Electrochemical Performance of LiTa2PO8-Based Succinonitrile Composite Solid Electrolyte without Sintering Process
Abstract
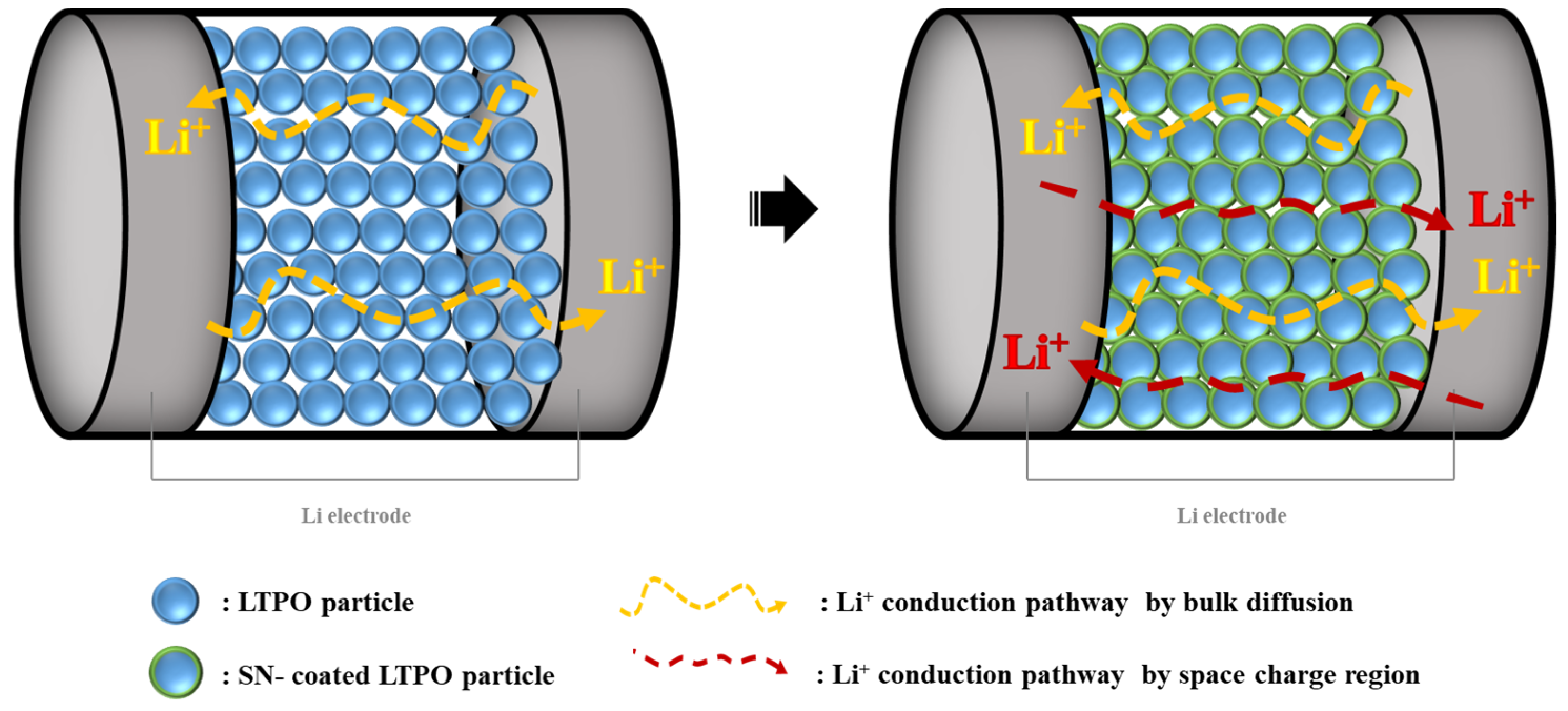
1. Introduction
2. Materials and Methods
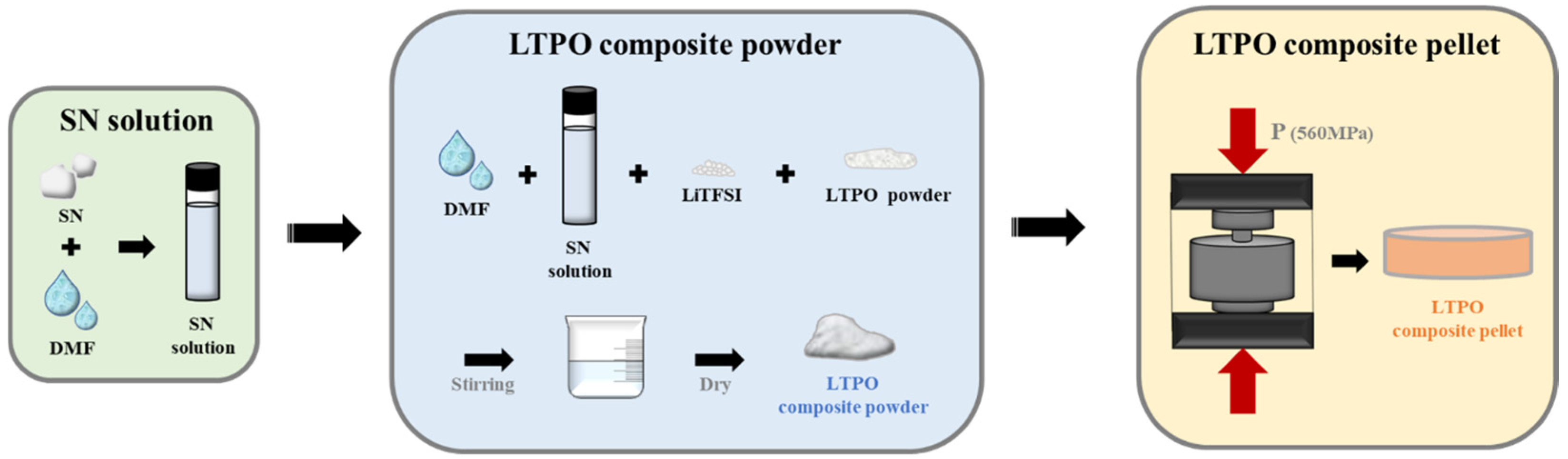
2.1. Synthesis of the LTPO and LTPO Composite Powder
2.2. Fabrication of LTPO Composite Pellets
2.3. Characterization
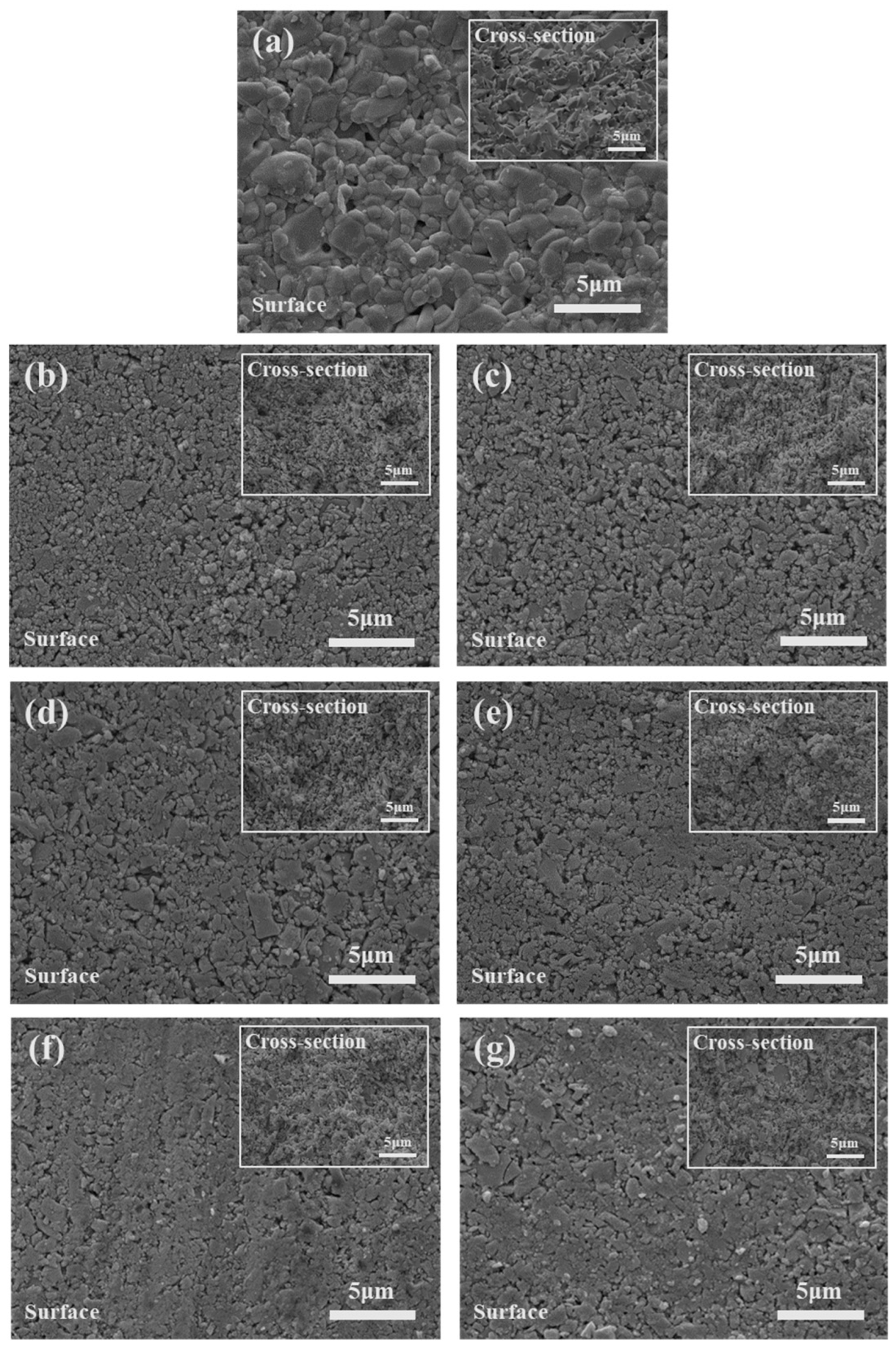
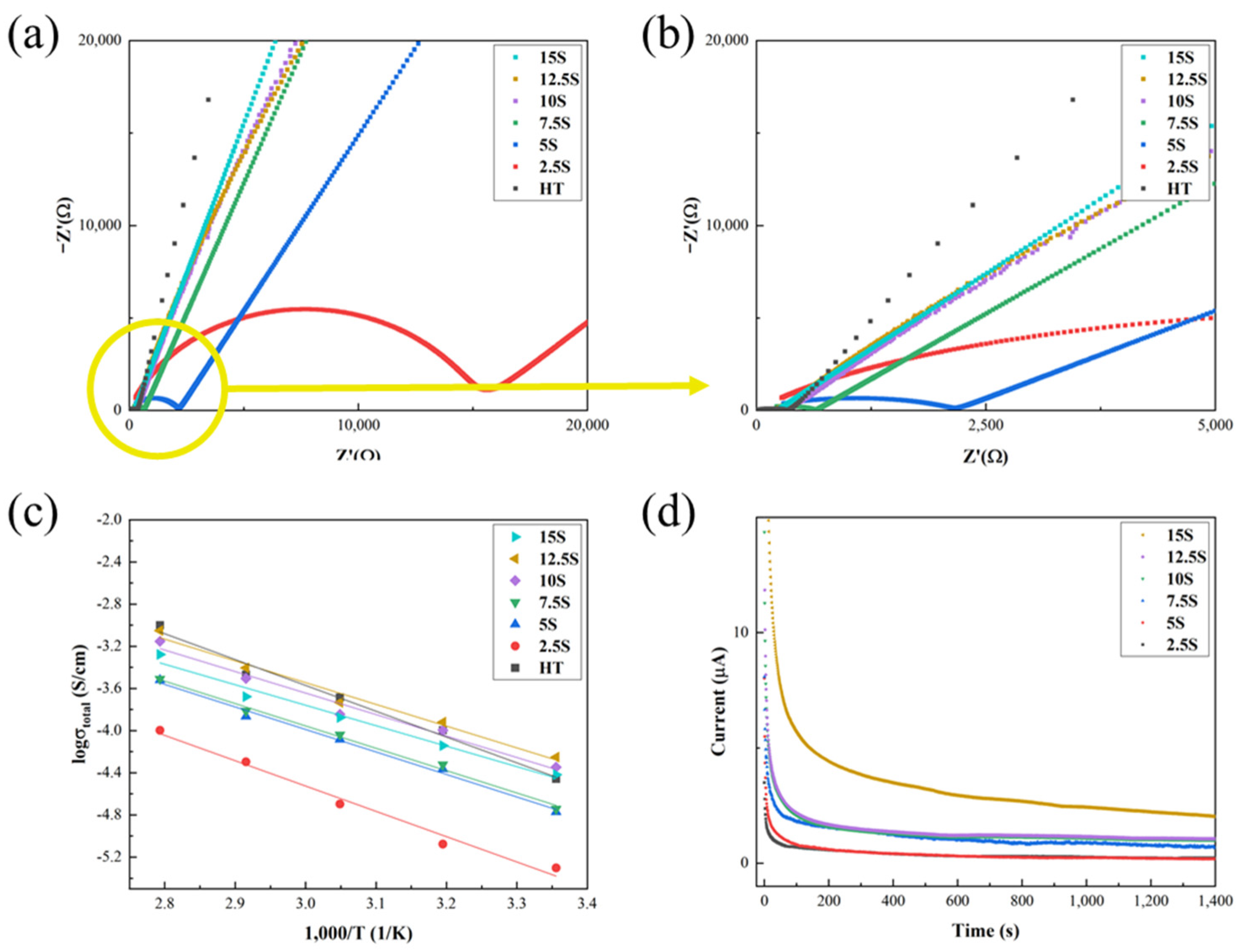
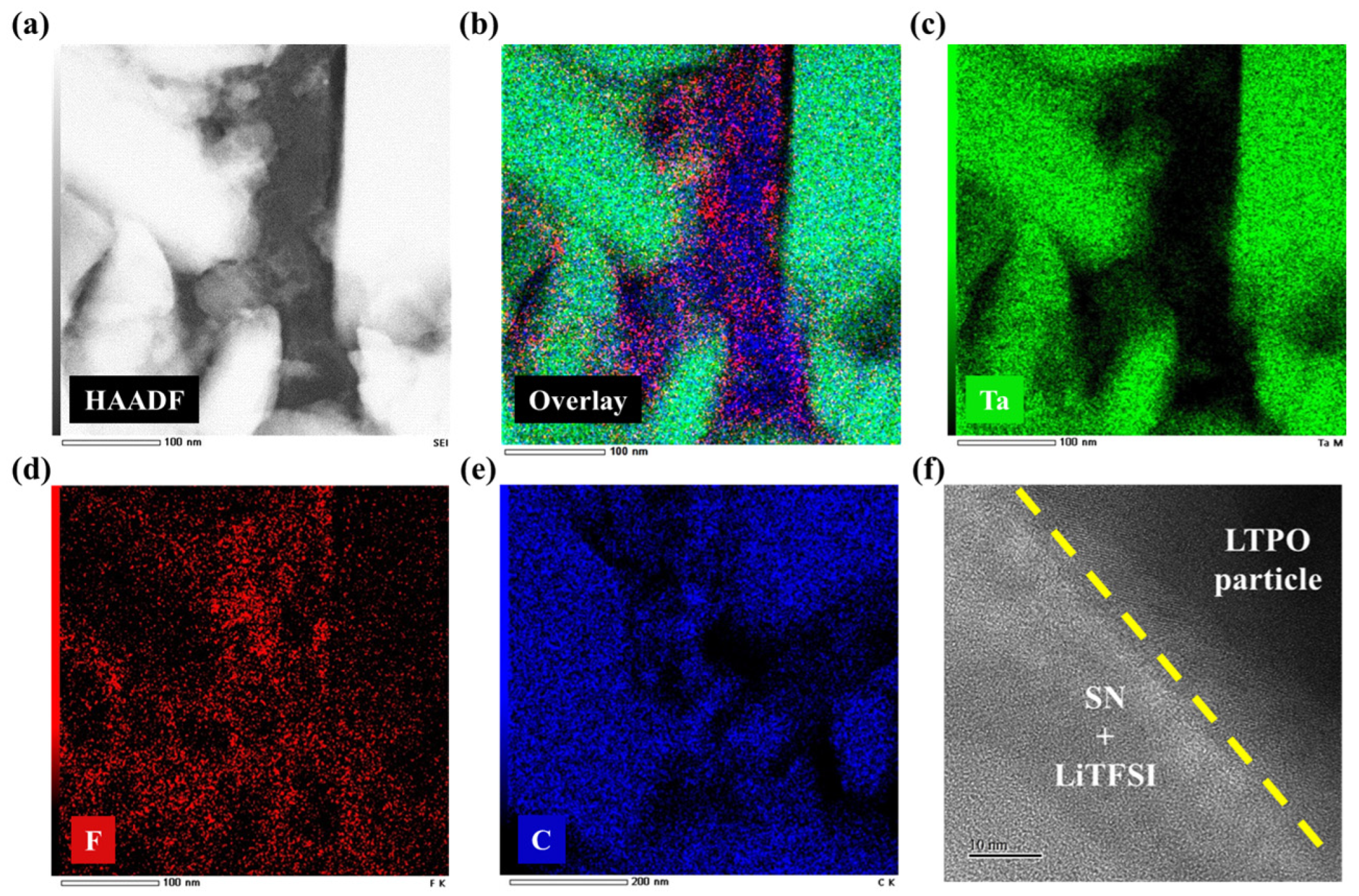
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, C. A review on lithium-ion batteries safety issues: Existing problems and possible solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Lim, H.-D.; Park, J.-H.; Shin, H.-J.; Jeong, J.; Kim, J.T.; Nam, K.-W.; Jung, H.-G.; Chung, K.Y. A review of challenges and issues concerning interfaces for all-solid-state batteries. Energy Storage Mater. 2020, 25, 224–250. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Raza, S.; Bashir, T.; Hayat, A.; Abd-Rabboh, H.S.; Shen, L.; Orooji, Y.; Lin, H. Recent progress and fundamentals of solid-state electrolytes for all solid-state rechargeable batteries: Mechanisms, challenges, and applications. J. Energy Storage 2024, 92, 112110. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Z.; Yoshida, A.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Utmost limits of various solid electrolytes in all-solid-state lithium batteries: A critical review. Renew. Sustain. Energy Rev. 2019, 109, 367–385. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, Y.; Li, X.; Huang, X.; Tian, B. Li5AlO4-Assisted Low-Temperature Sintering of Dense Li7La3Zr2O12 Solid Electrolyte with High Critical Current Density. ACS Appl. Mater. Interfaces 2024, 16, 5989–5998. [Google Scholar] [CrossRef]
- Takano, R.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Low temperature synthesis of Al-doped Li7La3Zr2O12 solid electrolyte by a sol–gel process. Solid State Ion. 2014, 255, 104–107. [Google Scholar] [CrossRef]
- Vinnichenko, M.; Waetzig, K.; Aurich, A.; Baumgaertner, C.; Herrmann, M.; Ho, C.W.; Kusnezoff, M.; Lee, C.W. Li-ion conductive Li1.3Al0.3Ti1.7(PO4)3 (LATP) solid electrolyte prepared by cold sintering process with various sintering additives. Nanomaterials 2022, 12, 3178. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Pu, Y.; Li, Y.; Xin, S.; Li, X.; Chen, J.; Goodenough, J.B. Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries. Adv. Mater. 2019, 31, 1805574. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Cui, Z.; Zhou, Q.; Shangguan, X.; Tian, S.; Zhou, X.; Cui, G. Siloxane-based polymer electrolytes for solid-state lithium batteries. Energy Storage Mater. 2019, 23, 466–490. [Google Scholar] [CrossRef]
- Duan, H.; Fan, M.; Chen, W.P.; Li, J.Y.; Wang, P.F.; Wang, W.P.; Shi, J.L.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for high-voltage lithium metal batteries. Adv. Mater. 2019, 31, 1807789. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Song, M.S.; Kong, B.; Cui, Y. Flexible and stretchable energy storage: Recent advances and future perspectives. Adv. Mater. 2017, 29, 1603436. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tang, S.; Cheng, Y.; Wang, K.; Liang, J.; Liu, C.; Cao, Y.-C.; Wei, F.; Mai, L. Interfaces in solid-state lithium batteries. Joule 2018, 2, 1991–2015. [Google Scholar] [CrossRef]
- Hussain, F.; Li, P.; Li, Z. Theoretical insights into Li-ion transport in LiTa2PO8. J. Phys. Chem. C 2019, 123, 19282–19287. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Avdeev, M.; Yun, H.; Kim, S.-J. LiTa2PO8: A fast lithium-ion conductor with new framework structure. J. Mater. Chem. A 2018, 6, 22478–22482. [Google Scholar] [CrossRef]
- Kim, H.; Choi, S.; Peck, D.-H.; Yoon, S.-Y. Influence of the cold sintering process and post annealing on the microstructure and Li-ion conductivity of LiTa2PO8 solid electrolyte. Ceram. Int. 2023, 49, 8718–8724. [Google Scholar] [CrossRef]
- Kim, H.; Nam, C.-Y.; Kim, N.; Lee, J.; Choi, S.; Yoon, S.-Y. Toxic solvent-free low-temperature fabrication and electrochemical performance of LiTa2PO8 ceramic matrix–based composite solid electrolytes containing polyvinylidene fluoride. Ceram. Int. 2024, 50, 1574–1580. [Google Scholar] [CrossRef]
- Kim, H.; Choi, S.; Nam, C.-Y.; Peck, D.-H.; Yoon, S.-Y. Effect of the Li2O–B2O3–Li2SO4 Amorphous Boundary Layer on the Ionic Conductivity and Humidity Stability of the LiTa2PO8 Solid Electrolyte. ACS Appl. Energy Mater. 2023, 6, 4810–4816. [Google Scholar] [CrossRef]
- Lei, J.; Liu, Z.; Wang, H.; Li, Z.; Liao, R.; Dmytro, S.; Zhang, Q. Te doping effect on the structure and ionic conductivity of LiTa2PO8 solid electrolyte. Ceram. Int. 2023, 49, 1980–1986. [Google Scholar] [CrossRef]
- Liu, Z.; Lei, J.; Liu, W.; Fang, B.; Xie, L.; Dmytro, S.; Zhang, Q. Enhanced grain boundary ionic conductivity of LiTa2PO8 solid electrolyte by 75Li2O-12.5B2O3-12.5SiO2 sintering additive. J. Eur. Ceram. Soc. 2023, 43, 4437–4442. [Google Scholar] [CrossRef]
- Abouimrane, A.; Davidson, I. Solid electrolyte based on succinonitrile and LiBOB: Interface stability and application in lithium batteries. J. Electrochem. Soc. 2007, 154, A1031. [Google Scholar] [CrossRef]
- Das, A.K.; Badole, M.; Vasavan, H.N.; Saxena, S.; Gami, P.; Dagar, N.; Kumar, S. LiTa2PO8-Based Polymer–Ceramic Electrolyte Paved the Way to High-Performance Solid-State Lithium Metal Batteries. Energy Fuels 2024, 38, 11253–11261. [Google Scholar] [CrossRef]
- Bi, J.; Mu, D.; Wu, B.; Fu, J.; Yang, H.; Mu, G.; Zhang, L.; Wu, F. A hybrid solid electrolyte Li0.33La0.557TiO3/poly (acylonitrile) membrane infiltrated with a succinonitrile-based electrolyte for solid state lithium-ion batteries. J. Mater. Chem. A 2020, 8, 706–713. [Google Scholar] [CrossRef]
- Zha, W.; Chen, F.; Yang, D.; Shen, Q.; Zhang, L. High-performance Li6.4La3Zr1.4Ta0.6O12/Poly (ethylene oxide)/Succinonitrile composite electrolyte for solid-state lithium batteries. J. Power Sources 2018, 397, 87–94. [Google Scholar] [CrossRef]
- Alarco, P.-J.; Abu-Lebdeh, Y.; Abouimrane, A.; Armand, M. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat. Mater. 2004, 3, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-Z.; Maier, J. Composite effects in poly (ethylene oxide)–succinonitrile based all-solid electrolytes. Electrochem. Commun. 2006, 8, 1753–1756. [Google Scholar] [CrossRef]
- Kasemchainan, J.; Zekoll, S.; Spencer Jolly, D.; Ning, Z.; Hartley, G.O.; Marrow, J.; Bruce, P.G. Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nat. Mater. 2019, 18, 1105–1111. [Google Scholar] [CrossRef]
- Rettenwander, D. Mechanism of Lithium Metal Penetration through Inorganic Solid Electrolyte. Adv. Energy Mater. 2017, 7, 1701003. [Google Scholar] [CrossRef]
- Cao, S.; Chen, F.; Shen, Q.; Zhang, L. Dual-coordination-induced poly (vinylidene fluoride)/Li6.4Ga0.2La3Zr2O12/succinonitrile composite solid electrolytes toward enhanced rate performance in all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 2023, 15, 37422–37432. [Google Scholar] [CrossRef]
- Noh, H.; Kim, D.; Lee, W.; Jang, B.; Ha, J.S.; Yu, J.H. Surface Modification of Ga-Doped-LLZO (Li7La3Zr2O12) by the Addition of Polyacrylonitrile for the Electrochemical Stability of Composite Solid Electrolytes. Energies 2023, 16, 7695. [Google Scholar] [CrossRef]
- Westerhoff, U.; Kurbach, K.; Lienesch, F.; Kurrat, M. Analysis of lithium-ion battery models based on electrochemical impedance spectroscopy. Energy Technol. 2016, 4, 1620–1630. [Google Scholar] [CrossRef]
- Zha, W.; Li, J.; Li, W.; Sun, C.; Wen, Z. Anchoring succinonitrile by solvent-Li+ associations for high-performance solid-state lithium battery. Chem. Eng. J. 2021, 406, 126754. [Google Scholar] [CrossRef]



| Composition (wt%) | Notation |
|---|---|
| 100 LTPO High-temperature sintering (1050 °C—6 h) | HT |
| 97.5 LTPO + 2.5 (SN + LiTFSI) | 2.5S |
| 95 LTPO + 5 (SN + LiTFSI) | 5S |
| 92.5 LTPO + 7.5 (SN + LiTFSI) | 7.5S |
| 90 LTPO + 10 (SN + LiTFSI) | 10S |
| 87.5 LTPO + 12.5 (SN + LiTFSI) | 12.5S |
| 85 LTPO + 15 (SN + LiTFSI) | 15S |
| Sample | Diameter (mm) | Thickness (mm) | Measured Density (g/cm3) | Theoretical Density (g/cm3) | Relative Density (%) |
|---|---|---|---|---|---|
| HT | 8.3 | 0.337 | 5.471 | 5.848 | 93.5 |
| 2.5S | 10 | 0.338 | 3.767 | 5.746 | 65.6 |
| 5S | 10 | 0.346 | 3.680 | 5.644 | 65.2 |
| 7.5S | 10 | 0.308 | 4.134 | 5.542 | 74.6 |
| 10S | 10 | 0.315 | 4.042 | 5.440 | 74.3 |
| 12.5S | 10 | 0.301 | 4.244 | 5.236 | 81.1 |
| 15S | 10 | 0.323 | 3.942 | 5.032 | 78.3 |
| Sample | Total Ionic Conductivity at RT (S/cm) | Activation Energy (eV) | Electronic Conductivity at RT (S/cm) |
|---|---|---|---|
| HT | 1.83 × 10−4 | 0.211 | 5.34 × 10−8 |
| 2.5S | 2.86 × 10−6 | 0.207 | 9.90 × 10−9 |
| 5S | 2.03 × 10−5 | 0.184 | 7.93 × 10−9 |
| 7.5S | 6.06 × 10−5 | 0.183 | 2.75 × 10−8 |
| 10S | 9.60 × 10−5 | 0.176 | 4.01 × 10−8 |
| 12.5S | 1.93 × 10−4 | 0.178 | 4.01 × 10−8 |
| 15S | 1.83 × 10−4 | 0.167 | 8.31 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Park, W.; Kim, H.; Yoon, S.-y. Electrochemical Performance of LiTa2PO8-Based Succinonitrile Composite Solid Electrolyte without Sintering Process. Materials 2024, 17, 4882. https://doi.org/10.3390/ma17194882
Kim N, Park W, Kim H, Yoon S-y. Electrochemical Performance of LiTa2PO8-Based Succinonitrile Composite Solid Electrolyte without Sintering Process. Materials. 2024; 17(19):4882. https://doi.org/10.3390/ma17194882
Chicago/Turabian StyleKim, Nayoung, Wongyeong Park, Hyeonjin Kim, and Seog-young Yoon. 2024. "Electrochemical Performance of LiTa2PO8-Based Succinonitrile Composite Solid Electrolyte without Sintering Process" Materials 17, no. 19: 4882. https://doi.org/10.3390/ma17194882
APA StyleKim, N., Park, W., Kim, H., & Yoon, S.-y. (2024). Electrochemical Performance of LiTa2PO8-Based Succinonitrile Composite Solid Electrolyte without Sintering Process. Materials, 17(19), 4882. https://doi.org/10.3390/ma17194882







