Improved HER/OER Performance of NiS2/MoS2 Composite Modified by CeO2 and LDH
Abstract
1. Introduction
2. Experiment
2.1. Nickel Foam Pretreatment
2.2. Preparation of Ni3S2/MoS2
2.3. Preparation of CeO2-Ni3S2/MoS2
2.4. Synthesis of CeO2-LDH/Ni3S2/MoS2
2.5. Material Characterization
2.6. Electrode Preparation and Electrochemical Measurement
3. Results and Discussion
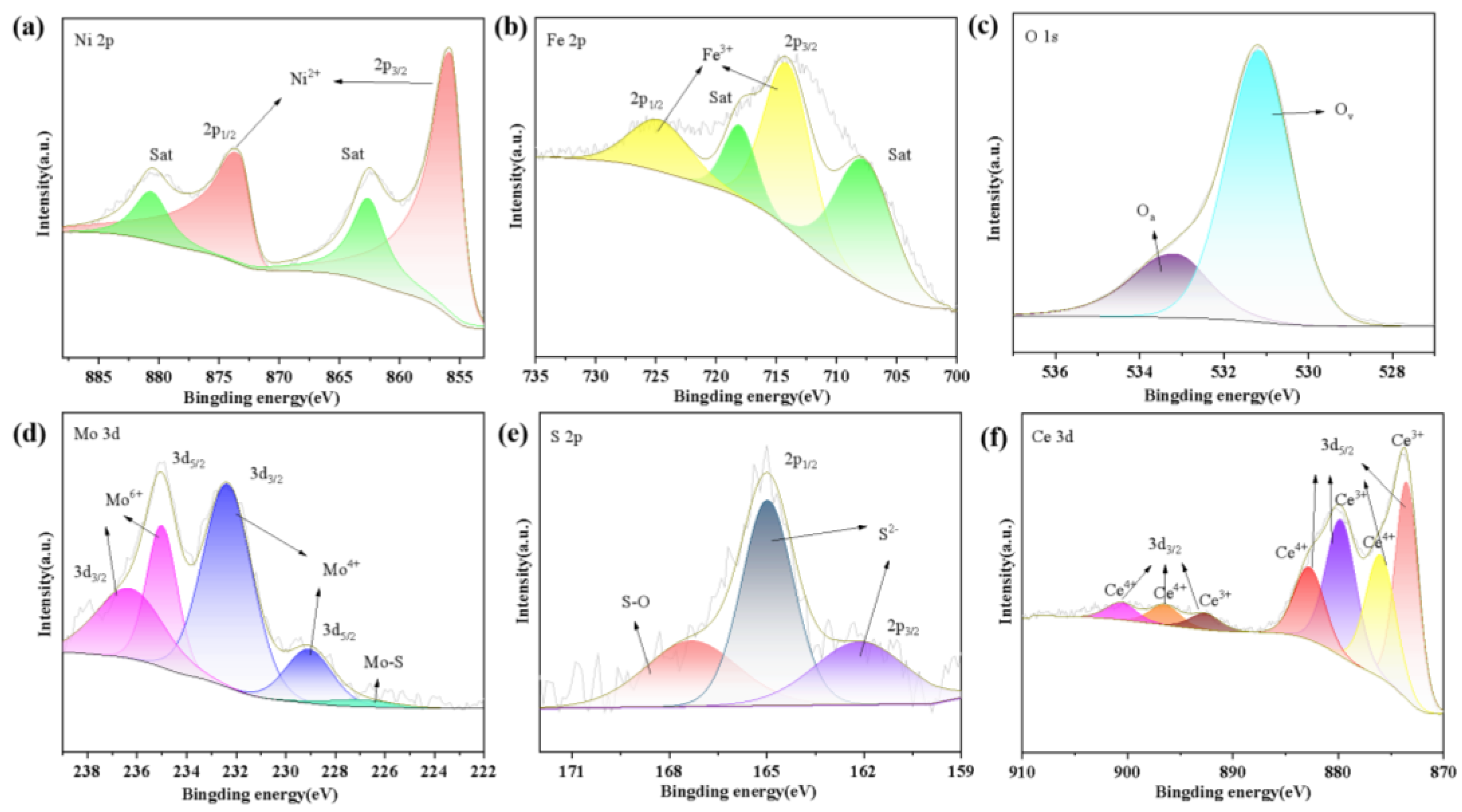
3.1. Structure and Morphology Characterization
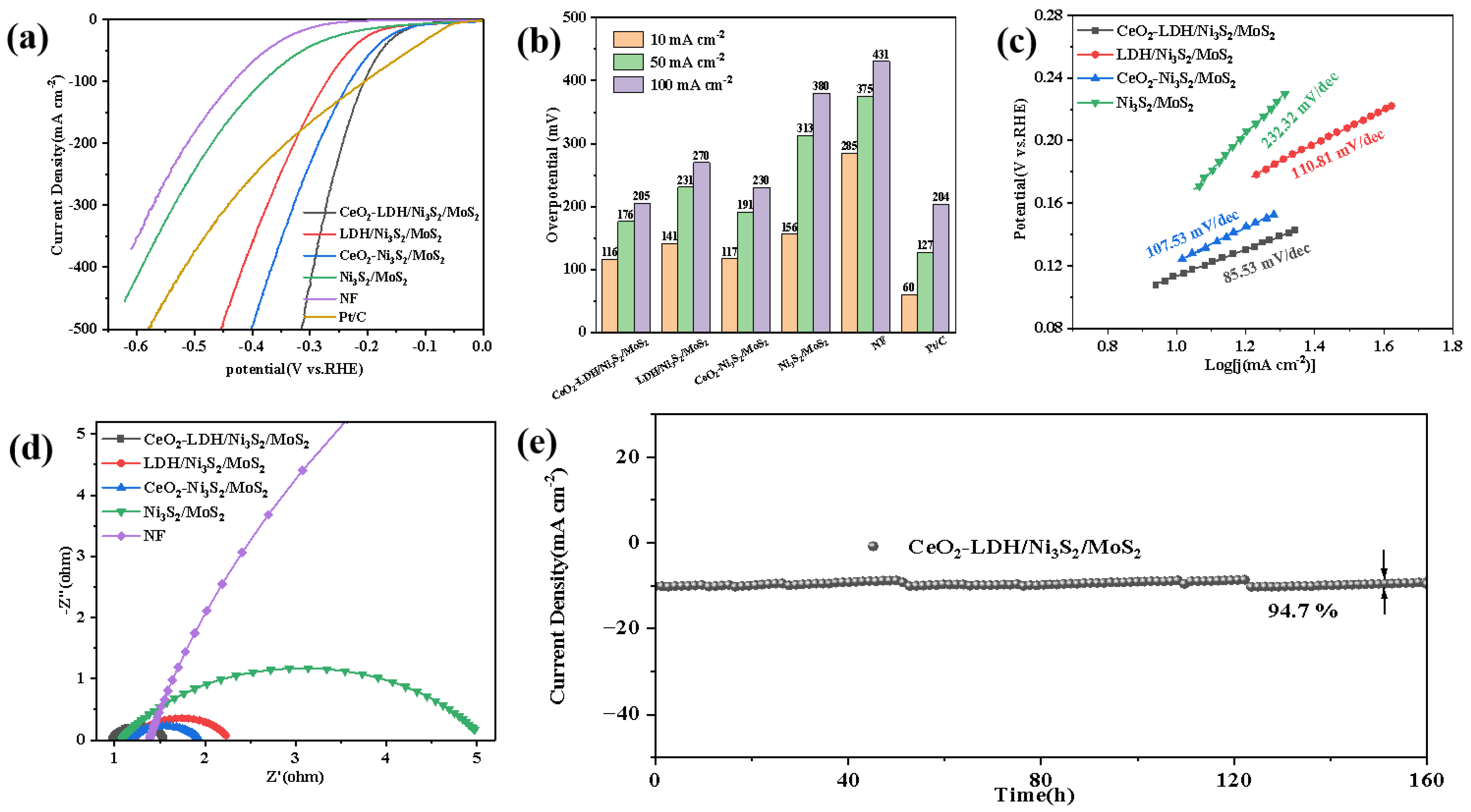
3.2. Electrochemical HER Performance
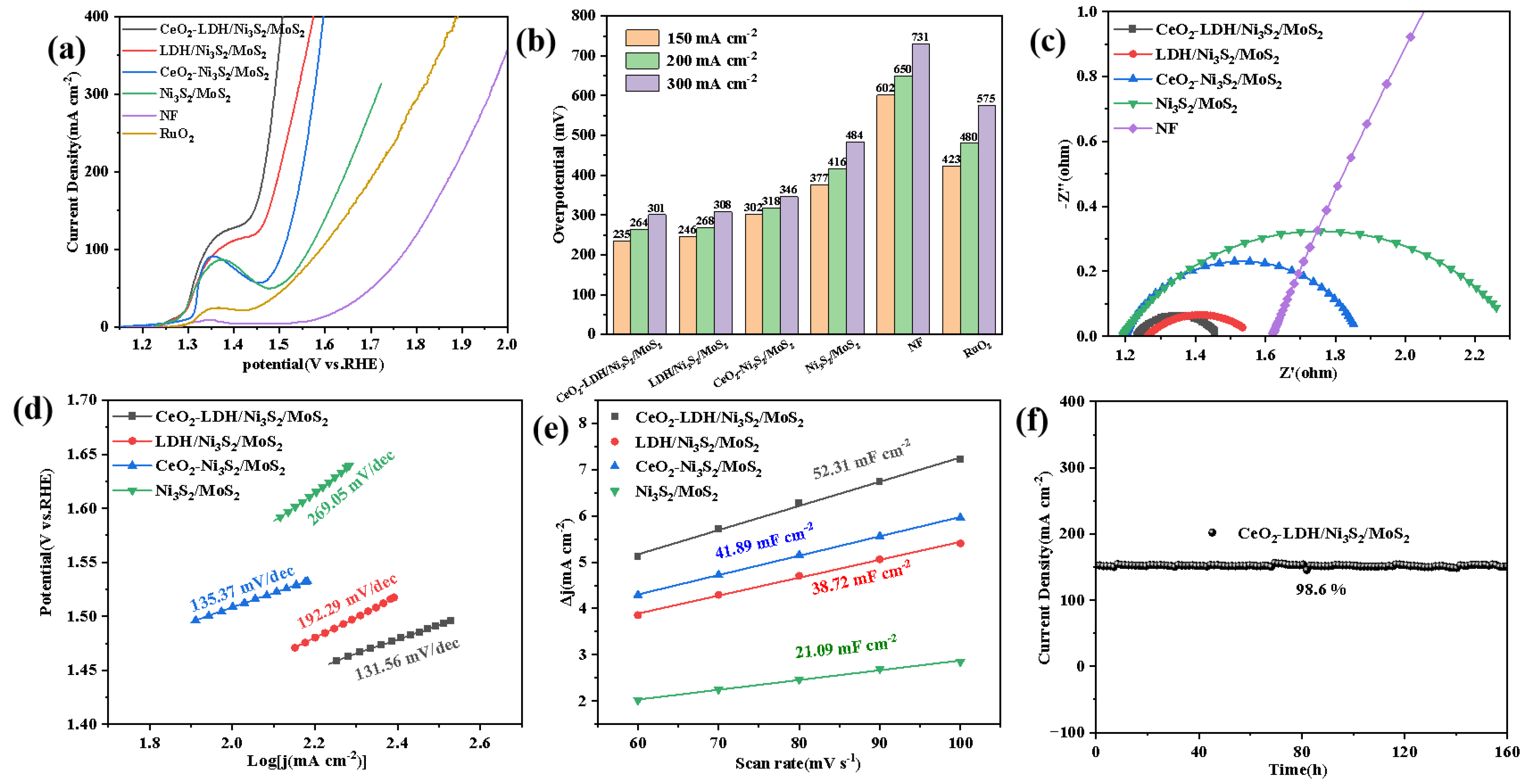
3.3. Electrochemical OER Performance
3.4. Overall Water Splitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, X.; Chen, Y.; Wang, Y.; Zhao, L.; Zhao, X.; Du, J.; Wu, H.; Chen, A. Next-Generation Green Hydrogen: Progress and Perspective from Electricity, Catalyst to Electrolyte in Electrocatalytic Water Splitting. Nano-Micro Lett. 2024, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lian, K.; Liu, Q.; Qi, G.; Zhang, S.; Luo, J.; Liu, X. High entropy alloy nanoparticles as efficient catalysts for alkaline overall seawater splitting and Zn-air batteries. J. Colloid Interface Sci. 2023, 646, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Liu, Y.; Cui, W.; Yang, B.; Li, Z.; Rodriguez, R.D.; Zhang, Q.; Dong, C.L.; Shang, X.; Lei, L.; et al. Electronic structure optimization of metal–phthalocyanine via confining atomic Ru for all-pH hydrogen evolution. Energy Environ. Sci. EES 2024, 17, 1540–1548. [Google Scholar] [CrossRef]
- Li, G.L.; Miao, Y.Y.; Deng, F.; Wang, S.; Wang, R.X.; Lu, W.H.; Chen, R.L. Highly-dispersed 2D NiFeP/CoP heterojunction trifunctional catalyst for efficient electrolysis of water and urea. J. Colloid Interface Sci. 2024, 667, 543–552. [Google Scholar] [CrossRef]
- Ling, X.; Du, F.; Zhang, Y.; Shen, Y.; Li, T.; Zhou, Y.; Zou, Z. Preparation of an Fe2Ni MOF on nickel foam as an efficient and stable electrocatalyst for the oxygen evolution reaction. RSC Adv. 2019, 9, 33558–33562. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zhang, Q.; Huang, B.; Wang, Z.; Liu, Y.; Zheng, Z.; Dai, Y.; Qin, X.; Zhang, X. Synthesis of MoS2/Ni3S2 heterostructure for efficient electrocatalytic hydrogen evolution reaction through optimizing the sulfur sources selection. Appl. Surf. Sci. 2018, 459, 422–429. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, B.; Zhang, B.; Ji, Y.; Xie, H.; Xie, Y.; Dong, Y.; Liu, Z.; Liu, Y.; Qiao, L. Bi-functional Ni3S2@MoS2 heterostructure with strong built-in field as highly-efficient electrolytic catalyst. J. Electroanal. Chem. 2023, 931, 117185. [Google Scholar] [CrossRef]
- Lin, T.; Xu, R.; Hu, Y.; Wang, J.; Liu, Y.; Zhou, W. The electrocatalytic HER activity of MoS2 decorated with adjustable-size ruthenium nanoparticles. Int. J. Hydrogen Energy 2024, 68, 688–695. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Wu, X.; Zhao, K.; Zhang, H.; Guo, J.; Jia, D. Effectively enhanced activity of hydrogen evolution through strong interfacial coupling on SnS2/MoS2/Ni3S2 heterostructured porous nanosheets. Colloids Surf. A Physicochem. Eng. Asp. 2023, 670, 131634. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, J.; Ding, L.; Lv, H.; Zhang, K.; Hu, A.; Yang, X.; Sun, W.; Mao, Y. Interfacial engineering for promoting charge transfer in MoS2/CoFeLDH heterostructure electrodes for overall water splitting. Int. J. Hydrogen Energy 2024, 49, 897–906. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Vedanarayanan, M.; Gopalakrishnan, S.M. Enhancement of Hydrogen and Oxygen Evolution Reaction Efficiencies by Coupling a MoS2 Nanoflower with a CoMnCr LDH Nanocube on Nickel Foam. Energ Fuel 2023, 37, 12204–12214. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Cao, D.; Gong, Y. Hierarchical Self-assembly of NiFe-LDH Nanosheets on CoFe2O4@Co3S4 Nanowires for Enhanced Overall Water Splitting. Sustain. Energy Fuels 2020, 4, 1933–1944. [Google Scholar] [CrossRef]
- Meng, C.; Chen, X.; Gao, Y.; Zhao, Q.; Kong, D.; Lin, M.; Chen, X.; Li, Y.; Zhou, Y. Recent Modification Strategies of MoS2 for Enhanced Electrocatalytic Hydrogen Evolution. Molecules 2020, 25, 1136. [Google Scholar] [CrossRef]
- Li, J.; Du, X.; Luo, Y.; Han, B.; Liu, G.; Li, J. MoS2/NiVFe crystalline/amorphous heterostructure induced electronic modulation for efficient neutral-alkaline hydrogen evolution. Electrochim. Acta 2023, 437, 141478. [Google Scholar] [CrossRef]
- Wang, Y.; Song, E.; Qiu, W.; Zhao, X.; Zhou, Y.; Liu, J.; Zhang, W. Recent progress in theoretical and computational investigations of structural stability and activity of single-atom electrocatalysts. Prog. Nat. Sci. Mater. Int. 2019, 29, 256–264. [Google Scholar] [CrossRef]
- Tian, J.; Shen, Y.; Liu, P.; Zhang, H.; Xu, B.; Song, Y.; Liang, J.; Guo, J. Recent advances of amorphous-phase-engineered metal-based catalysts for boosted electrocatalysis. J. Mater. Sci. Technol. 2022, 127, 1–18. [Google Scholar] [CrossRef]
- Xu, W.; Qiu, R.; Mao, X.; Yang, X.; Peng, B.; Shen, Y. One-step fabrication of amorphous Ni-Fe phosphated alloys as efficient bifunctional electrocatalysts for overall water splitting. J. Non-Cryst. Solids 2022, 587, 121598. [Google Scholar] [CrossRef]
- Zeng, S.P.; Shi, H.; Dai, T.Y.; Liu, Y.; Wen, Z.; Han, G.F.; Wang, T.H.; Zhang, W.; Lang, X.Y.; Zheng, W.T. Lamella-heterostructured nanoporous bimetallic iron-cobalt alloy/oxyhydroxide and cerium oxynitride electrodes as stable catalysts for oxygen evolution. Nat. Commun. 2023, 14, 1811. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, W.; Zhang, G. A general strategy for constructing transition metal Oxide/CeO2 heterostructure with oxygen vacancies toward hydrogen evolution reaction and oxygen evolution reaction. J. Power Sources 2021, 512, 230514. [Google Scholar] [CrossRef]
- Wenyu, Z.; Ruihua, G.; Quanxin, Y.; Yarong, H.; Guofang, Z.; Lili, G. Preparation of high-entropy alloy bifunctional catalysts with rare earth Ce coordination and Efficient water splitting research. Ionics 2024, 30, 3403–3416. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, S.; Huang, Y.; Yan, C.; Du, Y. Cerium contained advanced materials: Shining star under electrocatalysis. Coord. Chem. Rev. 2024, 518, 216111. [Google Scholar] [CrossRef]
- Bhosale, M.; Baby, N.; Magdum, S.S.; Murugan, N.; Kim, Y.A.; Thangarasu, S.; Oh, T.-H. Hierarchical nanoassembly of Ni3S2-MoS2 interconnected with CeO2 as a highly remarkable hybrid electrocatalyst for enhancing water oxidation and energy storage. J. Energy Storage 2024, 80, 110301. [Google Scholar] [CrossRef]
- Du, W.; Shi, Y.; Zhou, W.; Yu, Y.; Zhang, B. Unveiling the In Situ Dissolution and Polymerization of Mo in Ni4Mo Alloy for Promoting the Hydrogen Evolution Reaction. Angew. Chem. 2021, 60, 7051–7055. [Google Scholar] [CrossRef]
- Rner, L.; Schwarz, H.; Veremchuk, I.; Zerdoumi, R.; Armbrüster, M. Challenging the Durability of Intermetallic Mo–Ni Compounds in the Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 23616–23626. [Google Scholar]
- Wu, Q.; Gao, Q.; Sun, L.; Guo, H.; Tai, X.; Li, D.; Liu, L.; Ling, C.; Sun, X. Facilitating active species by decorating CeO2 on Ni3S2 nanosheets for efficient water oxidation electrocatalysis. Chin. J. Catal. 2021, 42, 482–489. [Google Scholar] [CrossRef]
- Gao, W.; Ma, F.; Wang, C.; Wen, D. Ce dopant significantly promotes the catalytic activity of Ni foam-supported Ni3S2 electrocatalyst for alkaline oxygen evolution reaction. J. Power Sources 2020, 450, 227654. [Google Scholar] [CrossRef]
- Jin, D.; Qiao, F.; Liu, W.; Liu, Y.; Xie, Y.; Li, H. One-step fabrication of MoS2/Ni3S2 with P-doping for efficient water splitting. CrystEngComm 2022, 24, 4057–4062. [Google Scholar] [CrossRef]
- Li, G.; Wang, P.; He, M.; Yuan, X.; Tang, L.; Li, Z. Cerium-based nanomaterials for photo/electrocatalysis. Sci. China Chem. 2023, 66, 2204–2220. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Chen, D.; Zhang, X.; Wang, Y.; Wang, Y. Enhanced electrocatalytic performance on FeOCl/CeO2 via synergy of oxygen vacancy and relative content of Ce3+. Surf. Interfaces 2022, 35, 102437. [Google Scholar] [CrossRef]
- Yao, Y.; He, J.; Ma, L.; Wang, J.; Peng, L.; Zhu, X.; Li, K.; Qu, M. Self-supported Co9S8-Ni3S2-CNTs/NF electrode with superwetting multistage micro-nano structure for efficient bifunctional overall water splitting. J. Colloid Interface Sci. 2022, 616, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Bo, X.; Guo, L. In-situ growth of iron-based metal-organic framework crystal on ordered mesoporous carbon for efficient electrocatalysis of p-nitrotoluene and hydrazine. Anal. Chim. Acta 2018, 1024, 73–83. [Google Scholar] [CrossRef]
- Li, C.; Yang, T.; Fan, J.; Liu, E.; Zhao, B.; Sun, T. NiFe LDH/MoS2/Ni3S2 p-n/Mott-Schottky heterojunction for efficient hydrogen generation coupled with electrochemical oxidation of organic molecules. J. Alloys Compd. 2024, 970, 172710. [Google Scholar] [CrossRef]
- Fan, J.; Du, X. Role of Ce in the enhanced performance of the water oxidation reaction and urea oxidation reaction for NiFe layered double hydroxides. Dalton Trans 2022, 51, 8240–8248. [Google Scholar] [CrossRef]
- Kitiphatpiboon, N.; Chen, M.; Feng, C.; Zhou, Y.; Liu, C.; Feng, Z.; Zhao, Q.; Abudula, A.; Guan, G. Modification of spinel MnCo2O4 nanowire with NiFe-layered double hydroxide nanoflakes for stable seawater oxidation. J. Colloid. Interface Sci. 2023, 632 Pt A, 54–64. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Xue, H.; Sun, J.; Guo, N.; Song, T.; Sun, J.; Hao, Y.R.; Wang, Q. Co-Construction of Sulfur Vacancies and Heterogeneous Interface into Ni3S2/MoS2 Catalysts to Achieve Highly Efficient Overall Water Splitting. Chin. J. Struct. Chem. 2022, 41, 37–43. [Google Scholar]
- Xiong, X.; Waller, G.; Ding, D.; Chen, D.; Rainwater, B.; Zhao, B.; Wang, Z.; Liu, M. Controlled synthesis of NiCo2S4 nanostructured arrays on carbon fiber paper for high-performance pseudocapacitors. Nano Energy 2015, 16, 71–80. [Google Scholar] [CrossRef]
- Cheng, Y.; Yuan, A.; Zhang, Y.; Liu, H.; Du, J.; Chen, L. Ce-doped multi-phase NiMo-based phosphorus/sulfide heterostructure for efficient photo-enhanced overall water splitting at high current densities. J. Colloid. Interface Sci. 2024, 660, 166–176. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Liu, Q.; Sun, X.; Ma, X.; Cheng, Z.; Wu, J.; Shi, M.; Zhu, J.; Qi, Y. Doping vacancy synergy engineering: Ce-doped FeNi-Sx micro-succulent ameliorating electrocatalytic oxygen evolution performance. Electrochim. Acta 2022, 431, 141133. [Google Scholar] [CrossRef]
- Ai, L.; Luo, Y.; Huang, W.; Tian, Y.; Jiang, J. Cobalt/cerium-based metal-organic framework composites for enhanced oxygen evolution electrocatalysis. Int. J. Hydrogen Energy 2022, 47, 12893–12902. [Google Scholar] [CrossRef]
- Heinritz, A.S.; Thomas, J. Asymmetric Butler-Volmer Kinetics of the Electrochemical Ce(III)/Ce(IV) Redox Couple on Polycrystalline Au Electrodes in Sulfuric Acid and the Dissociation Field Effect. ACS Catal. 2021, 11, 8140–8154. [Google Scholar] [CrossRef]
- Wu, Z.; Vagin, M.; Boyd, R.; Greczynski, G.; Ding, P.; Odén, M.; Bjrk, E. Bi-functional Mesoporous MOx (M = Cr, Fe, Co, Ni, Ce) Oxygen Electrocatalysts for PGM-free Oxygen Pumps. Energy Technol. 2022, 10, 2200927. [Google Scholar] [CrossRef]
- He, J.; Li, W.; Xu, P.; Sun, J. Tuning electron correlations of RuO2 by co-doping of Mo and Ce for boosting electrocatalytic water oxidation in acidic media. Appl. Catal. B Environ. 2021, 298, 1200528. [Google Scholar] [CrossRef]
- Pei, Z.; Zhang, H.; Wu, Z.; Lu, X.; Luan, D. Atomically dispersed Ni activates adjacent Ce sites for enhanced electrocatalytic oxygen evolution activity. Sci. Adv. 2023, 9, 1320. [Google Scholar] [CrossRef] [PubMed]
- Althubiti, N.A.; Aman, S.; Ahmad, N.; Farid, H.M.T.; Taha, T.A.M. Engineering in the morphology of Ni3S2 via doping of Ce with different concentration to improve the capacitive properties. J. Alloys Compd. 2023, 962, 171076. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, X.; Liu, R.; Zhang, H.; Peng, P.; Wu, W.; Li, Z.; Hou, Z.; Huang, K. Nano-modulated synthesis of NiCoP nanosheets coated by NiCoP nanoparticles for efficient water splitting. J. Taiwan Inst. Chem. Eng. 2023, 147, 104916. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, Z.; Li, Q.; Wan, H.; Chen, G.; Zhang, N.; Liu, X.; Ma, R. Boosting electrocatalytic water oxidation of NiFe layered double hydroxide via the synergy of 3d–4f electron interaction and citrate intercalation. J. Mater. Chem. A 2023, 11, 1944–1953. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, R.; Zheng, T.; Lu, Z.; Fang, Y.; Wang, W. Cerium decorated amorphous ternary Ni-Ce-B catalyst for enhanced electrocatalytic water oxidation. Surf. Interfaces 2021, 26, 101447. [Google Scholar] [CrossRef]
- Lu, W.; Yi, L.; Wei, X.C. 3D nanostructured Ce-doped CoFe-LDH/NF self-supported catalyst for high-performance OER. Dalton Trans. Int. J. Inorg. Chem. 2023, 52, 12038–12048. [Google Scholar]
- Zhang, Q.; Zhang, S.; Tian, Y.; Zhan, S. Ce-Directed Double-Layered Nanosheet Architecture of NiFe-Based Hydroxide as Highly Efficient Water Oxidation Electrocatalyst. ACS Sustain. Chem. Eng. 2018, 6, 15411–15418. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, S.; Zhang, H.; Li, Z.; Yang, C.; Cai, Z.; He, X.; Dong, K.; Luo, Y.; Wang, Y.; et al. Enhancing the stability of NiFe-layered double hydroxide nanosheet array for alkaline seawater oxidation by Ce doping. J. Energy Chem. 2024, 91, 306–312. [Google Scholar] [CrossRef]
- Ning, C.; Yuxuan, K.; Fei-Yan, Q.Y. (FeMnCe)-co-doped MOF-74 with significantly improved performance for overall water splitting. Dalton Trans. Int. J. Inorg. Chem. 2023, 52, 11601–11610. [Google Scholar]
- Li, R.Q.; Wang, C.; Xie, S.; Hang, T.; Wan, X.; Zeng, J.; Zhang, W. Coupling MoS2 nanosheets with CeO2 for efficient electrocatalytic hydrogen evolution at large current densities. Chem. Commun. 2023, 59, 11512–11515. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, K.; Li, X.; Li, H.; Li, S.; Ni, Y. Ce-Doped NiFe Layered Double Hydroxide/NiFe2O4 Nanosheet Catalysts for Water Oxidation. ACS Appl. Nano Mater. 2023, 6, 16938–16946. [Google Scholar] [CrossRef]
- Xiaomei, X.; Qiaoling, M.; Hu, Z.C. Multifunctional Ni3S2@NF-based electrocatalysts for efficient and durable electrocatalytic water splitting. Dalton Trans. Int. J. Inorg. Chem. 2023, 52, 12378–12389. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chen, F.; Wu, X.; Wang, D.; Ren, Y.; Li, Y. Improved HER/OER Performance of NiS2/MoS2 Composite Modified by CeO2 and LDH. Materials 2024, 17, 4876. https://doi.org/10.3390/ma17194876
Li H, Chen F, Wu X, Wang D, Ren Y, Li Y. Improved HER/OER Performance of NiS2/MoS2 Composite Modified by CeO2 and LDH. Materials. 2024; 17(19):4876. https://doi.org/10.3390/ma17194876
Chicago/Turabian StyleLi, Hao, Feng Chen, Xinyang Wu, Dandan Wang, Yongpeng Ren, and Yaru Li. 2024. "Improved HER/OER Performance of NiS2/MoS2 Composite Modified by CeO2 and LDH" Materials 17, no. 19: 4876. https://doi.org/10.3390/ma17194876
APA StyleLi, H., Chen, F., Wu, X., Wang, D., Ren, Y., & Li, Y. (2024). Improved HER/OER Performance of NiS2/MoS2 Composite Modified by CeO2 and LDH. Materials, 17(19), 4876. https://doi.org/10.3390/ma17194876






