Functional Silsesquioxanes—Tailoring Hydrophobicity and Anti-Ice Properties of Polylactide in 3D Printing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analyses
2.3. The Procedure for Synthesis of Spherosilicate-Based Derivatives
2.4. Preparation of PLA/OSS Composite Masterbatches
2.5. Preparation of Filament
2.6. 3D Printing (FDM)
3. Results and Discussion
3.1. Chemical Characterization of Modifiers
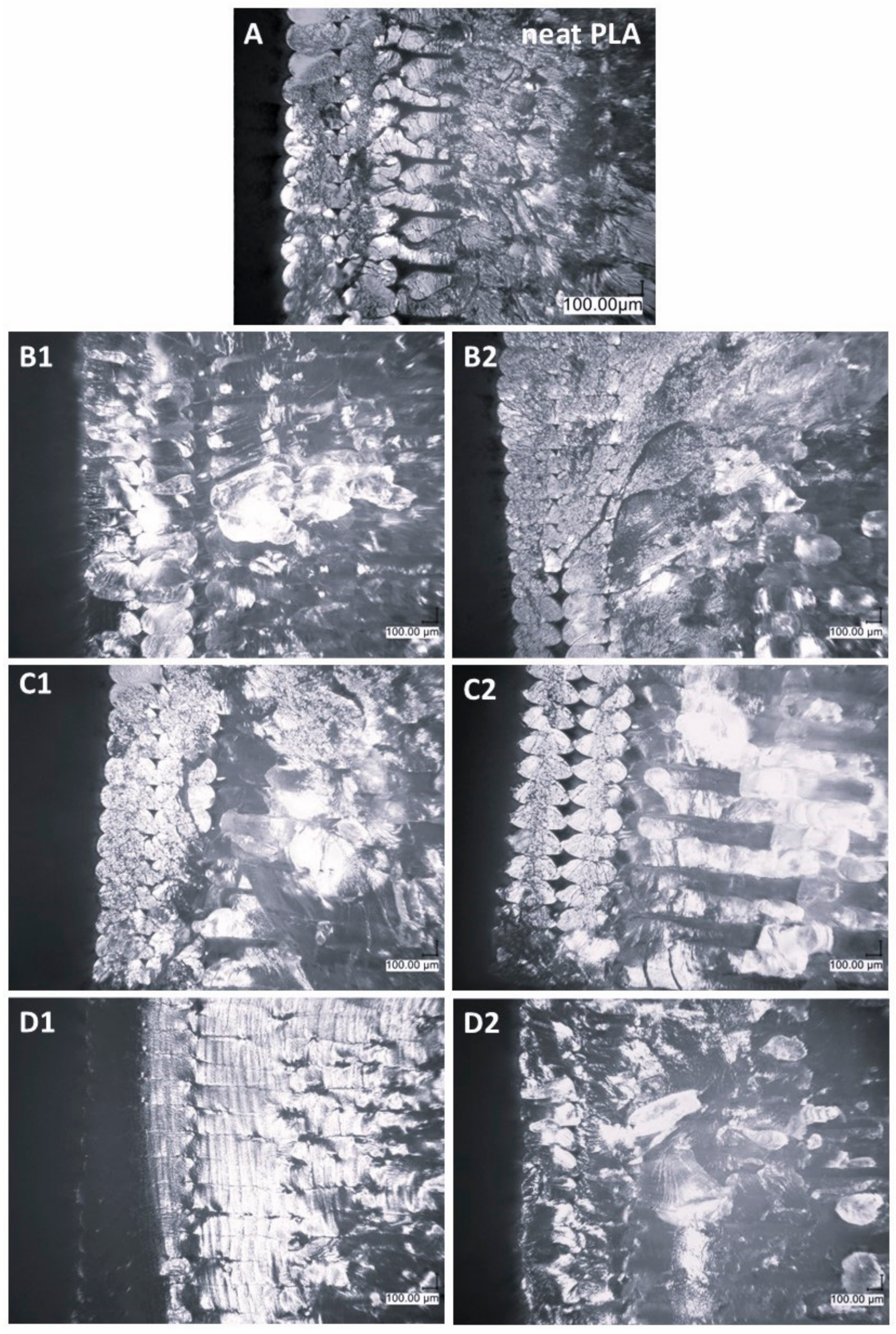
3.2. Microscopy
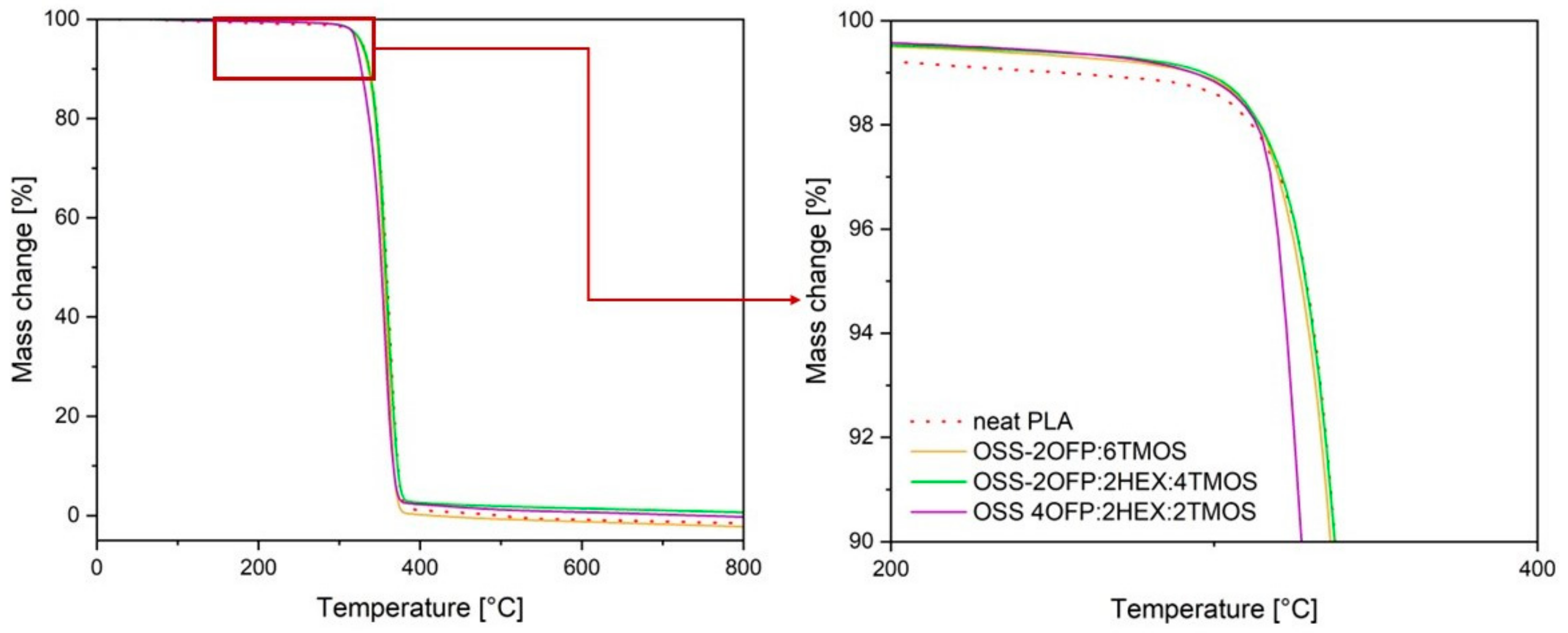
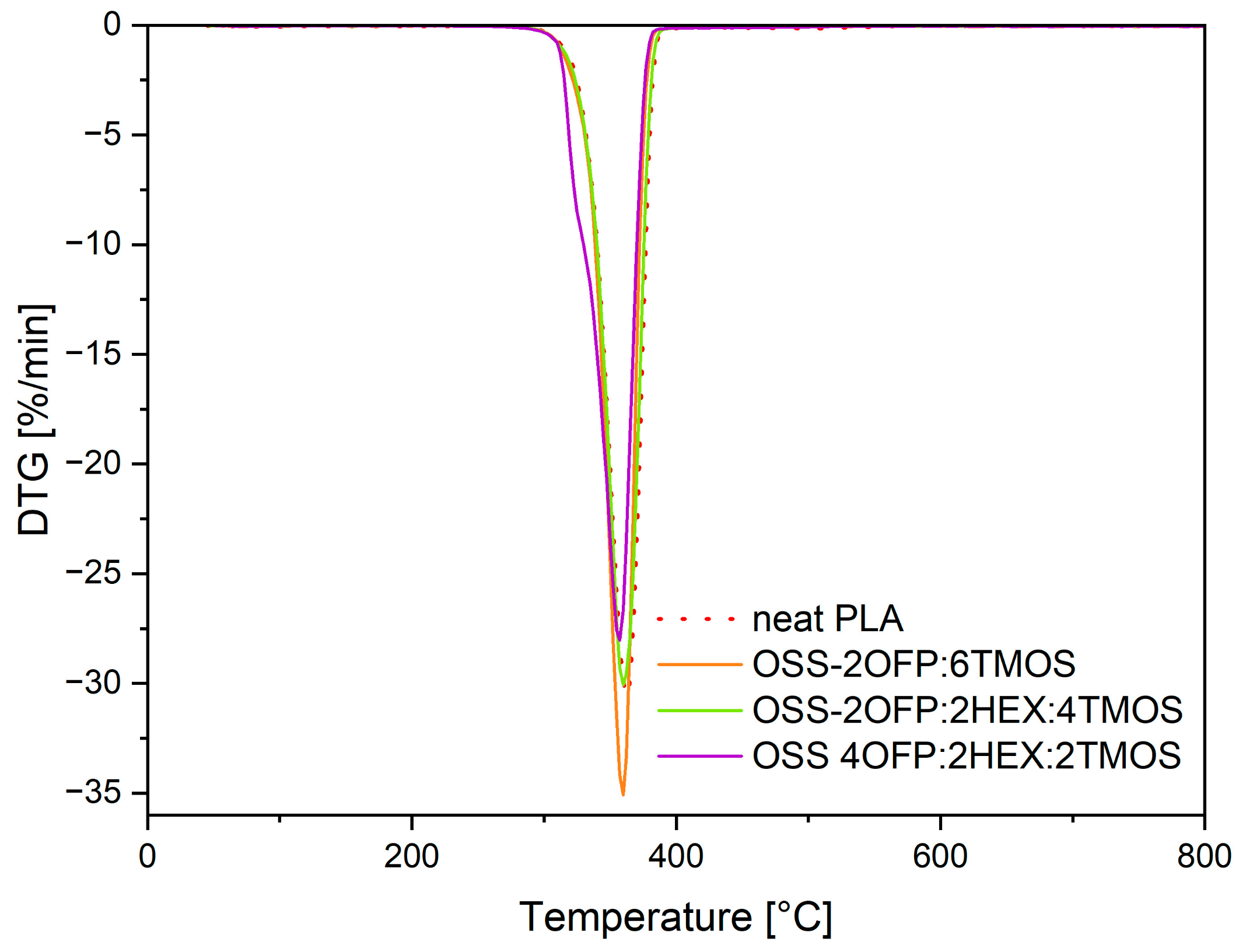
3.3. Thermal Analysis Results
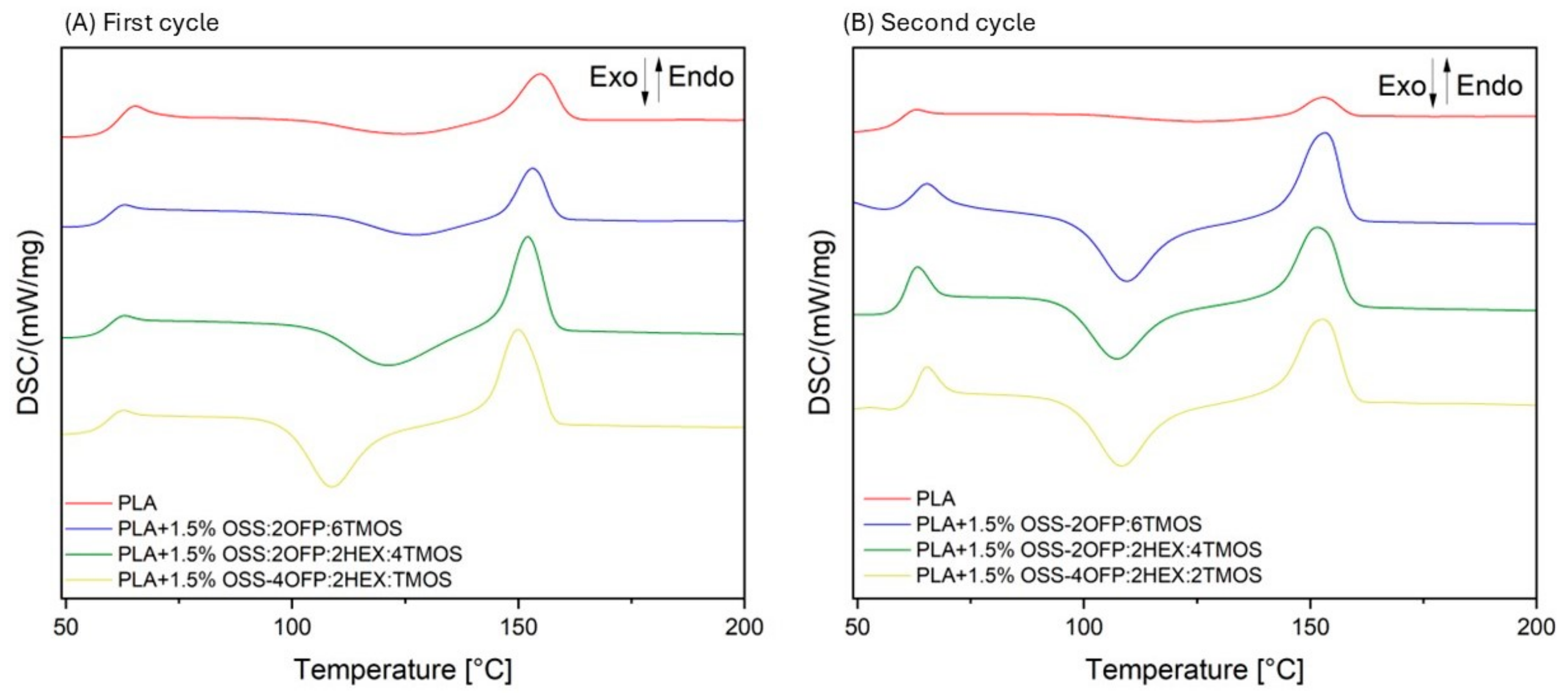
3.4. Differential Scanning Calorimetry (DSC)
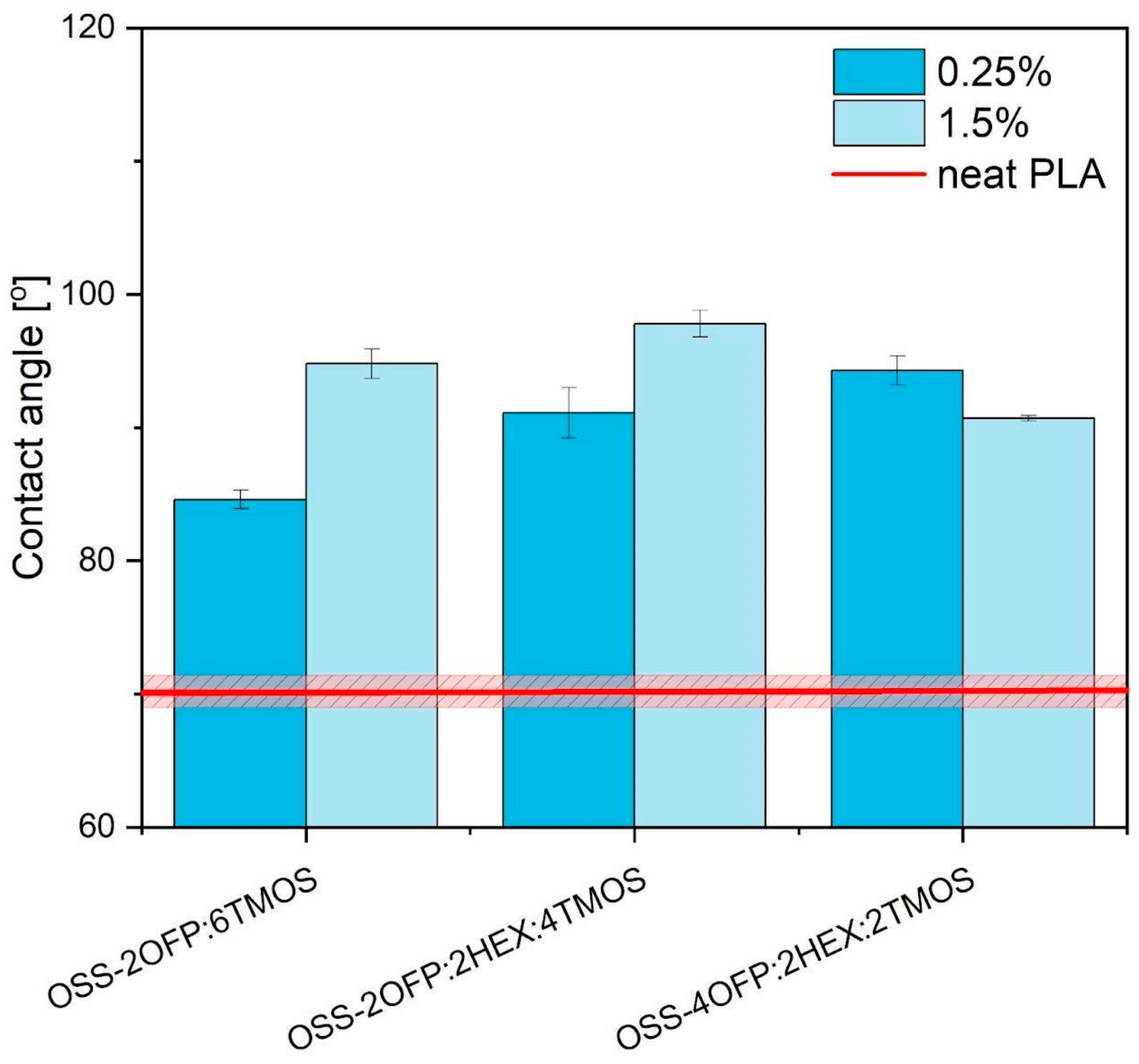
3.5. Water Contact Angle (WCA)
3.6. Ice Adhesion
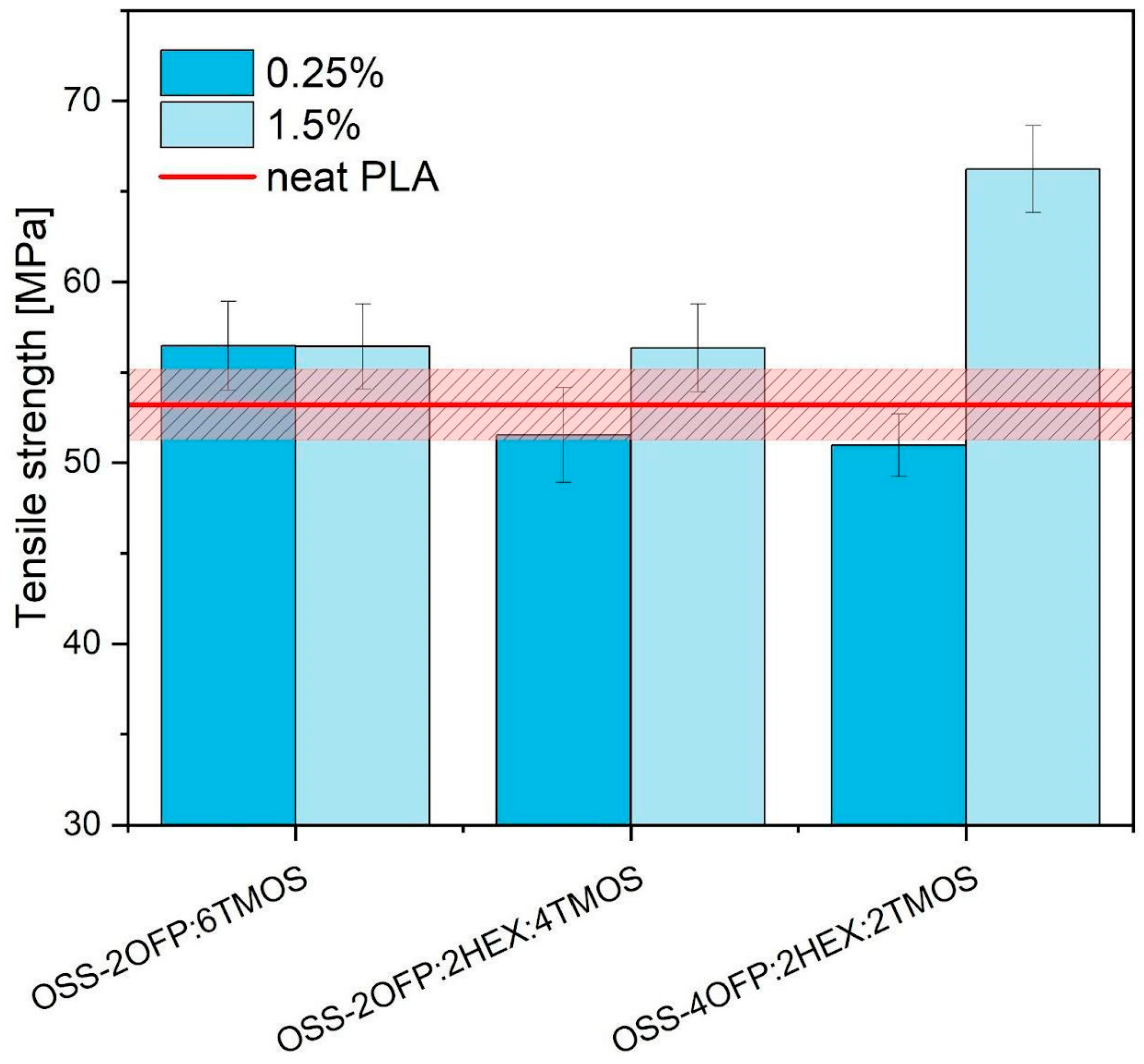
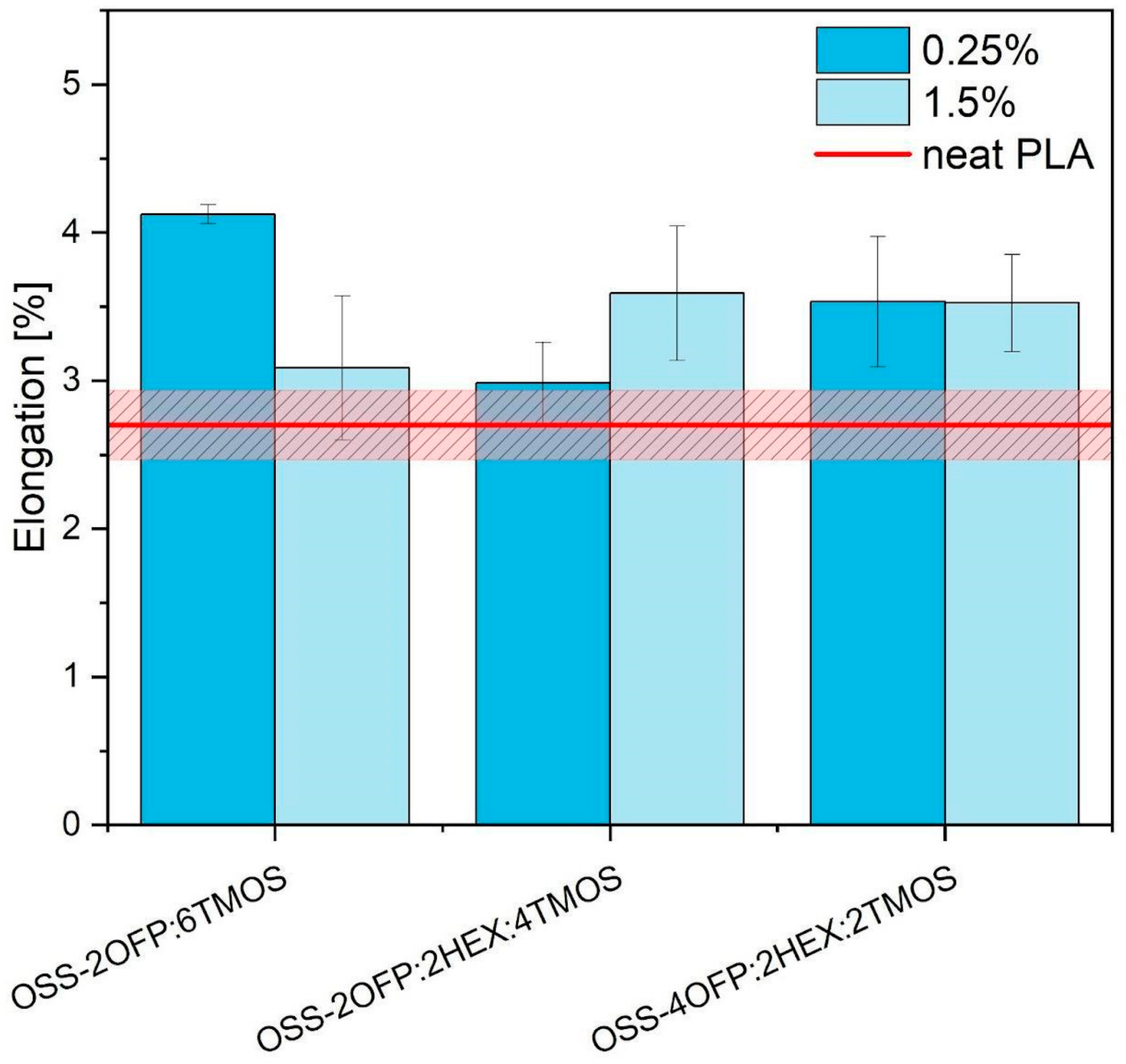
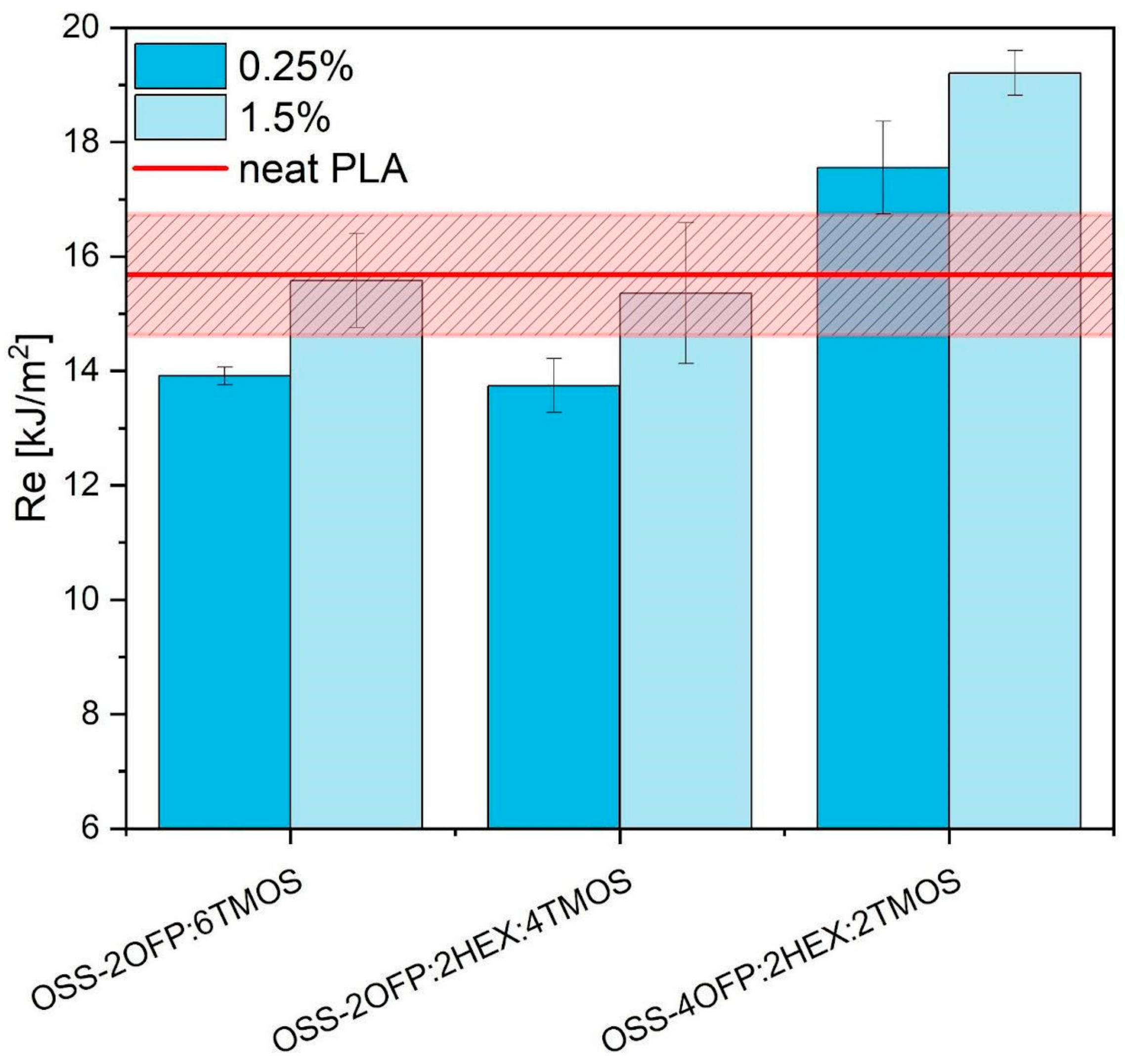
3.7. Mechanical Performance
3.7.1. Tensile Test Results
3.7.2. Impact Test
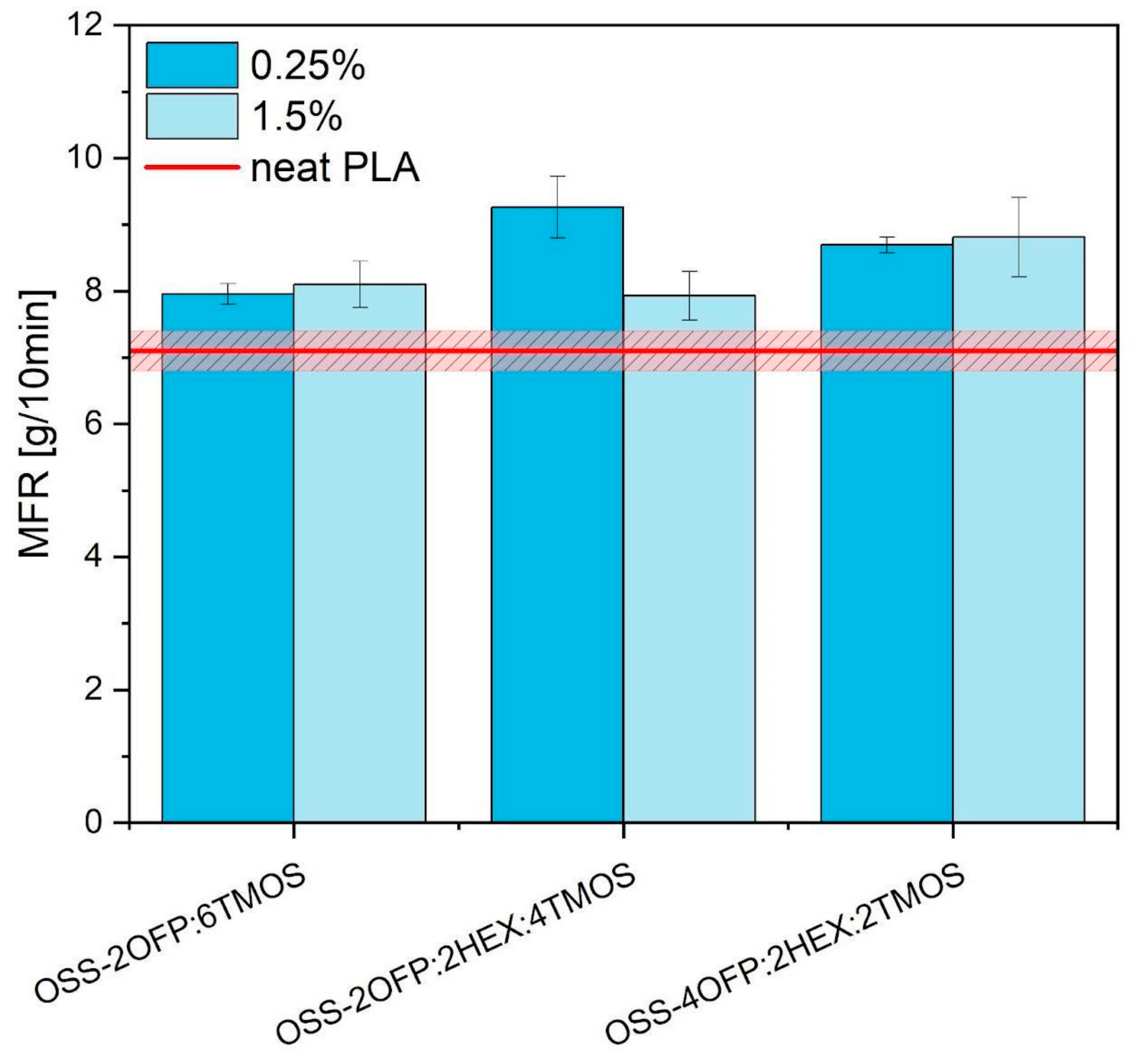
3.8. Rheology
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, N.; Leu, M.C. Additive Manufacturing: Technology, Applications and Research Needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Kristiawan, R.B.; Imaduddin, F.; Ariawan, D.; Ubaidillah; Arifin, Z. A Review on the Fused Deposition Modeling (FDM) 3D Printing: Filament Processing, Materials, and Printing Parameters. Open Eng. 2021, 11, 639–649. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A Review on Poly Lactic Acid (PLA) as a Biodegradable Polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Wang, X.; Huang, L.; Li, Y.; Wang, Y.; Lu, X.; Wei, Z.; Mo, Q.; Zhang, S.; Sheng, Y.; Huang, C.; et al. Research Progress in Polylactic Acid Processing for 3D Printing. J. Manuf. Process. 2024, 112, 161–178. [Google Scholar] [CrossRef]
- Ecker, J.V.; Haider, A.; Burzic, I.; Huber, A.; Eder, G.; Hild, S. Mechanical Properties and Water Absorption Behaviour of PLA and PLA/Wood Composites Prepared by 3D Printing and Injection Moulding. Rapid Prototyp. J. 2019, 25, 672–678. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic Materials: Fundamentals, Performance Evaluation, and Applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Huang, W.; Huang, J.; Guo, Z.; Liu, W. Icephobic/Anti-Icing Properties of Superhydrophobic Surfaces. Adv. Colloid Interface Sci. 2022, 304, 102658. [Google Scholar] [CrossRef]
- He, Z.; Zhuo, Y.; Zhang, Z.; He, J. Design of Icephobic Surfaces by Lowering Ice Adhesion Strength: A Mini Review. Coatings 2021, 11, 1343. [Google Scholar] [CrossRef]
- Cui, W.; Jiang, Y.; Mielonen, K.; Pakkanen, T.A. The Verification of Icephobic Performance on Biomimetic Superhydrophobic Surfaces and the Effect of Wettability and Surface Energy. Appl. Surf. Sci. 2019, 466, 503–514. [Google Scholar] [CrossRef]
- Zhuo, Y.; Xiao, S.; Amirfazli, A.; He, J.; Zhang, Z. Polysiloxane as Icephobic Materials—The Past, Present and the Future. Chem. Eng. J. 2021, 405, 127088. [Google Scholar] [CrossRef]
- Kohutiar, M.; Janík, R.; Krbata, M.; Bartosova, L.; Jus, M.; Timárová, Ľ. Study of the Effect of Pretreatment of 3D Printed PLA Filament Modified by Plasma Discharge and Changes in Its Dynamic-Mechanical Properties. Manuf. Technol. 2023, 23, 461–467. [Google Scholar] [CrossRef]
- Baran, E.H.; Erbil, H.Y. Surface Modification of 3D Printed PLA Objects by Fused Deposition Modeling: A Review. Colloids Interfaces 2019, 3, 43. [Google Scholar] [CrossRef]
- Johansson, K.S. Surface Modification of Plastics. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2017; pp. 443–487. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, Z.; Li, Z.; Ren, X. Dynamic Hydrophobic Behavior of Water Droplets Impact on the Cotton Fabrics Coated with POSS Block Copolymer. Cellulose. 2020, 27, 1705–1716. [Google Scholar] [CrossRef]
- Tao, C.; Li, X.; Liu, B.; Zhang, K.; Zhao, Y.; Zhu, K.; Yuan, X. Highly Icephobic Properties on Slippery Surfaces Formed from Polysiloxane and Fluorinated POSS. Prog. Org. Coat. 2017, 103, 48–59. [Google Scholar] [CrossRef]
- Drelich, J.; Marmur, A. Physics and Applications of Superhydrophobic and Superhydrophilic Surfaces and Coatings. Surf. Innov. 2014, 2, 211–227. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A Review on Fundamentals, Constraints and Fabrication Techniques of Superhydrophobic Coatings. Prog. Org. Coat. 2020, 142, 105557. [Google Scholar] [CrossRef]
- Erbil, H.Y. Practical Applications of Superhydrophobic Materials and Coatings: Problems and Perspectives. Langmuir 2020, 36, 2493–2509. [Google Scholar] [CrossRef]
- Park, J.H.; Olivares-Navarrete, R.; Baier, R.E.; Meyer, A.E.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Effect of Cleaning and Sterilization on Titanium Implant Surface Properties and Cellular Response. Acta Biomater. 2012, 8, 1966–1975. [Google Scholar] [CrossRef]
- Van Buren, T.; Smits, A.J. Substantial Drag Reduction in Turbulent Flow Using Liquid-Infused Surfaces. J. Fluid Mech. 2017, 827, 448–456. [Google Scholar] [CrossRef]
- Hazarika, A.; Deka, B.K.; Park, H.; Jae Hwang, Y.; Jaiswal, A.P.; Park, Y.-B.; Wook Park, H. Hierarchically Designed 3-D Printed Porous Nylon Fabric-Based Personal Thermoregulatory for Radiative and Directional Wick-Evaporative Cooling. Chem. Eng. J. 2023, 471, 144536. [Google Scholar] [CrossRef]
- Shams, H.; Basit, K.; Khan, M.A.; Mansoor, A.; Saleem, S. Scalable Wear Resistant 3D Printed Slippery Liquid Infused Porous Surfaces (SLIPS). Addit. Manuf. 2021, 48, 102379. [Google Scholar] [CrossRef]
- Bakhtiari, E. Super-Hydrophobicity Effects on Performance of a Dynamic Wind Turbine Blade Element under Yaw Loads. Renew. Energy 2019, 140, 539–551. [Google Scholar] [CrossRef]
- Xu, K.; Hu, J.; Jiang, X.; Meng, W.; Lan, B.; Shu, L. Anti-Icing Performance of Hydrophobic Silicone–Acrylate Resin Coatings on Wind Blades. Coatings 2018, 8, 151. [Google Scholar] [CrossRef]
- Kickelbick, G. Silsesquioxanes. In Functional Molecular Silicon Compounds I; Structure and Bonding; Scheschkewitz, D., Ed.; Springer International Publishing: Cham, Switzerland, 2013; Volume 155, pp. 1–28. ISBN 978-3-319-03619-9. [Google Scholar]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, C.; Li, X.; Zhang, K.; Zhao, Y.; Zhu, K.; Yuan, X. Submicron/nano-structured icephobic surfaces made from fluorinated polymethylsiloxane and octavinyl-POSS. Appl. Surf. Sci. 2016, 360, 113–120. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Zhao, Y.; Zhu, K.; Li, Y.; Tao, C.; Yuan, X. UV-curable POSS-fluorinated methacrylate diblock copolymers for icephobic coatings. Prog. Org. Coat. 2016, 93, 87–96. [Google Scholar] [CrossRef]
- Maciejewski, H.; Karasiewicz, J.; Marciniec, B. Efektywna synteza fluorofunkcyjnych (poli)siloksanów. Polimery 2012, 57, 449–456. [Google Scholar] [CrossRef]
- Dias Filho, N.L.; Aquino, H.A.D.; Pires, G.; Caetano, L. Relationship between the Dielectric and Mechanical Properties and the Ratio of Epoxy Resin to Hardener of the Hybrid Thermosetting Polymers. J. Braz. Chem. Soc. 2006, 17, 533–541. [Google Scholar] [CrossRef]
- ISO 1133-1:2022; Plastics—Determination of the melt mass-flow rate (MFR) and melt volume-flow rate (MVR) of thermoplastics—Part 1: Standard method. International Organization for Standardization: Geneva, Switzerland, 2022.
- PN-EN ISO 527:1996; Plastics—Determination of tensile properties. International Organization for Standardization: Geneva, Switzerland, 1996.
- PN-EN ISO 178:2019; Plastics—Determination of flexural properties. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 179-135:2020; Plastics—Determination of Charpy impact properties. International Organization for Standardization: Geneva, Switzerland, 2020.
- Kozera, R.; Przybyszewski, B.; Żołyńska, K.; Boczkowska, A.; Sztorch, B.; Przekop, R.E. Hybrid Modification of Unsaturated Polyester Resins to Obtain Hydro- and Icephobic Properties. Processes 2020, 8, 1635. [Google Scholar] [CrossRef]
- PN-EN ISO 527-2:2012; Plastics—Determination of tensile properties—Part 2: Test conditions for molding and extrusion plastics. International Organization for Standardization: Geneva, Switzerland, 2012.
- Khoo, R.Z.; Ismail, H.; Chow, W.S. Thermal and Morphological Properties of Poly (Lactic Acid)/Nanocellulose Nanocomposites. Procedia Chem. 2016, 19, 788–794. [Google Scholar] [CrossRef]
- Mecinović, J.; Snyder, P.W.; Mirica, K.A.; Bai, S.; Mack, E.T.; Kwant, R.L.; Moustakas, D.T.; Héroux, A.; Whitesides, G.M. Fluoroalkyl and Alkyl Chains Have Similar Hydrophobicities in Binding to the “Hydrophobic Wall” of Carbonic Anhydrase. J. Am. Chem. Soc. 2011, 133, 14017–14026. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Biswas, B.; Ghosh, N.; Singh, P.C.; Mondal, J.A. Hydrophobic Hydration of Fluoroalkyl (C–F) is Distinctly Different from That of Its Hydrogenated Counterpart (C–H), as Observed by Raman Difference with Simultaneous Curve Fitting Analysis. J. Phys. Chem. C 2019, 123, 27012–27019. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, X.; Xiong, J.; Shentu, B.; Weng, Z. Synthesis of fluorinated polyester used for hydrophobic coating: Influence of fluoroalkyl group length on enrichment behavior. Polym. Sci. Ser. B 2015, 57, 677–686. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Zhao, Y.; Zhu, K.; Yuan, X. Formation of icephobic film from POSS-containing fluorosilicone multi-block methacrylate copolymers. Prog. Org. Coat. 2015, 89, 150–159. [Google Scholar] [CrossRef]
- Zeybek, Y.M.; Kaynak, C. Behaviour of PLA/POSS Nanocomposites: Effects of Filler Content, Functional Group and Copolymer Compatibilization. Polym. Compos. 2021, 29, S485–S500. [Google Scholar] [CrossRef]
- Chi, H.; Zhang, G.; Wang, N.; Wang, Y.; Li, T.; Wang, F.; Ye, C. Enhancing the Mechanical Strength and Toughness of Epoxy Resins with Linear POSS Nano-Modifiers. Nanoscale Adv. 2022, 4, 1151–1157. [Google Scholar] [CrossRef]
- Kodal, M.; Sirin, H.; Ozkoc, G. Investigation of relationship between crystallization kinetics and interfacial interactions in plasticized poly (lactic acid)/POSS nanocomposites: “Effects of different POSS types”. Polym. Compos. 2018, 39, 2674–2684. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Zeng, G.; Liu, W. Biodegradable PLA-based composites modified by POSS particles. Polym.-Plast. Technol. Mater. 2020, 59, 998–1009. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, D.; Huang, L.; Li, W.; Tian, J.; Lu, L.; Zhou, C. Simultaneous improvement in toughness, strength and biocompatibility of poly (lactic acid) with polyhedral oligomeric silsesquioxane. Chem. Eng. J. 2018, 346, 649–661. [Google Scholar] [CrossRef]
- Srinivasan, S.A.; Hedrick, J.L.; Miller, R.D.; Di Pietro, R. Crosslinked networks based on trimethoxysilyl functionalized poly(amic ethyl ester) chain extendable oligomers. Polymer 1997, 38, 3129–3133. [Google Scholar] [CrossRef]

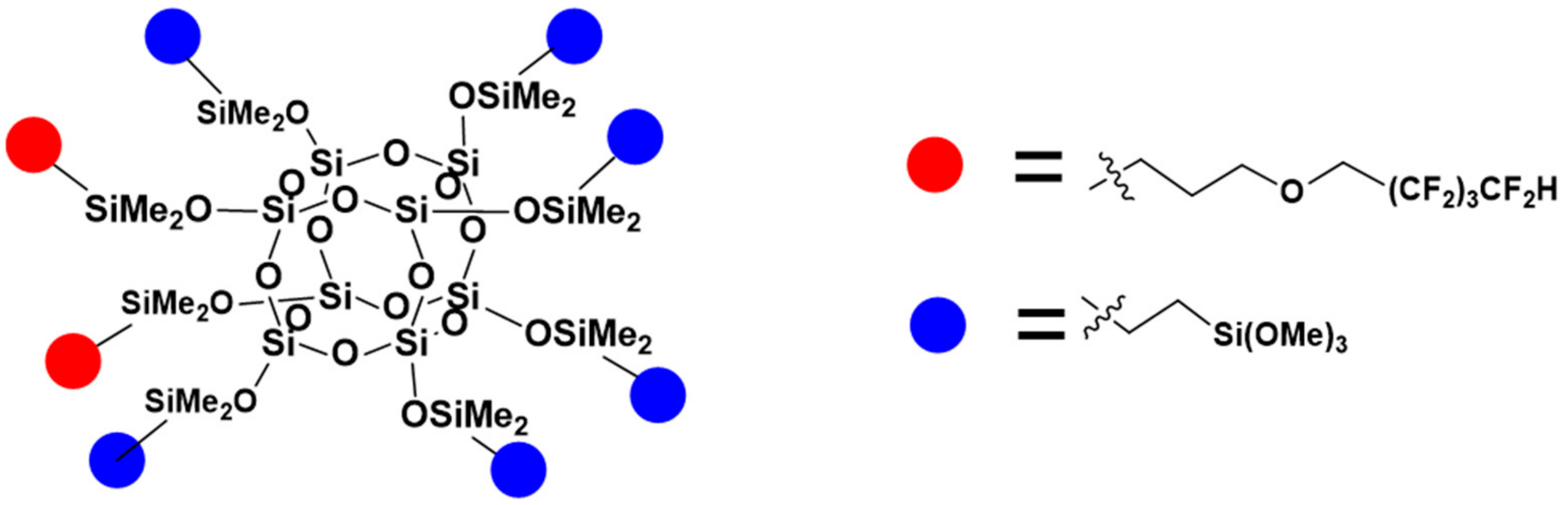
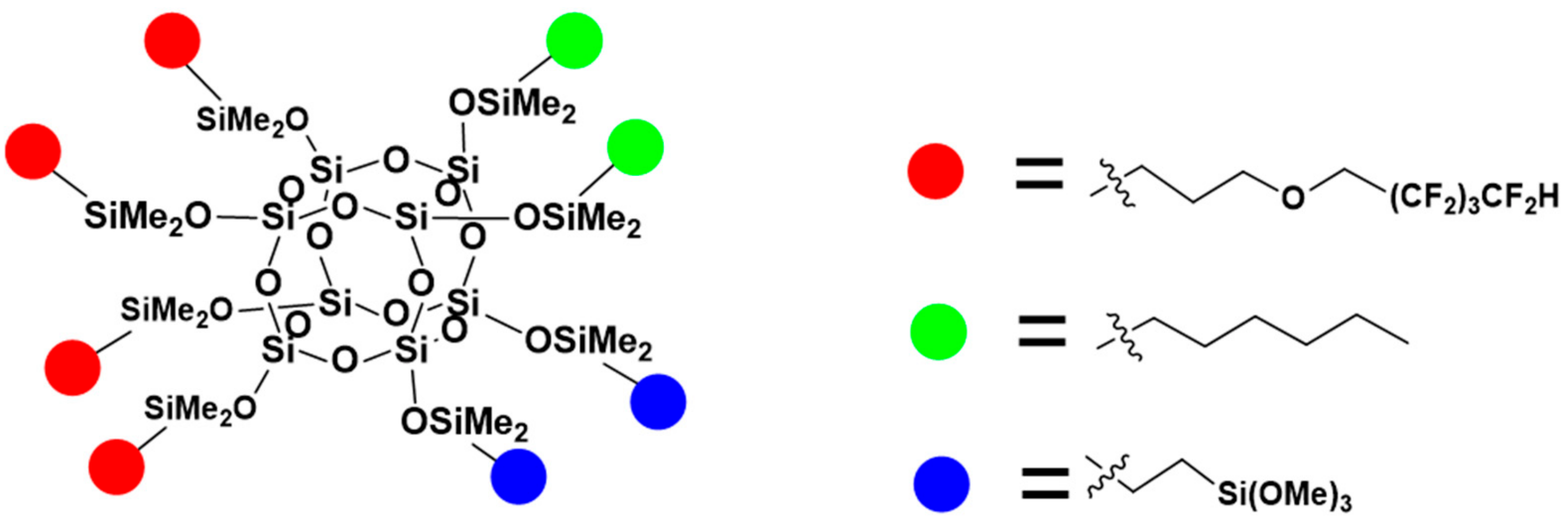

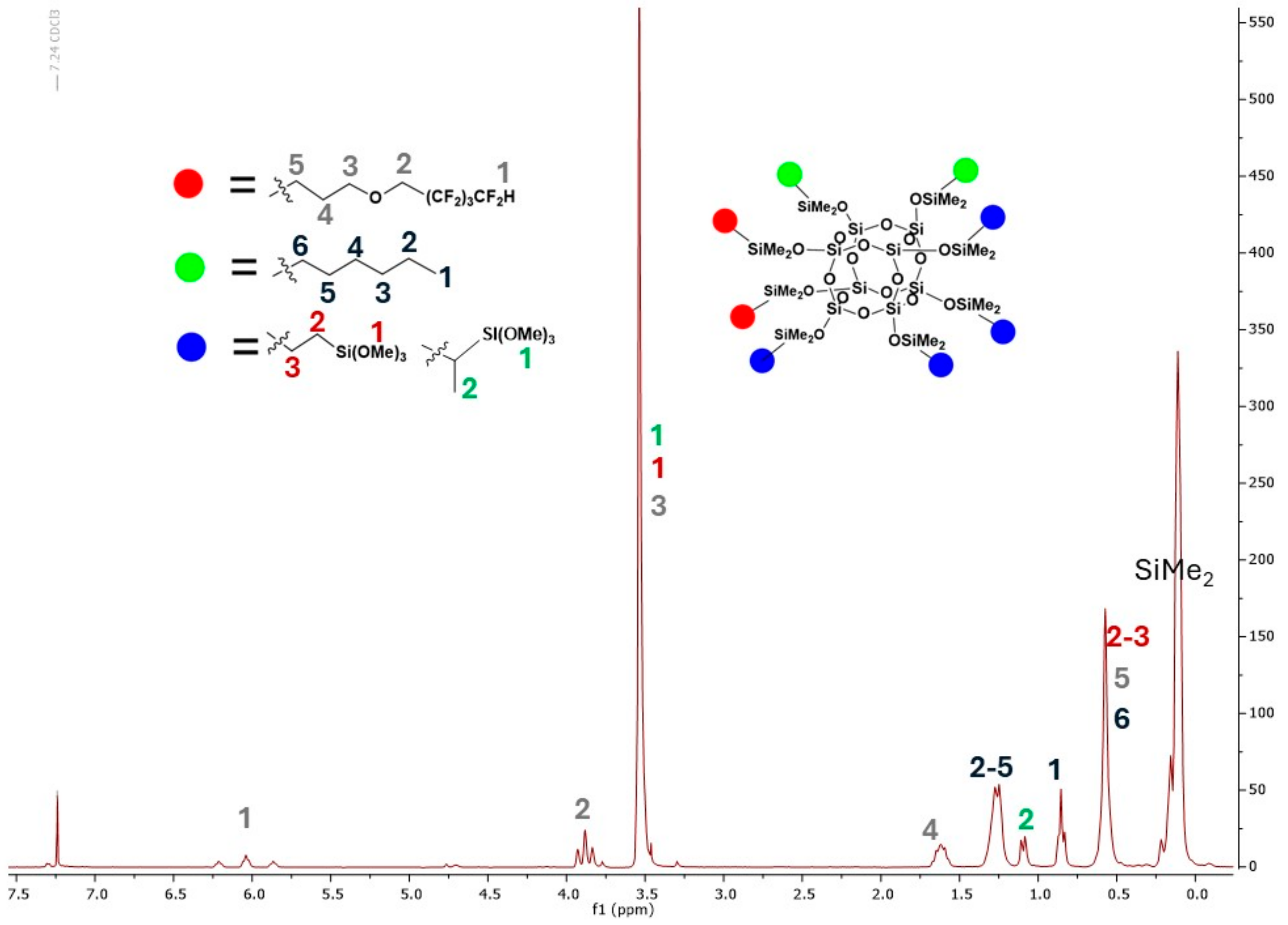

| Code | Amount of OFP/g | Amount of TMOS/g | Amount of HEX/g |
|---|---|---|---|
| 2OFP:6TMOS | 13.61 | 17.46 | - |
| 2OFP:2HEX:4TMOS | 13.61 | 11.64 | 3.30 |
| 4OFP:2HEX:2TMOS | 27.22 | 5.82 | 3.30 |
| Properties | Parameters |
|---|---|
| Nozzle diameter | 0.4 mm |
| Extruder temperature | 190 °C |
| Bed temperature | 60 °C |
| Layer height | 0.18 mm |
| Bottom and top layers number | 3 |
| Fill style | linear |
| Fill angle | 45° |
| Infill density | 100% |
| Printing speed | 80 mm/s |
| Code | Temperature at 1% Weight Loss/℃ | Temperature at 5% Weight Loss/℃ | Onset Temperature/°C | Temperature at Maximum Weight Change Rate/℃ |
|---|---|---|---|---|
| Neat PLA | 253.2 | 328.5 | 342.9 | 362.4 |
| PLA + 1.5% OSS-2OFP:6TMOS | 293.5 | 326.9 | 342.3 | 359.4 |
| PLA + 1.5% OSS-2OFP:2HEX:4TMOS | 296.8 | 328.3 | 342.6 | 360.0 |
| PLA + 1.5% OSS-4OFP:2HEX:2TMOS | 293.6 | 321.1 | 336.2 | 358.7 |
| First Cycle | Second Cycle | First Cycle | Second Cycle | First Cycle | Second Cycle | First Cycle | Second Cycle | |
|---|---|---|---|---|---|---|---|---|
| Tg | Tg | Tcc | Tcc | Tm | Tm | Degree of Crystallinity/% | Degree of Crystallinity/% | |
| Neat PLA | 64.7 | 62.4 | 124.3 | 123.1 | 155.2 | 153.2 | 14.5 | 5.4 |
| PLA + 1.5% OSS-2OFP:6TMOS | 65.7 | 62.2 | 123.9 | 109.0 | 153.8 | 153.4 | 13.6 | 28.9 |
| PLA + 1.5% OSS-2OFP:2HEX:4TMOS | 62.3 | 62.2 | 121.0 | 107.0 | 151.3 | 152.2 | 23.7 | 24.9 |
| PLA + 1.5% OSS-4OFP:2HEX:2TMOS | 65.4 | 62.1 | 108.2 | 108.8 | 154.2 | 149.5 | 27.6 | 27.4 |
| Surface Character | Concentration/% | Contact Angle/° | |
|---|---|---|---|
| Neat PLA | Hydrophilic | - | 70.2 ± 1.2 |
| OSS-2OFP:6TMOS | Hydrophobic | 0.25 | 84.6 ± 0.7 |
| 1.5 | 94.8 ± 1.1 | ||
| OSS-2OFP:2HEX:4TMOS | Hydrophobic | 0.25 | 91.1 ± 1.9 |
| 1.5 | 97.8 ± 1.0 | ||
| OSS-4OFP:2HEX:2TMOS | Hydrophobic | 0.25 | 94.3 ± 1.1 |
| 1.5 | 90.7 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konieczna, R.; Przekop, R.E.; Pakuła, D.; Głowacka, J.; Ziętkowska, K.; Kozera, R.; Sztorch, B. Functional Silsesquioxanes—Tailoring Hydrophobicity and Anti-Ice Properties of Polylactide in 3D Printing Applications. Materials 2024, 17, 4850. https://doi.org/10.3390/ma17194850
Konieczna R, Przekop RE, Pakuła D, Głowacka J, Ziętkowska K, Kozera R, Sztorch B. Functional Silsesquioxanes—Tailoring Hydrophobicity and Anti-Ice Properties of Polylactide in 3D Printing Applications. Materials. 2024; 17(19):4850. https://doi.org/10.3390/ma17194850
Chicago/Turabian StyleKonieczna, Roksana, Robert E. Przekop, Daria Pakuła, Julia Głowacka, Katarzyna Ziętkowska, Rafał Kozera, and Bogna Sztorch. 2024. "Functional Silsesquioxanes—Tailoring Hydrophobicity and Anti-Ice Properties of Polylactide in 3D Printing Applications" Materials 17, no. 19: 4850. https://doi.org/10.3390/ma17194850








