Leaching Thermodynamics of Low-Grade Copper Oxide Ore from [(NH4)2SO4]-NH3-H2O Solution
Abstract
:1. Introduction
2. Materials and Methods
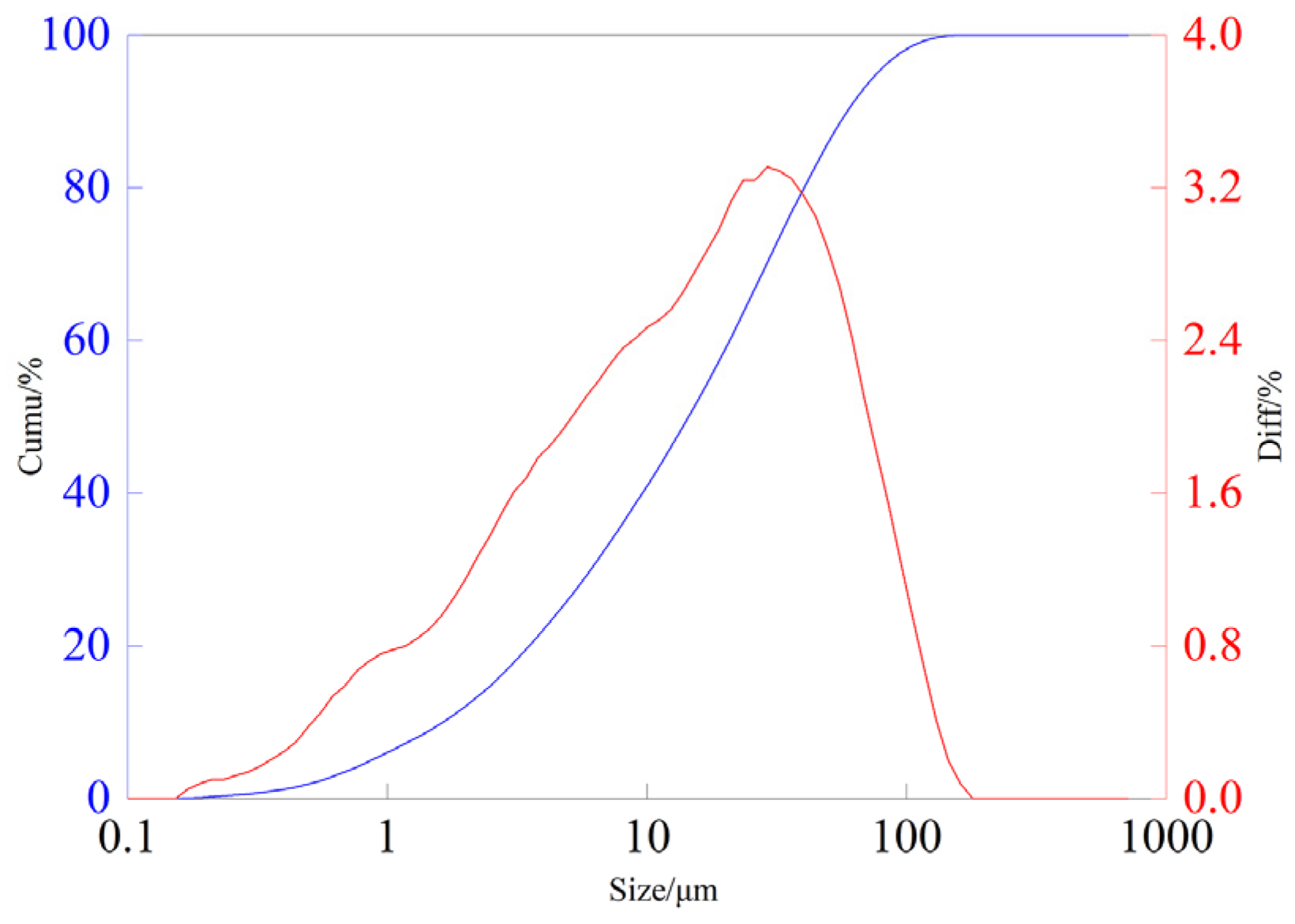
2.1. Experimental Material
2.2. Experimental Method
3. Results
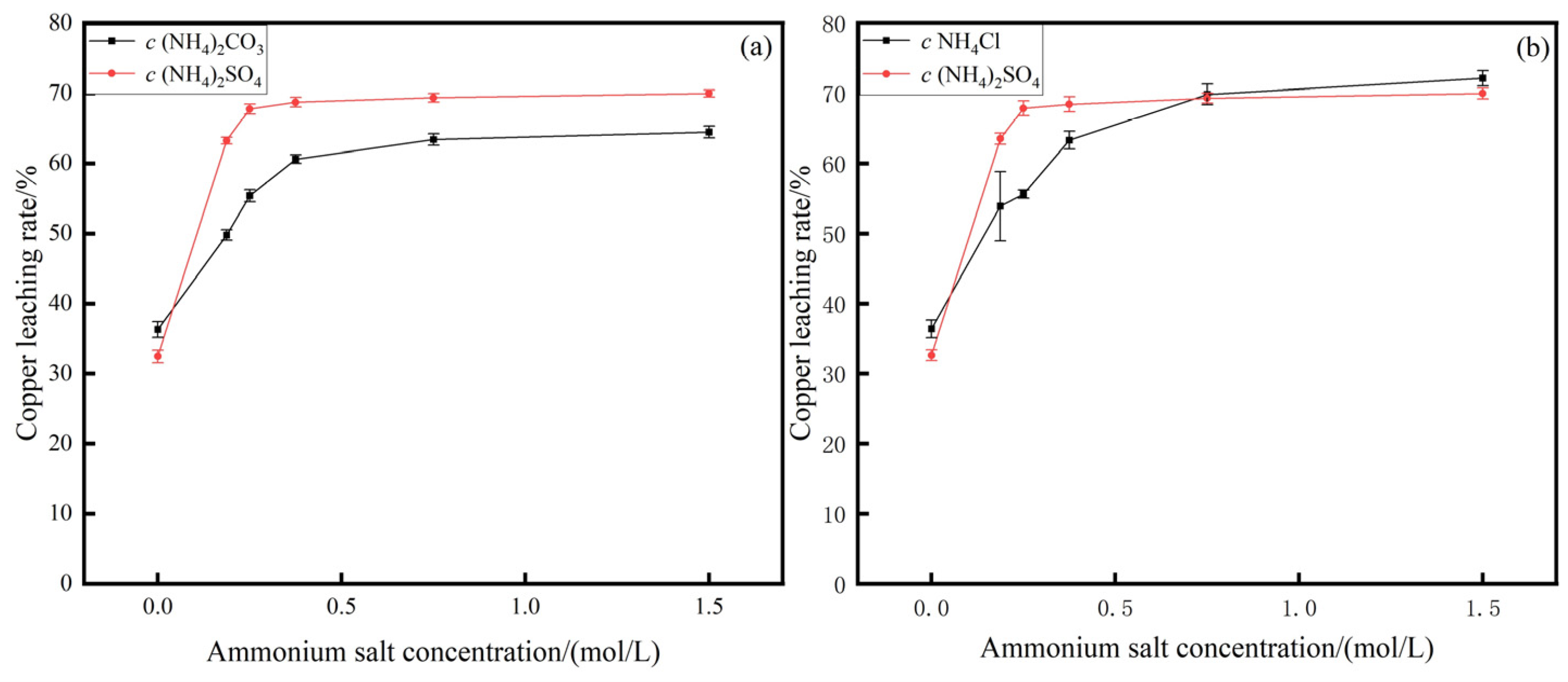
3.1. Influence of Ammonia Concentration on Copper Leaching Rate
3.2. Structure Characterization of Leaching Residue
4. Discussion
4.1. Leaching Principle
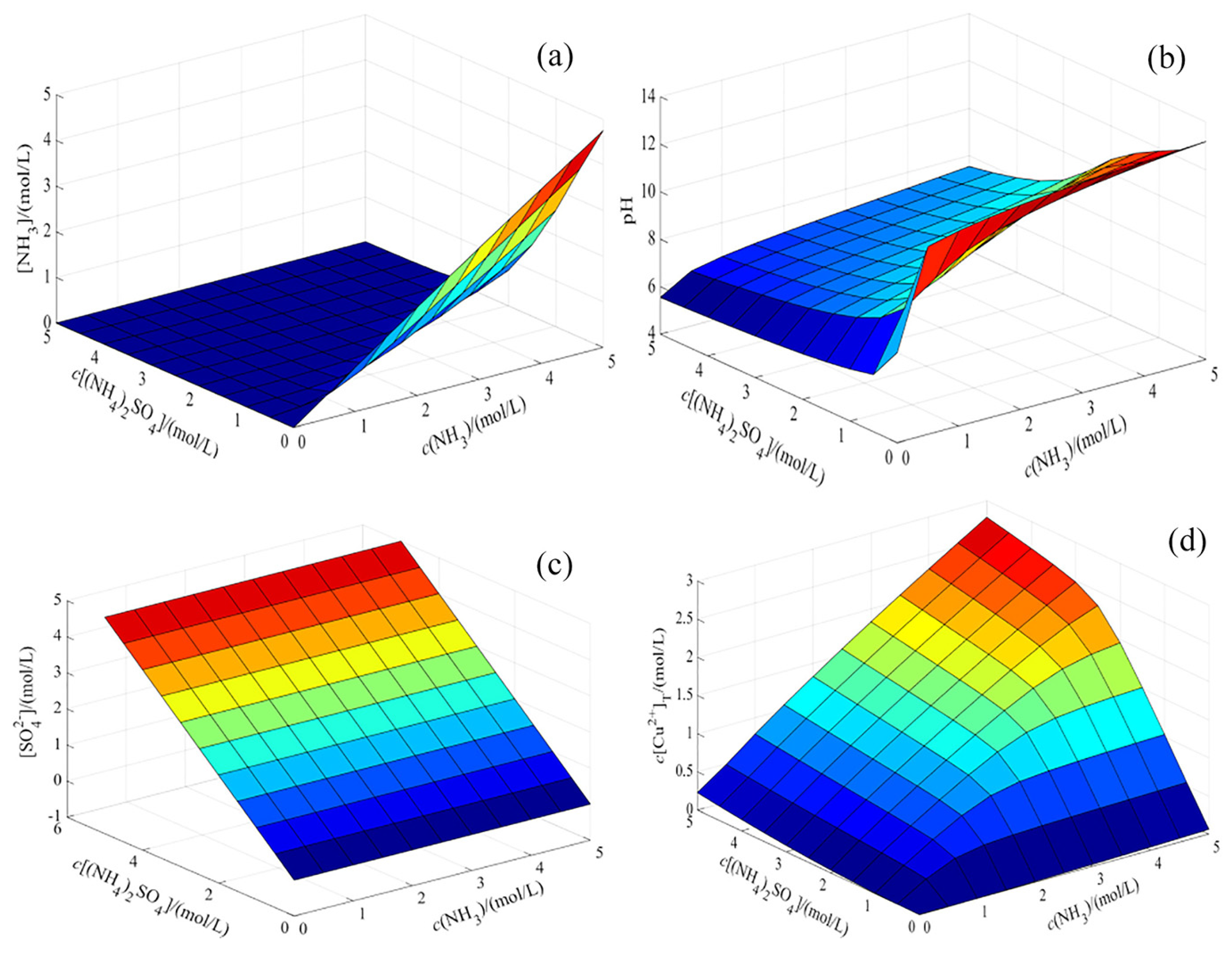
4.2. Thermodynamic Analysis
Establishment of Thermodynamic Model
- (1)
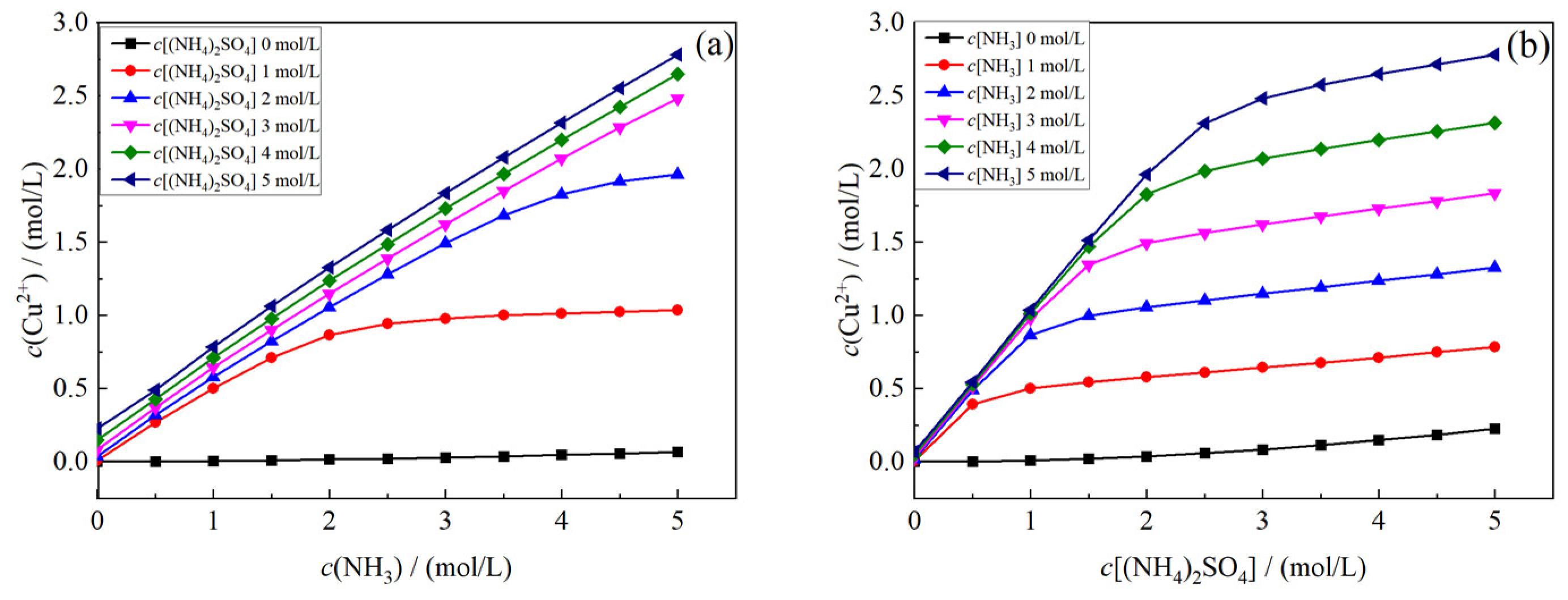
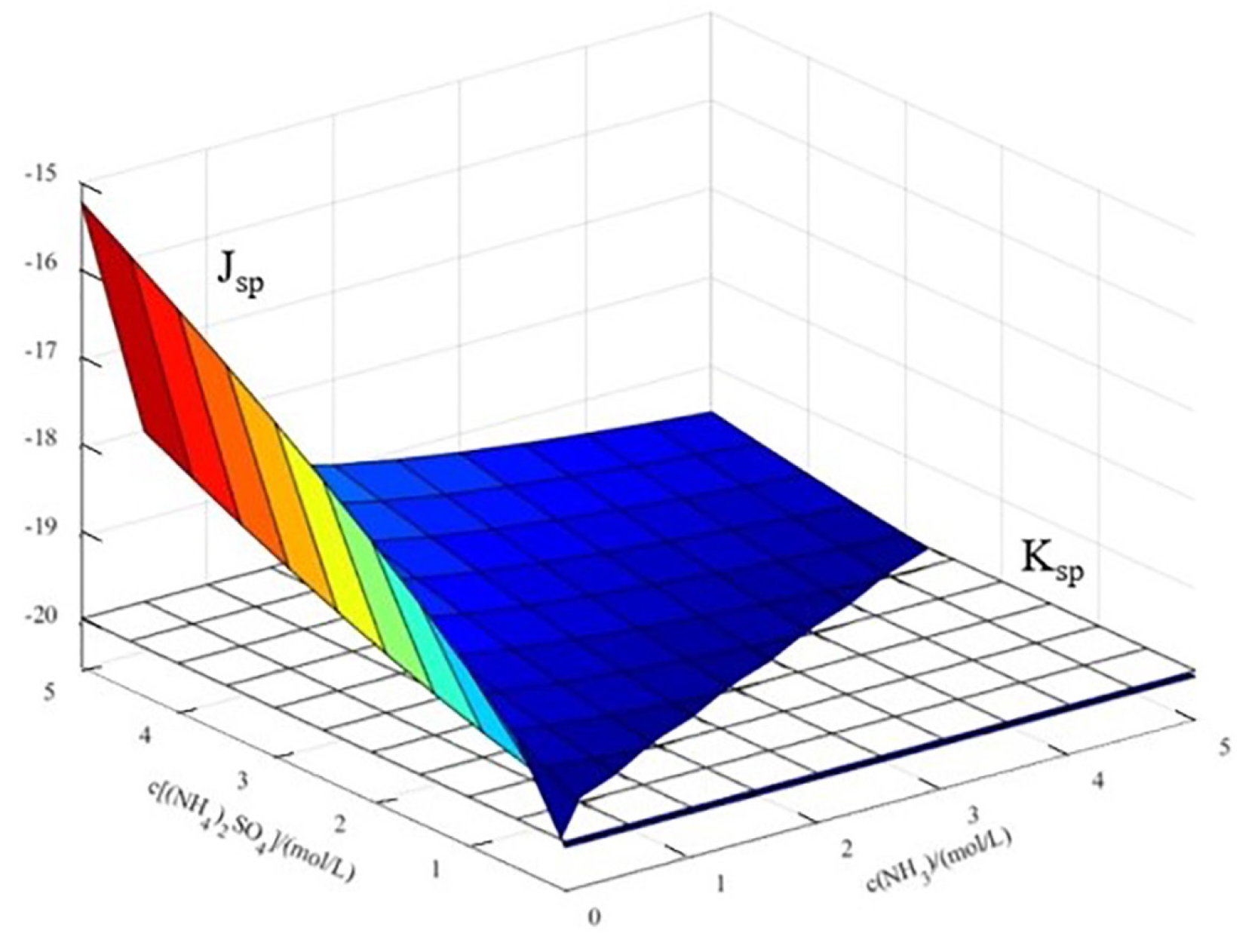
- Effects of c(NH3) and c[(NH4)2SO4] on the concentration of Cu2+
- (2)
- Stable region map
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, T.; Xu, J.Y. Review of development status of copper-cobalt mining in Congo. Chin. J. Nonferrous Met. 2021, 31, 3422. [Google Scholar]
- Pan, C.C.; Gaur, A.P.S. Enhanced electrical conductivity in graphene-copper multilayer composite. AIP Adv. 2022, 12, 6. [Google Scholar] [CrossRef]
- Liu, C.H.; Fan, F.Y. Copper resources supply and consumption patterns and trend in the Belt and Road Region. Conserv. Util. Miner. Resour. 2018, 2, 44. [Google Scholar]
- Han, J.; Xia, P. An analysis of China’s copper resources supply situation in the Post-COVID-19Era. Acta Geosci. Sin. 2021, 42, 223. [Google Scholar]
- Pietrzyk, S.; Tora, B. Trends in global copper mining—A review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Zawiercie, Poland, 2018. [Google Scholar]
- Chen, L.; He, H.P. Experimental study on benefication of one low-grade copper oxide in Xinjiang. Yunnan Metall. 2021, 50, 18. [Google Scholar]
- Jiang, S.Q. Distribution of copper resources in the word. World Nonferrous Met. 2018, 2, 1. [Google Scholar]
- Wang, C.Y. Situation and developing trend of copper hydrometallurgy in China. Xinjiang Geol. 2001, 19, 281. [Google Scholar]
- Hao, M.; Wang, P. Spatial distribution of copper in-use stocks and flows in China: 1978–2016. J. Clean Prod. 2020, 261, 8. [Google Scholar] [CrossRef]
- Han, J.W.; Liu, W. Influence of NH4HF2 activation on leaching of low-grade complex copper ore in NH3-NH4Cl solution. Sep. Purif. Technol. 2017, 181, 29. [Google Scholar] [CrossRef]
- Li, Q.; Gao, W. Direct reduction leaching of a low-grade copper-cobalt oxide ore. Min. Metall. 2019, 28, 60. [Google Scholar]
- Deng, J.S.; Wen, S.M. Extracting copper from copper oxide ore by a zwitterionic reagent and dissolution kinetics. Int. J. Min. Met. Mater. 2015, 22, 241. [Google Scholar] [CrossRef]
- Bingöl, D.; Canbazoğlu, M. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching. Hydrometallurgy 2005, 76, 55. [Google Scholar] [CrossRef]
- Gargul, K. Ammonia leaching of slag from direct-to-blister copper smelting technology. AIMS Mater. Sci. 2020, 7, 565. [Google Scholar] [CrossRef]
- Çalban, T.; Çolak, S. Optimization of leaching of copper from oxidized copper ore in NH3-(NH4)2SO4 medium. Chem. Eng. Commun. 2005, 192, 1515. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yin, Z.L.; Hu, H.P.; Chen, Q.Y. Leaching kinetics of low-grade copper ore containing calcium-magnesium carbonate in ammonia-ammonium sulfate solution with persulfate. Trans. Nonferrous Met. Soc. China 2012, 22, 2822. [Google Scholar] [CrossRef]
- Liu, W.; Tang, C.B.; He, J.; Yang, S.H.; Yang, J.G.; Chen, Y.M. Thermodynamic Research of Leaching Copper Oxide Materials with Ammonia-ammonium Chloride-water Solution. Can. Metall. Q. 2010, 49, 131–145. [Google Scholar]
- Cao, C.F.; Wang, X. Thermodynamic analysis of leaching malachite in CuO-CO2-NH3-H2O system. Nonferr. Met. Sci. Eng. 2014, 5, 82. [Google Scholar]
- Liu, W.; Tang, M.T.; Tang, C.B.; He, J.; Yang, S.H.; Yang, J.G. Thermodynamics of solubility of Cu2(OH)2CO3 in ammonia-ammonium chloride-ethylenediamine(En)-water system. Trans. Nonferrous Met. Soc. China 2010, 20, 336. [Google Scholar] [CrossRef]
- Robert, M.S.; Arthur, E.M. Critical Stability Constants, 1st ed.; Plenum Press: New York, NY, USA; London, UK, 1976; p. 31. [Google Scholar]
- Li, Y.; Kawashima, N. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface 2013, 197, 1. [Google Scholar] [CrossRef]
- Mu, W.; Yang, R.M.; Meng, J.J.; Li, M.; Lei, X.F.; Luo, S.H. Eco-friendly and selective separation of nickel and copper from low-grade nickel sulfide ore via sulfur fixation roasting with MgCO3 followed by sulfuric acid leaching. Sep. Purif. Technol. 2024, 127, 94. [Google Scholar] [CrossRef]
- Owen, N.D.; Ram, R.; Vollert, L.; Seaman, B.; Etschmann, B.; Xing, Y.L.; Rosemann, M.; Verdugo, L.; O’Callaghan, J.; Brugger, J. The deleterious role of gangue mineralogy in copper extraction: A case study of poor recovery in leaching low-grade Cu ores. Appl. Geochem. 2024, 105, 984. [Google Scholar] [CrossRef]
- Chen, W.; Tang, H.Y.; Yin, S.H. Bioleaching of low-grade copper sulfide enhanced by nutrients from sterilized medical waste. Process Saf. Environ. Prot. 2024, 188, 1527. [Google Scholar] [CrossRef]
- Cao, J.; Wang, R.B.; Zhang, Y.; Jia, W.B.; Zhong, C.G.; Chen, R.; Qu, J.H.; Feng, Y.Y.; Hao, J. MCNP simulation and experimental study in situ low-grade copper analysis based on PGNAA. Appl. Radiat. Isot. 2024, 206, 111224. [Google Scholar]
- Chen, W.; Tang, H.Y.; Yin, S.H.; Wang, L.M.; Zhang, M. Copper recovery from low-grade copper sulfides using bioleaching and its community structure succession in the presence of Sargassum. J. Environ. Manag. 2024, 349, 119549. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.X.; Peng, Y.; Sun, S.C.; Tu, G.F. Leaching of High-Alkaline Gangue Low-Grade Copper Oxide Ore in (NH4)2SO4-NH3-H2O Solution. J. Northeast. Univ. (Nat. Sci.) 2021, 42, 795. [Google Scholar]
- Lalegania, Z.; Ebrahimia, S.S.; Hamawandib, B.; Spadac, L.L.; Batilib, H.; Toprak, M.S. Targeted dielectric coating of silver nanoparticles with silica to manipulate optical properties for metasurface applications. Mater. Chem. Phys. 2022, 287, 126250. [Google Scholar] [CrossRef]

| SiO2 | CaO | MgO | Al2O3 | K2O | Fe2O3 | CuO |
|---|---|---|---|---|---|---|
| 56.1202 | 18.3875 | 11.6775 | 8.5244 | 1.6679 | 1.3900 | 1.2818 |
| TiO2 | P2O5 | Co2O3 | MnO | SO3 | ZrO2 | Na2O |
| 0.5054 | 0.1725 | 0.1199 | 0.1054 | 0.0308 | 0.0141 | 0.0023 |
| Type | Stability Constant lgβ |
|---|---|
| Cu(NH3)2+ | 4.24 |
| Cu(NH3)22+ | 7.83 |
| Cu(NH3)32+ | 10.80 |
| Cu(NH3)42+ | 13.00 |
| Cu(NH3)52+ | 12.43 |
| Cu(OH)+ | 6.30 |
| Cu(OH)2(aq) | 12.80 |
| Cu(OH)3− | 14.50 |
| Cu(OH)42− | 15.60 |
| Cu2(OH)22+ | 17.28 |
| Cu(NH3)3(OH)+ | 14.90 |
| Cu(NH3)2(OH)2(aq) | 15.70 |
| Cu(NH3)(OH)3− | 16.30 |
| HCO3− | 9.56 |
| H2CO3 | 15.98 |
| NH4+ | 9.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, F.; Cao, X.; Luo, X.; Tu, G.; Yang, C.; Peng, Y.; Li, H.; Xu, W.; Wang, S. Leaching Thermodynamics of Low-Grade Copper Oxide Ore from [(NH4)2SO4]-NH3-H2O Solution. Materials 2024, 17, 4821. https://doi.org/10.3390/ma17194821
Xiao F, Cao X, Luo X, Tu G, Yang C, Peng Y, Li H, Xu W, Wang S. Leaching Thermodynamics of Low-Grade Copper Oxide Ore from [(NH4)2SO4]-NH3-H2O Solution. Materials. 2024; 17(19):4821. https://doi.org/10.3390/ma17194821
Chicago/Turabian StyleXiao, Faxin, Xinyu Cao, Xuwei Luo, Ganfeng Tu, Cuixia Yang, Yu Peng, Hui Li, Wei Xu, and Shuo Wang. 2024. "Leaching Thermodynamics of Low-Grade Copper Oxide Ore from [(NH4)2SO4]-NH3-H2O Solution" Materials 17, no. 19: 4821. https://doi.org/10.3390/ma17194821






