Significantly Enhanced Corona Resistance of Epoxy Composite by Incorporation with Functionalized Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Surface Modification of GO
2.1.2. Preparation of GO/EP Composites
2.2. Corona Discharge Treatment
2.3. Characterization
3. Results and Discussion
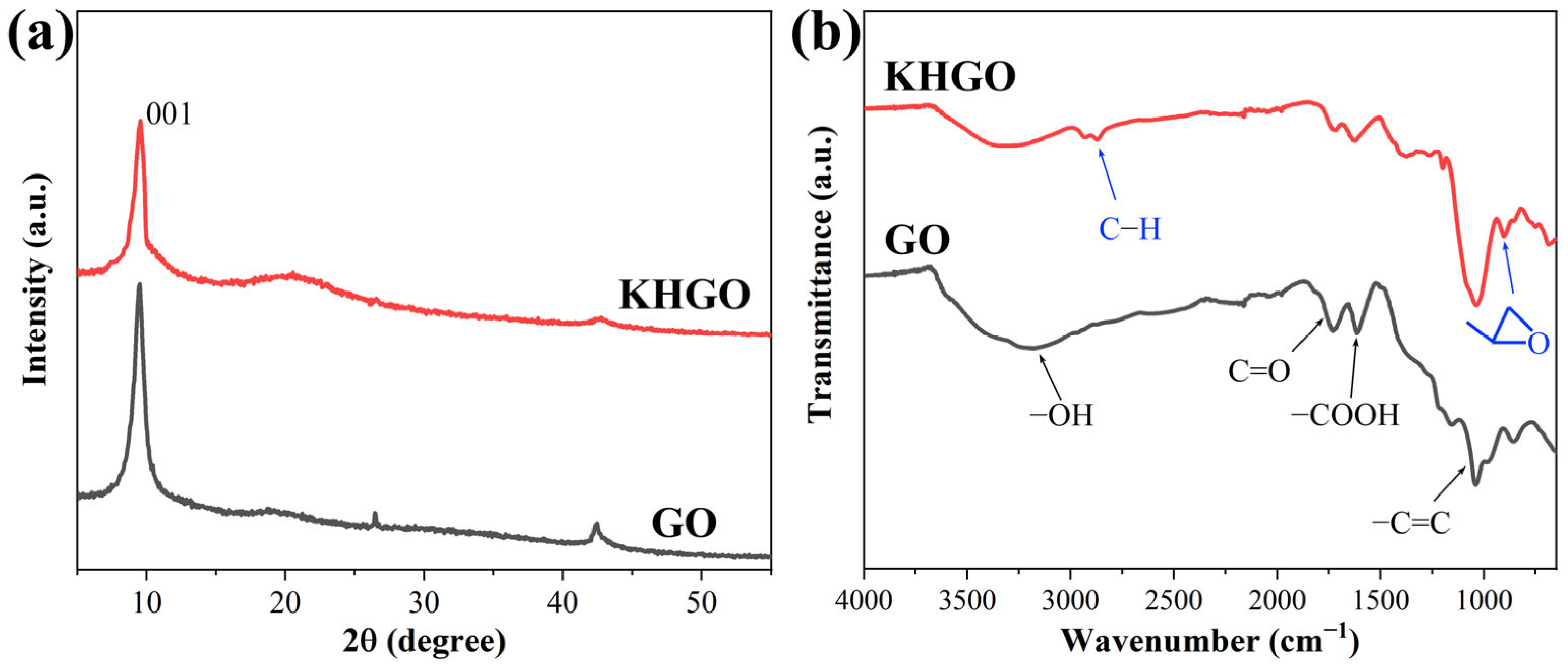
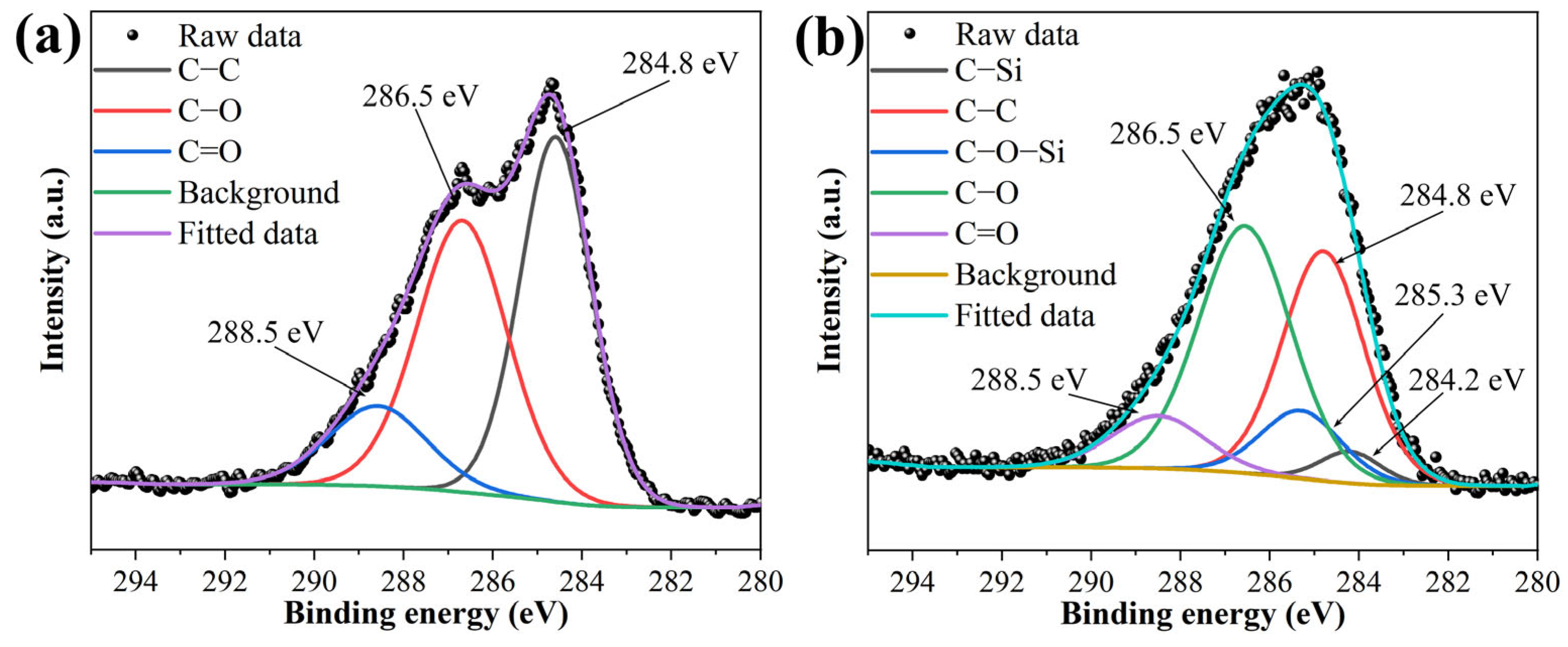
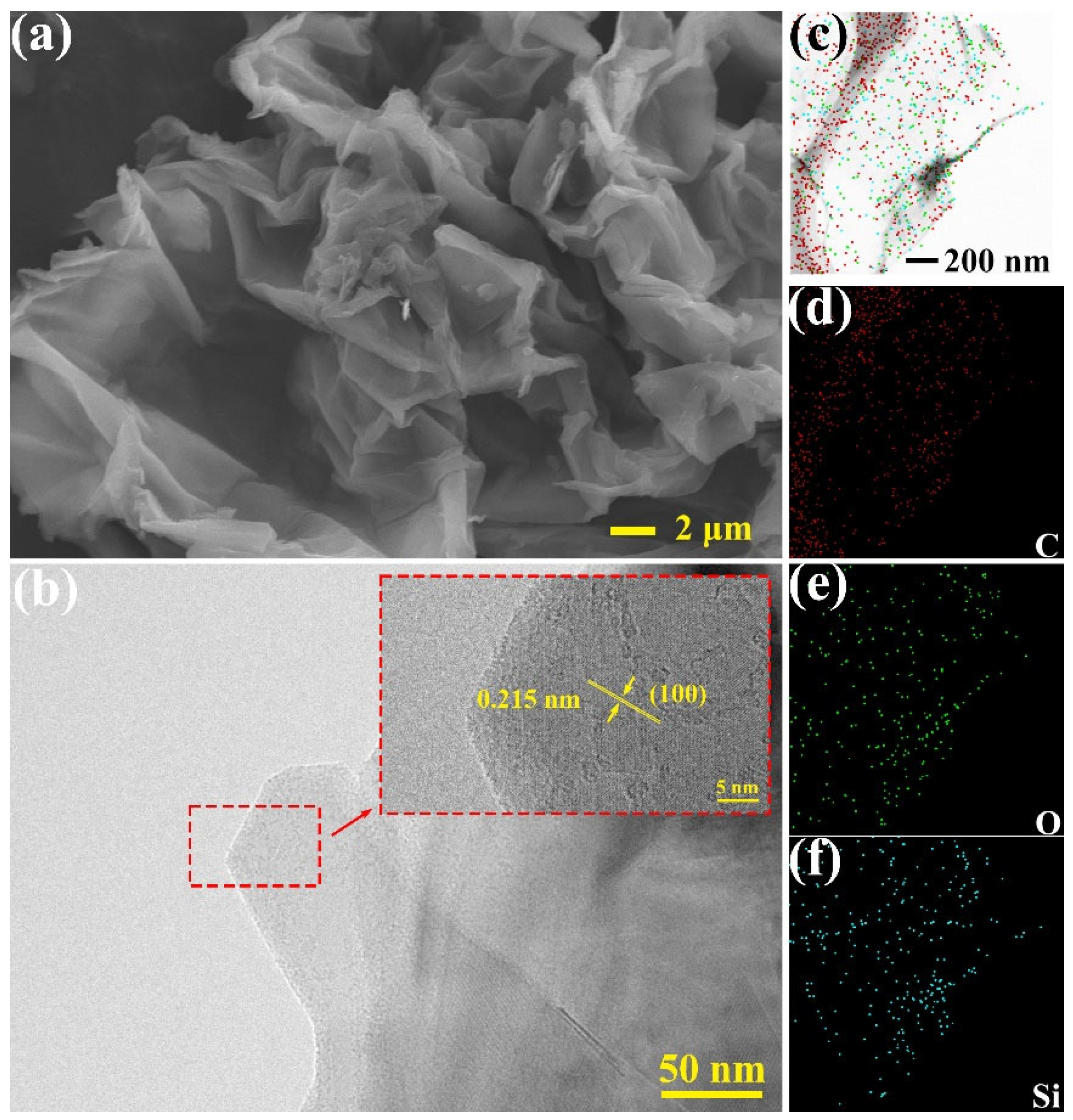
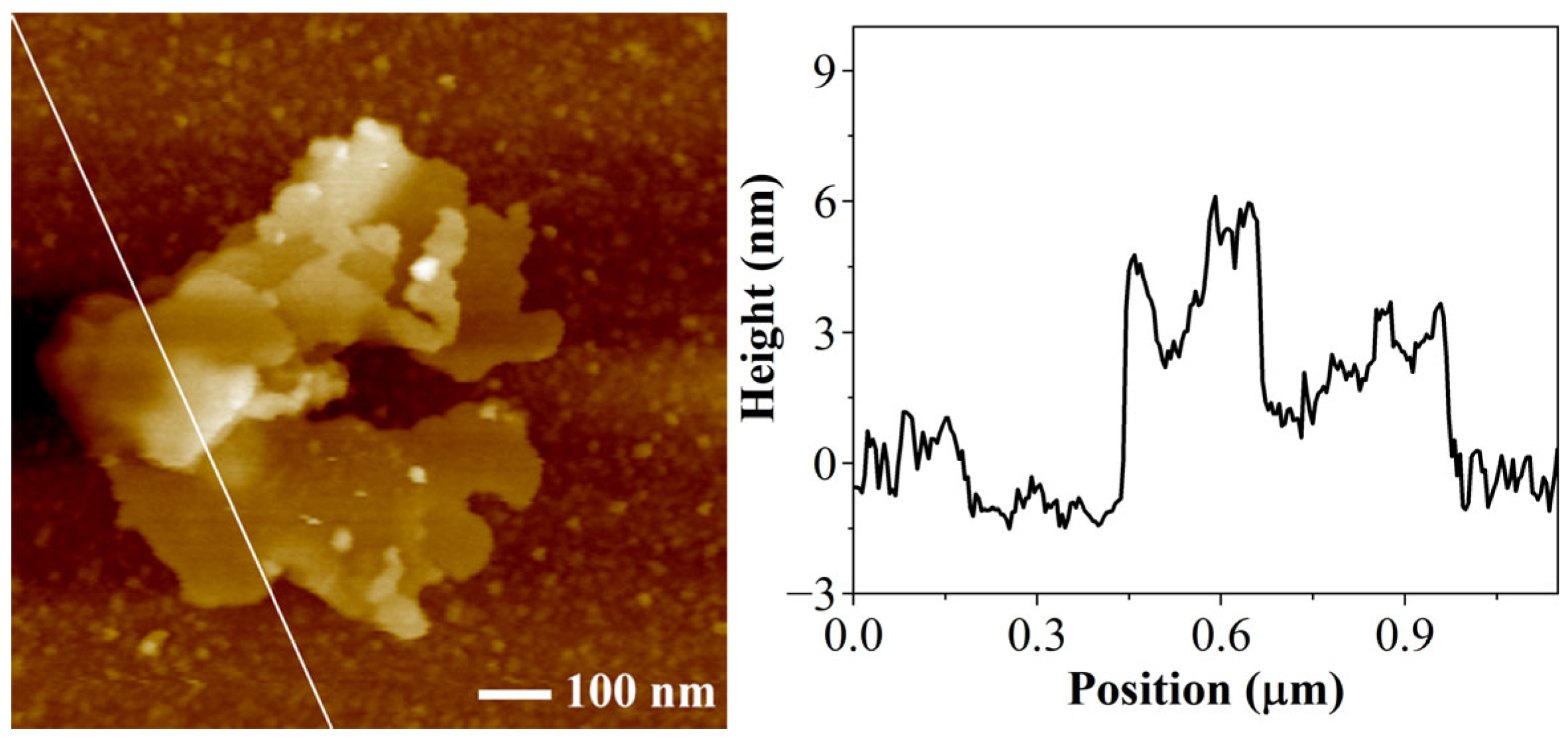
3.1. Chemical Structure and Morphology of Nanosheets
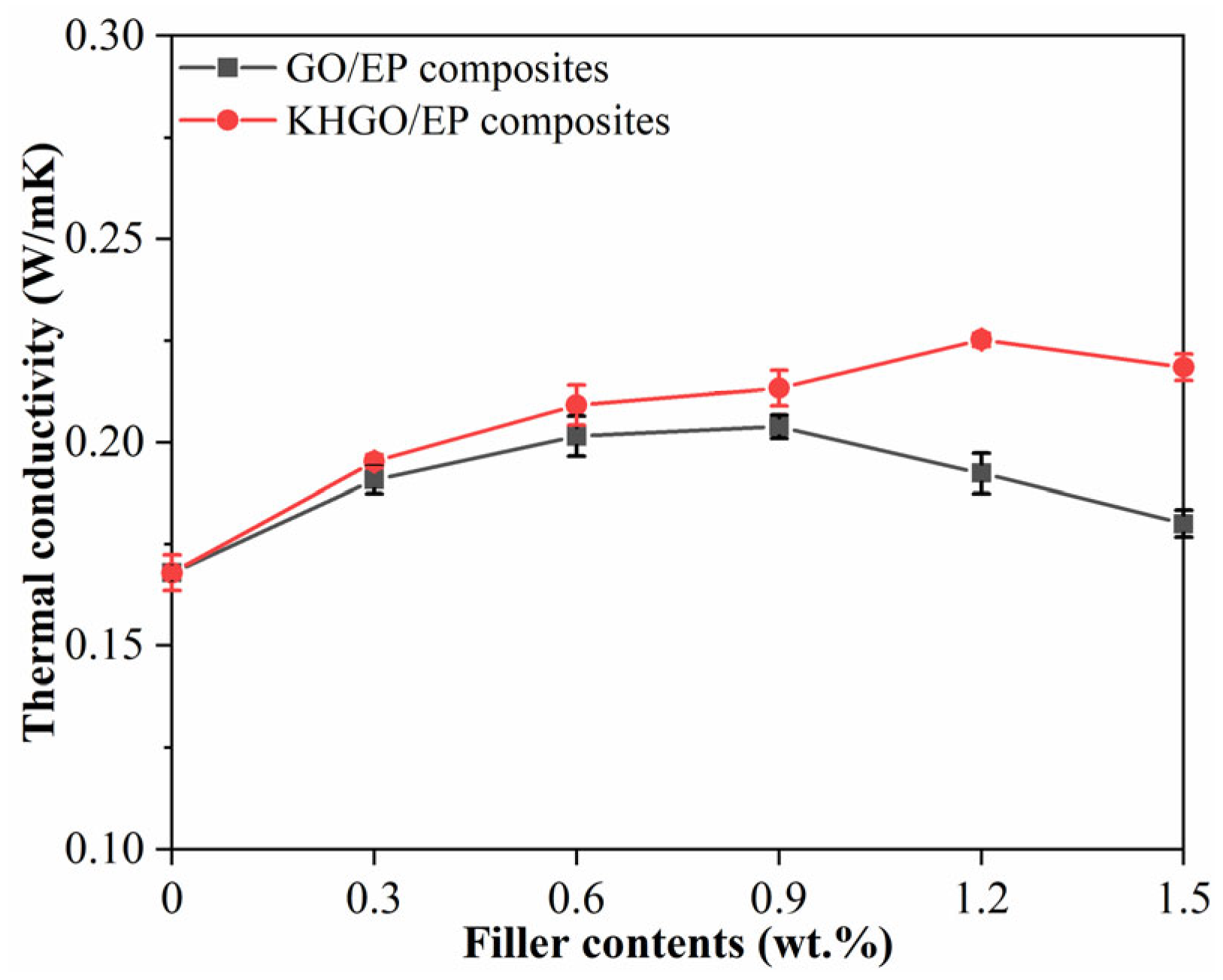
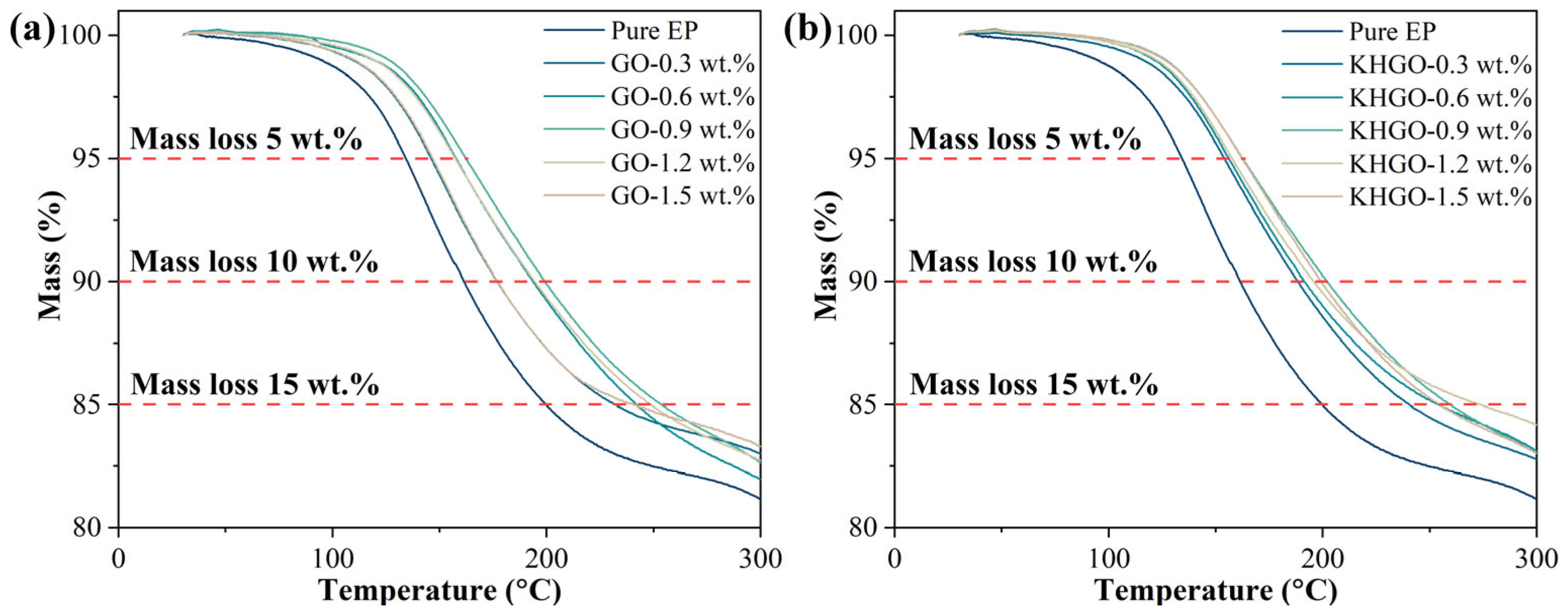
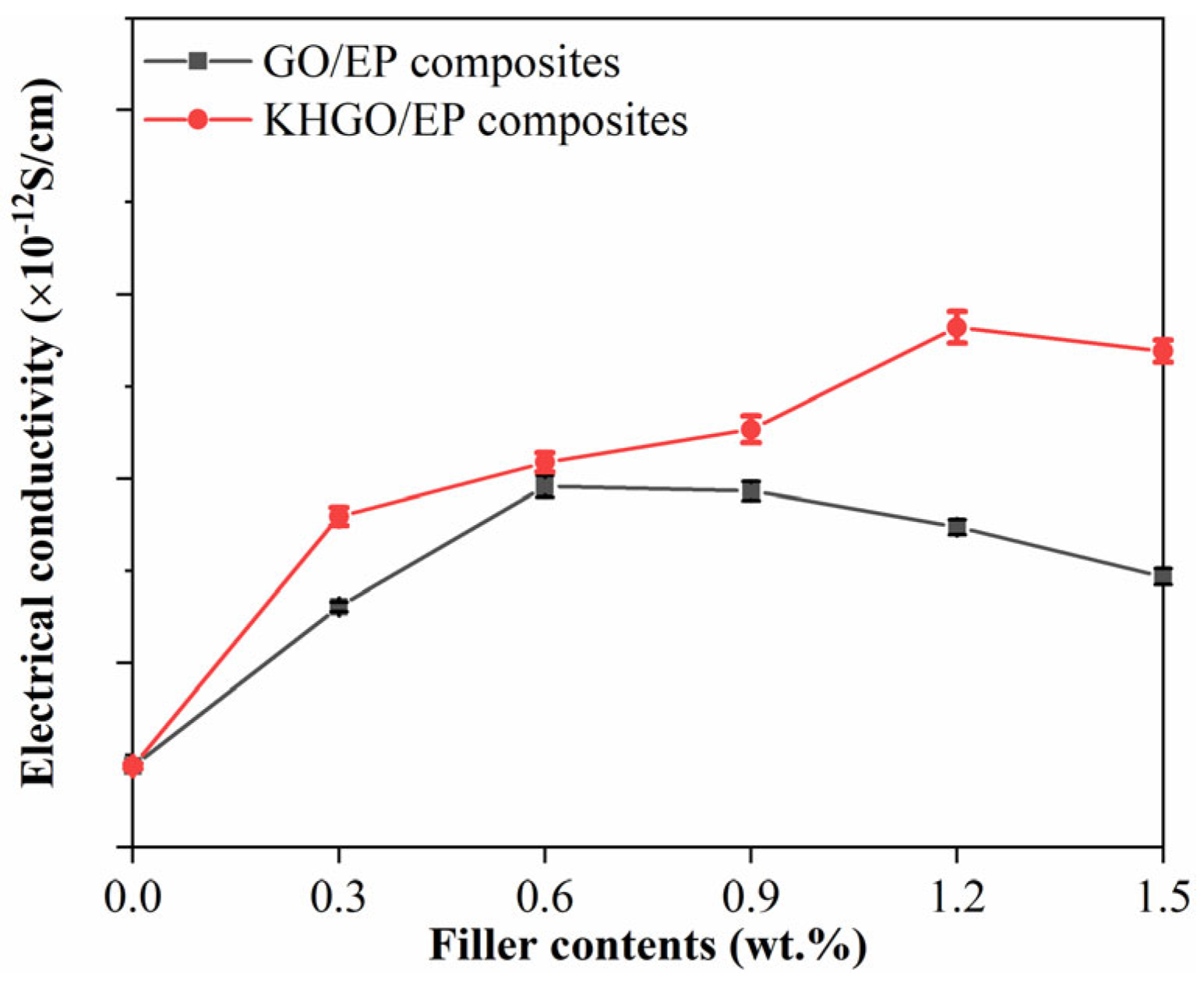
3.2. Thermal Properties and Volume Conductivity of Composites Samples
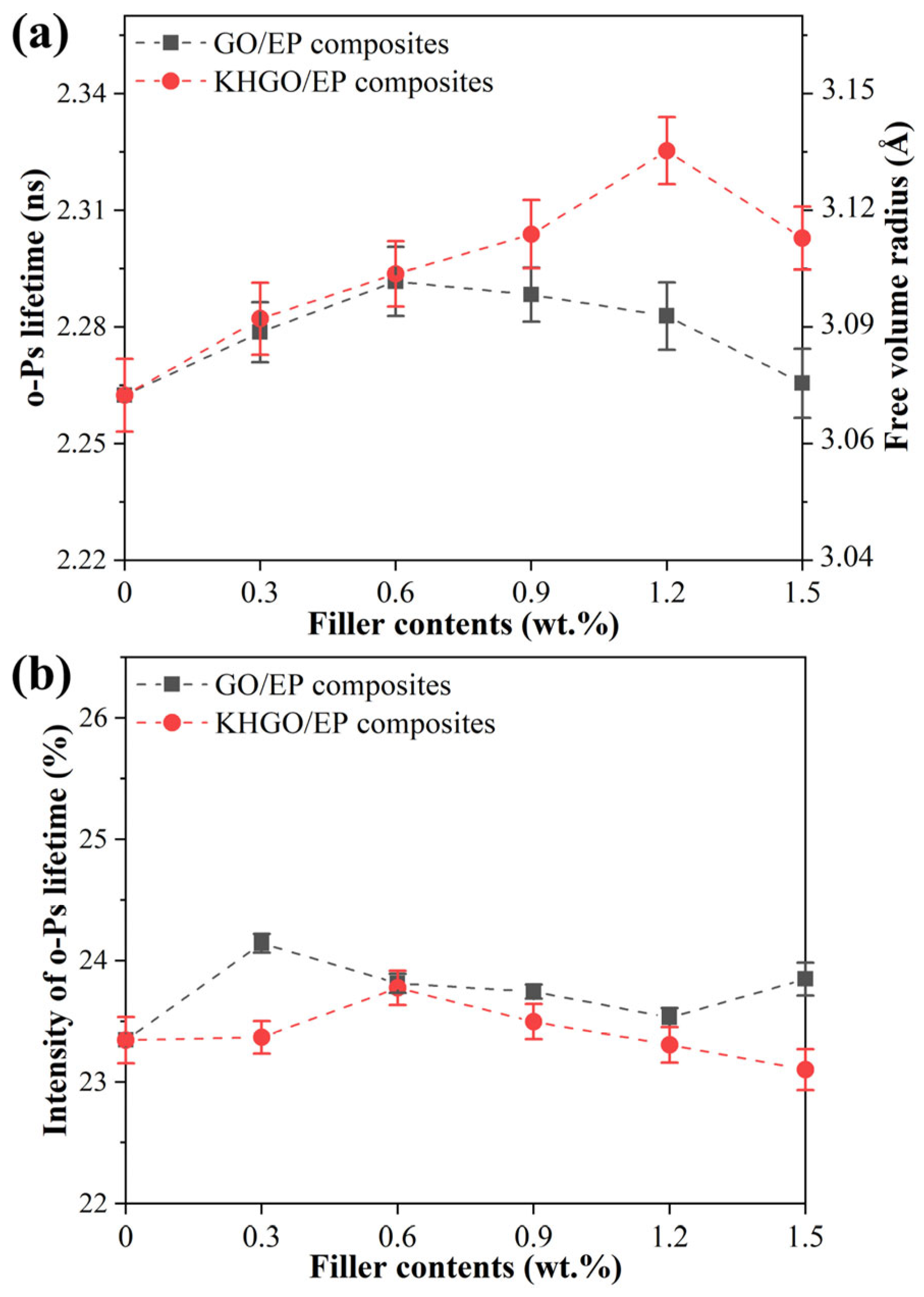
3.3. PALS Spectra of GO/EP Composites and KHGO/EP Composites
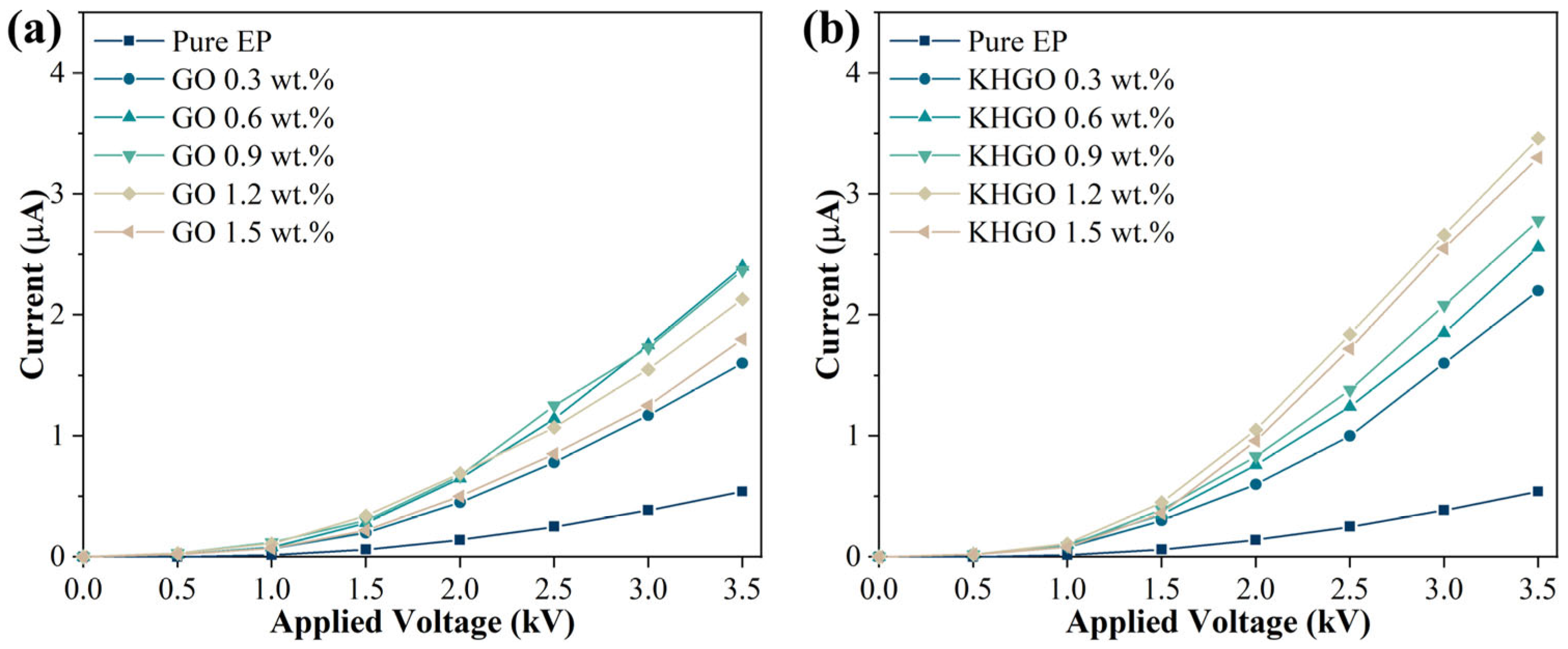
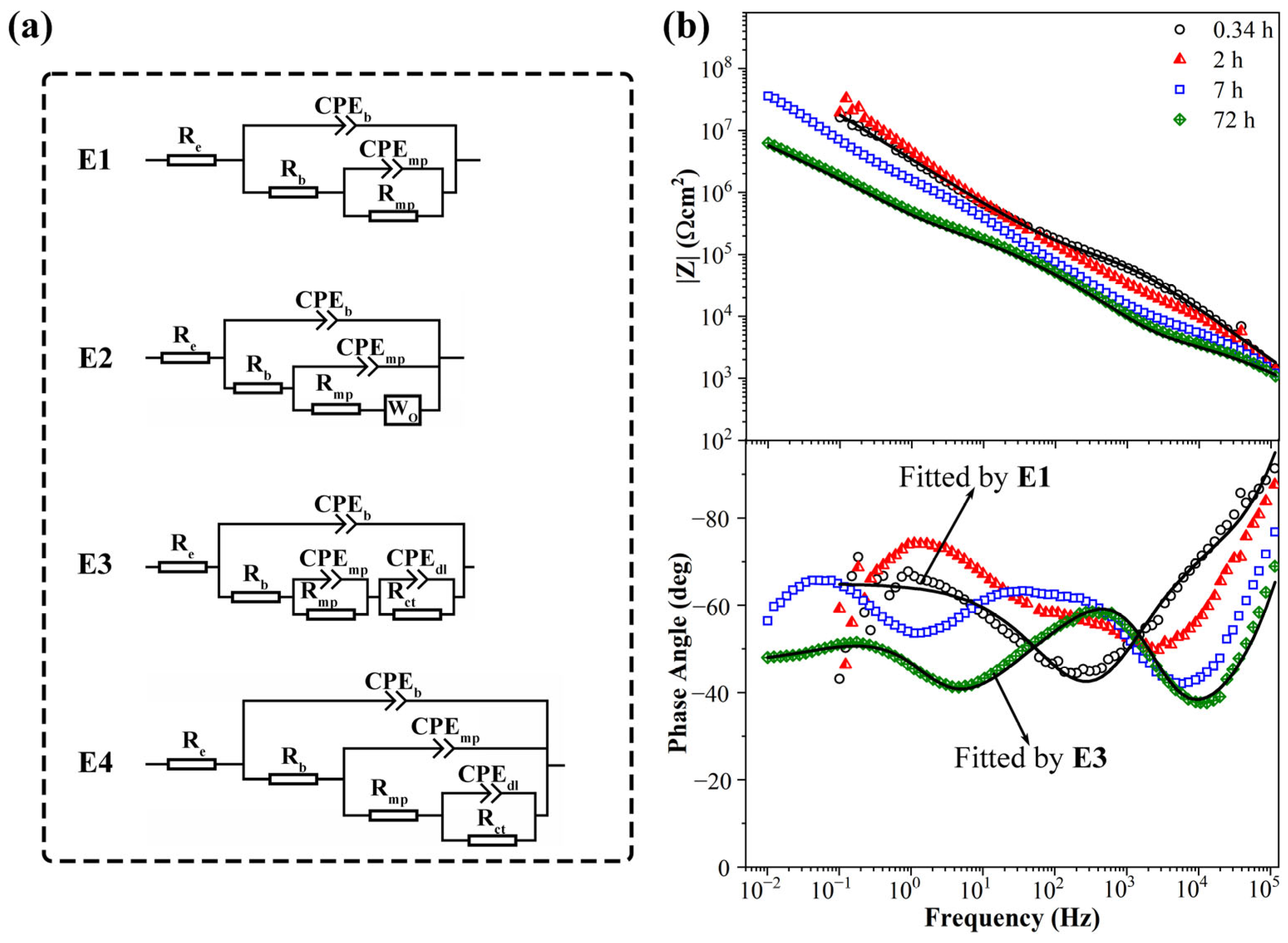
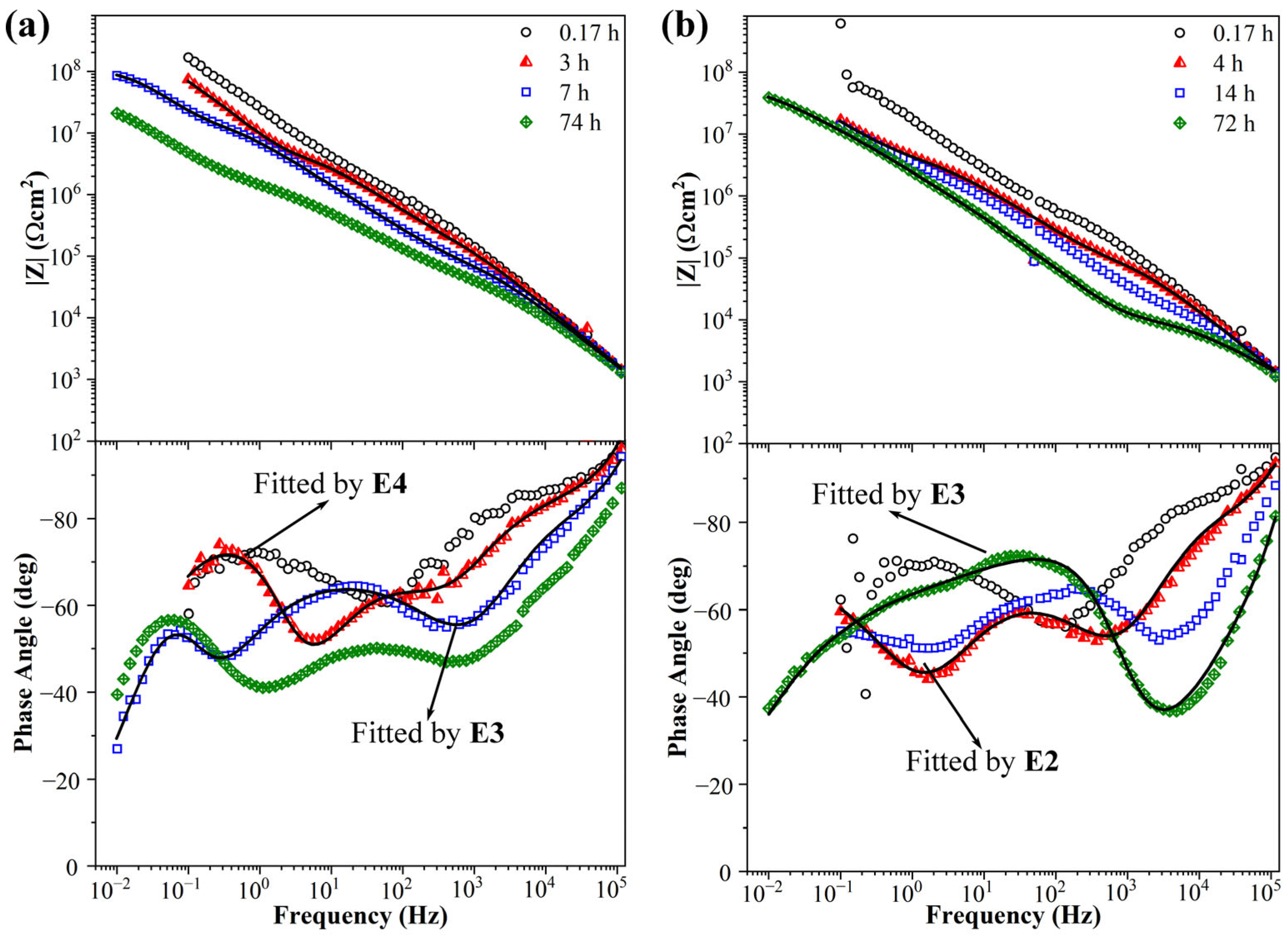
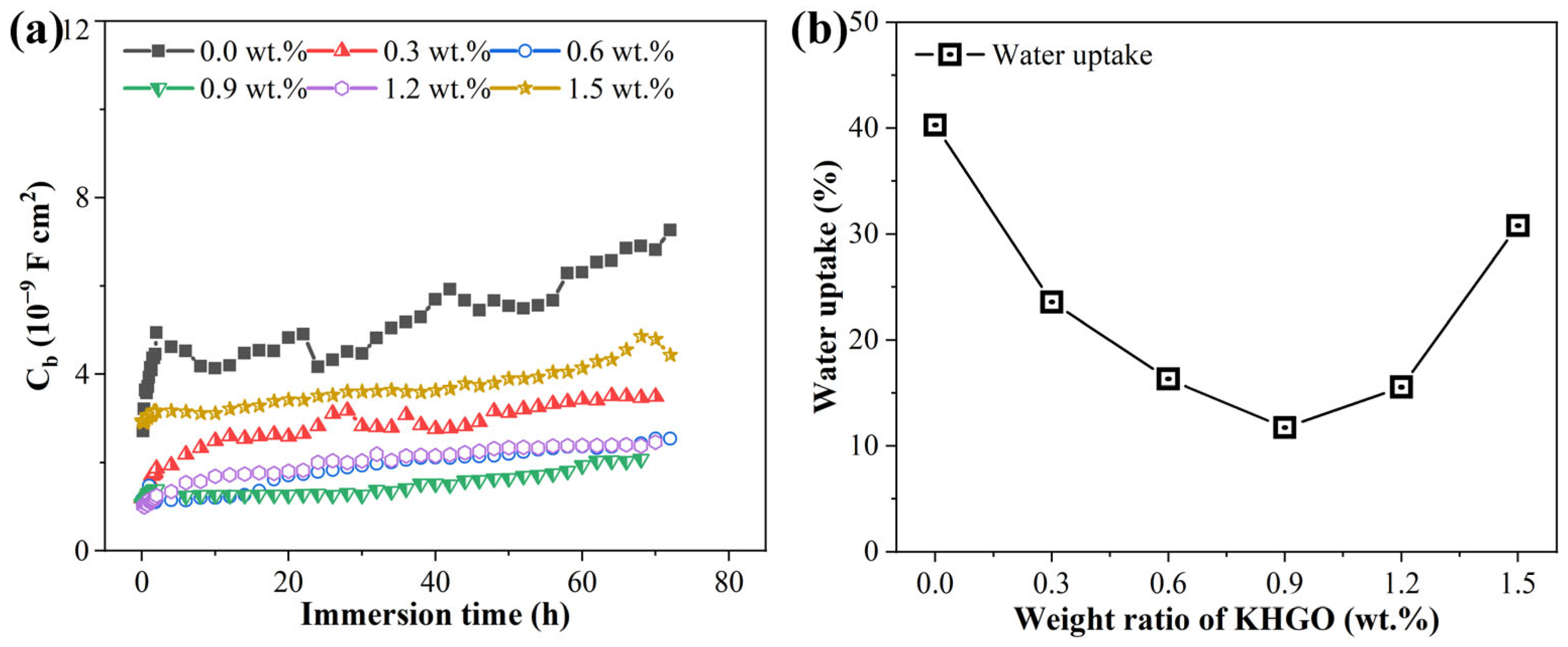
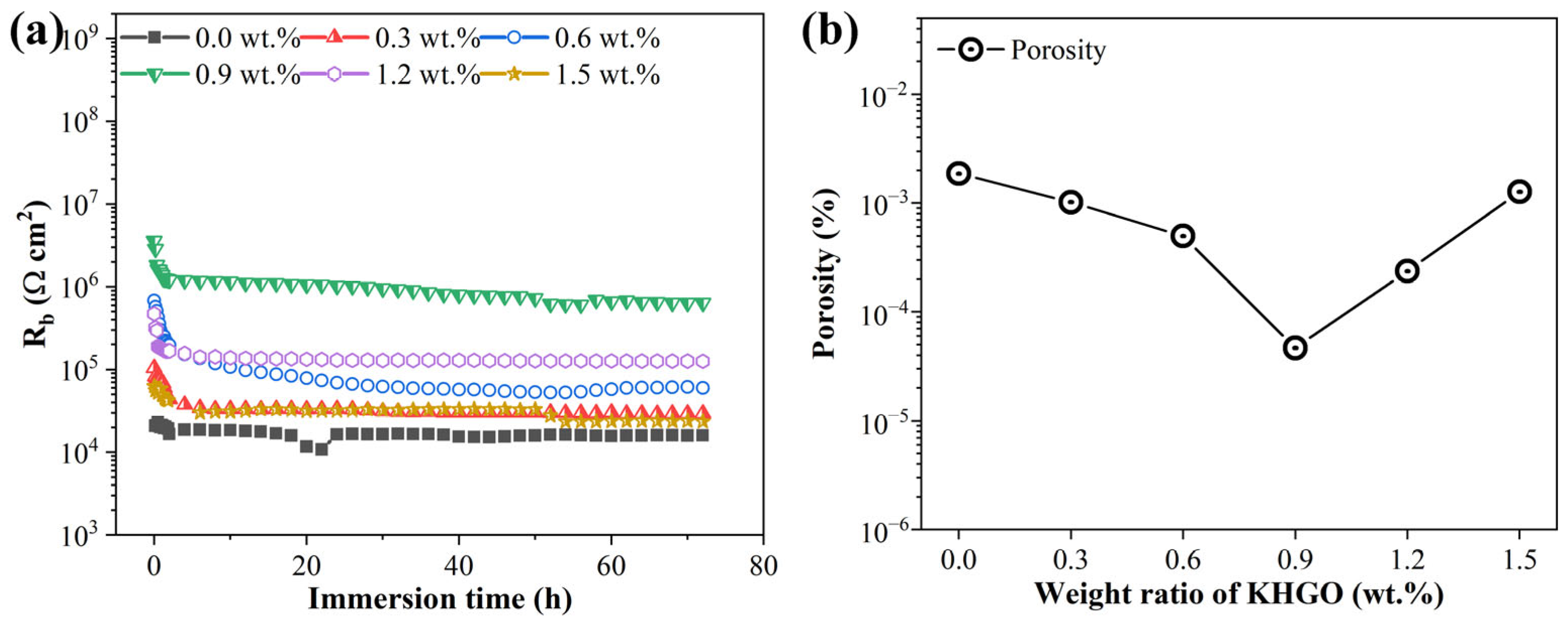
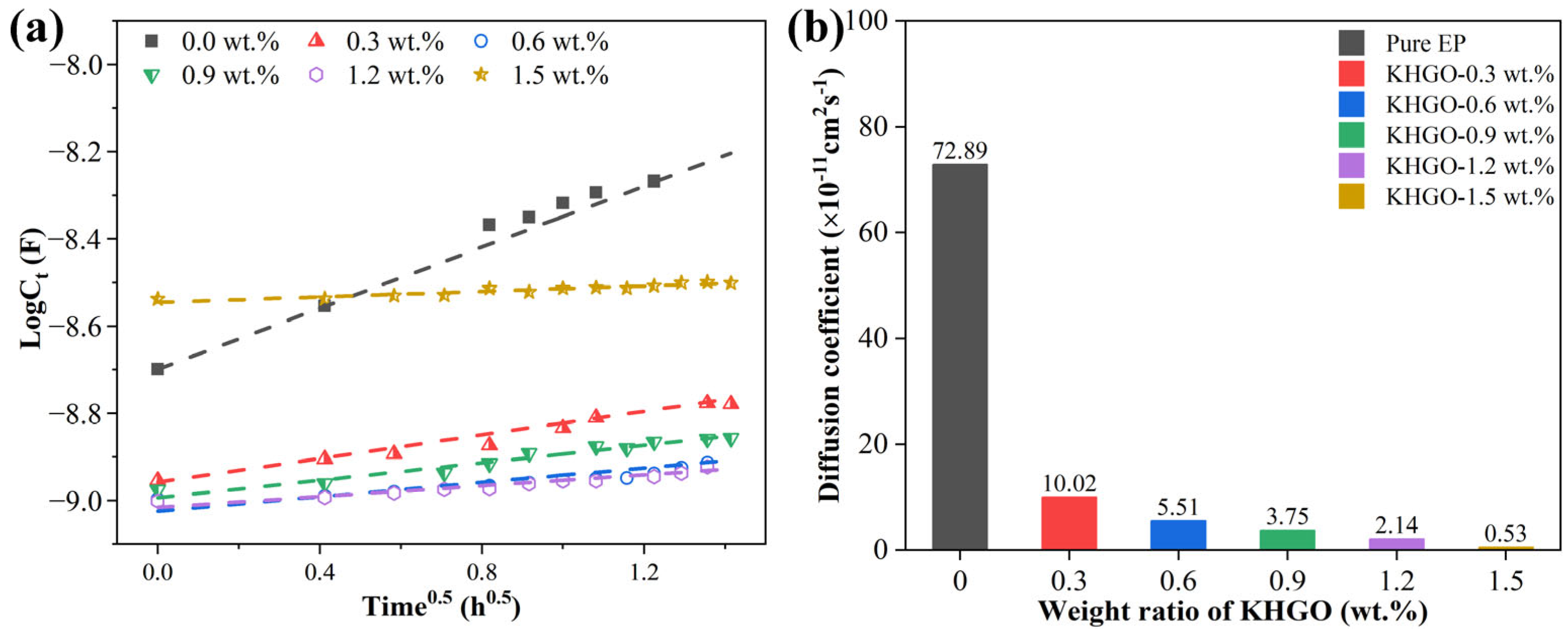
3.4. EIS Spectra of KHGO/EP Composites after Corona Discharge Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousavi, S.R.; Estaji, S.; Kiaei, H.; Mansourian-Tabaei, M.; Nouranian, S.; Jafari, S.H.; Ruckdäschel, H.; Arjmand, M.; Khonakdar, H.A. A review of electrical and thermal conductivities of epoxy resin systems reinforced with carbon nanotubes and graphene-based nanoparticles. Polym. Test 2022, 112, 107645. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, L.; Liang, Y.; Xu, J.; Gao, Y.; Luo, J.; Cheng, Y. Mechanically robust epoxy with electrical breakdown healing capability for power equipment insulation via dynamic networks. React. Funct. Polym. 2022, 176, 105307. [Google Scholar] [CrossRef]
- Zhang, C.; Xiang, J.; Chen, Z.; Wang, Z.; Su, Y.; Wang, S.; Li, J.; Li, S. Surface degradation of epoxy resin exposed to corona discharge under bipolar square wave field: From phenomenon to the insights. Polym. Degrad. Stab. 2024, 228, 110922. [Google Scholar] [CrossRef]
- Gao, C.; Peng, Y.; Zhang, S.; Zhang, B.; Li, W.; Li, L. Mechanism analysis of composite insulators deterioration and decay-like aging caused by internal discharge in transmission lines. IEEE Access 2023, 11, 114582–114589. [Google Scholar] [CrossRef]
- Nazir, M.T.; Phung, B.T.; Hoffman, M. Performance of silicone rubber composites with SiO2 micro/nano-filler under AC corona discharge. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2804–2815. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Huang, Z.; Shang, J. Preparation and characteristics of silicone-modified aging-resistant epoxy resin insulation material. J. Mater. Sci. 2022, 57, 3295–3308. [Google Scholar] [CrossRef]
- Belov, N.A.; Alentiev, A.Y.; Bogdanova, Y.G.; Vdovichenko, A.Y.; Pashkevich, D.S. Direct fluorination as method of improvement of operational properties of polymeric materials. Polymers 2020, 12, 2836. [Google Scholar] [CrossRef]
- Zha, J.; Chen, G.; Dang, Z.; Yin, Y. The influence of TiO2 nanoparticle incorporation on surface potential decay of corona-resistant polyimide nanocomposite films. J. Electrost. 2011, 69, 255–260. [Google Scholar] [CrossRef]
- Zha, J.-W.; Song, H.-T.; Dang, Z.-M.; Shi, C.-Y.; Bai, J. Mechanism analysis of improved corona-resistant characteristic in polyimide/TiO2 nanohybrid films. Appl. Phys. Lett. 2008, 93, 2911. [Google Scholar] [CrossRef]
- Hui, S.; Lizhu, L.; Ling, W.; Weiwei, C.; Xingsong, Z. Preparation and characterization of polyimide/Al2O3 nanocomposite film with good corona resistance. Polym. Compos. 2016, 37, 763–770. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Zhao, W.; Liu, C.; Song, J.; Huang, Z.; Lin, X.; Weng, L. Dielectric properties and corona resistance of Si–Mg–B/EP nano-composites. J. Inorg. Organomet. Polym. Mater. 2024, 34, 546–556. [Google Scholar] [CrossRef]
- Ogbonna, V.E.; Popoola, A.P.I.; Popoola, O.M.; Adeosun, S.O. A review on polyimide reinforced nanocomposites for mechanical, thermal, and electrical insulation application: Challenges and recommendations for future improvement. Polym. Bull. 2022, 79, 663–695. [Google Scholar] [CrossRef]
- Banerjee, S.; Prasad, D.S. AC corona degradation of silicone rubber nanocomposites at low air pressure. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 800–807. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, C.; Li, N.; Yin, J.; Feng, Y.; Liu, Y.; Li, J.; Li, Y.; Yue, D.; Zhu, C.; et al. Electrical and mechanical properties of polyimide composite films reinforced by ultralong titanate nanotubes. Surf. Coat. Technol. 2019, 360, 13–19. [Google Scholar] [CrossRef]
- An, Z.; Xiao, H.; Liu, F.; Zheng, F.; Lei, Q.; Zhang, Y. Improved resistance of epoxy resin to corona discharge by direct fluorination. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2278–2287. [Google Scholar] [CrossRef]
- An, Z.; Liu, F.; Tang, Y.; Zheng, F.; Zhang, Y. Resistance of surface fluorinated epoxy resin to corona discharge in SF6 gas. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3659–3667. [Google Scholar] [CrossRef]
- Preetha, P.; Alapati, S.; Singha, S.; Venkatesulu, B.; Thomas, M.J. Electrical discharge resistant characteristics of epoxy nanocomposites. In Proceedings of the 2008 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Quebec City, QC, Canada, 26–29 October 2008; 718-721. [Google Scholar]
- Yu, G.; Cheng, Y.; Duan, Z. Research progress of polymers/inorganic nanocomposite electrical insulating materials. Molecules 2022, 27, 7867. [Google Scholar] [CrossRef]
- Nazir, M.T.; Phung, B.T.; Yu, S.H.; Li, S.T. Resistance against AC corona discharge of micro-ATH/nano-Al2O3 co-filled silicone rubber composites. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 657–667. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.F.; Zhang, D.H.; Wang, H.S.; Chen, Y.; Chen, Y.F. Comparison of ATH and SiO2 fillers filled silicone rubber composites for HTV insulators. Compos. Sci. Technol. 2018, 155, 137–143. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, J.; Chen, M.; Liu, X.; Su, B.; Fei, W.; Lei, Q. Influence of interface on the electrical properties of polyimide/TiO2 composite films. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1501–1508. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, T.; Hu, R.; Jiang, S.; Zhang, C.; Hou, H. Thermal, mechanical and dielectric properties of polyimide composite films by in-situ reduction of fluorinated graphene. Molecules 2022, 27, 8896. [Google Scholar] [CrossRef]
- Teusdea, A.; Malaescu, I.; Sfirloaga, P.; Marin, C.N. Electric and dielectric properties in low-frequency fields of composites consisting of silicone rubber and Al particles for flexible electronic devices. Materials 2022, 15, 2309. [Google Scholar] [CrossRef]
- Ouyang, Y.; Yang, Z.; Long, T.; Tian, H.; Bai, L.; Li, X.; Yuan, F. Preparation of branched Al2O3 and its synergistic effect with carbon nanotubes on the enhancement of thermal conductive and electrical insulation properties of silicone rubber composites. Mater. Today Commun. 2023, 34, 105239. [Google Scholar] [CrossRef]
- Chen, M.H.; Yin, J.H.; Bu, W.B.; Liu, X.X.; Su, B.; Lei, Q.Q. Microstructure changes of polyimide/MMT-AlN composite hybrid films under corona aging. Appl. Surf. Sci. 2012, 263, 302–306. [Google Scholar] [CrossRef]
- Nguyen, H.; Mirza, A.Y.; Chen, W.; Ronzello, J.; Nasreen, S.; Chapman, J.; Bazzi, A.; Cao, Y. Discharge resistant epoxy/clay nanocomposite for high torque density electrical propulsion. In Proceedings of the 2018 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Cancun, Mexico, 21–24 October 2018; 171-174. [Google Scholar]
- Bu, W.; Yin, J.; Tian, F.; Li, G.; Lei, Q. Effect of corona ageing on the structure changes of polyimide and polyimide/Al2O3 nanocomposite films. J. Electrost. 2011, 69, 141–145. [Google Scholar] [CrossRef]
- Lu, H.; Lin, J.; Yang, W.; Liu, L.; Wang, Y.; Chen, G.; Huang, W. Effect of nano-TiO2 surface modification on polarization characteristics and corona aging performance of polyimide nano-composites. J. Appl. Polym. Sci. 2017, 134, 45101. [Google Scholar] [CrossRef]
- Zha, J.W.; Dang, Z.M.; Li, W.K.; Zhu, Y.H.; Chen, G. Effect of micro-Si3N4-nano-Al2O3 co-filled particles on thermal conductivity, dielectric and mechanical properties of silicone rubber composites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1989–1996. [Google Scholar] [CrossRef]
- Liu, P.; Xie, Z.; Pang, X.; Xu, T.; Zhang, S.; Morshuis, P.H.F.; Li, H.; Peng, Z. Space charge behavior in epoxy-based dielectrics: Progress and perspective. Adv. Electron. Mater. 2022, 8, 2200259. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Sun, D.; Yin, J.; Liu, Y.; Liu, X. The electrical and thermal properties of polyimide/boron nitride nanocomposite films. J. Polym. Res. 2016, 23, 254. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, H.; Dai, C.; Zhang, C.; Zhang, Y.; Chi, Q. Carbon nanotubes and hexagonal boron nitride nanosheets co-filled ethylene propylene diene monomer composites: Improved electrical property for cable accessory applications. High Volt. 2024, 9, 546–555. [Google Scholar] [CrossRef]
- Ren, M.; Wang, Y.; Liu, J.; Wu, C.; Hou, Z.; Konstantinou, A.; Zhou, J.; Nguyen, H.; Davis-Amendola, K.; Sun, L.; et al. Enhancing corona resistance in Kapton with self-assembled two-dimensional montmorillonite nanocoatings. Mater. Adv. 2022, 3, 3853–3861. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Sarath, P.S.; Thomas, S.; Haponiuk, J.T.; George, S.C. Fabrication, characterization and properties of silane functionalized graphene oxide/silicone rubber nanocomposites. J. Appl. Polym. Sci. 2022, 139, e52299. [Google Scholar] [CrossRef]
- Kiranakumar, H.V.; Thejas, R.; Naveen, C.S.; Khan, M.I.; Prasanna, G.D.; Reddy, S.; Oreijah, M.; Guedri, K.; Bafakeeh, O.T.; Jameel, M. A review on electrical and gas-sensing properties of reduced graphene oxide-metal oxide nanocomposites. Biomass Convers. Biorefinery 2024, 14, 12625–12635. [Google Scholar] [CrossRef]
- Zheng, L.; Shui, T.; Xiang, D.; Zhang, J.; Liu, L.; Sun, H.; Wu, Y.; Cheng, J.; Zhao, C.; Li, H. Effect of seawater corrosion on the mechanical properties of basalt fiber reinforced polymer composites modified by silane coupling agent KH560 and carboxylated carbon nanotubes. Mater. Today Commun. 2024, 40, 109753. [Google Scholar] [CrossRef]
- Bu, Q.; Yin, S.; Wan, W.; Zeng, S.; Zhou, S.; Yu, L.; Ouyang, Y. Effect of silane coupling agent KH550 on the dielectric properties of CaCu3Ti4O12/polyurethane composite films. Ceram. Int. 2024, 50 Pt B, 12389–12396. [Google Scholar] [CrossRef]
- Li, C.; Liao, H.; Gao, H.; Cheng, F. Enhancing interface compatibility in high-filled coal gangue/polyethylene composites through silane coupling agent-mediated interface modification. Compos. Sci. Technol. 2024, 251, 110546. [Google Scholar] [CrossRef]
- Surekha, G.; Krishnaiah, K.V.; Ravi, N.; Padma Suvarna, R. FTIR, Raman and XRD analysis of graphene oxide films prepared by modified Hummers method. J. Phys. Conf. Ser. 2020, 1495, 012012. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Chadha, N.; Sharma, R.; Saini, P. A new insight into the structural modulation of graphene oxide upon chemical reduction probed by Raman spectroscopy and X-ray diffraction. Carbon Lett. 2021, 31, 1125–1131. [Google Scholar] [CrossRef]
- Strankowski, M.; Włodarczyk, D.; Piszczyk, Ł.; Strankowska, J. Polyurethane nanocomposites containing reduced graphene oxide, FTIR, Raman, and XRD studies. J. Spectrosc. 2016, 2016, 7520741. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Haeri, Z.; Ramezanzadeh, M. A facile route of making silica nanoparticles-covered graphene oxide nanohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection performance. Chem. Eng. J. 2016, 303, 511–528. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Tuning the oxygen content of reduced graphene oxide and effects on its properties. ACS Omega 2021, 6, 6195–6205. [Google Scholar] [CrossRef]
- Maio, A.; Agnello, S.; Khatibi, R.; Botta, L.; Alessi, A.; Piazza, A.; Buscarino, G.; Mezzi, A.; Pantaleo, G.; Scaffaro, R. A rapid and eco-friendly route to synthesize graphene-doped silica nanohybrids. J. Alloys Compd. 2016, 664, 428–438. [Google Scholar] [CrossRef]
- Xu, Z.; Bando, Y.; Liu, L.; Wang, W.; Bai, X.; Golberg, D. Electrical conductivity, chemistry, and bonding alternations under graphene oxide to graphene transition as revealed by in situ TEM. ACS Nano 2011, 5, 4401–4406. [Google Scholar] [CrossRef]
- Rudajevová, A.; Staněk, M.; Lukáč, P. Determination of thermal diffusivity and thermal conductivity of Mg–Al alloys. Mater. Sci. Eng. A 2003, 341, 152–157. [Google Scholar] [CrossRef]
- Tao, S.J. Positronium annihilation in molecular substances. J. Chem. Phys. 1972, 56, 5499–5510. [Google Scholar] [CrossRef]
- Li, C.Y.; Qi, N.; Liu, Z.W.; Zhou, B.; Chen, Z.Q.; Wang, Z. Effect of synthesis temperature on the ordered pore structure in mesoporous silica studied by positron annihilation spectroscopy. Appl. Surf. Sci. 2016, 363, 445–450. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical note: Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Brasher, D.M.; Kingsbury, A.H. Electrical measurements in the study of immersed paint coatings on metal. I: Comparison between capacitance and gravimetric methods of estimating water-uptake. J. Appl. Chem. 1954, 4, 62–72. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Peng, X.; Huang, Z.; Fang, P. Influence of crosslinking extent on free volumes of silicone rubber and water diffusion after corona discharge. Materials 2022, 15, 6833. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xiong, J.; Tang, Y.; Zuo, Y. EIS study on failure process of two polyurethane composite coatings. Prog. Org. Coat. 2010, 69, 7–11. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Hou, B. Corrosion performance of epoxy coatings modified by nanoparticulate SiO2. Mater. Corros. 2012, 63, 44–53. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, Y.; He, C.; Wang, Z.; Peng, X.; Fang, P. Significantly Enhanced Corona Resistance of Epoxy Composite by Incorporation with Functionalized Graphene Oxide. Materials 2024, 17, 4864. https://doi.org/10.3390/ma17194864
Yang Y, Wang Y, He C, Wang Z, Peng X, Fang P. Significantly Enhanced Corona Resistance of Epoxy Composite by Incorporation with Functionalized Graphene Oxide. Materials. 2024; 17(19):4864. https://doi.org/10.3390/ma17194864
Chicago/Turabian StyleYang, Yue, Yumin Wang, Chunqing He, Zheng Wang, Xiangyang Peng, and Pengfei Fang. 2024. "Significantly Enhanced Corona Resistance of Epoxy Composite by Incorporation with Functionalized Graphene Oxide" Materials 17, no. 19: 4864. https://doi.org/10.3390/ma17194864






