Sintering and Tribological Properties of Ti3SiC2-TiSix Composite Sintered by High-Pressure High-Temperature Technology
Abstract
1. Introduction
2. Materials and Methods
3. Results
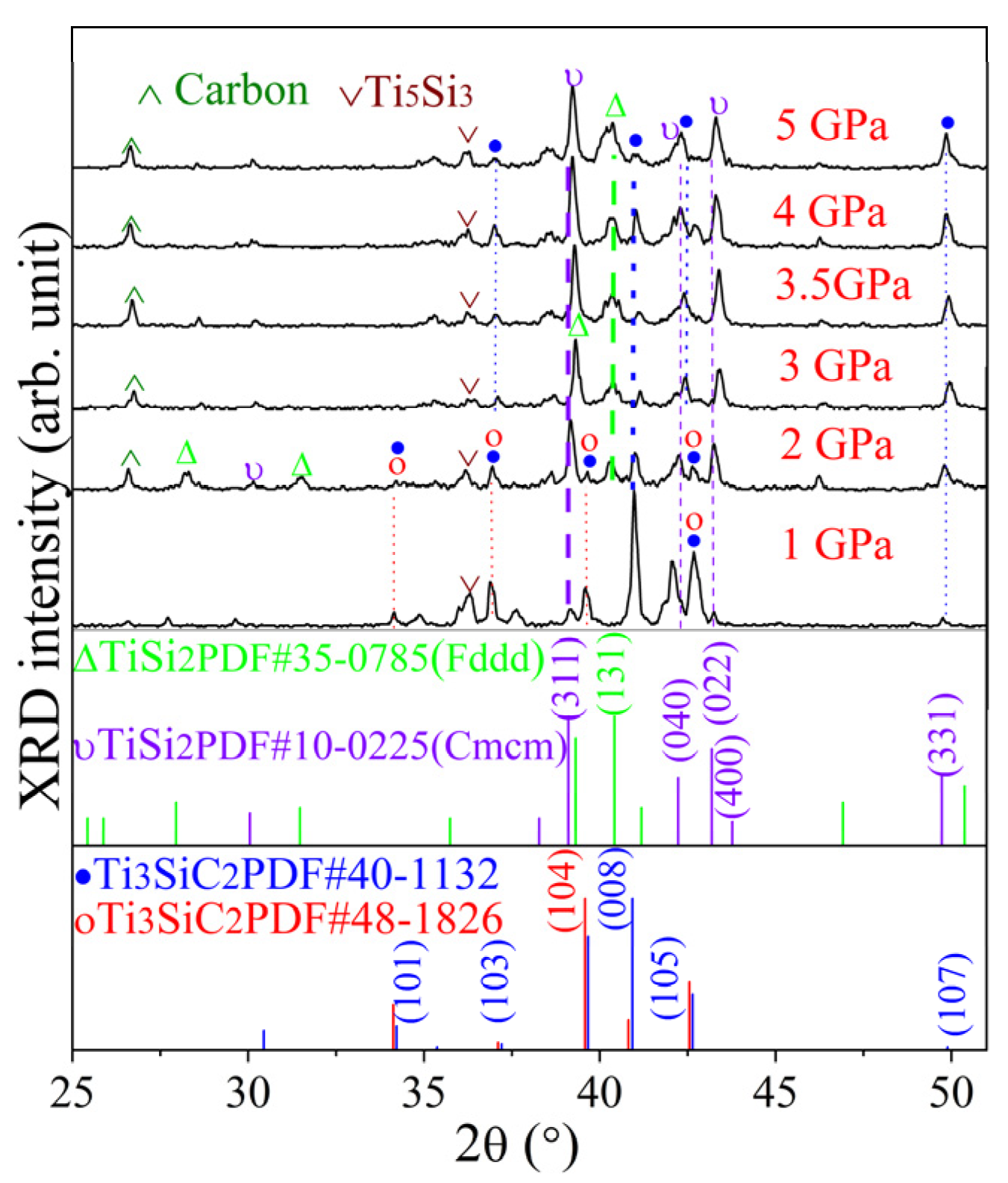
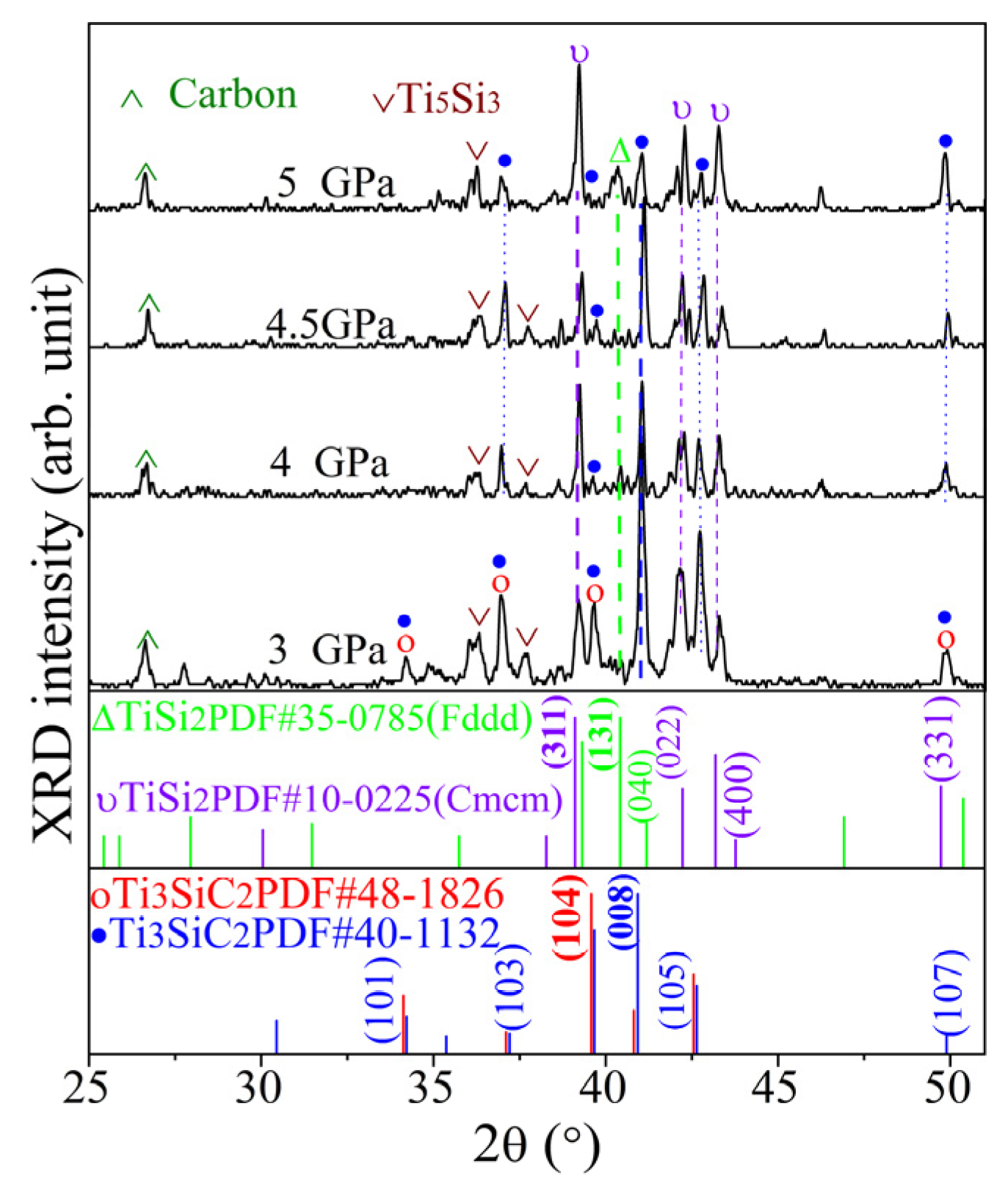
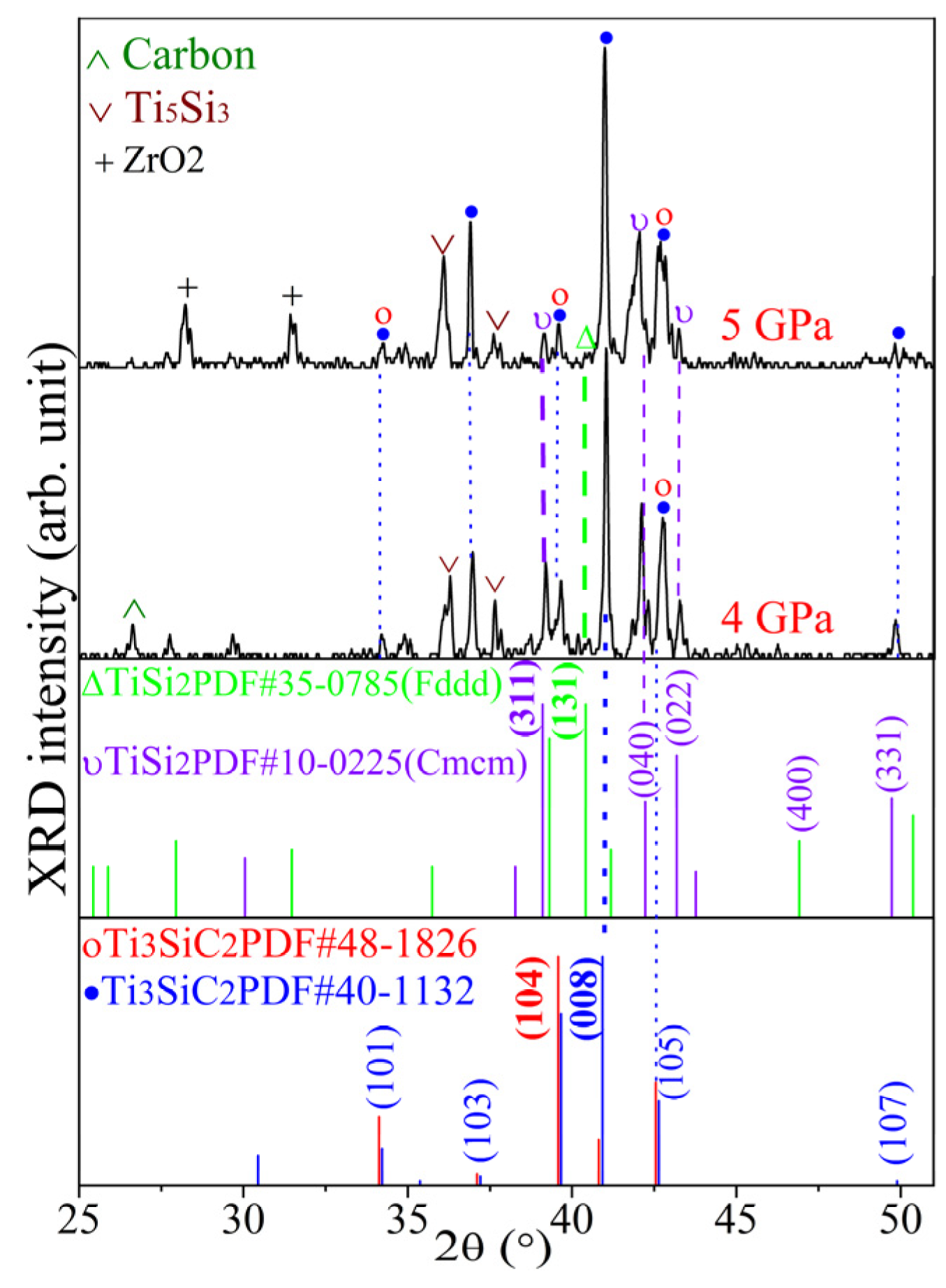
3.1. XRD Results of Ti3SiC2-TiSix Composite
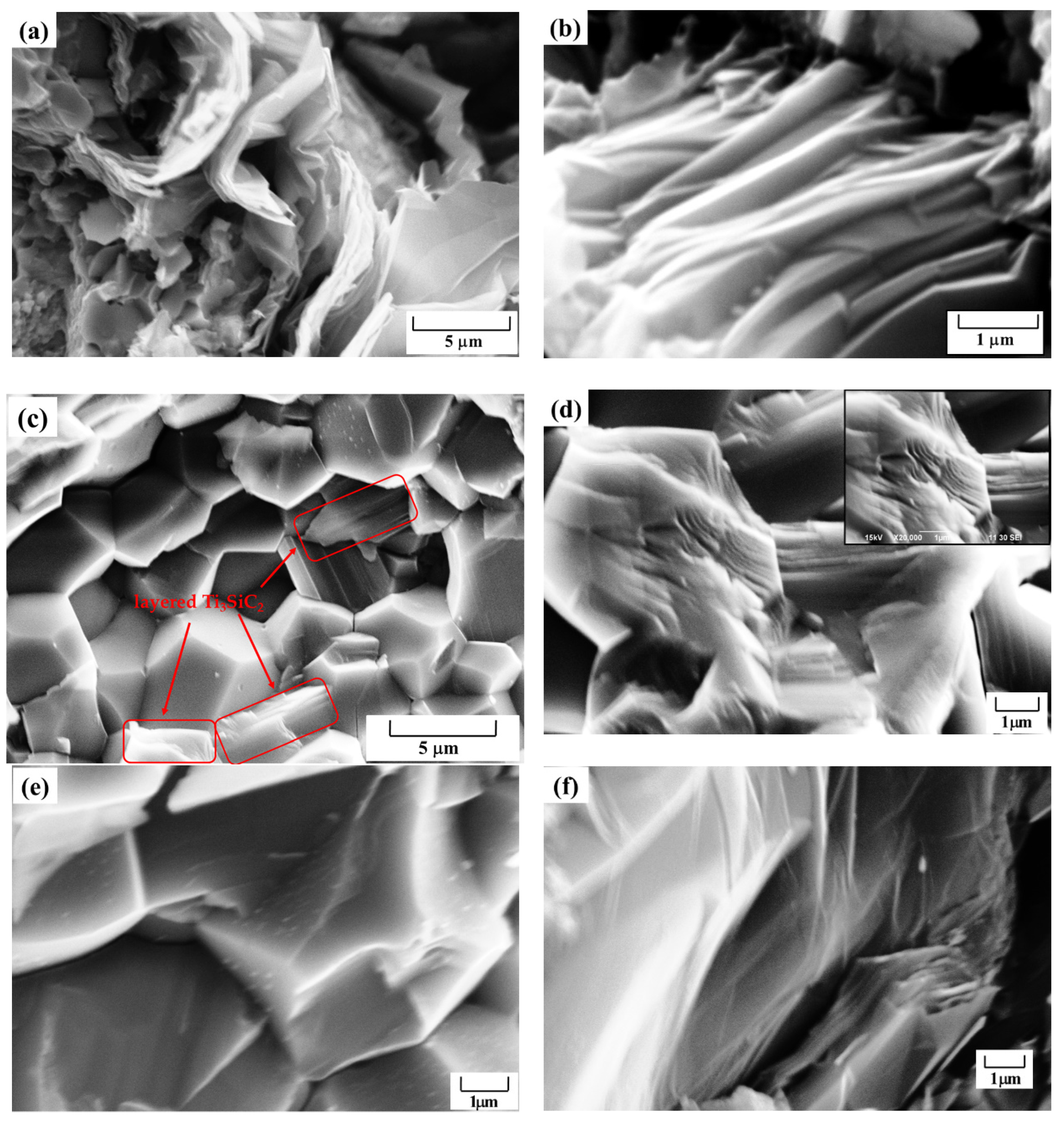
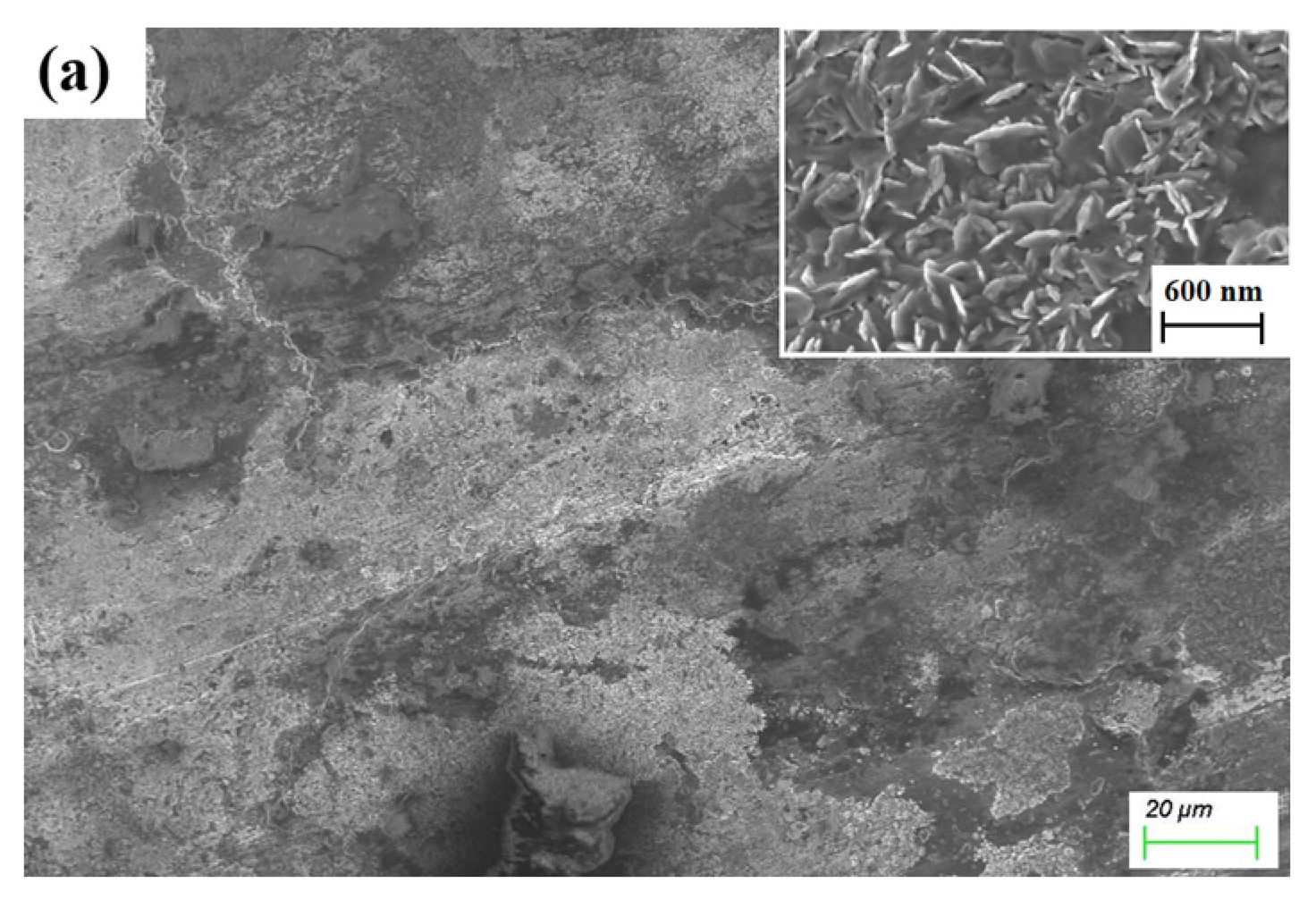
3.2. Microstructure of Ti3SiC2-TiSix Composites
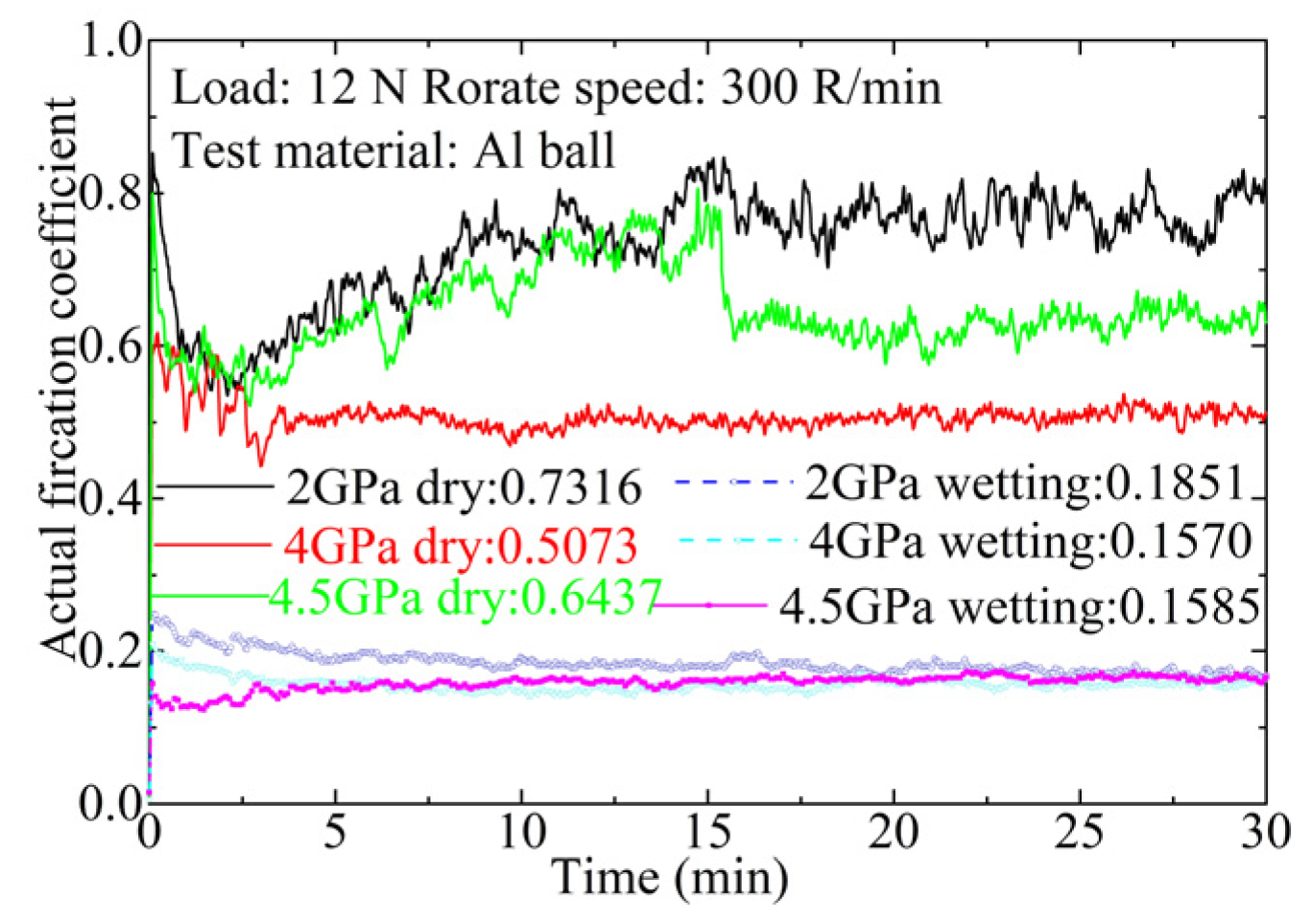
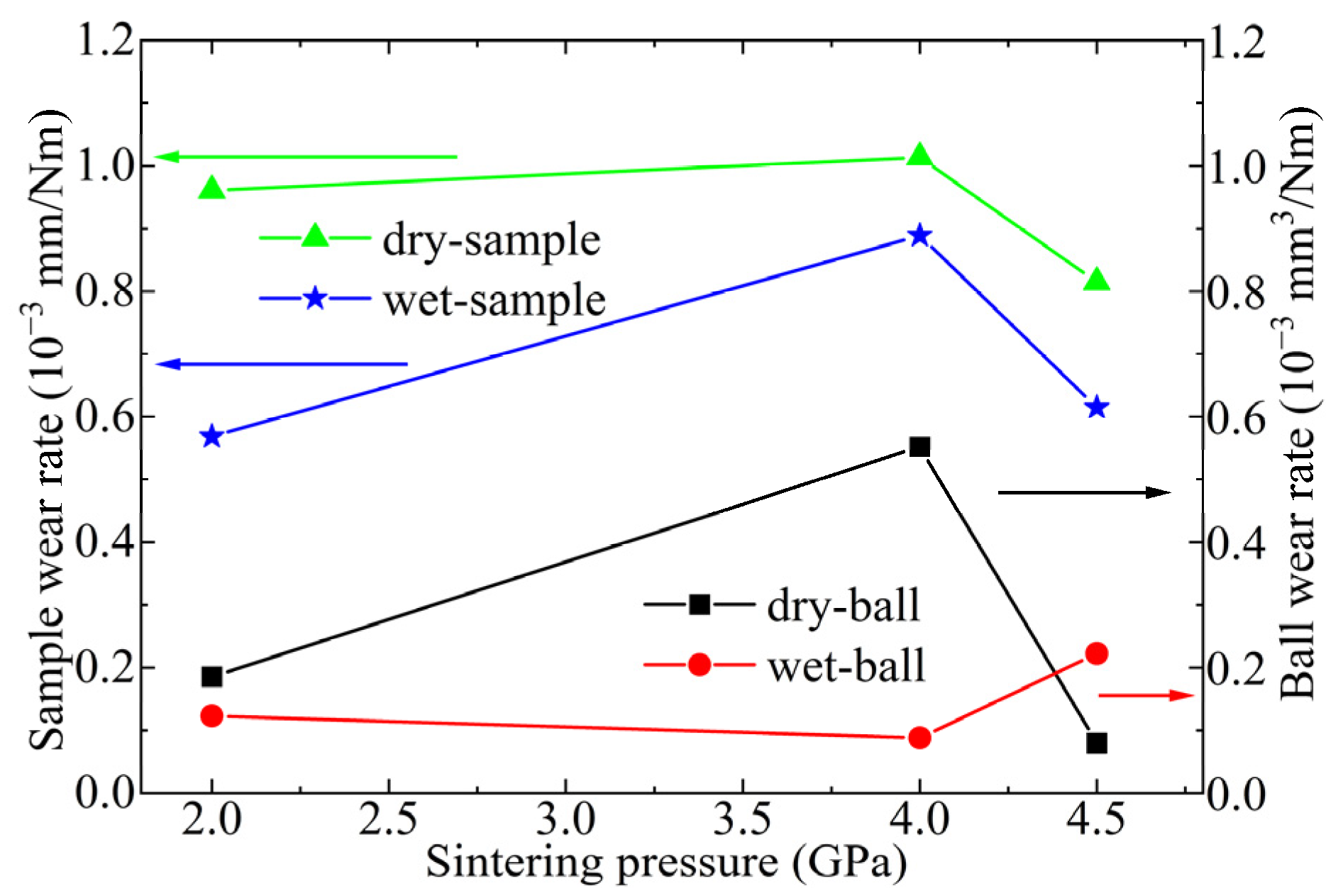
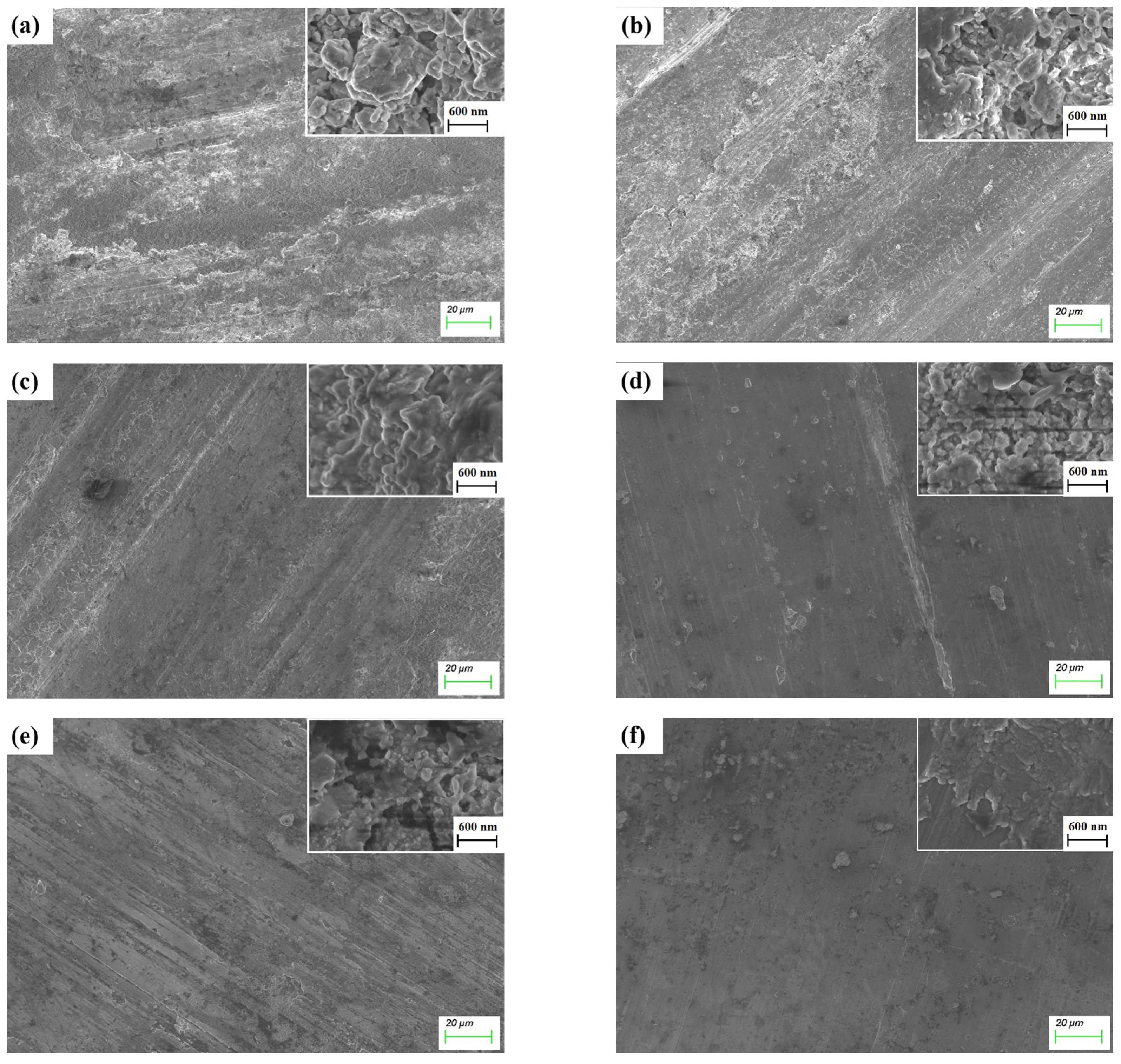
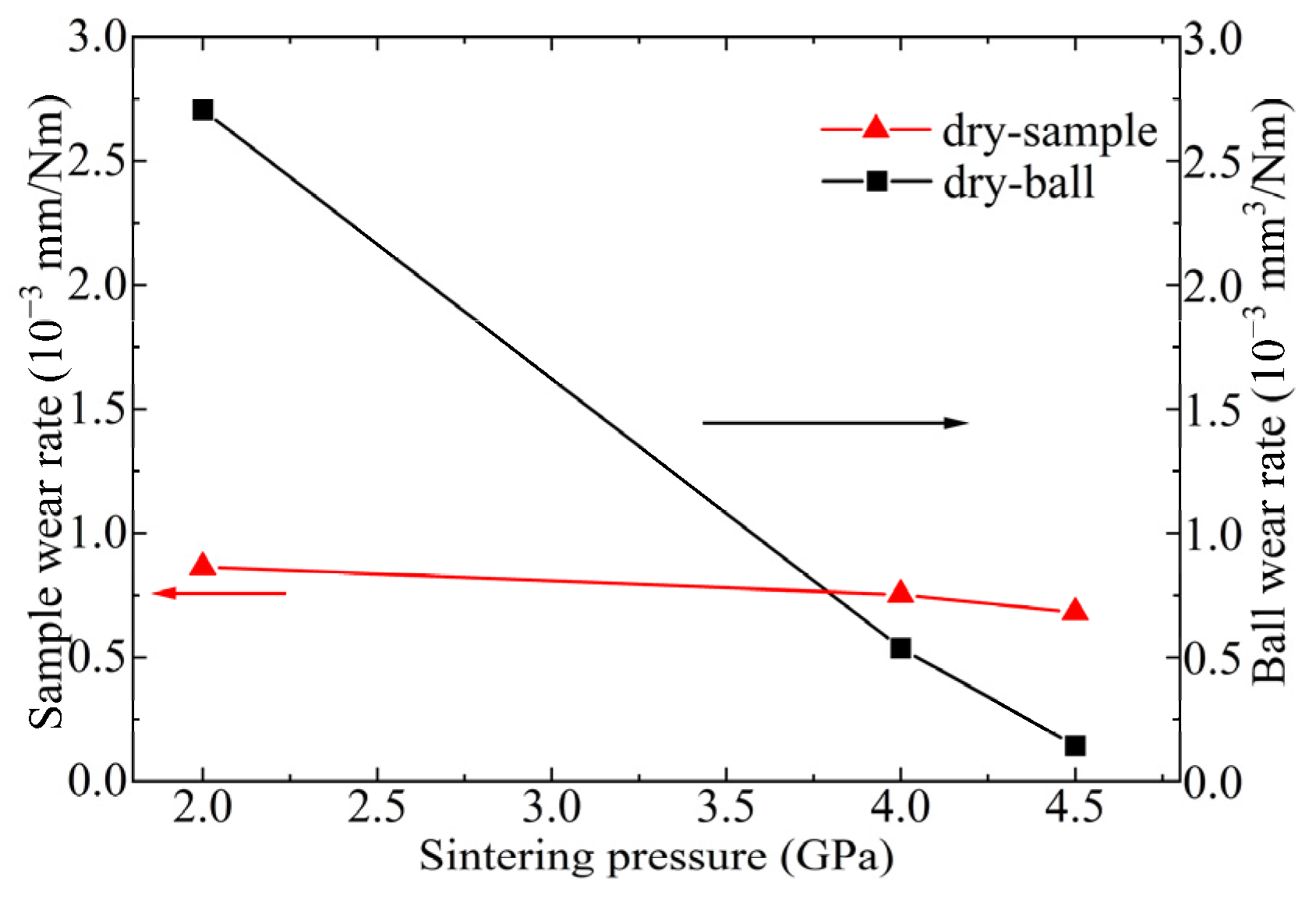
3.3. Friction Behavior of Ti3SiC2-TiSix Composites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.B.; Bao, Y.W.; Zhou, Y.C. Current status in layered ternary carbide Ti3SiC2, a review. J. Mater. Sci. Technol 2009, 25, 1–38. [Google Scholar]
- Ji, H.; Liang, Y.; Jiang, Z.; Li, Z.; Zhu, Y. Controllable HTHP sintering and property of cBN/diamond composites containing Ti3SiC2. Ceram. Int. 2020, 46, 13807–13812. [Google Scholar] [CrossRef]
- Lv, X.; Jian, Q.; Li, Z.; Sun, K.; Ji, H.; Zhu, Y. Effect of controllable decomposition of MAX phase (Ti3SiC2) on mechanical properties of rapidly sintered polycrystalline diamond by HPHT. Ceram. Int. 2019, 45, 16564–16568. [Google Scholar] [CrossRef]
- Li, L.; Zhou, A.; Wang, L.; Li, S.; Wu, D.; Yan, C. In situ synthesis of cBN–Ti3AlC2 composites by high-pressure and high-temperature technology. Diam. Relat. Mater. 2012, 29, 8–12. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, A.; Li, L.; Wang, L.; Hu, M.; Li, S.; Gupta, S. Synthesis and characterization of novel Ti3SiC2–cBN composites. Diam. Relat. Mater. 2014, 43, 29–33. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, X.; Li, Y.; Christian, P.; Sun, H.; Zhang, Y.; Fang, Y.; Shu, R. Microstructures and properties of graphene nanoplatelets reinforced Cu/Ti3SiC2/C nanocomposites with efficient dispersion and strengthening achieved by high-pressure torsion. Mater. Charact. 2022, 193, 112308. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, B.; Liu, F.; Sun, M.; Zhang, H.; Wu, C. Microstructure and mechanical properties of composites obtained by spark plasma sintering of Ti3SiC2-15 vol.% Cu mixtures. Materials 2022, 15, 2515. [Google Scholar] [CrossRef]
- Zhang, R.; Du, C.; Liu, F.; Wu, C. Electrochemical Corrosion Behavior and the Related Mechanism of Ti3SiC2/Cu Composites in a Strong Acid Environment. Materials 2024, 17, 4035. [Google Scholar] [CrossRef]
- Amiri, S.H.; Kakroudi, M.G.; Vafa, N.P.; Asl, M.S. Synthesis and sintering of Ti3SiC2–SiC composites through reactive hot-pressing of TiC and Si precursors. Silicon 2021, 14, 4227–4235. [Google Scholar] [CrossRef]
- He, G.; Xu, J.; Zhang, Z.; Qian, Y.; Zuo, J.; Li, M.; Liu, C. Interfacial reactions and mechanical properties of SiC fiber reinforced Ti3SiC2 and Ti3(SiAl)C2 composites. Mater. Sci. Eng. A 2021, 827, 142069. [Google Scholar] [CrossRef]
- Singh, J.; Wani, M. Fretting wear of spark plasma sintered Ti3SiC2/GNP ceramic composite against Si3N4. Ceram. Int. 2021, 47, 5648–5655. [Google Scholar] [CrossRef]
- Yang, J.; Ye, F.; Cheng, L. In-situ formation of Ti3SiC2 interphase in SiCf/SiC composites by molten salt synthesis. J. Eur. Ceram. Soc. 2021, 42, 1197–1207. [Google Scholar] [CrossRef]
- Giuranno, D.; Gambaro, S.; Bruzda, G.; Nowak, R.; Polkowski, W.; Sobczak, N.; Delsante, S.; Novakovic, R. Interface design in lightweight SiC/TiSi2 composites fabricated by reactive infiltration process: Interaction phenomena between liquid Si-rich Si-Ti alloys and glassy carbon. Materials 2021, 14, 3746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Feng, W.; Liu, F. Tribo-oxide competition and oxide layer formation of Ti3SiC2/CaF2 self-lubricating composites during the friction process in a wide temperature range. Materials 2021, 14, 7466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Feng, W.; Wei, Q.; Ma, S. Synthesis and tribological characterization of Ti3SiC2/ZnO composites. Materials 2021, 14, 6088. [Google Scholar] [CrossRef]
- Dang, W.; Ren, S.; Zhou, J.; Yu, Y.; Wang, L. The tribological properties of Ti3SiC2/Cu/Al/SiC composite at elevated temperatures. Tribol. Int. 2016, 104, 294–302. [Google Scholar] [CrossRef]
- Magnus, C.; Sharp, J.; Rainforth, W.M. The lubricating properties of spark plasma sintered (SPS) Ti3SiC2 MAX phase compound and composite. Tribol. Trans. 2020, 63, 38–51. [Google Scholar] [CrossRef]
- Yuqi, C.; Liang, L.; Shibang, M.; Chao, L.; Songhao, Z.; Wucheng, L.; Libo, W.; Aiguo, Z.; Xing, W. Preparation of Ti3Si0.8Al0.2C2 bonded diamond composites and their friction properties coupled with different counterfaces. Adv. Mater. Sci. Eng. 2023, 2023, 1740345. [Google Scholar] [CrossRef]
- Qin, J.; He, D. Phase stability of Ti3SiC2 at high pressure and high temperature. Ceram. Int. 2013, 39, 9361–9367. [Google Scholar] [CrossRef]
- Li, X.; Xu, L.; Chen, Q.; Cao, X.; Liu, L.; Wang, Y.; Zhang, H.; Meng, C.; Wu, Q. Investigation of formation mechanism of Ti3SiC2 by high pressure and high-temperature synthesis. High Press. Res. 2018, 38, 440–447. [Google Scholar] [CrossRef]
- Jaworska, L.; Stobierski, L.; Twardowska, A.; Królicka, D. Preparation of materials based on Ti–Si–C system using high temperature–high pressure method. J. Mater. Process. Technol. 2005, 162, 184–189. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, S.; Song, X.; Wang, Y.; Chen, Z. First principles study of stability, electronic structure and fracture toughness of Ti3SiC2/TiC interface. Vacuum 2021, 196, 110745. [Google Scholar] [CrossRef]
- Jung, Y.I.; Park, D.J.; Park, J.H.; Park, J.Y.; Kim, H.G.; Koo, Y.H. Effect of TiSi2/Ti3SiC2 matrix phases in a reaction-bonded SiC on mechanical and high-temperature oxidation properties. J. Eur. Ceram. Soc. 2016, 36, 1343–1348. [Google Scholar] [CrossRef]
- Wan, C.; Wang, Y.; Wang, N.; Koumoto, K. Low-thermal-conductivity (MS)1+ x(TiS2)2 (M = Pb, Bi, Sn) misfit layer compounds for bulk thermoelectric materials. Materials 2010, 3, 2606–2617. [Google Scholar] [CrossRef]
- Ursi, F.; Virga, S.; Garcìa-Espejo, G.; Masciocchi, N.; Martorana, A.; Giannici, F. Long-term stability of TiS2–Alkylamine hybrid materials. Materials 2022, 15, 8297. [Google Scholar] [CrossRef]
- Niranjan, M.K. Anisotropy in elastic properties of TiSi2 (C49, C40 and C54), TiSi and Ti5Si3: An ab-initio density functional study. Mater. Res. Express 2015, 2, 096302. [Google Scholar] [CrossRef]
- Li, C.; Yu, Z.; Liu, H.; Lü, T. The crystallographic stability and anisotropic compressibility of C54-type TiSi2 under high pressure. J. Phys. Chem. Solids 2013, 74, 1291–1294. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; Xu, B.; Meng, C.; Li, X. TiSi2-SiC agglomerates toughened TiC composites prepared by in-situ reaction under high pressure. High Press. Res. 2019, 39, 598–607. [Google Scholar] [CrossRef]
- Tian, W.; Sun, Z.; Hashimoto, H.; Du, Y. Synthesis, microstructure and mechanical properties of Ti3SiC2–TiC composites pulse discharge sintered from Ti/Si/TiC powder mixture. Mater. Sci. Eng. A 2009, 526, 16–21. [Google Scholar] [CrossRef]
- Raju, G.; Basu, B.; Tak, N.; Cho, S. Temperature dependent hardness and strength properties of TiB2 with TiSi2 sinter-aid. J. Eur. Ceram. Soc. 2009, 29, 2119–2128. [Google Scholar] [CrossRef]
- Frommeyer, G.; Rosenkranz, R. Structures and properties of the refractory silicides Ti5Si3 and TiSi2 and Ti-Si-(Al) eutectic alloys. In Metallic Materials with High Structural Efficiency; Springer: Berlin/Heidelberg, Germany, 2004; pp. 287–308. [Google Scholar]
- Racault, C.; Langlais, F.; Bernard, C. On the chemical vapour deposition of Ti3SiC2 from TiCl4-SiCl4-CH4-H2 gas mixtures: Part IA thermodynamic approach. J. Mater. Sci. 1994, 29, 5023–5040. [Google Scholar] [CrossRef]
- Abu, M.J.; Mohamed, J.J.; Ahmad, Z.A. Effect of excess silicon on the formation of Ti3SiC2 using free Ti/Si/C powders synthesized via arc melting. Int. Sch. Res. Not. 2012, 2012, 341285. [Google Scholar]
- El Saeed, M.; Deorsola, F.A.; Rashad, R. Optimization of the Ti3SiC2 MAX phase synthesis. Int. J. Refract. Met. Hard Mater. 2012, 35, 127–131. [Google Scholar] [CrossRef]
- Foratirad, H.; Baharvandi, H.; Maraghe, M.G. Effect of excess silicon content on the formation of nano-layered Ti3SiC2 ceramic via infiltration of TiC preforms. J. Eur. Ceram. Soc. 2017, 37, 451–457. [Google Scholar] [CrossRef]
- Hosseinizadeh, S.A.; Pourebrahim, A.; Baharvandi, H.; Ehsani, N. Synthesis of nano-layered Ti3SiC2 MAX phase through reactive melt infiltration (RMI): Metallurgical and thermodynamical parameters. Ceram. Int. 2020, 46, 22208–22220. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Sun, Z.M.; Hashimoto, H.; Abe, T. Effects of sintering temperature and Si content on the purity of Ti3SiC2 synthesized from Ti/Si/TiC powders. J. Alloys Compd. 2003, 352, 283–289. [Google Scholar] [CrossRef]
- Kero, I.; Antti, M.-L.; Odén, M. Synthesis of Ti3SiC2 by reaction of TiC and Si powders. In Proceedings of the International Conference on Advanced Ceramics and Composites: 27/01/2008–01/02/2008, Daytona Beach, FL, USA, 18–23 January 2009; pp. 21–30. [Google Scholar]
- Du, Y.; Schuster, J.C.; Seifert, H.J.; Aldinger, F. Experimental investigation and thermodynamic calculation of the titanium–silicon–carbon system. J. Am. Ceram. Soc. 2000, 83, 197–203. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y. The Influence of sintering pressure on the preparation, friction properties, and magnetic properties of Ti2AlC-TiC and Ti3AlC2-TiC composites under high-pressure and high-temperature. Adv. Mater. Sci. Eng. 2022, 2022, 9108736. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, A.; Ji, Y.; Jia, J.; Wang, L.; Wu, B.; Zan, Q. Tribological properties of Ti3SiC2 coupled with different counterfaces. Ceram. Int. 2015, 41, 6950–6955. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.; Liu, F. Microstructure and tribological properties of spark-plasma-sintered Ti3SiC2-Pb-Ag composites at elevated temperatures. Materials 2022, 15, 1437. [Google Scholar] [CrossRef]
- Zhimei, S.; Yanchun, Z.; Shu, L. Tribological behavior of Ti3SiC2-based material. J. Mater. Sci. Technol. 2002, 18, 142. [Google Scholar]
- Zhou, A.G.; Li, L.; Su, T.C.; Li, S.S. Synthesize Ti3SiC2 and Ti3SiC2-diamond composites at high pressure and high temperature. In Proceedings of the Key Engineering Materials, Singapore, 26–28 February 2012; pp. 671–675. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, L.; Han, M.; Sun, C.; Li, J. Sintering and Tribological Properties of Ti3SiC2-TiSix Composite Sintered by High-Pressure High-Temperature Technology. Materials 2024, 17, 4866. https://doi.org/10.3390/ma17194866
Chen Y, Li L, Han M, Sun C, Li J. Sintering and Tribological Properties of Ti3SiC2-TiSix Composite Sintered by High-Pressure High-Temperature Technology. Materials. 2024; 17(19):4866. https://doi.org/10.3390/ma17194866
Chicago/Turabian StyleChen, Yuqi, Liang Li, Ming Han, Chaofan Sun, and Jin Li. 2024. "Sintering and Tribological Properties of Ti3SiC2-TiSix Composite Sintered by High-Pressure High-Temperature Technology" Materials 17, no. 19: 4866. https://doi.org/10.3390/ma17194866
APA StyleChen, Y., Li, L., Han, M., Sun, C., & Li, J. (2024). Sintering and Tribological Properties of Ti3SiC2-TiSix Composite Sintered by High-Pressure High-Temperature Technology. Materials, 17(19), 4866. https://doi.org/10.3390/ma17194866







