Synergistic Effects of Ternary Microbial Self-Healing Agent Comprising Bacillus pasteurii, Saccharomyces cerevisiae, and Bacillus mucilaginosus on Self-Healing Performance in Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Medium
2.2. Preparation of Ternary Microorganism Self-Healing Agent
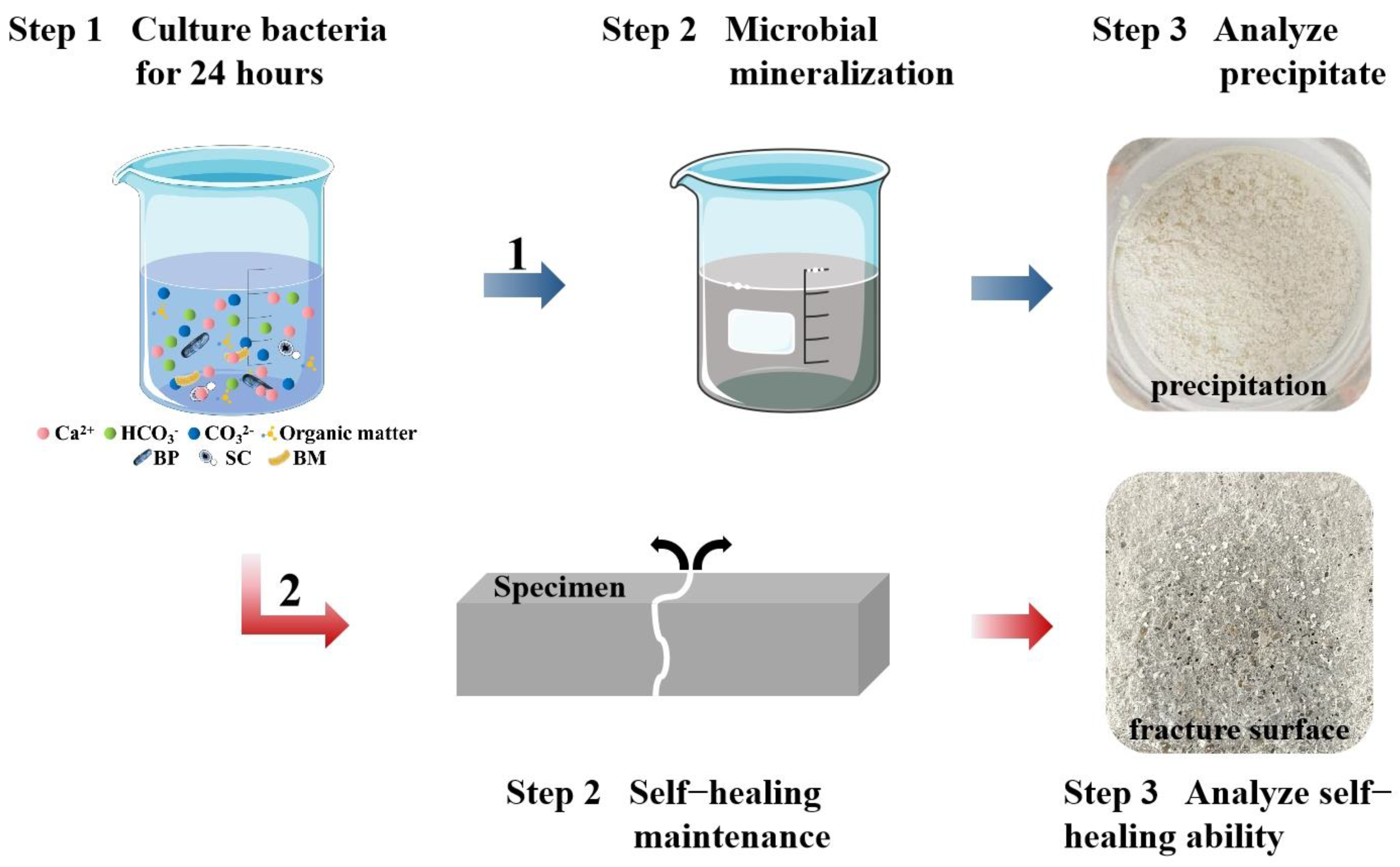
2.3. Microbial Mineralization
2.4. Preparation of Mortar Specimens and Cracks Creation
2.5. Characterization Methods
2.5.1. The pH Value and Cell Concentration of Microorganism Suspensions
2.5.2. Microbial Mineralization Products
2.5.3. Crack Self-Healing Ability of Ternary Microbial Self-Healing Agent
3. Results and Discussion
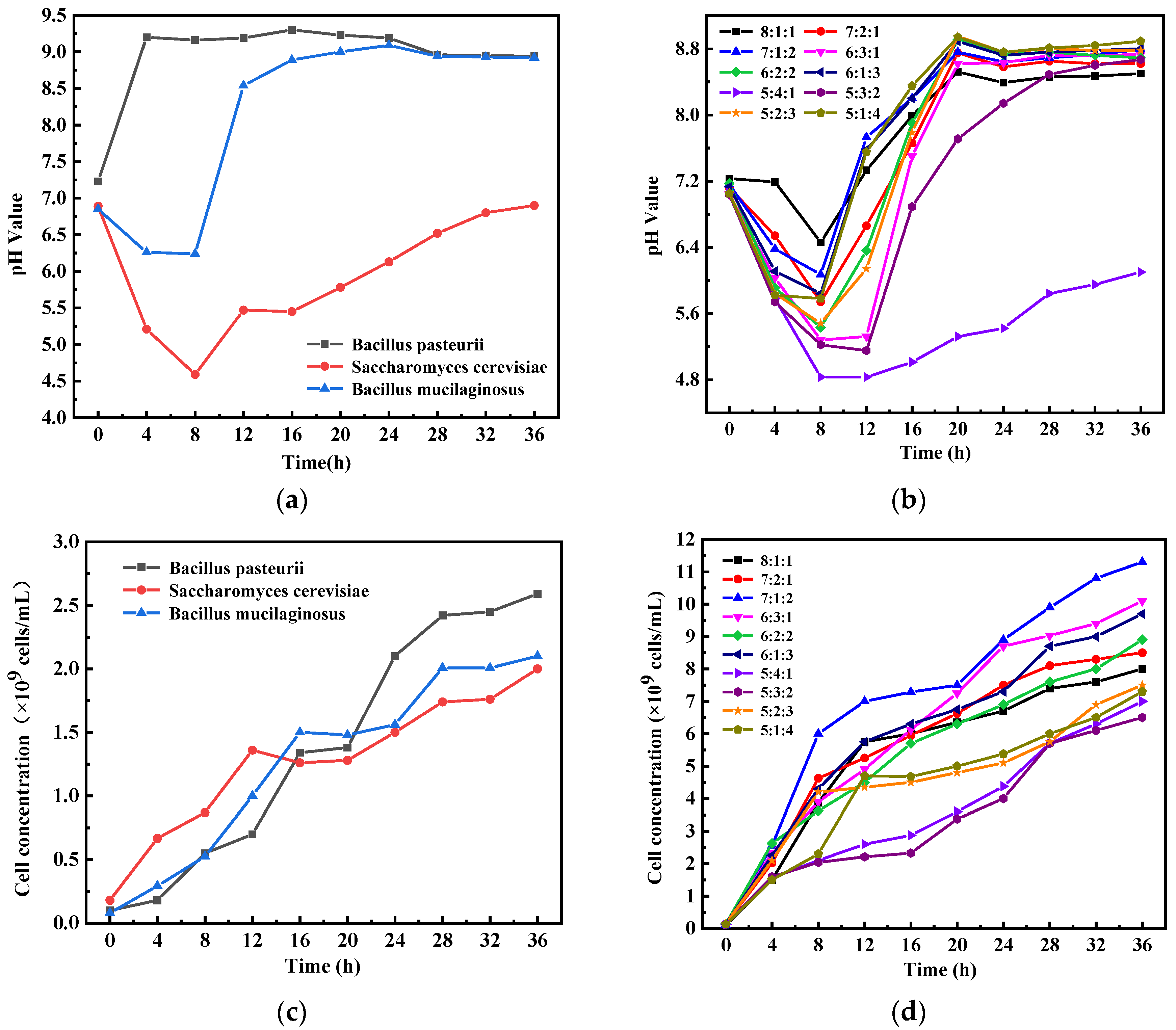
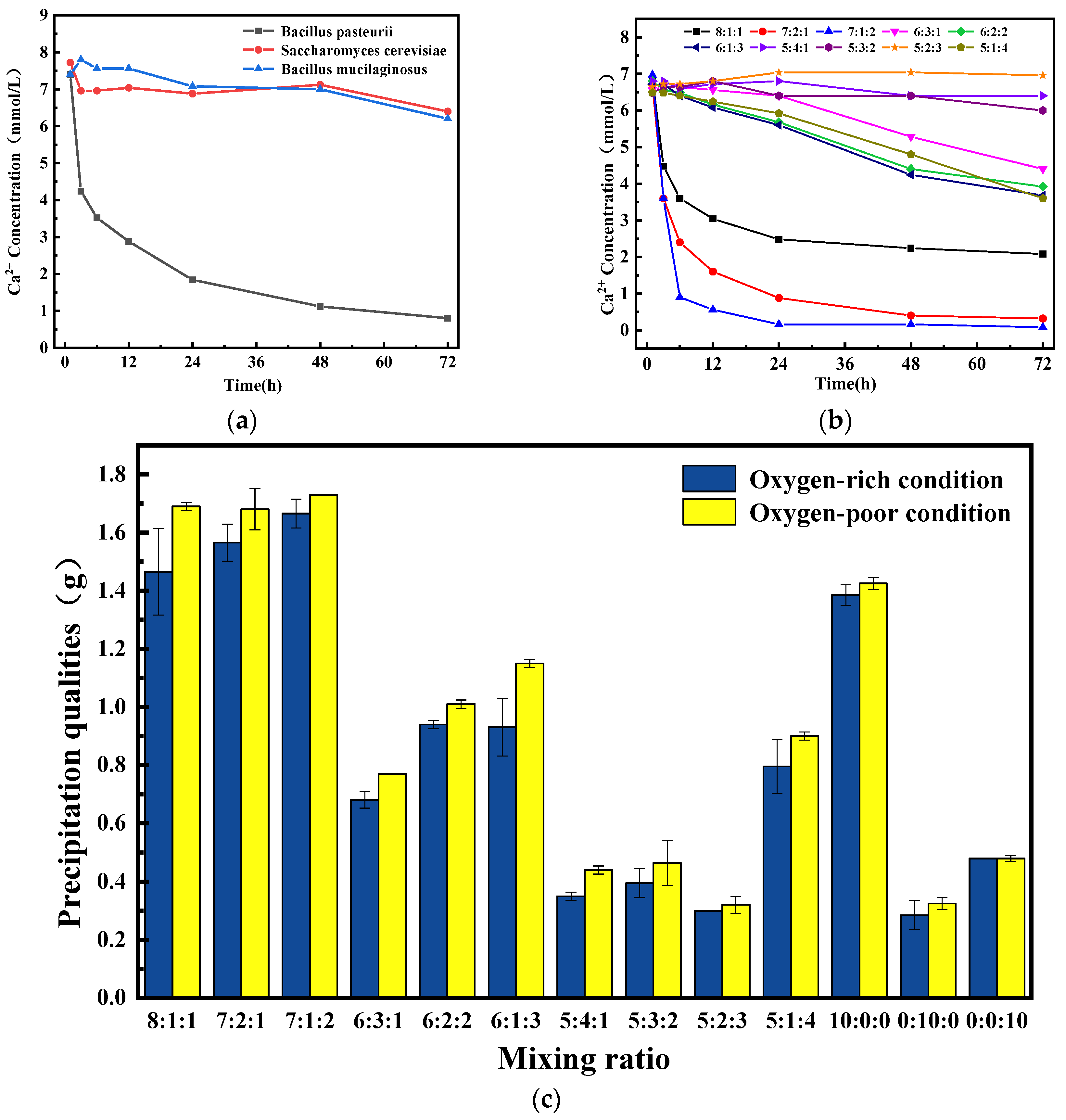
3.1. Optimal Mixing Ratio to Ternary Microorganism
3.1.1. Growth Law of Ternary Microorganism System
3.1.2. Microbial-Induced CaCO3 Precipitation
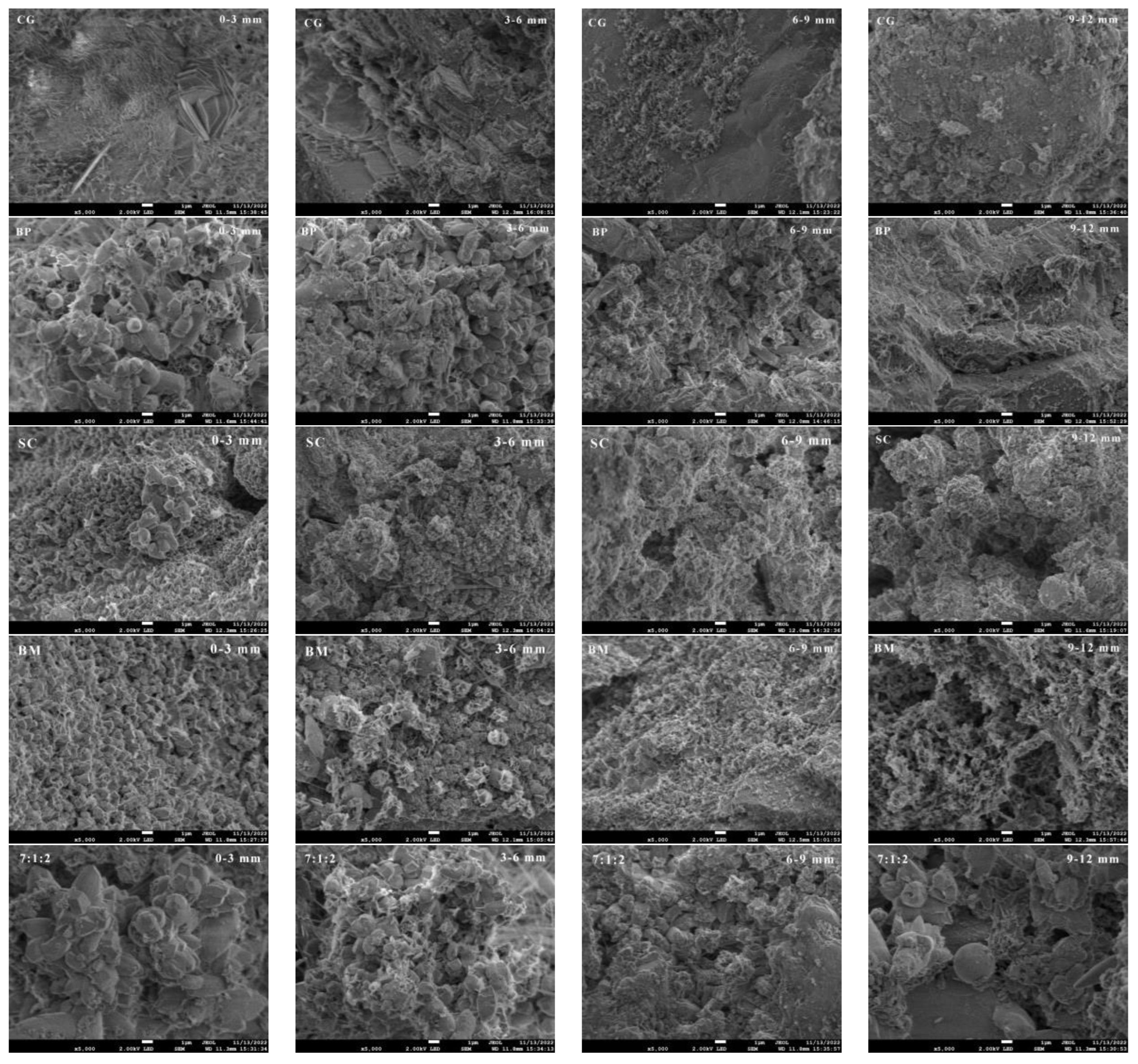
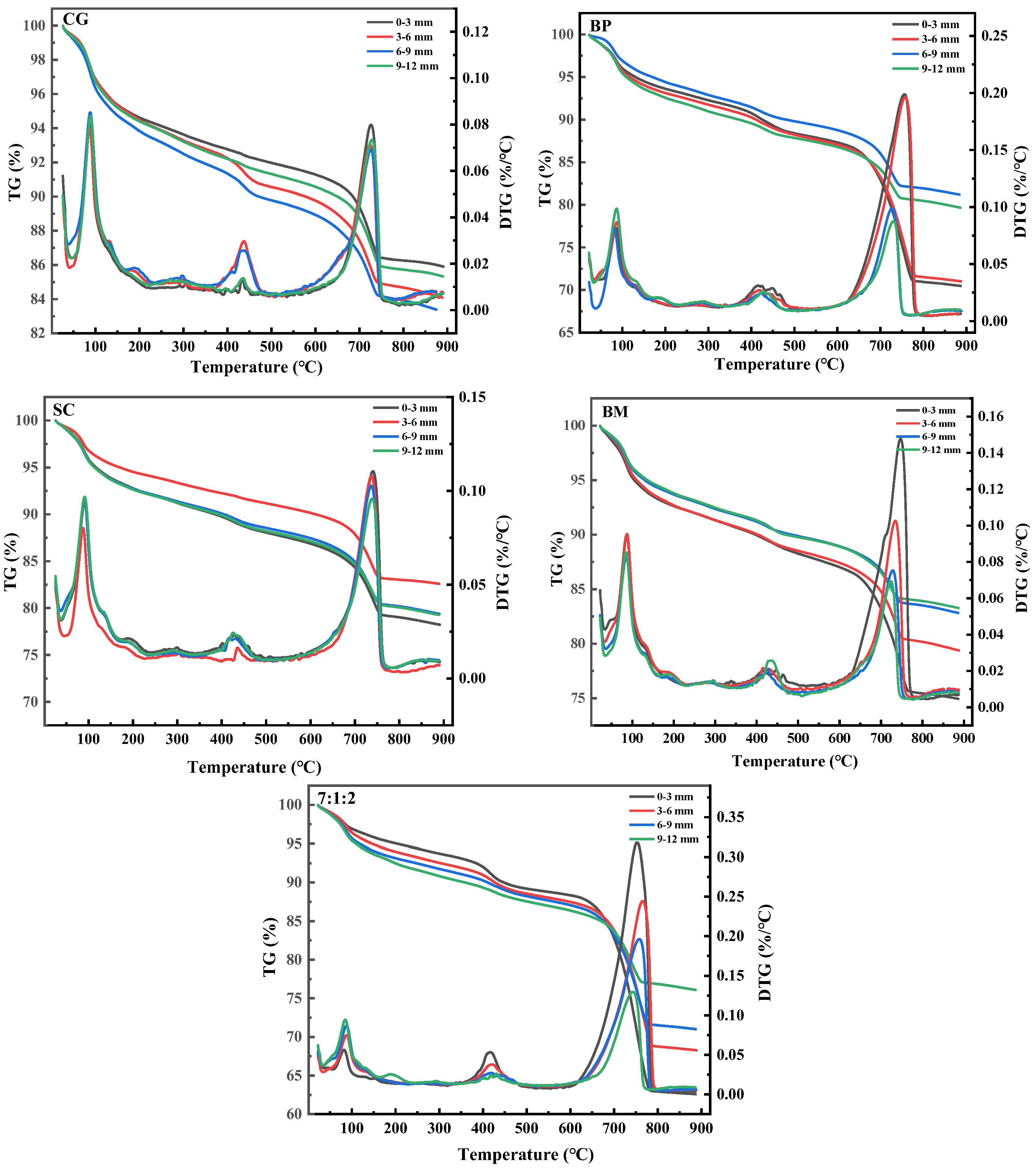
3.1.3. Composition and Microstructures of Microbial Mineralization Products
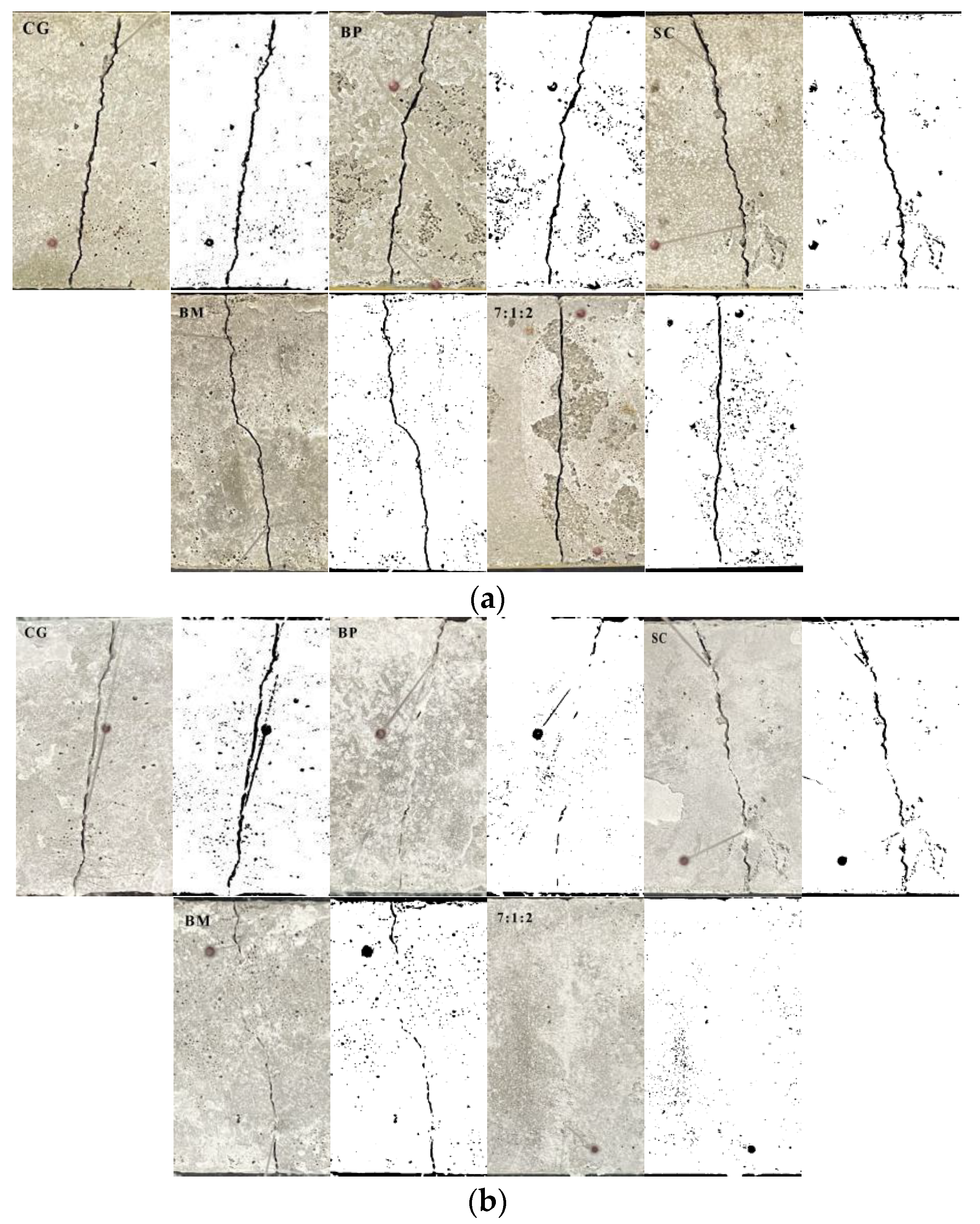
3.2. Self-Healing of Cracks with Ternary Microorganism System
3.2.1. Percentage of Repair Surface Area
3.2.2. Effect of Self-Healing Cracks at the Depth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, F.B.D.; Belie, N.D.; Boon, N.; Verstraete, W. Production of non-axenic ureolytic spores for self-healing concrete applications. Constr. Build. Mater. 2015, 93, 1034–1041. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X. Self-healing of concrete cracks by use of bacteria-containing low alkali cementitious material. Constr. Build. Mater. 2018, 167, 1–14. [Google Scholar] [CrossRef]
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Belie, N.D.; Gruyaert, E.; Tabbaa, A.A. A review of self-healing concrete for damage management of structures. Adv. Mater. Interfaces 2018, 5, 1800074. [Google Scholar] [CrossRef]
- Sangadji, S.; Wiktor, V.; Jonkers, H.; Schlangen, E. The use of alkaliphilic bacteria-based repair solution for porous network concrete healing mechanism. Procedia Eng. 2021, 171, 606–613. [Google Scholar] [CrossRef]
- Chetty, K.; Garbe, U.; McCarthy, T.; Hai, F.S.; Jiang, G.M. Long-term self-healing efficiency of bioconcrete based on integrated sulfate- and nitrate-reducing bacterial granules. J. Mater. Civ. Eng. 2023, 35, 04023305. [Google Scholar] [CrossRef]
- Rosewitz, J.A.; Wang, S.; Scarlata, S.F.; Nima, R. An enzymatic self-healing cementitious material. Appl. Mater. Today 2021, 23, 101035. [Google Scholar] [CrossRef]
- Luo, J.; Chen, X.B.; Crump, J.; Zhou, H.; Davies, D.G.; Zhou, G.W.; Zhang, N.; Jin, C.R. Interactions of fungi with concrete: Significant importance for bio-based self-healing concrete. Constr. Build. Mater. 2018, 164, 275–285. [Google Scholar] [CrossRef]
- Seifan, M.; Ebrahiminezhad, A.; Ghasemi, Y.; Berenjian, A. Microbial calcium carbonate precipitation with high affinity to fill the concrete pore space: Nanobiotechnological approach. Bioproc. Biosyst. Eng. 2019, 42, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Ghorbel, E.; Fares, H.; Cousture, A. Bacterial self-healing of concrete and durability assessment. Cem. Concr. Comp. 2019, 104, 103340. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Comp. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Rong, H.; Wei, G.; Ma, G.; Zhang, Y.; Zheng, X.; Zhang, L.; Xu, R. Influence of bacterial concentration on crack self-healing of cement-based materials. Constr. Build. Mater. 2020, 244, 118372. [Google Scholar] [CrossRef]
- Zhang, J.L.; Mai, B.X.; Cai, T.G.; Luo, J.Y.; Wu, W.H.; Liu, B.; Han, N.X.; Xing, F.; Deng, X. Optimization of a binary concrete crack self-healing system containing bacteria and oxygen. Materials 2017, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Wang, C.G.; Wang, Q.L.; Feng, J.L.; Pan, W.; Zheng, X.C.; Liu, B.; Han, N.X.; Xing, F.; Deng, X. A binary concrete crack self-healing system containing oxygen-releasing tablet and bacteria and its Ca2+-precipitation performance. Appl. Microbiol. Biotechnol. 2016, 100, 10295–10306. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Belie, N.D.; Boon, N. Complementing urea hydrolysis and nitrate reduction for improved microbially induced calcium carbonate precipitation. Appl. Microbiol. Biotechnol. 2019, 103, 8825–8838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Zhou, A.J.; Liu, Y.Z.; Zhao, B.W.; Luan, Y.B.; Wang, S.F.; Yue, X.P.; Li, Z. Microbial network of the carbonate precipitation process induced by microbial consortia and the potential application to crack healing in concrete. Sci. Rep. 2017, 7, 10–19. [Google Scholar] [CrossRef]
- Jiang, L.; Xia, H.; Hu, S.S.; Zhao, X.B.; Wang, W.J.; Zhang, Y.; Li, Z. Crack-healing ability of concrete enhanced by aerobic-anaerobic bacteria. Cem. Concr. Comp. 2024, 183, 107585. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, L.; Zhang, Y.; Xu, R.; Zheng, X.G.; Rong, H.; Yue, C.S. A Binary Microorganism self-healing agent for concrete cracks comprising Bacillus pasteurii and Saccharomyces cerevisiae. J. Mater. Civ. Eng. 2024, 36, 04023601. [Google Scholar] [CrossRef]
- Qian, C.X.; Chen, H.C.; Ren, L.F.; Luo, M. Self-healing of early age cracks in cement-based materials by mineralization of carbonic anhydrase microorganism. Front. Microbiol. 2015, 6, 1225. [Google Scholar] [CrossRef]
- Zheng, T.W.; Qian, C.X. Influencing factors and formation mechanism of CaCO3 precipitation induced by microbial carbonic anhydrase. Process Biochem. 2020, 91, 271–281. [Google Scholar] [CrossRef]
- Chen, H.C.; Qian, C.X.; Huang, H.L. Self-healing cementitious materials based on bacteria and nutrients immobilized respectively. Constr. Build. Mater. 2016, 126, 297–303. [Google Scholar] [CrossRef]
- Qian, C.X.; Rui, Y.F.; Wang, C.Y.; Wang, X.M.; Xue, B.; Yi, H.H. Bio-mineralization induced by Bacillus mucilaginosus in crack mouth and pore solution of cement-based materials. Mat. Sci. Eng. C 2021, 126, 112120. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Zhang, Q.; Hu, X. Investigation on mineralization performance and spore germination conditions of calcium carbonate mineralizing bacteria. Mater. Res. Express. 2022, 9, 65403. [Google Scholar] [CrossRef]
- Rui, Y.; Qian, C. The regulation mechanism of bacteria on the properties of biominerals. J. Cryst. Growth. 2021, 570, 126214. [Google Scholar] [CrossRef]
- Osman, K.M.; Taher, F.M.; EL-Tawab, A.A.; Faried, A.S. Role of different microorganisms on the mechanical characteristics, self-healing efficiency, and corrosion protection of concrete under different curing conditions. J. Build. Eng. 2021, 41, 102414. [Google Scholar] [CrossRef]
- Zheng, T.W.; Qian, C.X.; Su, Y.L. Influences of different calcium sources on the early age cracks of self-healing cementitious mortar. Biochem. Eng. J. 2021, 166, 107849. [Google Scholar] [CrossRef]
- Carvalho, Â.R.; Bazana, L.C.G.; Ferrão, M.F.; Fuentefria, A.M. Curve fitting and linearization of UV-Vis spectrophotometric measurements to estimate yeast in inoculum preparation. Anal. Biochem. 2021, 625, 114216. [Google Scholar] [CrossRef]
- Jiang, L.; Jia, G.H.; Jiang, C.; Li, Z. Sugar-coated expanded perlite as a bacterial carrier for crack-healing concrete applications. Constr. Build. Mater. 2020, 232, 117222. [Google Scholar] [CrossRef]
- Konstantinou, C.; Biscontin, G.; Jiang, N.; Soga, K. Application of microbially induced carbonate precipitation to form bio-cemented artificial sandstone. J. Rock. Mech. Geotech. Eng. 2021, 13, 579–592. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, R.; Su, H.; Xu, H.; Zhang, L.; Huang, D.; Liang, Z.; Hu, Y.; Zhao, L.; Lian, X. Synthesis and characterization of porous CaCO3 microspheres templated by yeast cells and the application as pH value-sensitive anticancer drug carrier. Colloid. Surf. B. 2021, 199, 111545. [Google Scholar] [CrossRef] [PubMed]


| Beef Extract | Tryptone | Glucose | Yeast Extract | Na2HPO4 | KCl | Sucrose | (NH4)2SO4 | MgSO4 |
|---|---|---|---|---|---|---|---|---|
| 3.00 | 15.00 | 10.00 | 5.60 | 5.00 | 0.28 | 20.00 | 1.04 | 1.01 |
| Cement/g | Water/g | Sand/g | Microorganism Suspension/mL | Substrates/g |
|---|---|---|---|---|
| 650.00 | 300.00 | 1300.00 | 25.00 | 21.02 |
| Calcium Acetate | Urea | Beef Extract | Tryptone | Glucose | Yeast Extract | Na2HPO4 | KCl | (NH4)2SO4 | MgSO4 |
|---|---|---|---|---|---|---|---|---|---|
| 15.00 | 2.90 | 0.24 | 1.14 | 0.75 | 0.42 | 0.39 | 0.02 | 0.08 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Li, J.; Wang, T.; Zhang, L.; Peng, B.; Yue, C. Synergistic Effects of Ternary Microbial Self-Healing Agent Comprising Bacillus pasteurii, Saccharomyces cerevisiae, and Bacillus mucilaginosus on Self-Healing Performance in Mortar. Materials 2024, 17, 4834. https://doi.org/10.3390/ma17194834
Wu Z, Li J, Wang T, Zhang L, Peng B, Yue C. Synergistic Effects of Ternary Microbial Self-Healing Agent Comprising Bacillus pasteurii, Saccharomyces cerevisiae, and Bacillus mucilaginosus on Self-Healing Performance in Mortar. Materials. 2024; 17(19):4834. https://doi.org/10.3390/ma17194834
Chicago/Turabian StyleWu, Zhaoyun, Jiaxuan Li, Tianlei Wang, Lei Zhang, Ben Peng, and Changsheng Yue. 2024. "Synergistic Effects of Ternary Microbial Self-Healing Agent Comprising Bacillus pasteurii, Saccharomyces cerevisiae, and Bacillus mucilaginosus on Self-Healing Performance in Mortar" Materials 17, no. 19: 4834. https://doi.org/10.3390/ma17194834
APA StyleWu, Z., Li, J., Wang, T., Zhang, L., Peng, B., & Yue, C. (2024). Synergistic Effects of Ternary Microbial Self-Healing Agent Comprising Bacillus pasteurii, Saccharomyces cerevisiae, and Bacillus mucilaginosus on Self-Healing Performance in Mortar. Materials, 17(19), 4834. https://doi.org/10.3390/ma17194834





