Chemical Bonds Formed in Solid Wood by Reaction with Maleic Anhydride and Sodium Hypophosphite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Specimens
2.3. Analysis with Solid-State Nuclear Magnetic Resonance (SS-NMR)
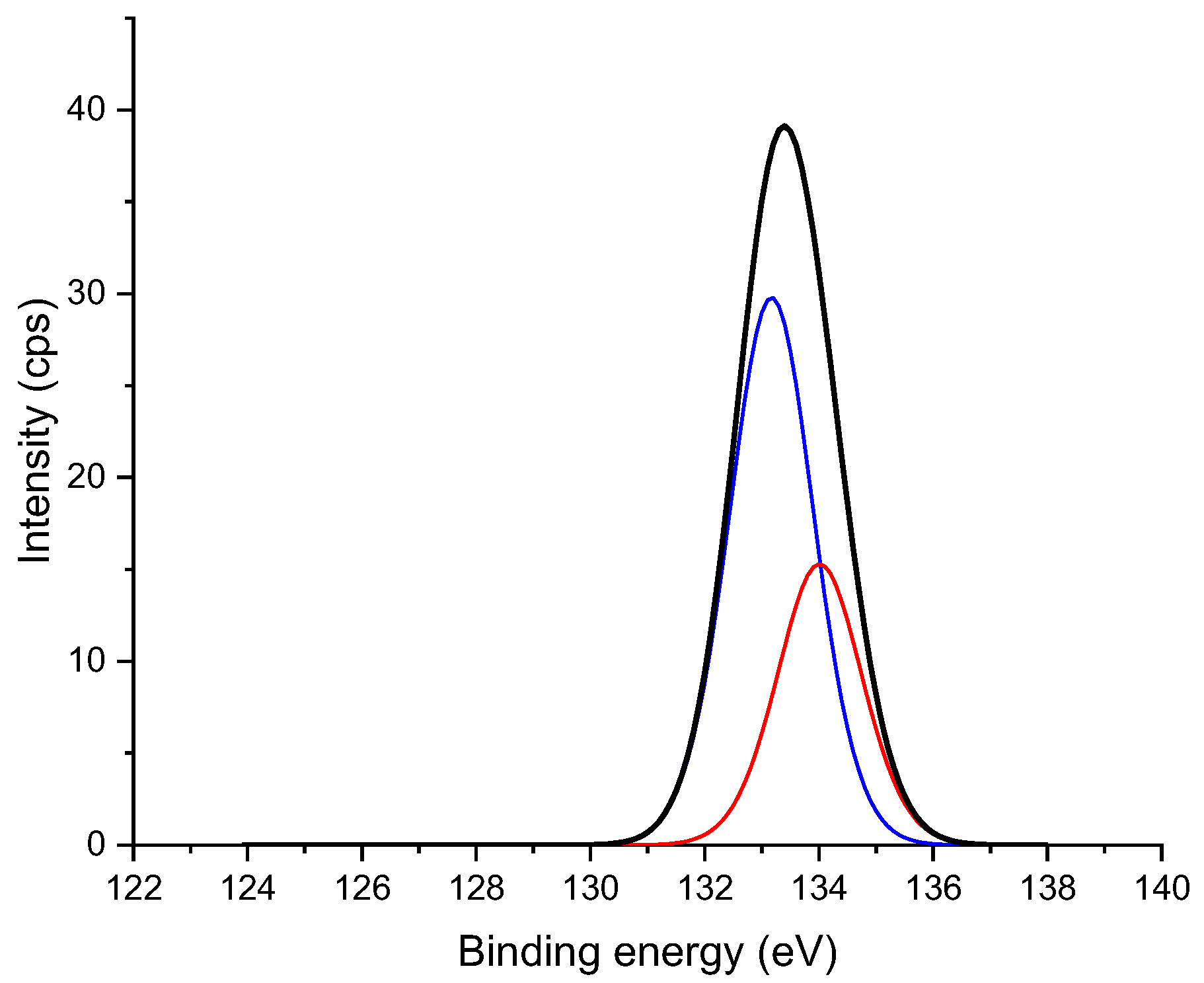
2.4. Analysis with X-ray Photoelectron Spectroscopy (XPS)
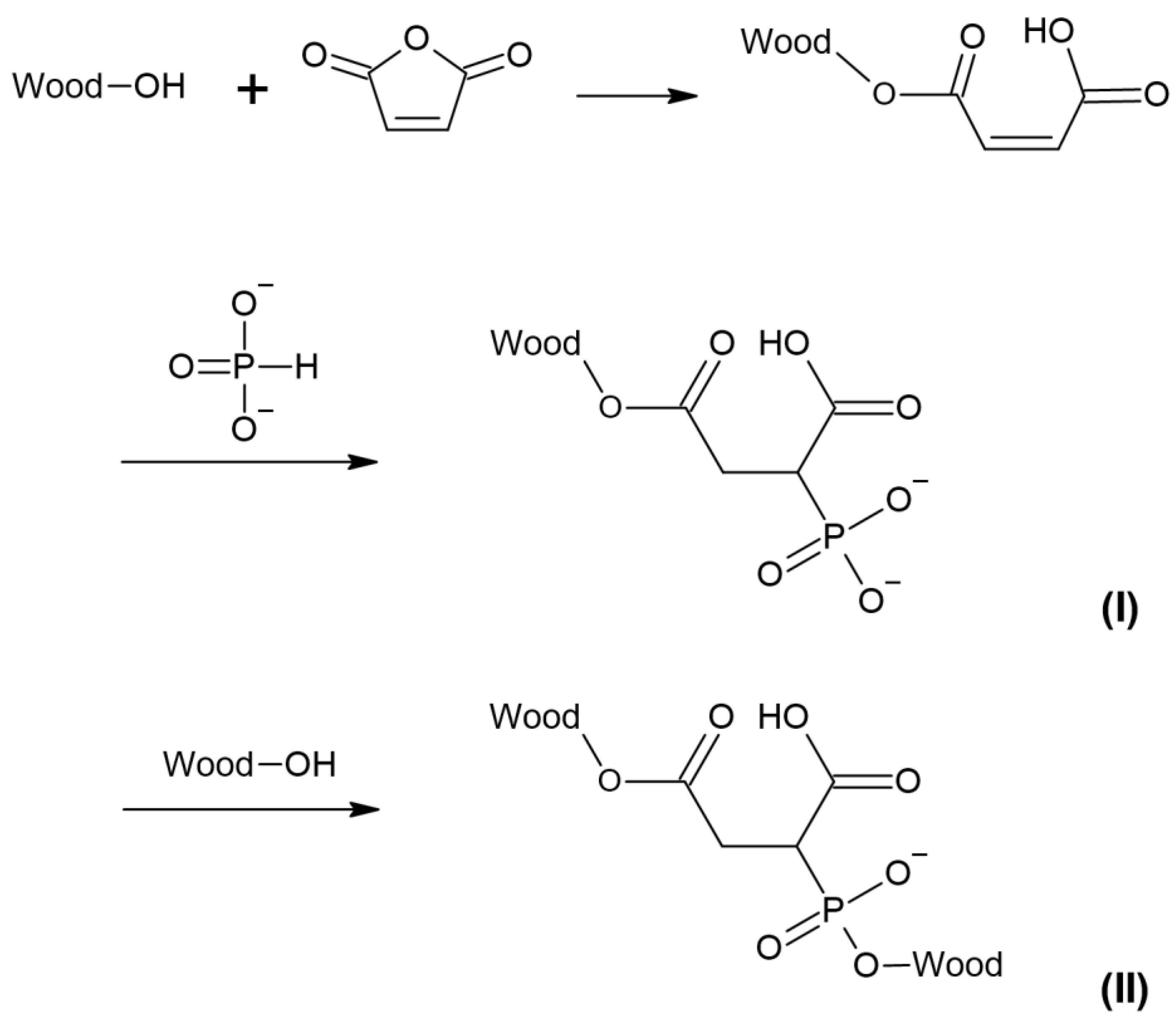
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.A.S. Wood Modification—Chemical, Thermal and Other Processes. In Wiley Series in Renewable Resources; Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Sandberg, D.; Kutnar, A.; Karlsson, O.; Jones, D. Wood Modification Technologies: Principles, Sustainability, and the Need for Innovation; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Thybring, E.E.; Fredriksson, M. Wood modification as a tool to understand moisture in wood. Forests 2021, 12, 372. [Google Scholar] [CrossRef]
- Mai, C.; Militz, H. Wood Modification. In Springer Handbook of Wood Science and Technology; Niemz, P., Teischinger, A., Sandberg, D., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Yasuda, R.; Minato, K. Chemical modification of wood by non-formaldehyde cross-linking reagents: Part 2. Moisture adsorption and creep properties. Wood Sci. Technol. 1994, 28, 209–218. [Google Scholar] [CrossRef]
- Williams, F.C.; Hale, M.D. The resistance of wood chemically modified with isocyanates: The role of moisture content in decay suppression. Int. Biodeterior. Biodegrad. 2003, 52, 215–221. [Google Scholar] [CrossRef]
- Emmerich, L.; Bollmus, S.; Militz, H. Wood modification with DMDHEU (1.3-dimethylol-4.5-dihydroxyethyleneurea)—State of the art, recent research activities and future perspectives. Wood Mater. Sci. Eng. 2019, 14, 3–18. [Google Scholar] [CrossRef]
- Matsuda, H.; Ueda, M.; Murakami, K. Oligoesterified woods based on anhydride and epoxide (I). Preparation and dimensional stability of oligoesterified woods by stepwise addition reactions. Mokuzai Gakkaishi 1988, 34, 140–148. (In Japanese) [Google Scholar]
- Iwamoto, Y.; Ito, T. Vapor phase reaction of wood with maleic anhydride (I): Dimensional stability and durability of treated wood. J. Wood Sci. 2005, 51, 595–600. [Google Scholar] [CrossRef]
- Essoua Essoua, G.G.; Blanchet, P.; Landry, V.; Beauregard, R. Maleic anhydride treated wood: Effects of drying time and esterification temperature on properties. BioResources 2015, 10, 6830–6860. [Google Scholar] [CrossRef]
- Kim, I.; Karlsson, O.; Jones, D.; Mantanis, G.; Sandberg, D. Dimensional stabilisation of Scots pine (Pinus sylvestris L.) sapwood by reaction with maleic anhydride and sodium hypophosphite. Eur. J. Wood Wood Prod. 2021, 79, 589–596. [Google Scholar] [CrossRef]
- Wu, X.; Yang, C.Q. Flame retardant finishing of cotton fleece fabric- part III: The combination of maleic acid and sodium hypophosphite. J. Fire Sci. 2008, 26, 351–368. [Google Scholar]
- Hameed, S.; Hussain, M.A.; Masood, R.; Haseeb, M.T. Cross-linking of cotton fabric using maleic anhydride and sodium hypophosphite. Cellul. Chem. Technol. 2016, 50, 321–328. [Google Scholar]
- Peng, H.; Yang, C.Q.; Wang, S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydr. Polym. 2012, 87, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Thybring, E.E.; Karlsson, O.; Jones, D.; Mantanis, G.I.; Sandberg, D. Characterisation of moisture in Scots pine (Pinus sylvestris L.) sapwood modified with maleic anhydride and sodium hypophosphite. Forests 2021, 12, 1333. [Google Scholar] [CrossRef]
- Boonstra, M.G.; Pizzi, A.; Tekely, P.; Pendlebury, J. Chemical Modification of Norway Spruce and Scots Pine. A 13C NMR CP-MAS Study of the Reactivity and Reactions of Polymeric Wood Components with Acetic Anhydride. Holzforschung 1996, 50, 215–220. [Google Scholar] [CrossRef]
- Chang, S.-T.; Chang, H.-T. Comparisons of the photostability of esterified wood. Polym. Degrad. Stab. 2001, 71, 261–266. [Google Scholar] [CrossRef]
- Wikberg, H. Advanced Solid State NMR Spectroscopic Techniques in the Study of Thermally Modified Wood. Ph.D. Thesis, Department of Chemistry, University of Helsinki, Helsinki, Finland, 2004. [Google Scholar]
- Martha, R.; Mubarok, M.; Batubara, I.; Rahayu, I.S.; Setiono, L.; Darmawan, W.; Akong, F.O.; George, B.; Gérardin, C.; Gérardin, P. Errect of furfurylation treatment on technological properties of short rotation teak wood. J. Mater. Res. Technol. 2021, 12, 1689–1699. [Google Scholar] [CrossRef]
- Gil, A.M.; Neto, C.P. Solid-state NMR studies of wood and other lignocellulosic materials. Annu. Rep. NMR Spectrosc. 1999, 37, 75–117. [Google Scholar]
- Capanema, E.A.; Balakshin, M.Y.; Kadla, J.F. A comprehensive approach for quantitative lignin characterization by NMR spectroscopy. J. Agric. Food Chem. 2004, 52, 1850–1860. [Google Scholar] [CrossRef]
- Sievers, C.; Marzialetti, T.; Hoskins, T.J.; Olarte, M.B.V.; Agrawal, P.K.; Jones, C.W. Quantitative solid state NMR analysis of residues from acid hydrolysis of loblolly pine wood. Bioresour. Technol. 2009, 100, 4758–4765. [Google Scholar] [CrossRef]
- Tjeerdsma, B.F.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Characterisation of thermally modified wood: Molecular reasons for wood performance improvement. Holz Roh-Werkst. 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Bonneaud, C.; Decostanzi, M.; Burgess, J.; Trusiano, G.; Burgess, T.; Bongiovanni, R.; Joly-Duhamel, C.; Friesen, C.M. Synthesis of α,β-unsaturated esters of perfluoropolyalkylethers (PFPAEs) based on hexafluoropropylene oxide units for photopolymerization. RSC Adv. 2018, 8, 32664. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Y.; Kanan, M.W. Hypophosphite addition to alkenes under solvent-free and non-acidic aqueous conditions. Chem. Commun. 2022, 58, 2180–2183. [Google Scholar] [CrossRef]
- Harris, R.K.; Merwin, L.H.; Hägele, G. Solid-state nuclear magnetic resonance study of a series of phosphonic and phosphinic acids. J. Chem. Soc. Faraday Trans. 1989, 85, 1409–1423. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Socha, R.P.; Gurgul, J.; Wisniewski, M. XPS and NMR studies of phosphoric acid activated carbons. Carbon 2008, 46, 2113–2123. [Google Scholar] [CrossRef]
- Singh, A.S.; Advani, J.H.; Biradar, A.V. Phosphonate functionalized carbon spheres as a Brønsted acid catalysts for valorization of bio-renewable α-pinene oxide to trans-carveol. Dalton Trans. 2020, 49, 7210. [Google Scholar] [CrossRef] [PubMed]
- Dorris, G.M.; Gray, D.G. The surface analysis of paper and wood fibres by ESCA. II. Surface composition of mechanical pulps. Cellul. Chem. Technol. 1978, 12, 721–734. [Google Scholar]
- Baillie, A.C.; Cornell, C.L.; Wright, B.J.; Wright, K. Synthesis of potential inhibitors of the enzyme pantothenate synthetase. Tetrahedron Lett. 1992, 33, 5133–5136. [Google Scholar] [CrossRef]
- Baylis, E.K. 1,1-Diethoxyethylphosphinates and phosphonites. Intermediates for the synthesis of functional phosphorus acids. Tetrahedron Lett. 1995, 36, 9385–9388. [Google Scholar] [CrossRef]
- Froestl, W.; Mickel, S.J.; von Sprecher, G.; Diel, P.J.; Hall, R.G.; Maier, L.; Strub, D.; Melillo, V.; Baumann, P.A.; Bernasconi, R.; et al. Phosphinic Acid Analogs of GABA. 1. New Potent and Selective GABAB Agonists. Med. Chem. 1995, 38, 3313. [Google Scholar] [CrossRef]
- Hall, R.G.; Kane, P.D.; Bittiger, H.; Froestl, W. Phosphinic acid analogues of γ-aminobutyric acid (GABA). Synthesis of a new radioligand. J. Label. Compd. Radiopharm. 1995, 36, 129–135. [Google Scholar] [CrossRef]
- Abrunhosa-Homas, I.; Sellers, C.E.; Montchamp, J.-L. Alkylation of H-phosphinate esters under basic conditions. J. Org. Chem. 2007, 72, 2851–2856. [Google Scholar] [CrossRef]
- Montchamp, J.-L. Phosphinate chemistry in the 21st century: A viable alternative to the use of phosphorus trichloride in organophosphorus synthesis. Acc. Chem. Res. 2014, 47, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Lehrhofer, L.; Fliri, L.; Bacher, M.; Budischovwsky, D.; Sulaeva, I.; Hummel, M.; Rosenau, T.; Hettegger, H. A mechanistic study on the alleged cellulose cross-linking system: Maleic acid/sodium hypophosphite. Carbohydr. Polym. 2024, 346, 122653. [Google Scholar] [CrossRef] [PubMed]
- Dorez, G.; Otazaghine, B.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.M. Use of Py-GC/MS and PCFC to characterize the surface modification of flax fibres. J. Anal. Appl. Pyrolysis 2014, 105, 122–130. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J.M. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Gledhill, W.E.; Feijtel, T.C. Environmental Properties and Safety Assessment of Organic Phosphonates Used for Detergent and Water Treatment Applications. In The Handbook of Environmental Chemistry, Part F; Hutzinger, O.E., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; Volume 3, pp. 261–285. [Google Scholar]
- Jaworska, J.; Van Genderen-Takken, H.; Hanstveit, A.; van de Plassche, E.; Feijtel, T. Environmental risk assessment of phosphonates used in domestic laundry and cleaning agents in the Netherlands. Chemosphere 2002, 47, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B. Environmental chemistry of phosphonates. Water Res. 2003, 37, 2533–2546. [Google Scholar] [CrossRef]
- Lesueur, C.; Pfeffer, M.; Fuerhacker, M. Photodegradation of phosphonates in water. Chemosphere 2005, 59, 685–691. [Google Scholar] [CrossRef]
- Feng, X.; Xiao, Z.; Sui, S.-J.; Wang, Q.; Xie, Y. Esterification of wood with citric acid: The catalytic effects of sodium hypophosphite (SHP). Holzforschung 2014, 68, 427–433. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Y.; Li, H. Synthesis and performance evaluation of carboxyl-rich low phosphorus copolymer scale inhibitor. J. App. Polym. Sci. 2023, 140, e53333. [Google Scholar] [CrossRef]



| Atom % | Atomic Ratio | ||||
|---|---|---|---|---|---|
| C | O | P | N | O/C | |
| Untreated | 68.5 | 31.1 | - | 0.5 | 0.45 |
| MS05 | 72.3 | 27.3 | 0.2 | 0.2 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.; Antzutkin, O.N.; Shah, F.U.; Karlsson, O.; Jones, D.; Sandberg, D. Chemical Bonds Formed in Solid Wood by Reaction with Maleic Anhydride and Sodium Hypophosphite. Materials 2024, 17, 4856. https://doi.org/10.3390/ma17194856
Kim I, Antzutkin ON, Shah FU, Karlsson O, Jones D, Sandberg D. Chemical Bonds Formed in Solid Wood by Reaction with Maleic Anhydride and Sodium Hypophosphite. Materials. 2024; 17(19):4856. https://doi.org/10.3390/ma17194856
Chicago/Turabian StyleKim, Injeong, Oleg N. Antzutkin, Faiz Ullah Shah, Olov Karlsson, Dennis Jones, and Dick Sandberg. 2024. "Chemical Bonds Formed in Solid Wood by Reaction with Maleic Anhydride and Sodium Hypophosphite" Materials 17, no. 19: 4856. https://doi.org/10.3390/ma17194856
APA StyleKim, I., Antzutkin, O. N., Shah, F. U., Karlsson, O., Jones, D., & Sandberg, D. (2024). Chemical Bonds Formed in Solid Wood by Reaction with Maleic Anhydride and Sodium Hypophosphite. Materials, 17(19), 4856. https://doi.org/10.3390/ma17194856







