRef]
- Prabhudessai, A.G.; Balaji, S.; Biswas, K.; Molla, A.R.; Vinoth, S.; Ramesh, K.; Dutta, S.; Chauhan, A.K.; Singh, S.; Dasgupta, R.; et al. Purification and Investigation of Tellurium Rich Te-As-Se Chalcogenide Glass for Extended Far-Infrared Transmission. J. Non-Cryst. Solids. 2024, 642, 123093. [Google Scholar] [CrossRef]
- Wang, P.; Bei, J.; Ahmed, N.; Ng, A.K.L.; Ebendorff-Heidepriem, H. Development of Low-Loss Lead-Germanate Glass for Mid-Infrared Fiber Optics: I. Glass Prep. Optim. J. Am. Ceram. Soc. 2021, 104, 860–876. [Google Scholar] [CrossRef]
- Ding, S.; Liu, Z.; Dai, S.; Liu, C.; Cao, Z. Novel Acousto-Optic Material Based on Ge-Te-AgI Chalcohalide Glasses. Ceram. Int. 2021, 47, 12072–12077. [Google Scholar] [CrossRef]
- Liu, K.; Kang, Y.; Tao, H.; Zhang, X.; Xu, Y. Effect of Se on Structure and Electrical Properties of Ge-As-Te Glass. Materials 2022, 15, 1797. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Vincenti, M.A.; Frantz, J.; Clabeau, A.; Qiao, X.; Feng, L.; Scalora, M.; Litchinitser, N.M. Near-Infrared to Ultra-Violet Frequency Conversion in Chalcogenide Metasurfaces. Nat. Commun. 2021, 12, 5833. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, R.; Brandová, D.; Chromčíková, M.; Setnička, M.; Chovanec, J.; Černá, A.; Liška, M.; Málek, J. Se-Doped GeTe4 Glasses for Far-Infrared Optical Fibers. J. Alloys Compd. 2017, 695, 2434–2443. [Google Scholar] [CrossRef]
- Noé, P.; Verdy, A.; d’Acapito, F.; Dory, J.-B.; Bernard, M.; Navarro, G.; Jager, J.-B.; Gaudin, J.; Raty, J.-Y. Toward Ultimate Nonvolatile Resistive Memories: The Mechanism behind Ovonic Threshold Switching Revealed. Sci. Adv. 2020, 6, 2830. [Google Scholar] [CrossRef]
- Raja, A.; Jauhar, R.M.; Ramachandran, K.; Vediyappan, S.; Kumar Raji, R.; Pandian, M.S.; Perumalsamy, R. A Quintuple-Layered Binary Chalcogenide Sb2Te3 Single Crystal and Its Transport Properties for Thermoelectric Applications. ACS Omega 2022, 7, 27798–27803. [Google Scholar] [CrossRef]
- Jauhar, R.M.; Raja, A.; Rajkumar, R.; Arulraj, A.; Almansour, A.I.; Deepapriya, S.; Era, P.; Senthilpandian, M.; Ramachandran, T.; Mangalaraja, R.V.; et al. Thermoelectric Potential: Role of Bismuth in CuSb1−xBixSe2 for Improved Transport Properties. J. Mater Sci. Mater. Electron. 2024, 35, 1195. [Google Scholar] [CrossRef]
- Vaney, J.-B.; Carreaud, J.; Morin, C.; Delaizir, G.; Piarristeguy, A.; Colas, M.; Cornette, J.; Le Parc, R.; Alleno, E.; Monnier, J.; et al. Thermoelectric Properties and Stability of Glasses in the Cu-As-Te System. J. Am. Ceram. Soc. 2017, 100, 2840–2851. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Yu, P.; Wang, L.-M.; Li, G. Preparation and Thermoelectric Properties of Novel Tellurium-Based Glassy Semiconductors. Scr. Mater. 2021, 203, 114038. [Google Scholar] [CrossRef]
- He, S.; Li, Y.; Liu, L.; Jiang, Y.; Feng, J.; Zhu, W.; Zhang, J.; Dong, Z.; Deng, Y.; Luo, J. Semiconductor Glass with Superior Flexibility and High Room Temperature Thermoelectric Performance. Sci. Adv. 2020, 6, 8423. [Google Scholar] [CrossRef]
- Kang, S.; Fu, Y.; Gu, H.; Lin, C. Chalcogenide Glass for Thermoelectric Application. J. Non-Cryst. Solids X 2022, 15, 100111. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Chen, C.; Yu, P.; Wang, L.-M.; Li, G. High-Conductivity Chalcogenide Glasses in Ag-Ga2Te3-SnTe Systems and Their Suitability as Thermoelectric Materials. ACS Appl. Mater. Interfaces 2023, 15, 19170–19177. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Boussard-Plédel, C.; Lucas, J.; Bureau, B. Te-Based Glass Fiber for Far-Infrared Biochemical Sensing up to 16 um. Opt. Express 2014, 22, 21253–21262. [Google Scholar] [CrossRef]
- Černošek, Z.; Černošková, E.; Hejdová, M.; Holubová, J.; Todorov, R. The Properties and Structure of GeSeTe Glasses and Thin Films. J. Non-Cryst. Solids 2017, 460, 169–177. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Li, G. A Comprehensive Study of Sn-Ga2Te3-SnTe Amorphous Alloys: Glass Formation and Crystallization Kinetics. Metals 2023, 13, 532. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, Z.; Jin, J.; Deng, L.; Gong, P.; Wang, X. Densification Mechanism of Zr-Based Bulk Metallic Glass Prepared by Two-Step Spark Plasma Sintering. J. Alloys Compd. 2021, 850, 156724. [Google Scholar] [CrossRef]
- Bao, W.; Yan, H.; Chen, J.; Xie, G. High Strength Conductive Bulk Cu-Based Alloy/Metallic Glass Composites Fabricated by Spark Plasma Sintering. Mater. Sci. Eng. A 2021, 825, 141919. [Google Scholar] [CrossRef]
- Chang, Z.; Wang, W.; Ge, Y.; Zhou, J.; Dong, P.; Cui, Z. Micro-Mechanical Properties and Corrosion Resistance of Zr55Cu30Al10Ni5 Bulk Metallic Glass Fabricated by Spark Plasma Sintering. J. Alloys Compd. 2019, 780, 220–227. [Google Scholar] [CrossRef]
- Paul, T.; Chawake, N.; Kottada, R.S.; Harimkar, S.P. Pressure Controlled Micro-Viscous Deformation Assisted Spark Plasma Sintering of Fe-Based Bulk Amorphous Alloy. J. Alloys Compd. 2018, 738, 10–15. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Li, X.-S.; Yu, J.-H.; Zhao, H.; Zhou, J.-H.; Yang, J.-M.; Li, J.-Z.; Wang, J.-G.; Chang, C.-T.; et al. High-Temperature Malleable Ta-Co Metallic Glass Developed by Combinatorial Method. Scr. Mater. 2022, 219, 114883. [Google Scholar] [CrossRef]
- Li, H.; Yang, W.; Ma, Y.; Kong, F.; Wan, Y.; Chen, C.; Liu, H.; Li, H.; Inoue, A. Plastic TiZrHfCoNiCu High Entropy Alloy via Stable B2 Phase. J. Alloys Compd. 2023, 935, 167897. [Google Scholar] [CrossRef]
- Huang, L.; Tan, W.; Li, S.; Li, Y. Effect of Loading Pressure on Mechanical Properties and Interface Characteristics of 7056 Al Alloy Particle Reinforced Zr-Al-Ni-Cu Bulk Metallic Glass Matrix Composite Prepared by Spark Plasma Sintering. J. Alloys Compd. 2020, 816, 152605. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.; Luo, J.; Yin, N.; Shi, Q.; Gleiter, H.; Hahn, H. Enhanced Specific Heat of the Sc79Fe21 Nanoglass Compared to the Sc79Fe21 Amorphous Melt-Spun Ribbon in a Temperature Range of 150–300 K. Mater. Lett. 2023, 349, 134706. [Google Scholar] [CrossRef]
- Khalaf, K.A.M. Calorimetric Investigation of the Low Temperature Specific Heat of the Amorphous RE-Cu-Y Metallic Glasses. Mater. Chem. Phys. 2020, 252, 123079. [Google Scholar] [CrossRef]
- Sharma, A.; Mehta, N. Thermo-Physical Properties of Multi-Component Se78−xTe20Sn2Pbx Chalcogenide Glasses. Mater. Chem. Phys. 2015, 161, 35–42. [Google Scholar] [CrossRef]
- Gonçalves, A.P.; Lopes, E.B.; Delaizir, G.; Vaney, J.B.; Lenoir, B.; Piarristeguy, A.; Pradel, A.; Monnier, J.; Ochin, P.; Godart, C. Semiconducting Glasses: A New Class of Thermoelectric Materials? J. Solid State Chem. 2012, 193, 26–30. [Google Scholar] [CrossRef]
- Gonçalves, A.P.; Lopes, E.B.; Rouleau, O.; Godart, C. Conducting Glasses as New Potential Thermoelectric Materials: The Cu-Ge-Te Case. J. Mater. Chem. 2010, 20, 1516–1521. [Google Scholar] [CrossRef]
- Lonergan, J.; Lonergan, C.; McCloy, J.; Richardson, K.A. Modeling and Experimental Determination of Physical Properties of GexGaySe1-x-y Chalcogenide Glasses II: Optical and Thermal Properties. J. Non-Cryst. Solids 2019, 511, 115–124. [Google Scholar] [CrossRef]
- Mishra, P.K.; Singh, K.; Upadhyay, A.N.; Kumar, H. Compositional Dependence of Thermal Transport and Optical Properties of Se85Ge15-xPbx (0 ≤ x ≤ 10) Chalcogenide Glassy Alloys. Opt. Mater. 2019, 97, 109395. [Google Scholar] [CrossRef]
- Yang, Z.; Wilhelm, A.A.; Lucas, P. High-Conductivity Tellurium-Based Infrared Transmitting Glasses and Their Suitability for Bio-Optical Detection. J. Am. Ceram. Soc. 2010, 93, 1941–1944. [Google Scholar] [CrossRef]
- Vaney, J.-B.; Carreaud, J.; Piarristeguy, A.; Morin, C.; Delaizir, G.; Viennois, R.; Colas, M.; Cornette, J.; Alleno, E.; Monnier, J.; et al. Stabilization of Metastable Thermoelectric Crystalline Phases by Tuning the Glass Composition in the Cu-As-Te System. Inorg. Chem. 2018, 57, 754–767. [Google Scholar] [CrossRef]
- Lucas, P.; Conseil, C.; Yang, Z.; Hao, Q.; Cui, S.; Boussard-Pledel, C.; Bureau, B.; Gascoin, F.; Caillaud, C.; Gulbiten, O.; et al. Thermoelectric Bulk Glasses Based on the Cu-As-Te-Se System. J. Mater. Chem. A 2013, 1, 8917–8925. [Google Scholar] [CrossRef]
- Zheng, J.; Li, L.; Yin, H.; Wang, Y.; Wei, J.; Zeng, H.; Xia, F.; Chen, G. Mutual Effects of Ag Doping and Non-Stoichiometric Glass Forming Units on the Structural, Thermal, and Electrical Properties of Ag30+xAs28-xSe21Te21 Chalcogenide Glasses. Ceram. Int. 2020, 46, 22826–22830. [Google Scholar] [CrossRef]
- Zheng, J.; Li, L.; Yin, H.; Wang, Y.; Wei, J.; Chen, G. Effects of the Se Substitution for Te on the Structure and Properties of Glasses in the Ag30As28(SexTe100-x)42 System. Ceram. Int. 2019, 45, 9136–9139. [Google Scholar] [CrossRef]
- Cui, S.; Boussard-plédel, C.; Calvez, L.; Rojas, F.; Chen, K.; Ning, H.; Reece, M.J.; Guizouarn, T.; Bureau, B. Comprehensive Study of Tellurium Based Glass Ceramics for Thermoelectric Application. Adv. Appl. Ceram. 2015, 114, 42–47. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Guo, F.; Zhang, H.; Zhang, M.; Su, J.; Li, Z. Preparation and Electrothermal Transport Behavior of Sn8[(Ga2Te3)34(SnTe)66]92 Bulk Glass. Materials 2024, 17, 4809. https://doi.org/10.3390/ma17194809
Zhang Y, Guo F, Zhang H, Zhang M, Su J, Li Z. Preparation and Electrothermal Transport Behavior of Sn8[(Ga2Te3)34(SnTe)66]92 Bulk Glass. Materials. 2024; 17(19):4809. https://doi.org/10.3390/ma17194809
Chicago/Turabian StyleZhang, Yaqi, Feng Guo, Huan Zhang, Mingming Zhang, Jianxiu Su, and Zhengxin Li. 2024. "Preparation and Electrothermal Transport Behavior of Sn8[(Ga2Te3)34(SnTe)66]92 Bulk Glass" Materials 17, no. 19: 4809. https://doi.org/10.3390/ma17194809





