Enhancing the Mechanical Properties of Regenerated Cellulose through High-Temperature Pre-Gelation
Abstract
1. Introduction
2. Materials and Characterization
3. Results
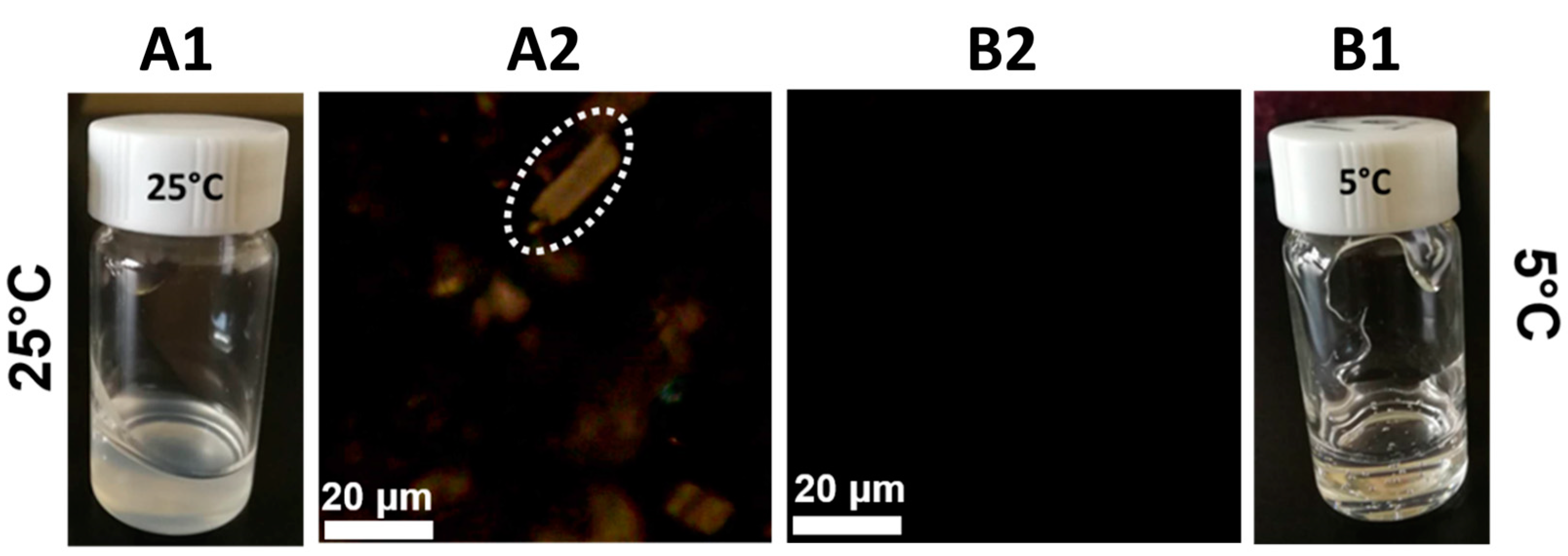
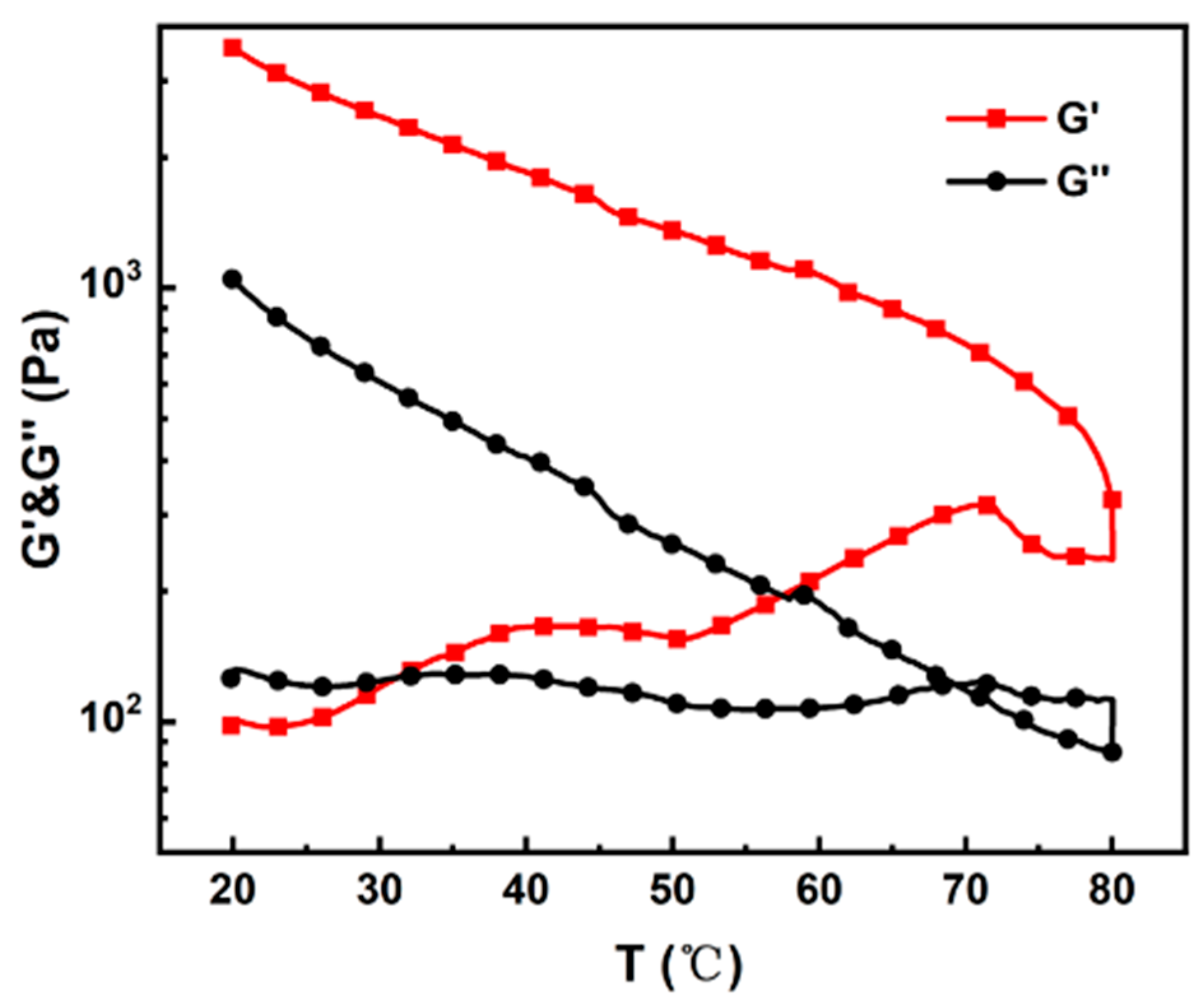
3.1. Effect of Pre-Gelation on MCC Solution
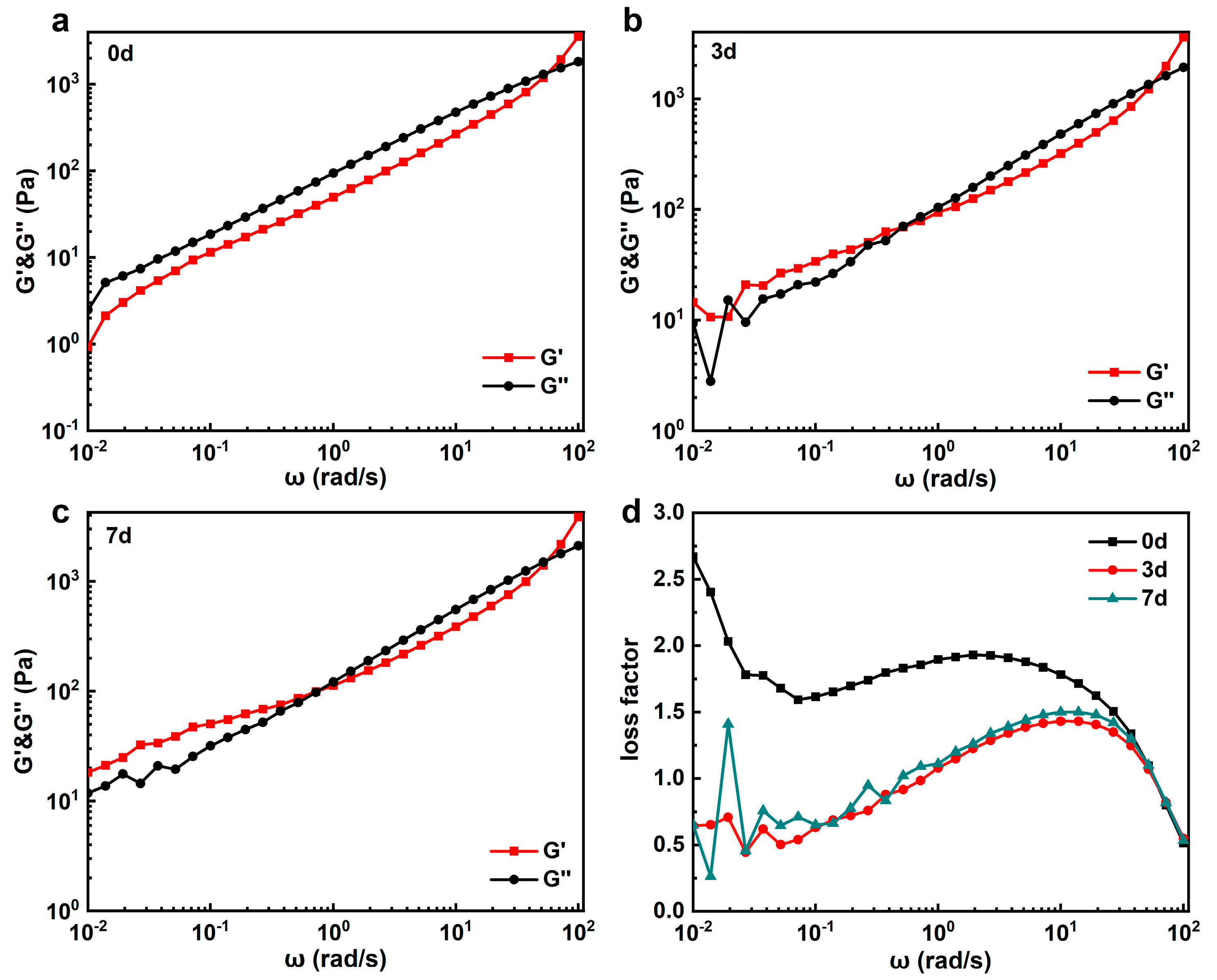
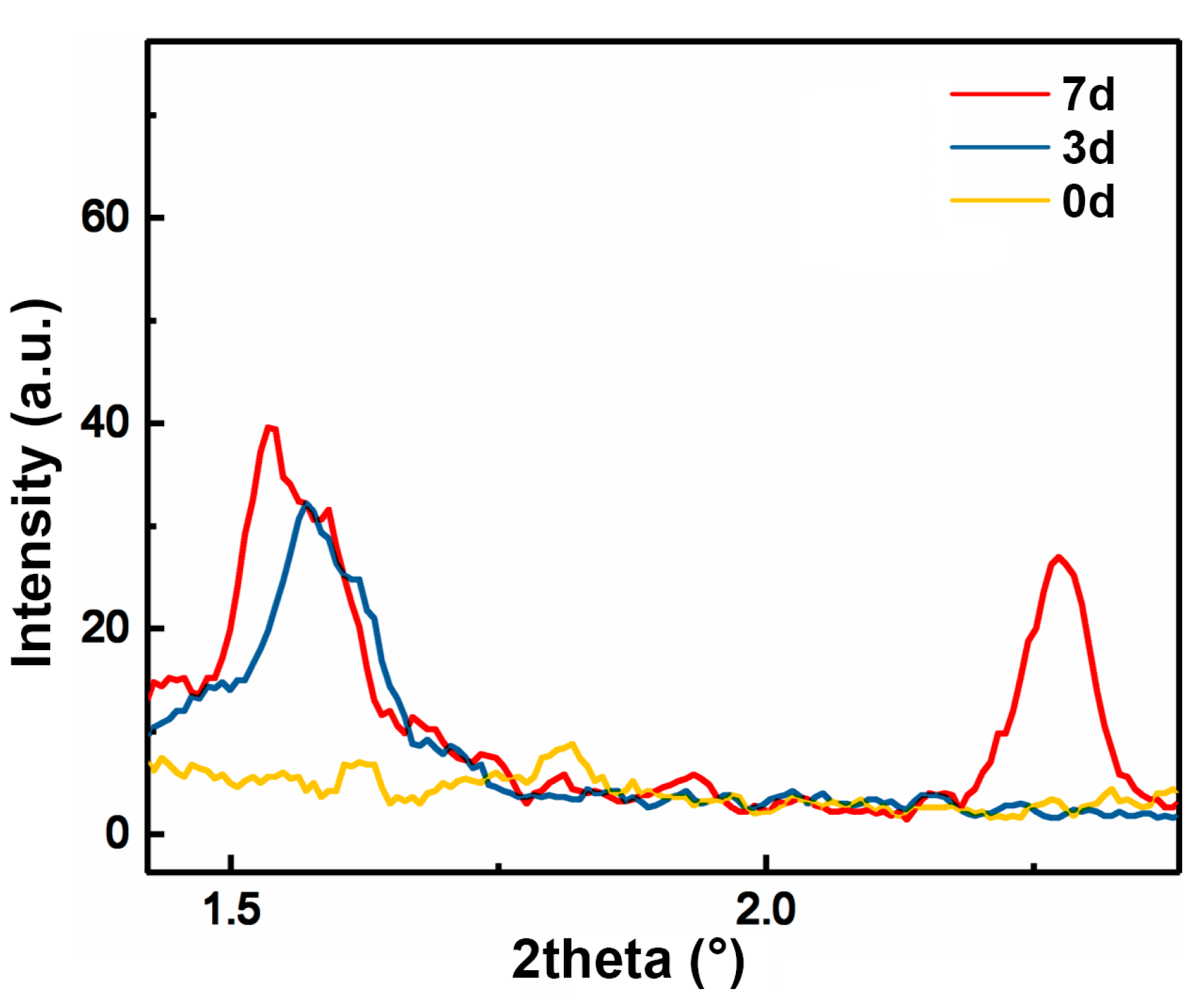
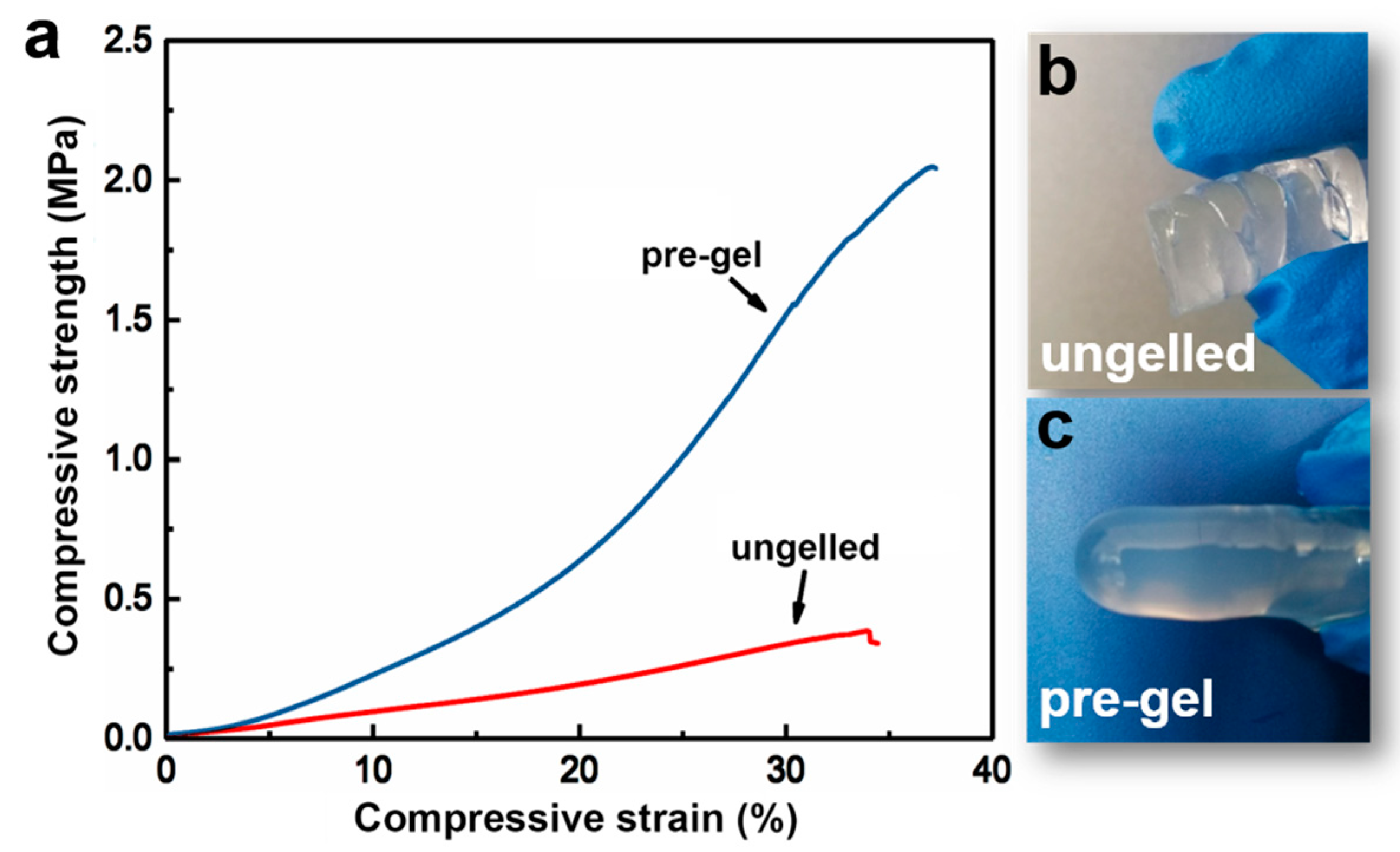
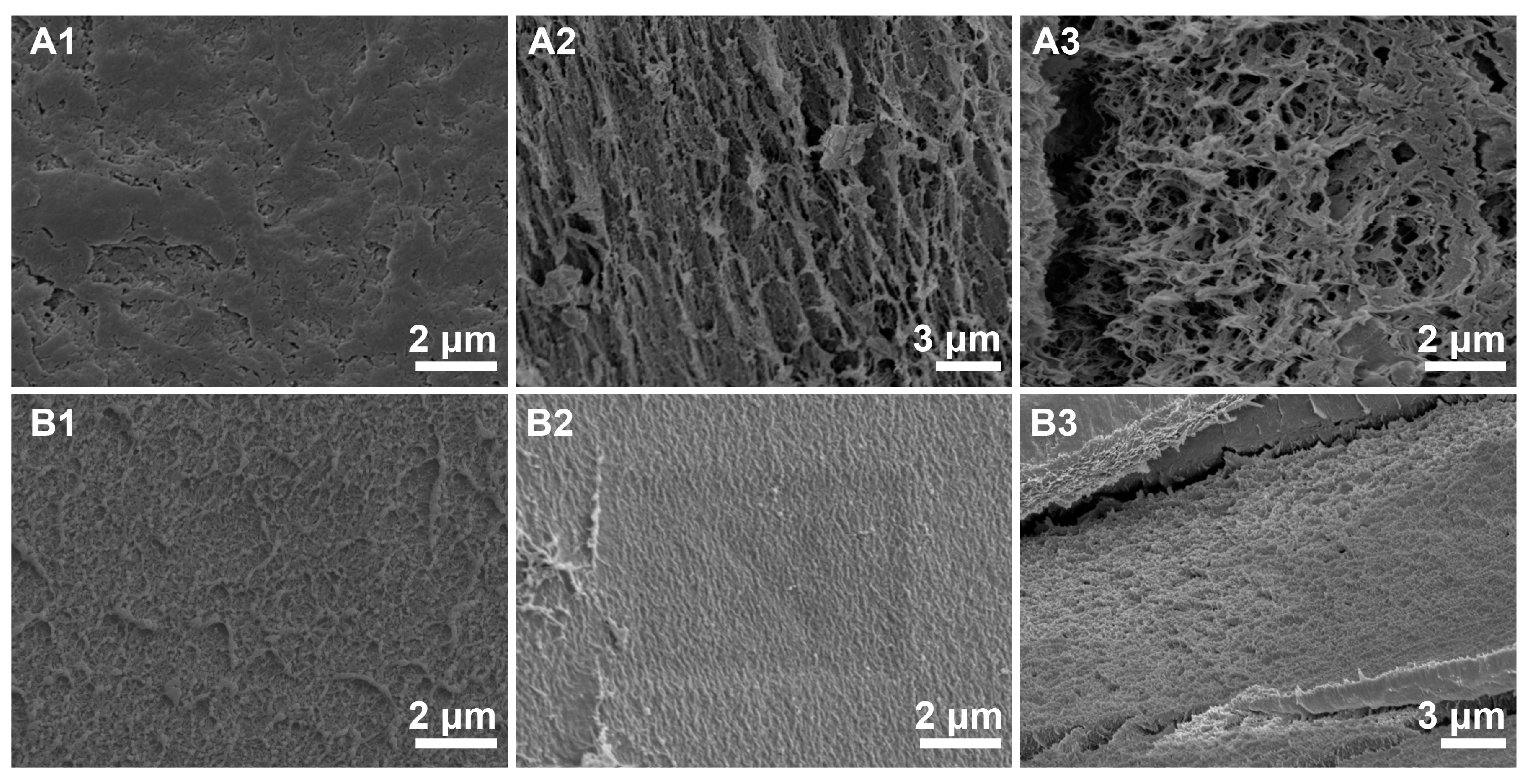
3.2. Effect of Pre-Gelation on Regenerated Hydrogel
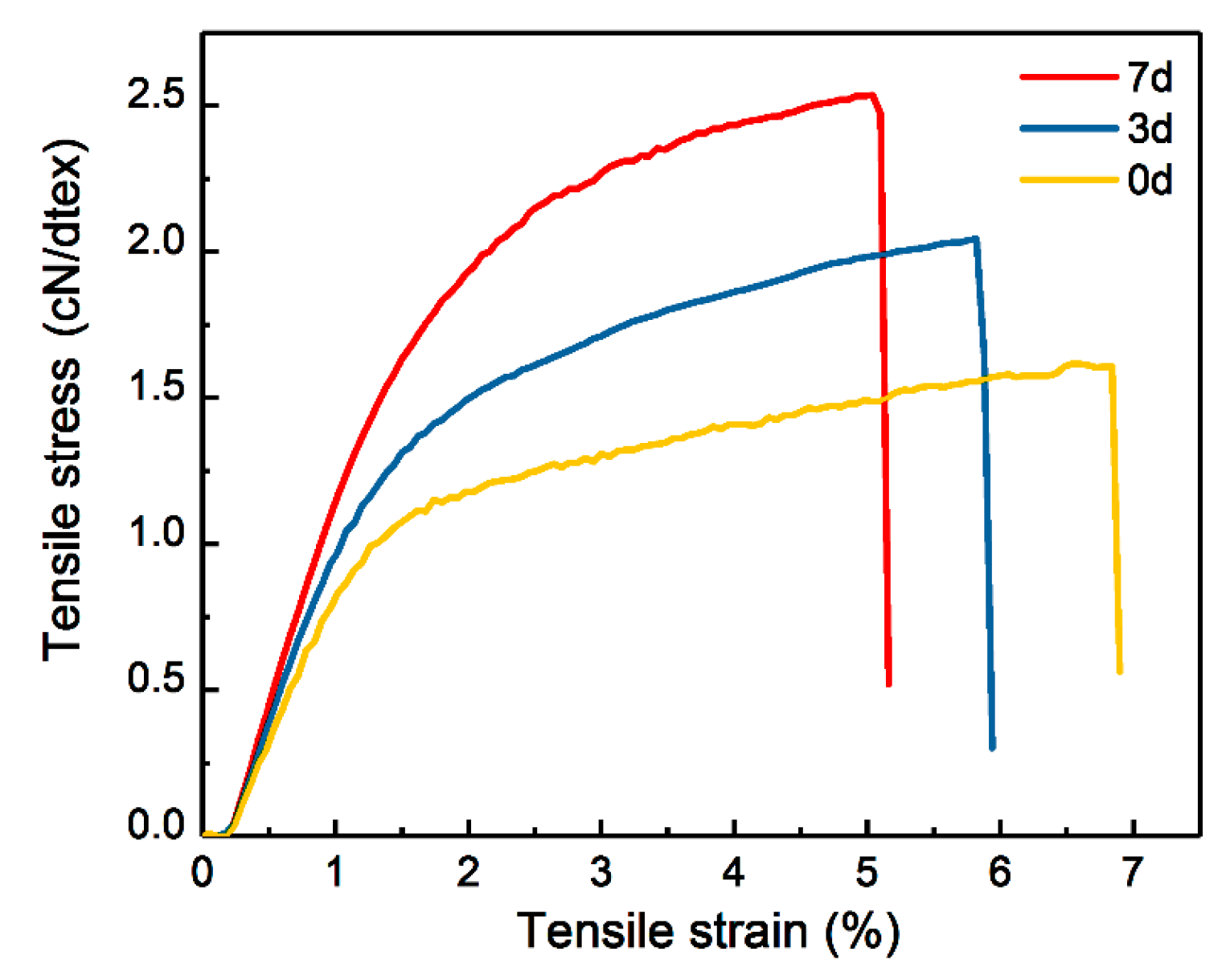
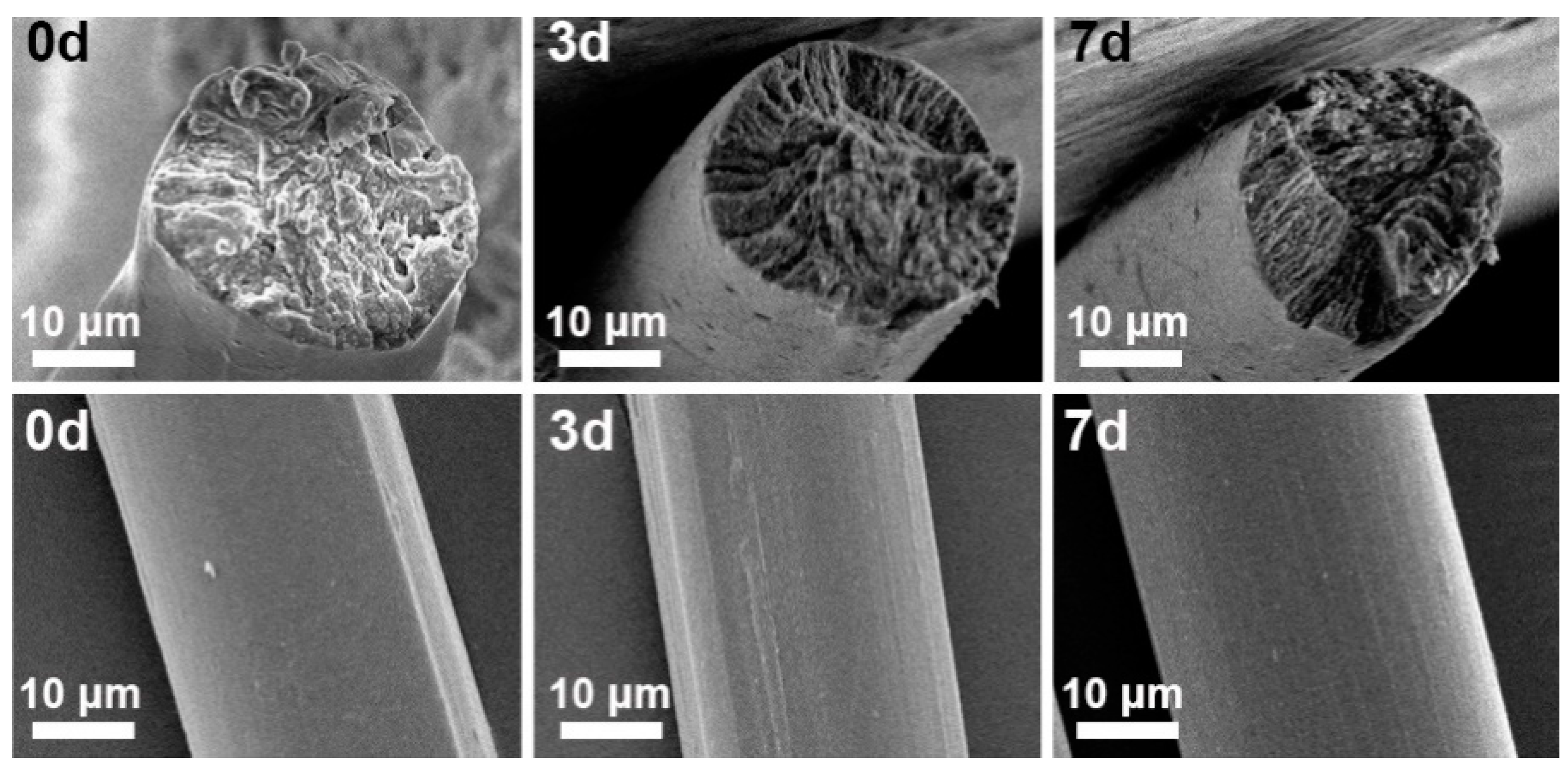
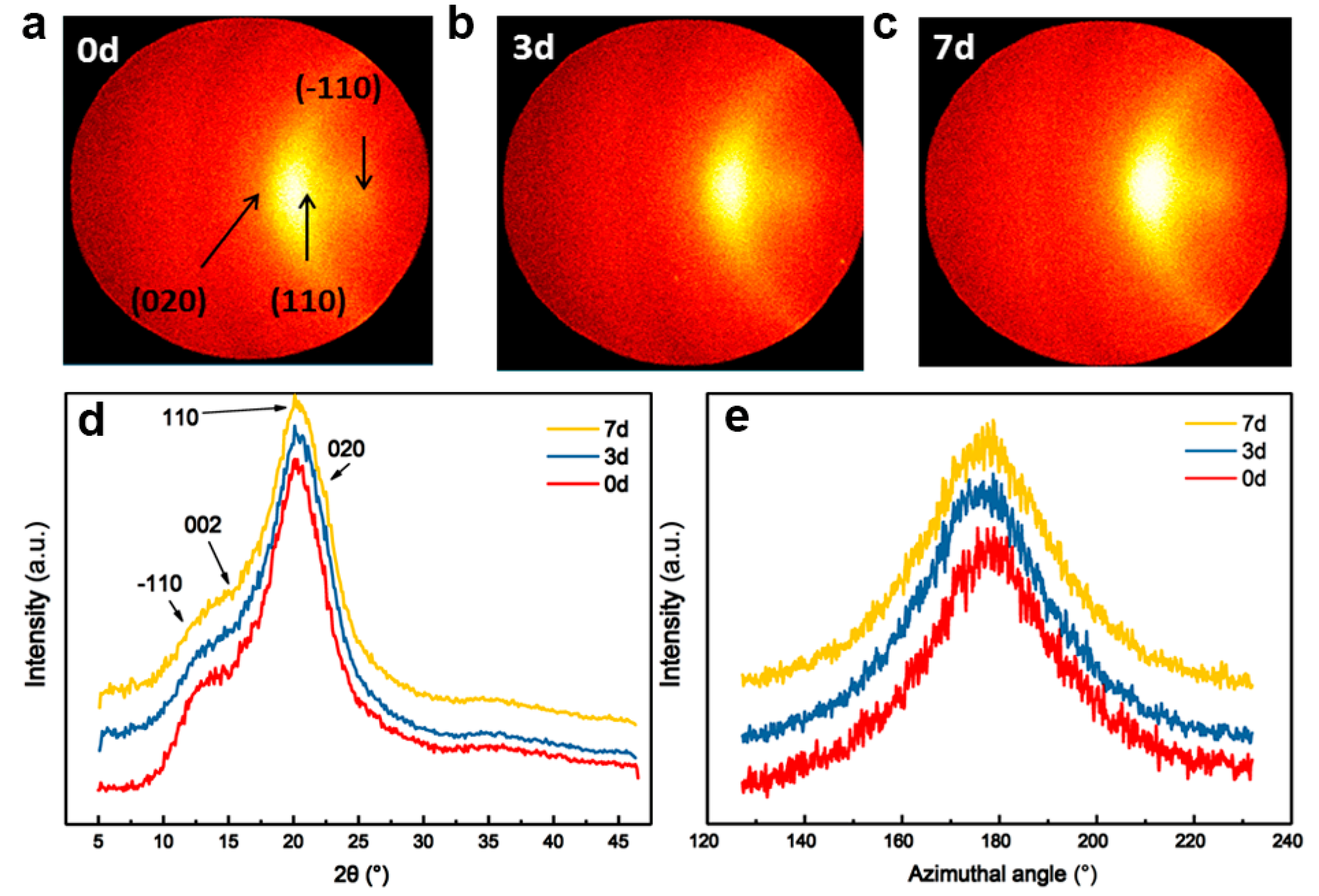
3.3. Effect of Pre-Gelation on Regenerated Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.K.; Brown Jr, R.M. Cellulose I Microfibril Assembly: Computational Molecular Mechanics Energy Analysis Favours Bonding by Van Der Waals forces as the Initial Step in Crystallization. Polymer 1995, 36, 3885–3888. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- White, R.J.; Brun, N.; Budarin, V.L.; Clark, J.H.; Titirici, M.M. Always Look on The “Light” Side of Life: Sustainable Carbon Aerogels. ChemSusChem 2014, 7, 670–689. [Google Scholar] [CrossRef]
- Cuculo, J.; Smith, C.; Sangwatanaroj, U.; Stejskal, E.; Sankar, S. A Study on the Mechanism of Dissolution of the Cellulose/NH3/NH4SCN System. I. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 229–239. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Jin, H.; Zha, C.; Gu, L. Direct Dissolution of Cellulose in NaOH/Thiourea/Urea Aqueous Solution. Carbohydr. Res. 2007, 342, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L. Solubility of Cellulose in NaOH/Urea Aqueous Solution. Polym. J. 2000, 32, 866–870. [Google Scholar] [CrossRef]
- El-Kafrawy, A. Investigation of the Cellulose/LiCl/Dimethylacetamide and Cellulose/LiCl/N-Methyl-2-Pyrrolidinone Solutions by 13C NMR Spectroscopy. J. Appl. Polym. Sci. 1982, 27, 2435–2443. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; Xiang, J.; Kang, H.; Liu, Z.; Huang, Y. Dissolution Mechanism of Cellulose in N, N-Dimethylacetamide/Lithium Chloride: Revisiting Through Molecular Interactions. J. Phys. Chem. B 2014, 118, 9507–9514. [Google Scholar] [CrossRef]
- Belousova, T.; Shablygin, M.; Belousov, Y.Y.; Golova, L.; Papkov, S. Spectral Features of the N-Methylmorpholine-N-Oxide-Water-Cellulose System. Polym. Sci. USSR 1986, 28, 1115–1122. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tatsumi, D.; Tamai, N.; Takaki, T. Solution properties of celluloses from different biological origins in LiCl· DMAc. Cellulose 2001, 8, 275–282. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Unique Gelation Behavior of Cellulose in NaOH/Urea Aqueous Solution. Biomacromolecules 2006, 7, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Fang, Y.; Ma, Y.; Liu, M.; Liu, R.; Guo, H.; Lu, A.; Zhang, L. Dissolution and Metastable Solution of Cellulose in NaOH/Thiourea at 8 C for Construction of Nanofibers. J. Phys. Chem. B 2017, 121, 1793–1801. [Google Scholar] [CrossRef]
- Weijie, L.; Fengying, Z.; Weiku, W.; Yinhui, L.; Yaodong, L.; Chunxiang, L.; Zhengfang, Z. Rheological Transitions and in-situ IR Characterizations of Cellulose/LiCl·DMAc Solution as a Function of Temperature. Cellulose 2018, 25, 4955–4968. [Google Scholar]
- Ono, Y.; Hou, G.; Chitbanyong, K.; Takeuchi, M.; Isogai, A. Molar Masses and Molar Mass Distributions of Commercial Regenerated Cellulose Materials and Softwood Dissolving Pulp Determined by SEC/MALLS. Cellulose 2023, 30, 8221–8233. [Google Scholar] [CrossRef]
- Zhu, C.; Richardson, R.M.; Potter, K.D.; Koutsomitopoulou, A.F.; Duijneveldt, J.S.V.; Vincent, S.R.; Wanasekara, N.D.; Eichhorn, S.J.; Rahatekar, S.S. High Modulus Regenerated Cellulose Fibers Spun from a Low Molecular Weight Microcrystalline Cellulose Solution. ACS Sustain. Chem. Eng. 2016, 4, 4545–4553. [Google Scholar] [CrossRef]
- Tan, L.; Pan, D.; Pan, N. Thermodynamic Study of a Water-Dimethylformamide-Polyacrylonitrile Ternary System. J. Appl. Polym. Sci. 2008, 110, 3439–3447. [Google Scholar] [CrossRef]
- Ouyang, Q.; Chen, Y.-S.; Zhang, N.; Mo, G.-M.; Li, D.-H.; Yan, Q. Effect of Jet Swell and Jet Stretch on the Structure of Wet-Spun Polyacrylonitrile Fiber. J. Macromol. Sci. Part B Phys. 2011, 50, 2417–2427. [Google Scholar] [CrossRef]
- Tan, L.; Wan, A.; Pan, D. Pregelled Gel Spinning of Polyacrylonitrile Precursor Fiber. Mater. Lett. 2011, 65, 887–890. [Google Scholar] [CrossRef]
- GB/T 14337-2022; Shanghai Textile Industry Technical Supervision Institute. Test Method for Tensile Properties of Chemical Fiber Short Fibers. State Administration for Market Regulation; National Standardization Management Committee: Beijing, China, 2022; p. 16.
- Hermans, J.J.; Hermans, P.H.; Vermaas, D.; Weidinger, A. Quantitative Evaluation of Orientation in Cellulose Fibres from the X-ray Fibre Diagram. Recl. Des Trav. Chim. Des Pays-Bas-J. R. Neth. Chem. Soc. 1946, 65, 427–447. [Google Scholar] [CrossRef]
- Wan, Y.; An, F.; Zhou, P.; Li, Y.; Liu, Y.; Lu, C.; Chen, H. Regenerated Cellulose I from LiCl·DMAc Solution. Chem. Commun. 2017, 53, 3595–3597. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Li, W.; Zhang, B.; Liu, Y. Effect of Solvent Pre-treatment on the Structures and Dissolution of Microcrystalline Cellulose in Lithium Chloride/Dimethylacetamide. Cellulose 2019, 26, 3095–3109. [Google Scholar] [CrossRef]
- Geng, H. A One-step Approach to Make Cellulose-based Hydrogels of Various Transparency and Swelling Degrees. Carbohydr. Polym. 2018, 186, 208–216. [Google Scholar] [CrossRef]
- Mao, Y.-Z.; Wang, H.; Wang, D.; Shen, H.; Yang, S.-G.; Ma, J.-H.; Zhao, N.; Liu, R.-G.; Xu, J. Studies on Properties of Regenerated Cellulose Hydrogel Membranes Prepared by Solution Pre-gelation. Acta Polym. Sin. 2014, 7, 1023–1028. [Google Scholar]
- Miri, S.; Raghuwanshi, V.S.; Andrews, P.C.; Batchelor, W. Composites of Mesoporous Silica Precipitated on Nanofibrillated Cellulose and Microfibrillated Cellulose: Effect of Fibre Diameter and Reaction Conditions on Particle Size and Mesopore Diameter. Microporous Mesoporous Mater. 2021, 311, 110701. [Google Scholar] [CrossRef]
- Wu, J.; Liang, S.; Dai, H.; Zhang, X.; Yu, X.; Cai, Y.; Zhang, L.; Wen, N.; Jiang, B.; Xu, J. Structure and Properties of Cellulose/Chitin Blended Hydrogel Membranes Fabricated via a Solution Pre-gelation Technique. Carbohydr. Polym. 2010, 79, 677–684. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, H.; Hu, X. The chain orientation of Cellulose flat and tubular films prepared from N-methylmorpholine N-oxide solutions. Polym. J. 2002, 34, 666–673. [Google Scholar] [CrossRef][Green Version]
- Gindl, W.; Martinschitz, K.J.; Boesecke, P.; Keckes, J. Changes in the molecular orientation and tensile properties of uniaxially drawn cellulose films. Biomacromolecules 2006, 7, 3146–3150. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, W.; Liu, Y. Enhancing the Mechanical Properties of Regenerated Cellulose through High-Temperature Pre-Gelation. Materials 2024, 17, 4886. https://doi.org/10.3390/ma17194886
Yu Y, Wang W, Liu Y. Enhancing the Mechanical Properties of Regenerated Cellulose through High-Temperature Pre-Gelation. Materials. 2024; 17(19):4886. https://doi.org/10.3390/ma17194886
Chicago/Turabian StyleYu, Yuxiu, Weiku Wang, and Yaodong Liu. 2024. "Enhancing the Mechanical Properties of Regenerated Cellulose through High-Temperature Pre-Gelation" Materials 17, no. 19: 4886. https://doi.org/10.3390/ma17194886
APA StyleYu, Y., Wang, W., & Liu, Y. (2024). Enhancing the Mechanical Properties of Regenerated Cellulose through High-Temperature Pre-Gelation. Materials, 17(19), 4886. https://doi.org/10.3390/ma17194886





