Characterization and Growth Kinetics of Borides Layers on Near-Alpha Titanium Alloys
Abstract
:1. Introduction
2. Experimental Procedure
3. Results
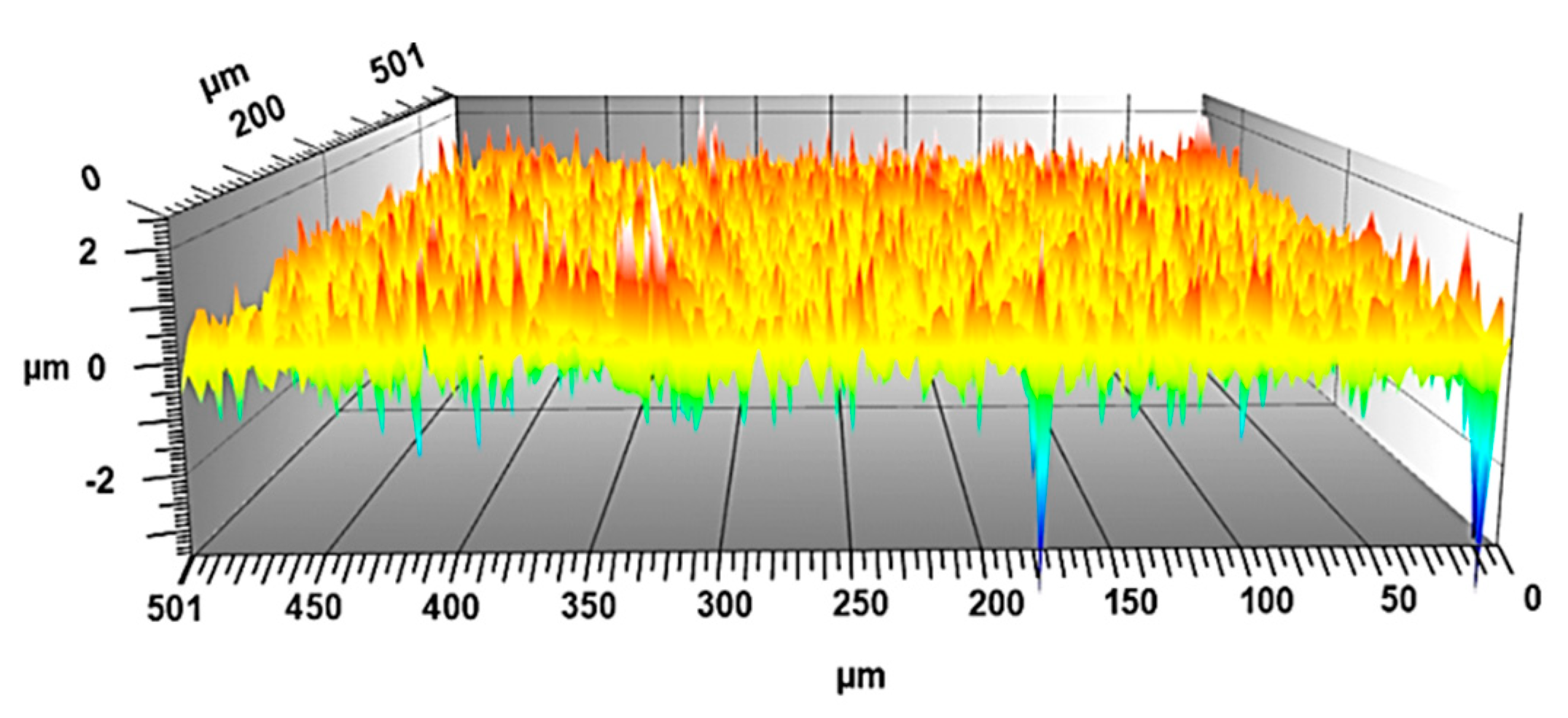
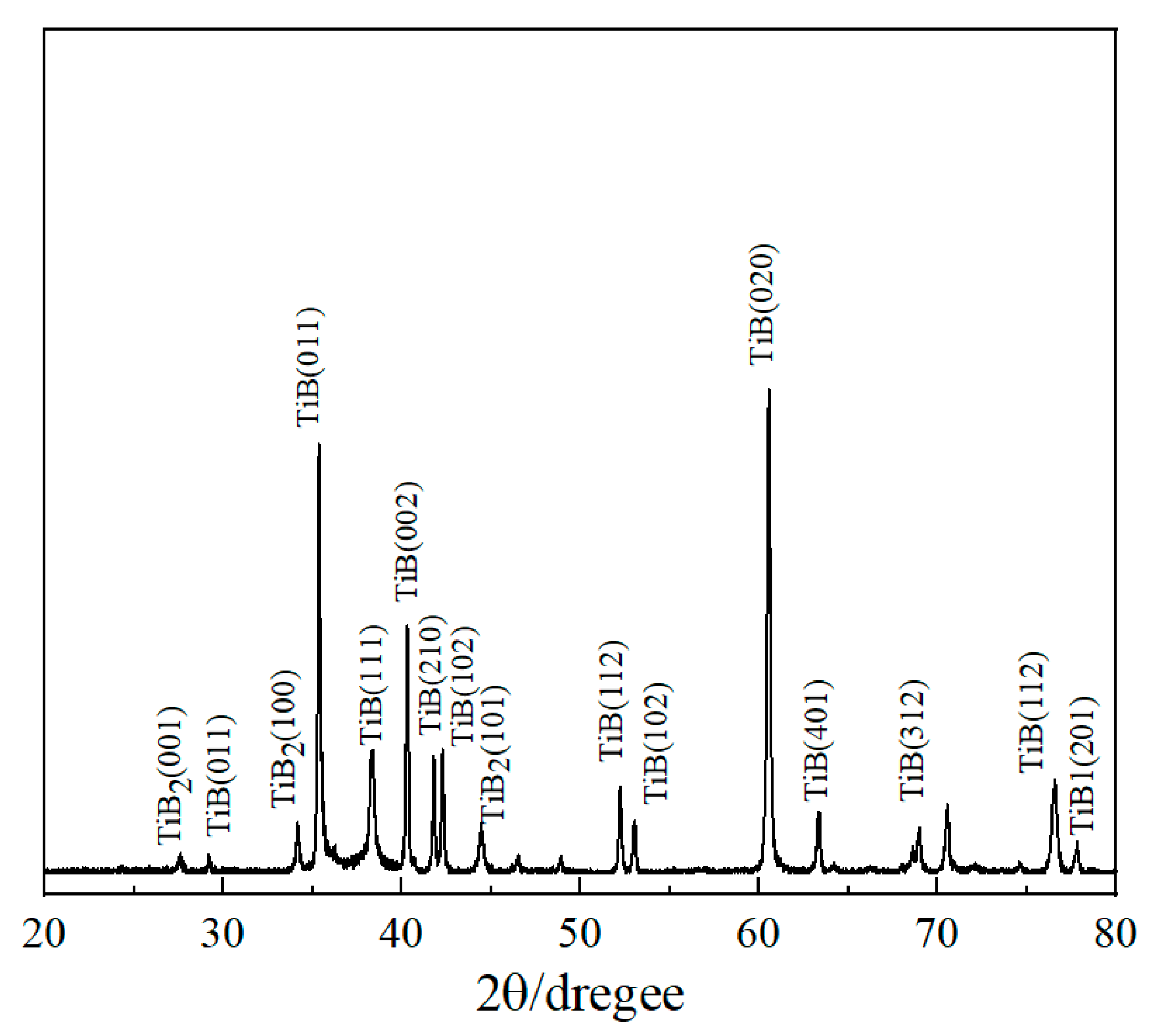
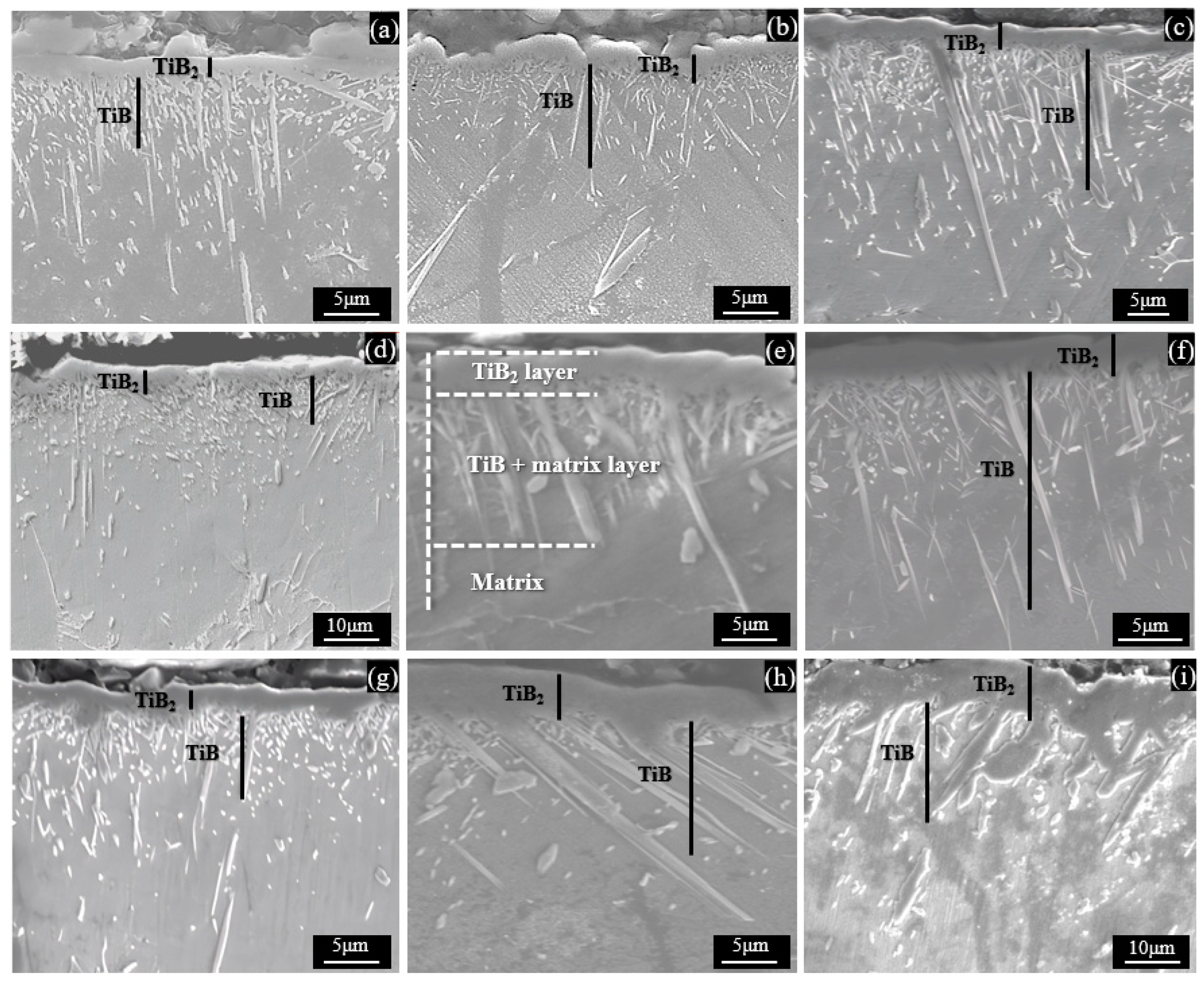
3.1. Characterization of Titanium Boride Layers
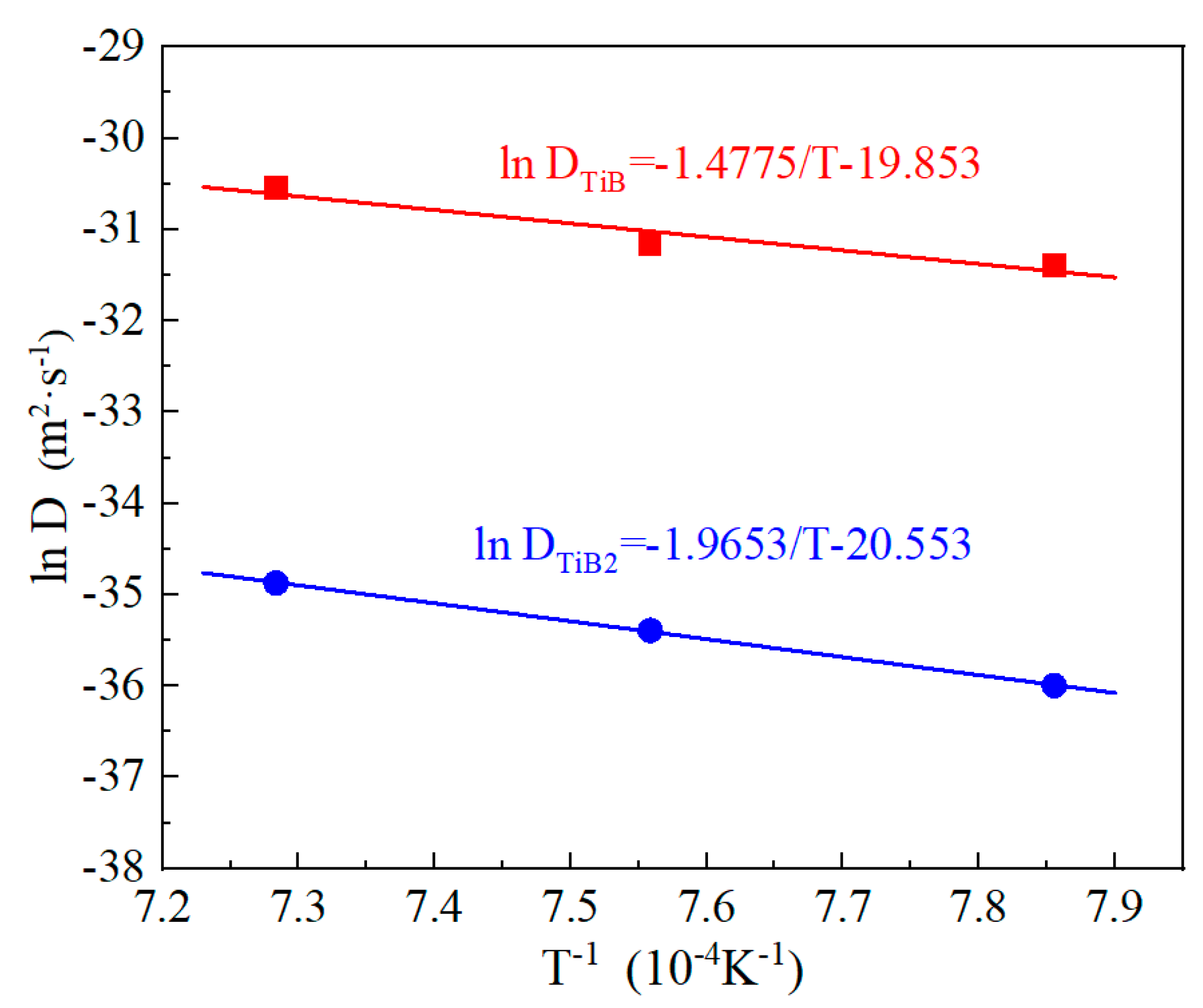
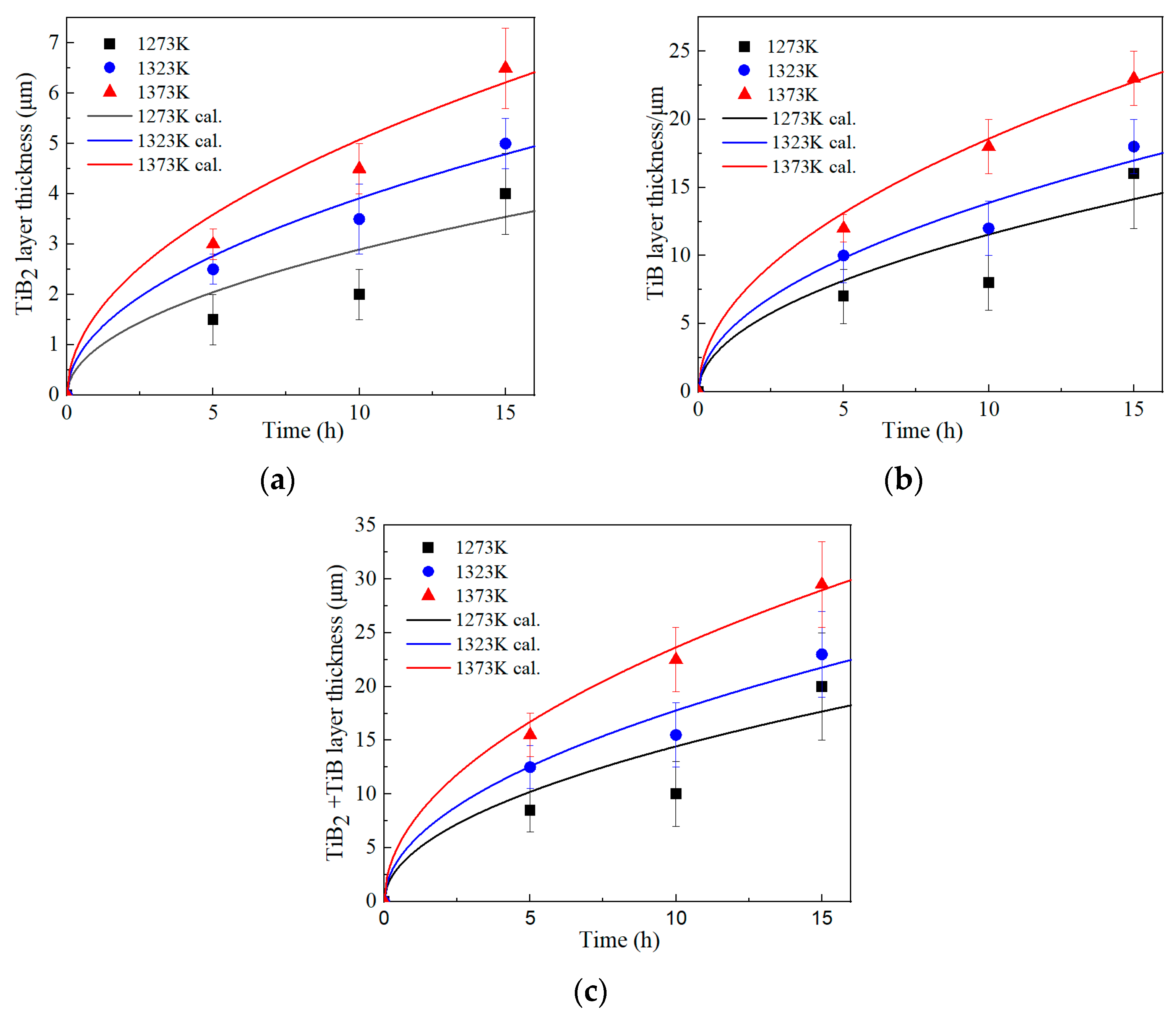
3.2. Growth Kinetics of Boride Layers
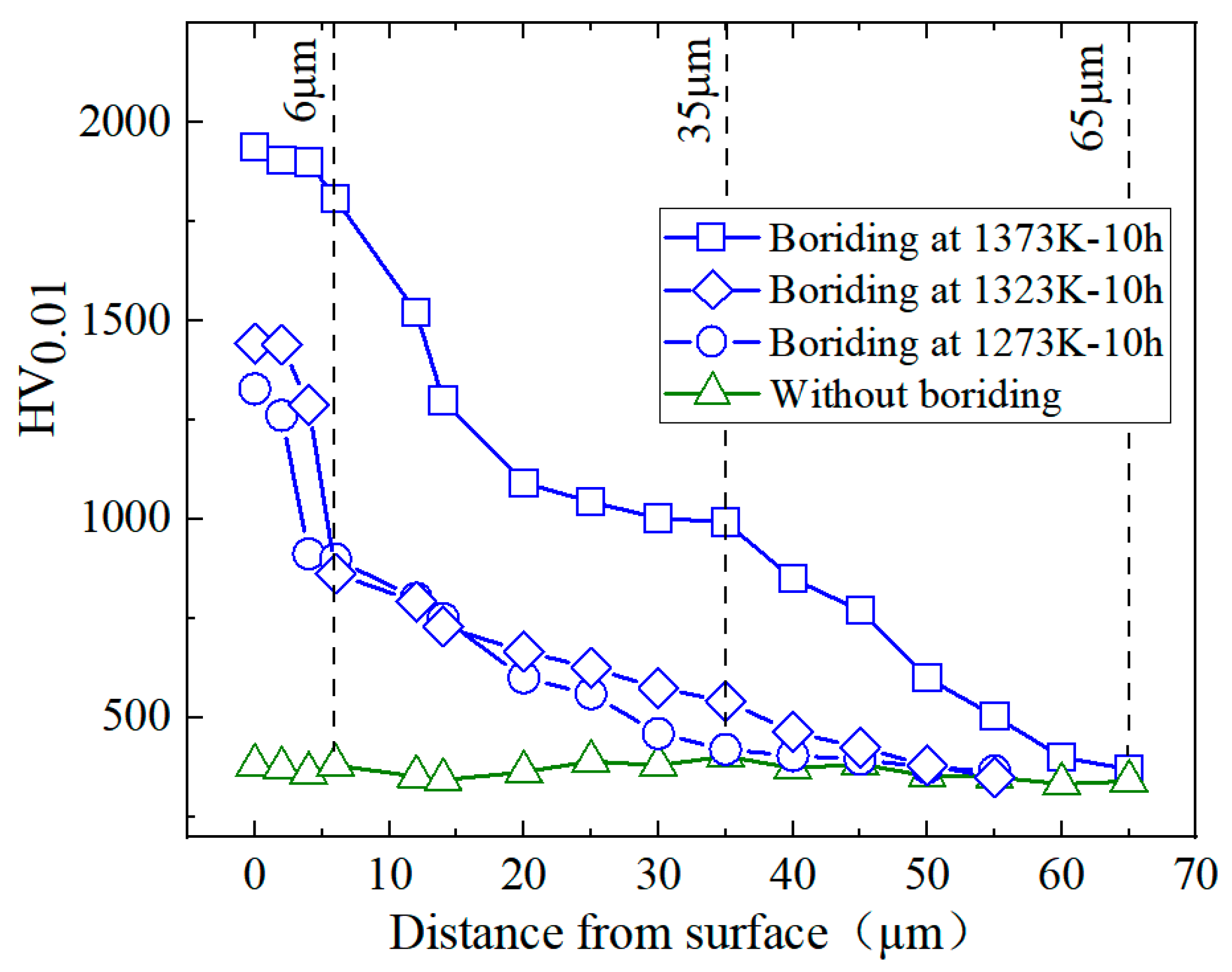
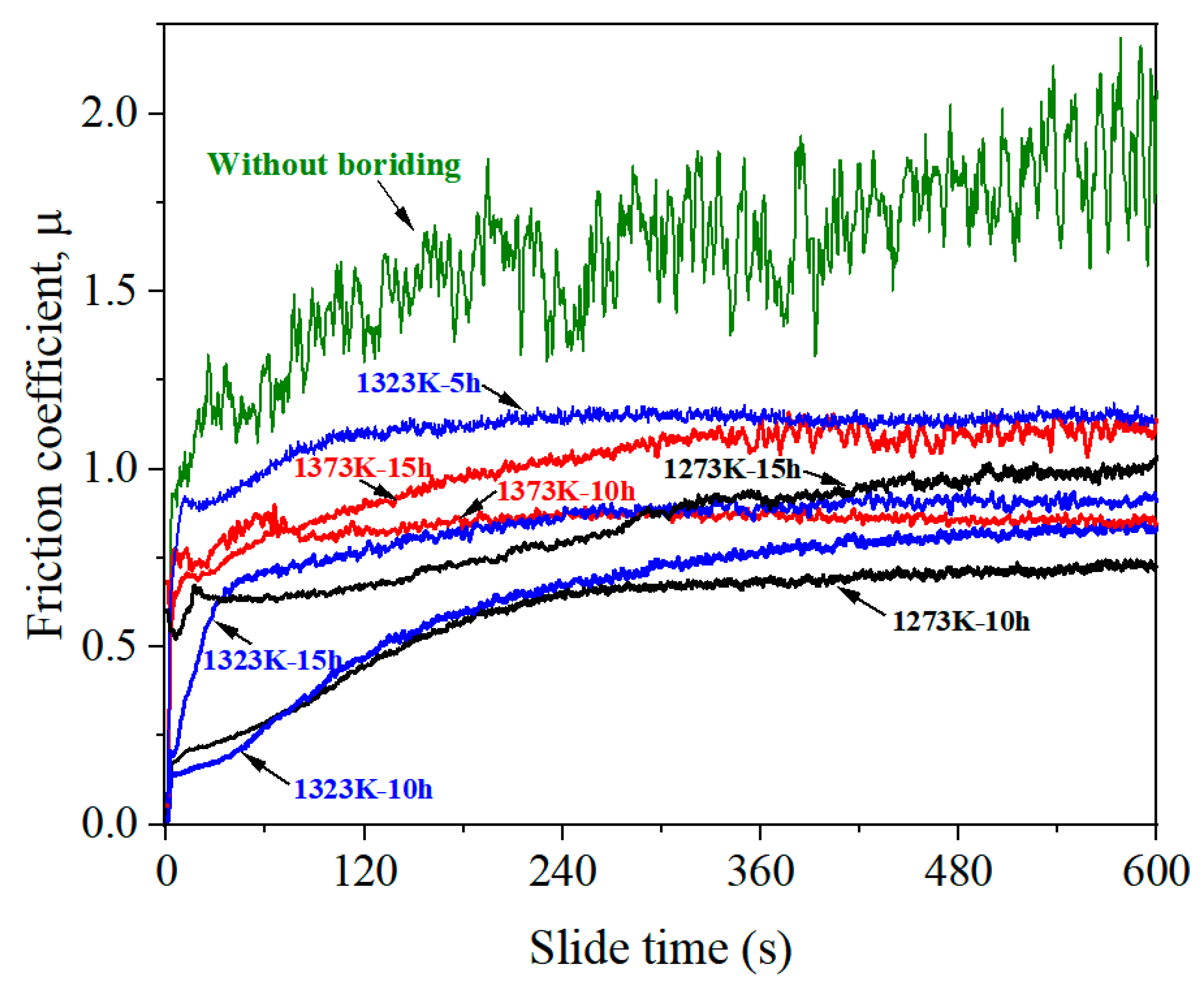
3.3. Microhardenss and Wear Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, Z.N.; Gao, P.F.; Li, H.W.; Cai, Y. On the fracture behavior and toughness of TA15 titanium alloy with tri-modal microstructure. Mater. Sci. Eng. A 2019, 753, 238–246. [Google Scholar] [CrossRef]
- Williams, J.C.; Starke, E.A. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Ouyang, D.L.; Hu, S.W.; Tao, C.; Cui, X.; Zhu, Z.; Lu, S. Experiment and modeling of TiB2/TiB boride layer of Ti6Al2Zr1Mo1V alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 3752–3761. [Google Scholar] [CrossRef]
- Sarma, B.; Chandran, K.S.R. Accelerated kinetics of surface hardening by diffusion near phase transition temperature: Mechanism of growth of boride layers on titanium. Acta Mater. 2011, 59, 4216–4228. [Google Scholar] [CrossRef]
- Li, C.Z.; Fu, B.G.; Dong, T.S.; Liu, J.; Song, Y.; Zhao, X.; Li, G. Microstructure and dry sliding wear behavior of as-cast TiCp/Ti-1100-0.5Nb titanium matrix composite at elevated temperatures. China Foundry 2020, 6, 17. [Google Scholar] [CrossRef]
- Blau, P.J.; Jolly, B.C.; Qu, J.; Peter, W.H.; Blue, C.A. Tribological investigation of titanium-based materials for brakes. Wear 2007, 263, 1202–1211. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, H.; Zheng, X.; Tu, Y.; Ran, X.; Wang, W.; Wang, Q.y.; Lin, Y.H. Preparation and wear resistance of B–Al co-permeation layers on TC4 titanium alloy surface. Mater. Today Commun. 2024, 39, 108697. [Google Scholar]
- Lv, X.J.; Hu, L.Y.; Liu, J.H.; Lai, Y.Q.; Jiang, X.L.; Li, J. The growth behavior of titanium boride layers in α and β phase fields of titanium. Metall. Mater. Trans. A 2016, 47, 3573–3579. [Google Scholar] [CrossRef]
- Peretti, V.; Ferraris, S.; Gautier, G.; Hellmich, C.; Lahayne, O.; Stella, B.; Yamaguchi, S. Surface treatments for boriding of Ti6Al4V alloy in view of applications as a biomaterial. Tribol. Int. 2018, 126, 21–28. [Google Scholar] [CrossRef]
- Keddam, M.; Taktak, S. Characterization and diffusion model for the titanium boride layers formed on the Ti6Al4V alloy by plasma paste boriding. Appl. Surf. Sci. 2017, 399, 229–236. [Google Scholar] [CrossRef]
- Kaestner, P.; Olfe, J.; Rie, K.T. Plasma-assisted boriding of pure titanium and TiAl6V4. Surf. Coat. Technol. 2001, 142–144, 248–252. [Google Scholar] [CrossRef]
- Monisha, K.; Shariff, S.M.; Sekar, A.; Raju, R.; Manonmani, J.; Senthilselvan, J. Titanium boride coating by high power diode laser alloying of amorphous boron with titanium and its surface property investigations. Opt. Laser Technol. 2024, 170, 110159. [Google Scholar] [CrossRef]
- Li, P.; Liu, D.; Bao, Z.W.; Ma, L.S.; Duan, Y.H. Surface characterization and diffusion model of pack borided TB2 titanium alloy. Ceram. Int. 2018, 44, 18429–18437. [Google Scholar] [CrossRef]
- Keddam, M.; Chentouf, S.M. A diffusion model for describing the bilayer growth (FeB/Fe2B) during the iron powder-pack boriding. Appl. Surf. Sci. 2005, 252, 393–399. [Google Scholar] [CrossRef]
- Keddam, M.; Taktak, S.; Tasgetiren, S. A diffusion model for the titanium borides on pure titanium. Surf. Eng. 2016, 32, 802–808. [Google Scholar] [CrossRef]
- Li, F.; Yi, X.; Zhang, J.; Fan, Z.; Gong, D.; Xi, Z. Growth kinetics of titanium boride layers on the surface of Ti6Al4V. Acta Metall. Sin.-Engl. Lett. 2010, 23, 293–300. [Google Scholar]
- Liu, D.; Duan, Y.; Bao, W.; Peng, M. Characterization and growth kinetics of boride layers on Ti-5Mo-5V-8Cr-3Al alloy by pack boriding with CeO2. Mater. Charact. 2020, 164, 110362. [Google Scholar] [CrossRef]
- Sarman, B.; Tikekar, N.M.; Ravi Chandran, K.S. Kinetics of growth of superhard boride layers during solid state diffusion of boron into titanium. Ceram. Int. 2012, 38, 6795–6805. [Google Scholar] [CrossRef]
- Xing, Z.; Feng, Z.; Wu, Y.; Lu, Y.; Duan, Y.; Zheng, S.; Peng, M.; Li, M.; Wu, Y. Growth kinetics and corrosion properties of pack-borided Ti-5Al-2.5Sn alloy. Surf. Coat. Technol. 2023, 473, 130003. [Google Scholar] [CrossRef]
- Piao, R.; Zhu, W.J.; Ma, L.; Zhao, P.; Hu, B. Characterization of Hot Deformation of near alpha Titanium Alloy Prepared by TiH2-Based Powder Metallurgy. Materials 2022, 15, 5932. [Google Scholar] [CrossRef]
- Duan, Y.H.; Li, P.; Chen, Z.Z.; Shi, J.; Ma, L.S. Surface evolution and growth kinetics of Ti6Al4V alloy in pack boriding. J. Alloys Compd. 2018, 742, 690–701. [Google Scholar] [CrossRef]
- Yang, A.; Wu, Y.; Duan, Y.H.; Peng, M.J.; Zheng, S.J.; Li, M.N.; Yu, J. The effect of alloying elements in Ti-5Mo-5V-8Cr-3Al alloy on growth kinetics of TiB whiskers in boride layer. Mater. Des. 2023, 225, 111478. [Google Scholar] [CrossRef]
- Cui, G.; Yang, B.; Hou, J.; Chen, W.; Zhang, W. The Sticking Out Mechanism and Growth of TiB Whiskers from TiBw/Ti-6Al-4V Composites during Sintering. Metals 2020, 11, 1467–1478. [Google Scholar] [CrossRef]
- Singh, H.; Hayat, M.; He, Z.; Peterson, V.K.; Das, R.; Cao, R. In situ neutron diffraction observations of Ti-TiB composites. Compos. Part A 2019, 124, 105501. [Google Scholar] [CrossRef]
- Ataibis, V.; Taktak, S. Characteristics and growth kinetics of plasma paste borided Cp–Ti and Ti6Al4V alloy. Surf. Coat. Technol. 2015, 279, 65–71. [Google Scholar] [CrossRef]
- Makuch, N.; Kulka, M.; Keddam, M.; Taktak, S.; Ataibis, V.; Dziarski, P. Growth kinetics and some mechanical properties of two-phase boride layers produced on commercially pure titanium during plasma paste boriding. Thin Solid Film. 2017, 626, 25–37. [Google Scholar] [CrossRef]
- Kara, G.; Purcek, G. Boriding behaviour of titanium alloys with different crystalline structures. Surf. Eng. 2019, 35, 611–617. [Google Scholar] [CrossRef]
- Keddam, M. Simulation of the growth kinetics of the (FeB/Fe2B) bilayer obtained on a borided stainless steel. Appl. Surf. Sci. 2011, 257, 2004–2010. [Google Scholar] [CrossRef]
- Keddam, M.; Kulka, M.; Makuch, N.; Pertek, A.; Małdzinski, L. A kinetic model for estimating the boron activation energies in the FeB and Fe2B layers during the gas-boriding of Armco iron: Effect of boride incubation times. Appl. Surf. Sci. 2014, 298, 155–163. [Google Scholar] [CrossRef]
- Duan, Y.H.; Wu, Y.; Peng, M.J.; Qi, H.R. The interstitial diffusion behaviors and mechanisms of boron in a-Ti and b-Ti: A first-principles calculation. Comp. Mater. Sci. 2020, 184, 109866. [Google Scholar] [CrossRef]
- Kara, G.; Purcek, G. Growth kinetics and mechanical characterization of boride layers formed on β-type Ti−45Nb alloy. Surf. Coat. Technol. 2018, 352, 201–212. [Google Scholar] [CrossRef]
- Kartal, G.; Timur, S. Growth kinetics of titanium borides produced by CRTD-Bor method. Surf. Coat. Technol. 2013, 215, 440–446. [Google Scholar] [CrossRef]
- Fay, Z.; Guo, Z.X.; Cantor, B. The kinetics and mechanism of interfacial reaction in sigma fibre-reinforced Ti MMCs. Compos. A 1997, 28, 131–140. [Google Scholar]
- Munro, R.G. Material properties of titanium diboride. J. Res. Natl. Inst. Stand. Technol. 2000, 105, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Madtha, S.; Lee, C.; Chandran, K.S.R. Physical and mechanical properties of nanostructured titanium boride (TiB) ceramic. J. Am. Ceram. Soc. 2008, 91, 1319–1321. [Google Scholar] [CrossRef]
- Liu, Y.F.; Liskiewicz, T.W.; Beake, B.D. Dynamic changes of mechanical properties induced by friction in the Archard wear model. Wear 2019, 428–429, 366–375. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Liu, Y.F.; Wei, X.N.; Sun, D.; Lu, W.Z.; Ko, T.J. Tribological characteristics of the dual titanium boride layers (TiB2+TiB) on titanium alloy. Ceram. Int. 2021, 47, 13957–13969. [Google Scholar] [CrossRef]
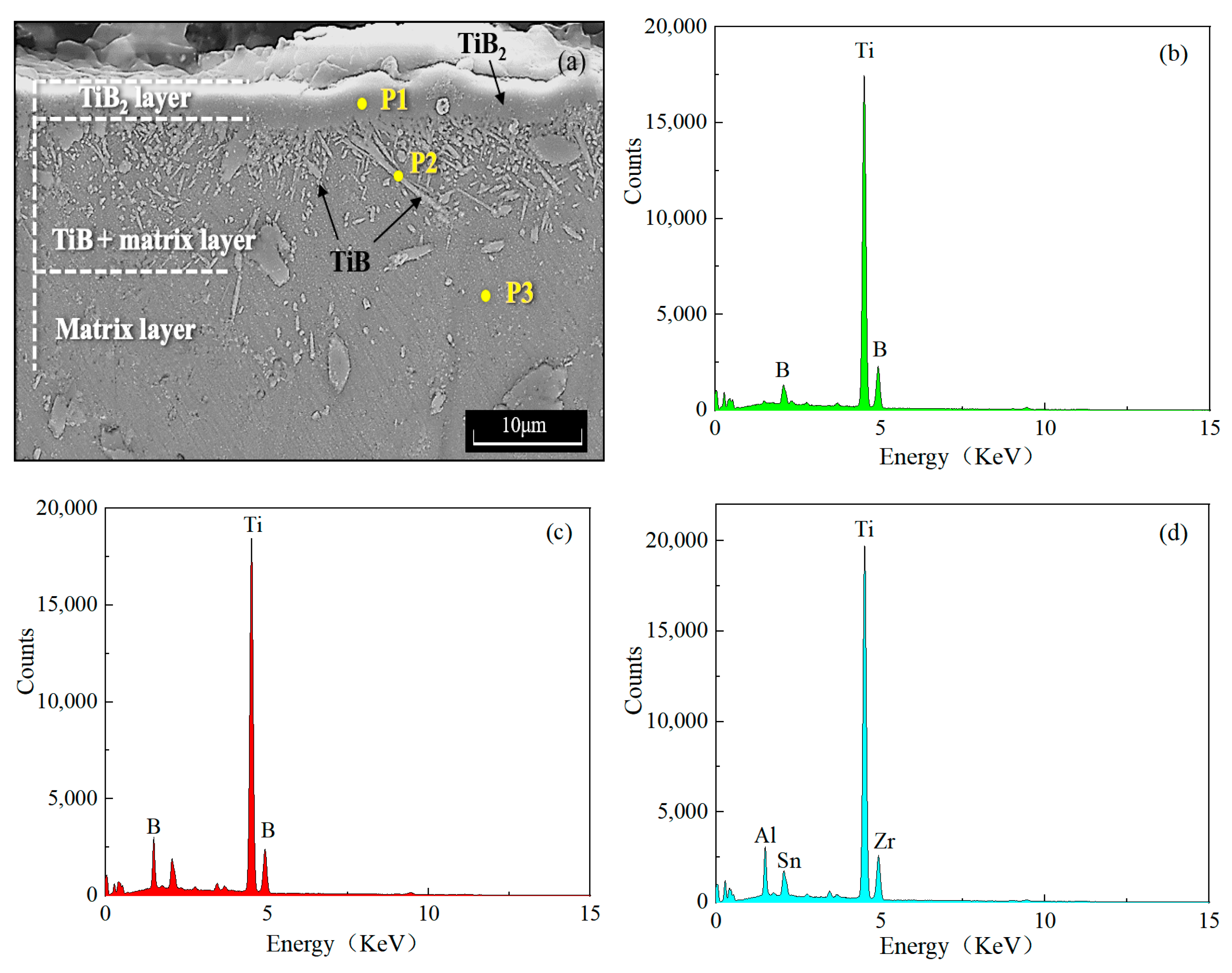



| Al | Sn | Zr | Mo | Si | Ti |
|---|---|---|---|---|---|
| 5.05 | 1.96 | 3.689 | 0.32 | 0.29 | Bal. |
| T (K) | t (h) | d (μm) | ||
|---|---|---|---|---|
| TiB2 | TiB | TiB2 + TiB | ||
| 1273 | 5 | 1.5 ± 0.2 | 7 ± 4 | 8.5 ± 4 |
| 1273 | 10 | 2 ± 0.5 | 8 ± 2 | 10 ± 3 |
| 1273 | 15 | 4 ± 0.8 | 16 ± 4 | 20 ± 5 |
| 1323 | 5 | 2.5 ± 0.3 | 10 ± 4 | 12.5 ± 4 |
| 1323 | 10 | 3.5 ± 0.7 | 12 ± 5 | 15.3 ± 5 |
| 1323 | 15 | 5 ± 0.5 | 18 ± 7 | 23 ± 8 |
| 1373 | 5 | 3 ± 0.3 | 12 ± 2 | 15 ± 2 |
| 1373 | 10 | 4.5 ± 0.5 | 18 ± 2 | 22.5 ± 3 |
| 1373 | 15 | 6.5 ± 0.5 | 23 ± 5 | 29.8 ± 5 |
| T (K) | (μm × s−0.5) | (μm × s−0.5) | ) (μm × s−0.5) | (m2 × s−1) | (m2 × s−1) |
|---|---|---|---|---|---|
| 1273 | 0.015 | 0.076 | 0.061 | 2.33 × 10−16 | 2.33 × 10−14 |
| 1323 | 0.021 | 0.094 | 0.073 | 4.25 × 10−16 | 2.92 × 10−14 |
| 1373 | 0.027 | 0.125 | 0.098 | 7.16 × 10−16 | 5.45 × 10−14 |
| Substrate | Boriding Methods | Tβ | T(K) | (m2 × s−1) | (m2 × s−1) |
|---|---|---|---|---|---|
| Ti5Al2Sn3.7Zr0.3Mo0.3Si (near-α type) [Present] | Pack boriding | 1293 [20] | 1273 | 2.33 × 10−16 | 2.33 × 10−14 |
| 1323 | 4.25 × 10−16 | 2.92 × 10−14 | |||
| 1373 | 7.16 × 10−16 | 5.45 × 10−14 | |||
| Cp−Ti [18] (α-type) | Pack boriding | 1183 | 1123 | 1.34 × 10−16 | 6.13 × 10−15 |
| 1223 | 6.92 × 10−16 | 3.25 × 10−14 | |||
| 1323 | 2.78 × 10−15 | 1.34 × 10−13 | |||
| Ti6Al2Zr1Mo1V [3] (α-type) | Pack boriding | 1253 | 1193 | 3.72 × 10−17 | 9.64 × 10−16 |
| 1273 | 1.25 × 10−16 | 3.94 × 10−15 | |||
| 1313 | 2.89 × 10−16 | 9.33 × 10−15 | |||
| 1353 | 5.22 × 10−16 | 1.81 × 10−14 | |||
| Ti5Al2.5Sn [19] (α-type, TA7) | Pack boriding | 1298 | 1248 | 2.45 × 10−14 | 1.09 × 10−15 |
| 1273 | 3.31 × 10−14 | 2.26 × 10−15 | |||
| 1298 | 3.87 × 10−14 | 3.49 × 10−15 | |||
| 1323 | 4.42 × 10−14 | 8.05 × 10−15 | |||
| 1348 | 5.22 × 10−14 | 1.10 × 10−14 | |||
| Ti6Al4V [10] (α + β type, TC4) | Plasma paste boriding | 1183 [4] | 973 | 5.08 × 10−15 | 1.90 × 10−15 |
| 1023 | 7.25 × 10−15 | 4.32 × 10−15 | |||
| 1073 | 10.18 × 10−15 | 9.13 × 10−15 | |||
| Ti5Mo5V8Cr3Al (β-type,TB2) [13] | Pack boriding | 1223 | 6.47 × 10−15 | 2.79 × 10−14 | |
| - | 1273 | 9.93 × 10−15 | 5.01 × 10−14 | ||
| 1323 | 1.27 × 10−14 | 9.24 × 10−14 | |||
| 1373 | 1.88 × 10−14 | 1.50 × 10−13 | |||
| Ti5Mo5V8Cr3Al (β-type, TB2) [17] | Pack boriding | - | 1223 | 4.20 × 10−14 | 7.35 × 10−14 |
| 1273 | 8.42 × 10−14 | 1.06 × 10−13 | |||
| 1323 | 1.39 × 10−13 | 1.55 × 10−13 | |||
| 1373 | 2.05 × 10−13 | 1.93 × 10−13 |
| Materials | Bording Method | Model | T (K) | (kJ/mol) | (kJ/mol) |
|---|---|---|---|---|---|
| Ti5Al2Sn3.7Zr0.3Mo0.3Si (near α-type) [present study] | Pack boriding | Parabolic model | 1273–1373 | 166.4 | 122.8 |
| Cp−Ti [15] (α-type) | Plasma paste boriding | Parabolic model | 973–1073 | 137.6 | 55.2 |
| Cp−Ti [32] (α-type) | CRTD based boriding | 1173–1373 | 189.9 | - | |
| Ti6Al2Zr1Mo1V [3] (near α-type) | Pack boriding | 1193–1353 | 223.1 | 246.9 | |
| Ti5Al2.5Sn [19] (α-type, TA7) | Pack boriding | 1248–1348 | 94.8 | 221.2 | |
| Ti6Al4V [16] (α + β type, TC4) | Pack boriding | 1273–1373 | |||
| Ti6Al4V [10] (α + β type, TC4) | Plasma paste bording | Parabolic model | 973–1073 | 136.2 | 63.8 |
| Ti6Al4V [33] (α + β type, TC4) | Sigma fiber composites | 1143–1243 | 187 | 190 | |
| Ti5Mo5V8Cr3Al [17] (β-type, TB2) | Pack boriding | Parabolic model | 1223–1373 | 158.1 | 96.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, R.; Wang, W.; Hu, B.; Hu, H. Characterization and Growth Kinetics of Borides Layers on Near-Alpha Titanium Alloys. Materials 2024, 17, 4815. https://doi.org/10.3390/ma17194815
Piao R, Wang W, Hu B, Hu H. Characterization and Growth Kinetics of Borides Layers on Near-Alpha Titanium Alloys. Materials. 2024; 17(19):4815. https://doi.org/10.3390/ma17194815
Chicago/Turabian StylePiao, Rongxun, Wensong Wang, Biao Hu, and Haixia Hu. 2024. "Characterization and Growth Kinetics of Borides Layers on Near-Alpha Titanium Alloys" Materials 17, no. 19: 4815. https://doi.org/10.3390/ma17194815







