Towards More Sustainable Schiff Base Carboxylate Anodes for Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
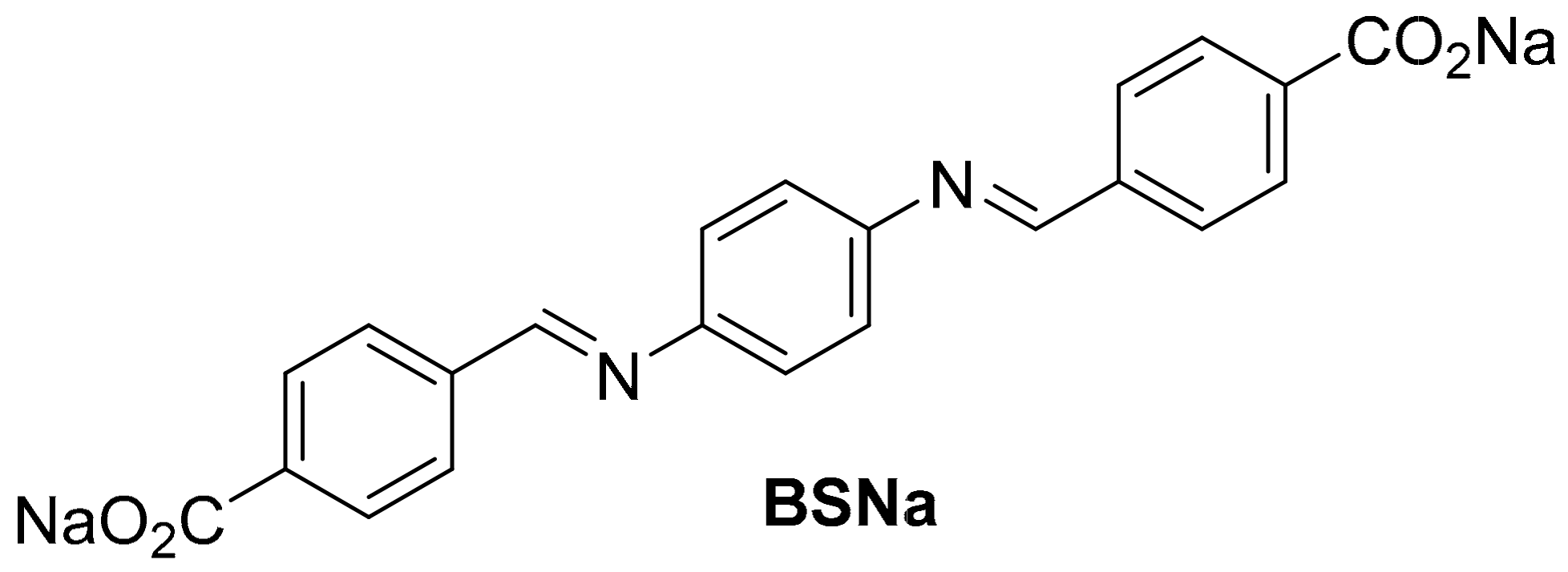
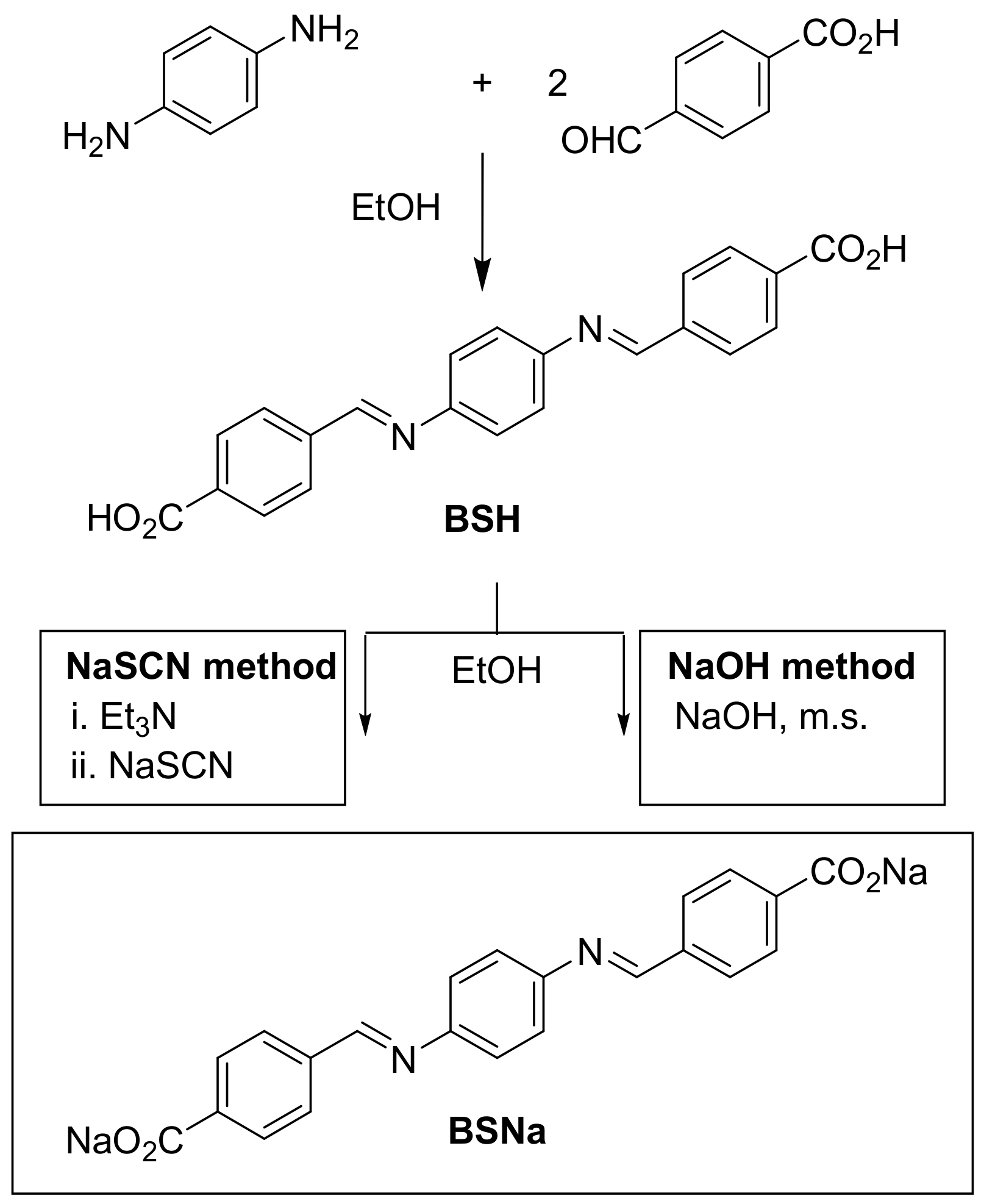
2.1. Synthesis
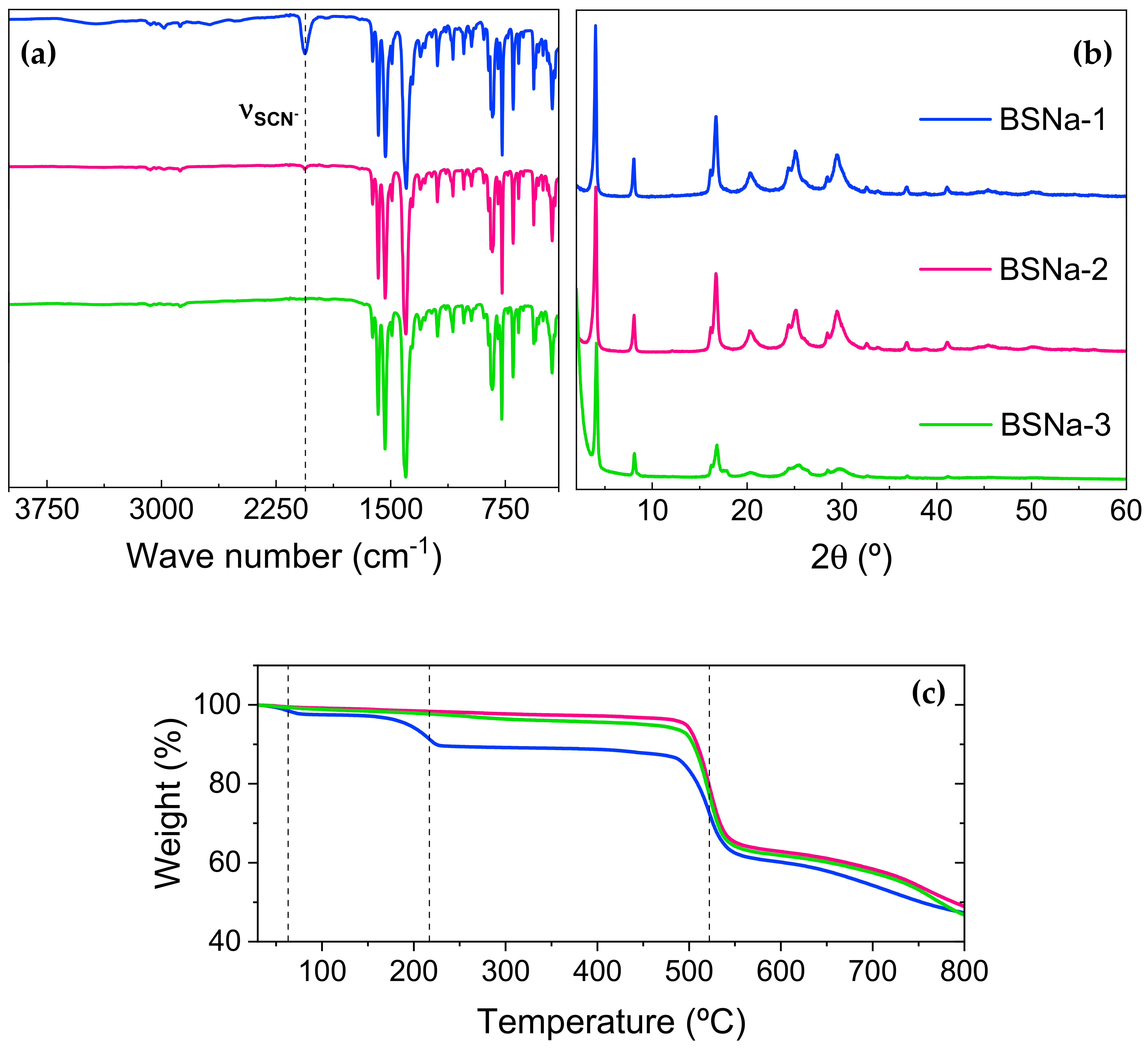
- NaSCN method: BSH (1 equiv. mol) was reacted with triethylamine (Aldrich, 2 equiv. mol) in a vial with absolute ethanol. After a few minutes of stirring, NaSCN (Aldrich, 4 equiv. mol) was added to the suspension. The mixture was magnetically stirred for 24 h. The resulting yellow powder was centrifuged at 8000 rpm, washed six times with absolute ethanol, and dried at 60 °C for 12 h to yield BSNa-1. This yellow solid was further washed seven more times and dried at 60 °C for 12 more hours, yielding BSNa-2.
- NaOH method: BSH (1 equiv. mol) and NaOH (Scharlau, 3 equiv. mol) were stirred magnetically in a flask with absolute ethanol under reflux at 80 °C for 24 h. To remove the water formed during the reaction (as vapor in the azeotropic mixture with ethanol), molecular sieve (Alfa Aesar (Heysham, UK), 3 Å, 3–4 mm) was suspended over the reaction mixture in a Soxhlet-type device. The resultant yellow powder was centrifuged at 8000 rpm, washed four times with absolute ethanol, and dried at 80 °C for 12 h, yielding BSNa-3.
2.2. Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Optimization of the Synthesis
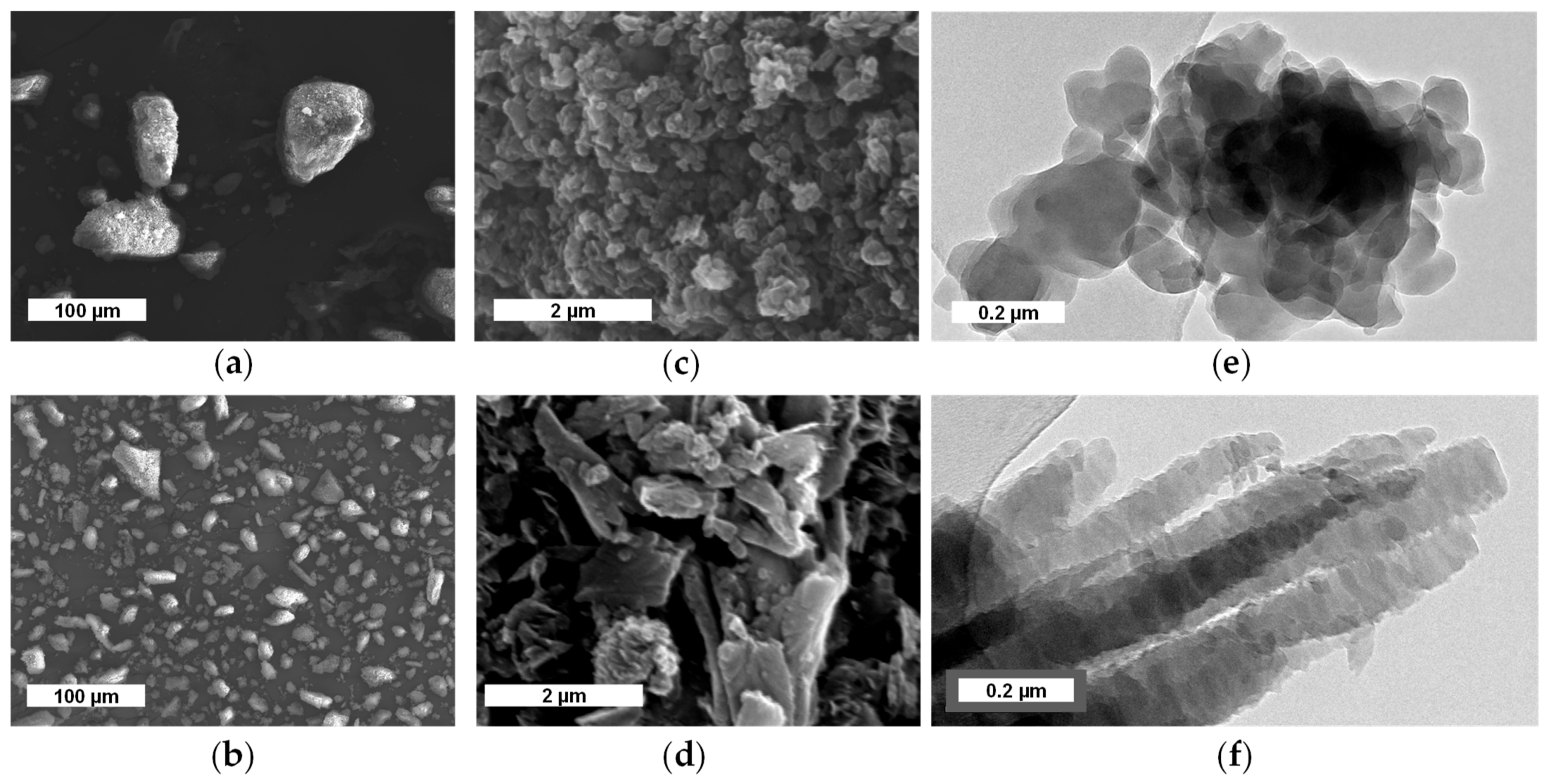
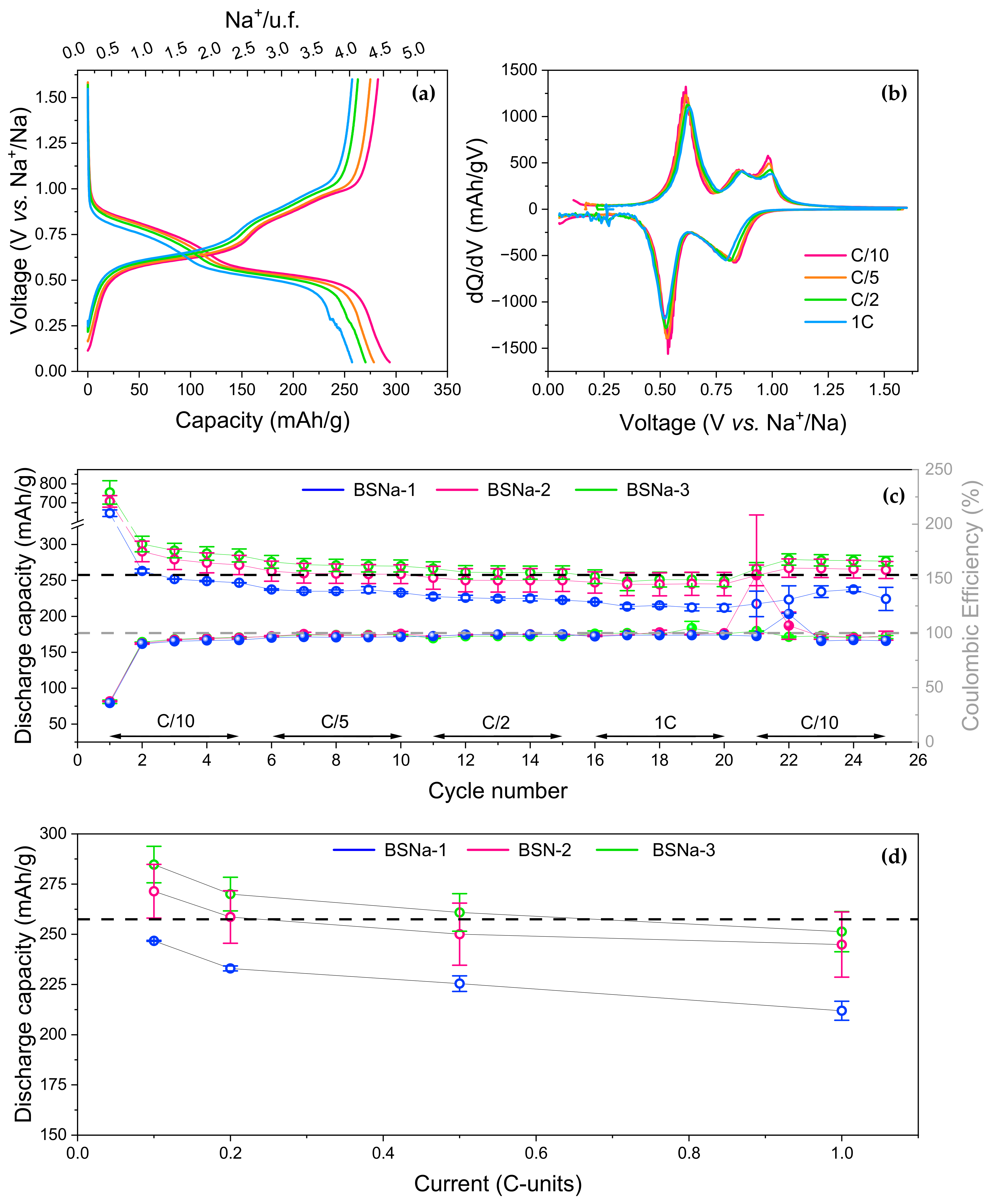
3.1.1. Synthesis and Characterization
3.1.2. Electrochemical Performance
3.2. Electrode Processing
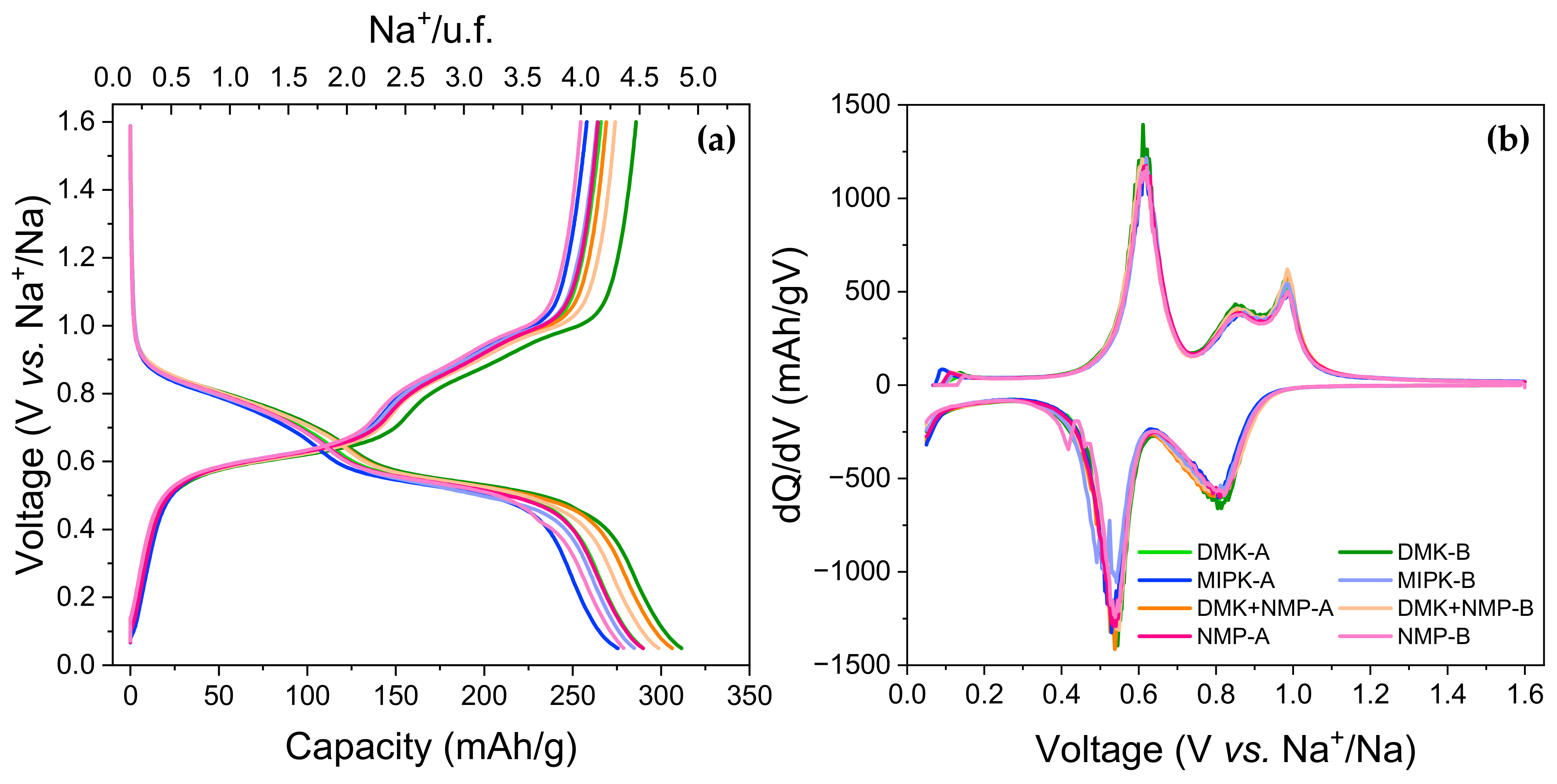
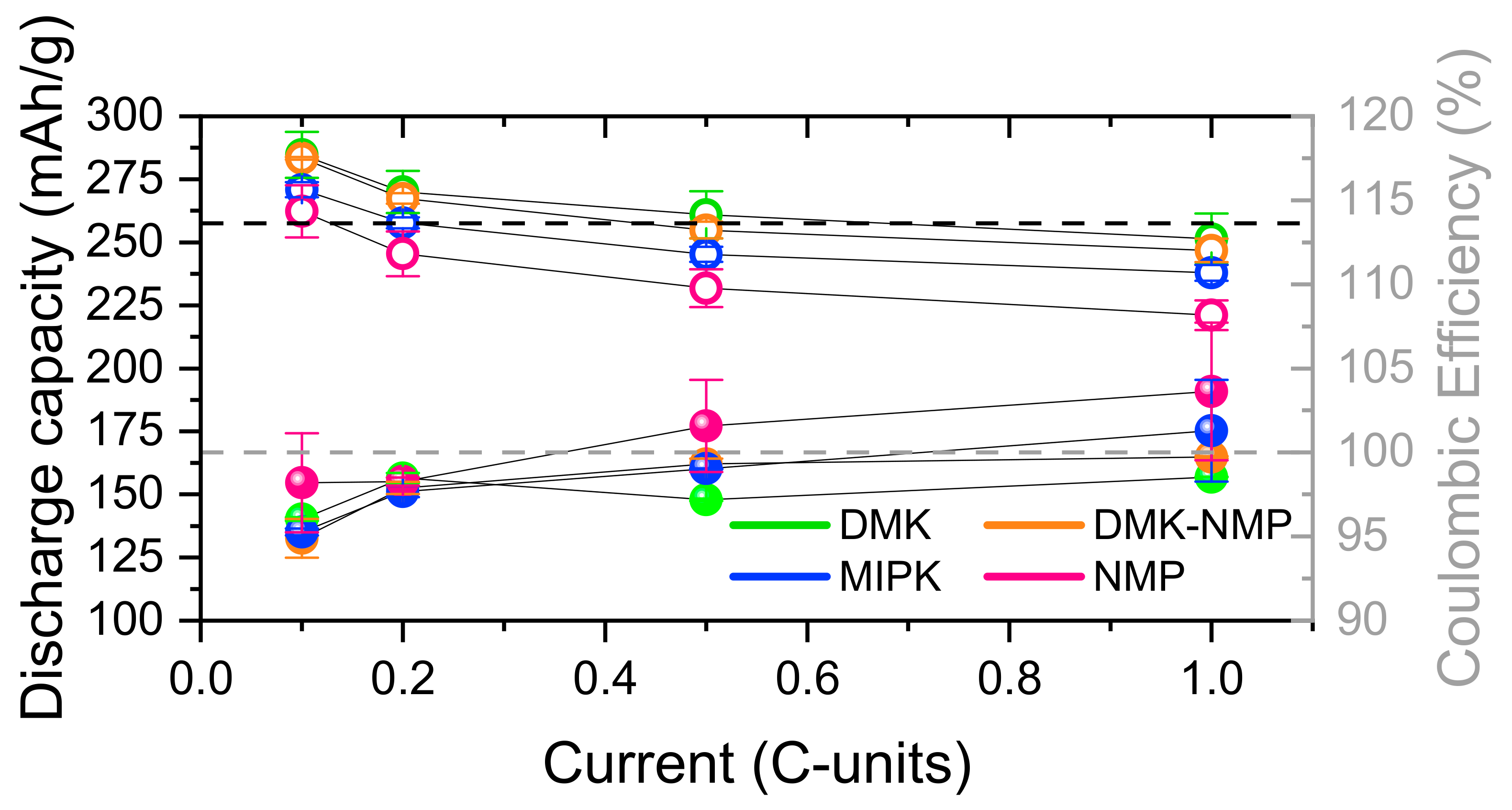
3.2.1. Solvents
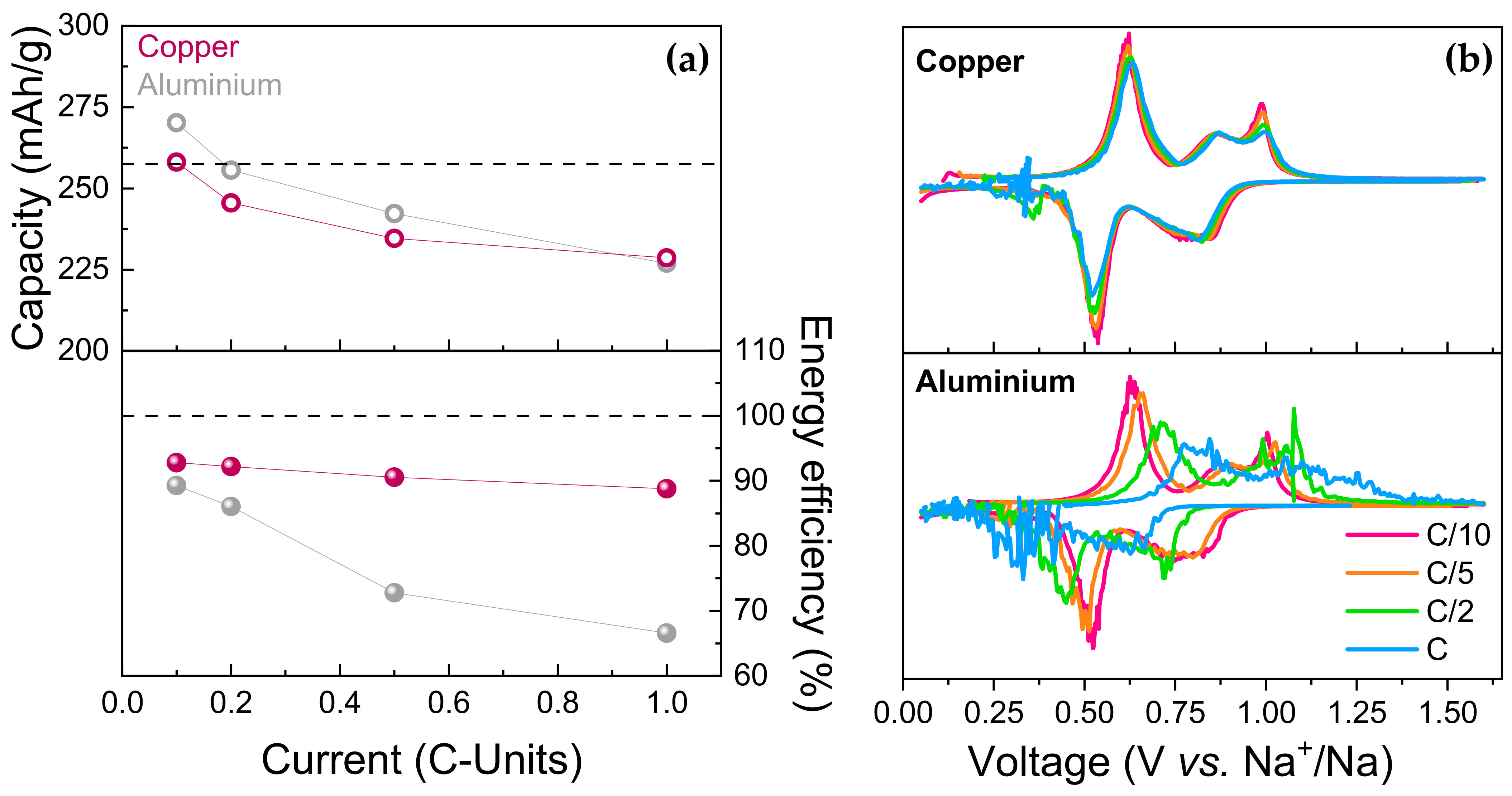
3.2.2. Current Collectors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-Ion Batteries, Recent Advances and Present Challenges to Become Low Cost Energy Storage Systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Myung, S.T.; Hitoshi, Y.; Sun, Y.K. Electrochemical Behavior and Passivation of Current Collectors in Lithium-Ion Batteries. J. Mater. Chem. 2011, 21, 9891–9911. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, T.; Li, W.; Li, T.; Zhang, L.; Zhang, X.; Wang, Z. Engineering of Sodium-Ion Batteries: Opportunities and Challenges. Engineering 2023, 24, 172–183. [Google Scholar] [CrossRef]
- Gester, A.; Wagner, G.; Pöthig, P.; Bergmann, J.P.; Fritzsche, M. Analysis of the Oscillation Behavior during Ultrasonic Welding of EN AW-1070 Wire Strands and EN CW004A Terminals. Weld. World 2022, 66, 567–576. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Vazquez-Santos, M.B.; Tartaj, P.; Morales, E.; Amarilla, J.M. TiO2 Nanostructures as Anode Materials for Li/Na-Ion Batteries. Chem. Rec. 2018, 18, 1178–1191. [Google Scholar] [CrossRef]
- Chen, J.; Adit, G.; Li, L.; Zhang, Y.; Chua, D.H.C.; Lee, P.S. Optimization Strategies Toward Functional Sodium-Ion Batteries. Energy Environ. Mater. 2023, 6, e12633. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Lei, Y. Organic Materials for Rechargeable Sodium-Ion Batteries. Mater. Today 2018, 21, 60–78. [Google Scholar] [CrossRef]
- Hari Prasad, P.M.; Malavika, G.; Pillai, A.; Sadan, S.; Pillai, Z.S. Emerging Organic Electrode Materials for Sustainable Batteries. NPG Asia Mater. 2024, 16, 37. [Google Scholar] [CrossRef]
- Desai, A.V.; Morris, R.E.; Armstrong, A.R. Advances in Organic Anode Materials for Na-/K-Ion Rechargeable Batteries. ChemSusChem 2020, 13, 4866–4884. [Google Scholar] [CrossRef]
- Yin, X.; Sarkar, S.; Shi, S.; Huang, Q.A.; Zhao, H.; Yan, L.; Zhao, Y.; Zhang, J. Recent Progress in Advanced Organic Electrode Materials for Sodium-Ion Batteries: Synthesis, Mechanisms, Challenges and Perspectives. Adv. Funct. Mater. 2020, 30, 1908445. [Google Scholar] [CrossRef]
- Luo, C.; Shea, J.J.; Huang, J. A Carboxylate Group-Based Organic Anode for Sustainable and Stable Sodium Ion Batteries. J. Power Sources 2020, 453, 227904. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Hu, Y.S.; Li, H.; Zhou, Z.; Armand, M.; Chen, L. Disodium Terephthalate (Na2C8H4O4) as High Performance Anode Material for Low-Cost Room-Temperature Sodium-Ion Battery. Adv. Energy Mater. 2012, 2, 962–965. [Google Scholar] [CrossRef]
- Park, Y.; Shin, D.S.; Woo, S.H.; Choi, N.S.; Shin, K.H.; Oh, S.M.; Lee, K.T.; Hong, S.Y. Sodium Terephthalate as an Organic Anode Material for Sodium Ion Batteries. Adv. Mater. 2012, 24, 3562–3567. [Google Scholar] [CrossRef]
- Castillo-Martínez, E.; Carretero-González, J.; Armand, M. Polymeric Schiff Bases as Low-Voltage Redox Centers for Sodium-Ion Batteries. Angew. Chem. 2014, 126, 5445–5449. [Google Scholar] [CrossRef]
- López-Herraiz, M.; Castillo-Martínez, E.; Carretero-González, J.; Carrasco, J.; Rojo, T.; Armand, M. Oligomeric-Schiff Bases as Negative Electrodes for Sodium Ion Batteries: Unveiling the Nature of Their Active Redox Centers. Energy Environ. Sci. 2015, 8, 3233–3241. [Google Scholar] [CrossRef]
- Hoffmann, A.; Heider, E.A.; Dreer, C.; Pfeifer, C.; Wohlfahrt-Mehrens, M. Influence of the Mixing and Dispersing Process on the Slurry Properties and the Microstructure and Performance of Ultrathick Cathodes for Lithium-Ion Batteries. Energy Technol. 2023, 11, 2200484. [Google Scholar] [CrossRef]
- Vidal, E.; Rojo, J.M.; García-Alegre, M.C.; Guinea, D.; Soto, E.; Amarilla, J.M. Effect of Composition, Sonication and Pressure on the Rate Capability of 5 V-LiNi0.5Mn1.5O4 Composite Cathodes. Electrochim. Acta 2013, 108, 175–181. [Google Scholar] [CrossRef]
- Gonçalves, R.; Lanceros-Méndez, S.; Costa, C.M. Electrode Fabrication Process and Its Influence in Lithium-Ion Battery Performance: State of the Art and Future Trends. Electrochem. Commun. 2022, 135, 107–210. [Google Scholar] [CrossRef]
- Xu, J.; Chou, S.L.; Gu, Q.F.; Liu, H.K.; Dou, S.X. The Effect of Different Binders on Electrochemical Properties of LiNi 1/3Mn1/3Co1/3O2 Cathode Material in Lithium Ion Batteries. J. Power Sources 2013, 225, 172–178. [Google Scholar] [CrossRef]
- Lazarraga, M.G.; Mandal, S.; Ibañez, J.; Manuel Amarilla, J.; Rojo, J.M. LiMn2O4-Based Composites Processed by a Chemical-Route: Microstructural, Electrical, Electrochemical, and Mechanical Characterization. J. Power Sources 2003, 115, 315–322. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, M.; Majeed, I.; Fan, W.; Zhao, J.; Zeng, Z. Toward Superior Low-Temperature Performance and Safe Cathode Slurry Fabrication via 3-Methoxy-N,N-Dimethylpropionamide as a Low-Toxicity and Facile Solvent. ACS Sustain. Chem. Eng. 2023, 11, 14582–14590. [Google Scholar] [CrossRef]
- Hunt, A.; Dale, N. Economic Valuation in 1-Methyl-2-Pyrrolidone (NMP) Regulation. OECD Environ. Work. Pap. 2018, 13, 1–32. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer Binders: Characterization and Development toward Aqueous Electrode Fabrication for Sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Sliz, R.; Valikangas, J.; Silva Santos, H.; Vilmi, P.; Rieppo, L.; Hu, T.; Lassi, U.; Fabritius, T. Suitable Cathode NMP Replacement for Efficient Sustainable Printed Li-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 4047–4058. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, C.; Liu, H.; Huang, Q.; Fu, H. Fabrication and Properties of PVDF and PVDF-HFP Microfiltration Membranes. J. Appl. Polym. Sci. 2018, 135, 46711. [Google Scholar] [CrossRef]
- Castillo-Martínez, E.; Solana-Madruga, E.; Ebrahimi-Koodehi, S.; Leskes, M.; del Burgo-Olivares, C.; Linage, M.; Martín, C.; Sanchez-Ahijón, E.; Gómez-Herrero, A.; Cascos, V.; et al. Hybrid Carboxylate-Schiff-Bases as Electroactive Anode Materials for Potassium-Ion Batteries. Sustain. Mater. Technol. 2024, 40, e00840. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley Publishing Company: Boston, MA, USA, 1978. [Google Scholar]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and Improving the Initial Coulombic Efficiency of High-Capacity Anode Materials for Practical Sodium Ion Batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Sarkar, A.; Manohar, C.V.; Mitra, S. A Simple Approach to Minimize the First Cycle Irreversible Loss of Sodium Titanate Anode towards the Development of Sodium-Ion Battery. Nano Energy 2020, 70, 104520. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Shu, C.; Hua, W.; Wang, X.; Zhao, Y.; Luo, J.; Tang, Z.; Wu, Y.; Tang, W. Research Progress and Perspectives on Pre-Sodiation Strategies for Sodium-Ion Batteries. Adv. Funct. Mater. 2024, 2409628. [Google Scholar] [CrossRef]
- Barker, J.; Heap, R.J.; Roche, N.; Tan, C.; Sayers, R.; Whitley, J.; Lui, Y. 3rd International Conference on Sodium Batteries on Na Batteries, Geelong. 2016. Available online: https://faradion.co.uk/wp-content/uploads/2018/04/Faradion-Limited-3rd-International-Meeting-on-Sodium-Batteries.pdf (accessed on 16 August 2024).
- Hjelm, A.-K.; Lindbergh, G. Experimental and theoretical analysis of LiMn2O4 cathodes for use in rechargeable lithium batteries by electrochemical impedance spectroscopy (EIS). Electrochem. Acta 2002, 47, 1747. [Google Scholar] [CrossRef]
- LANDT Battery Test Equipment & Supplies. Available online: https://landtinst.com/carbon-coated-aluminum-foil/ (accessed on 16 August 2024).



| Cycle 1 | Cycle 2 | |||
|---|---|---|---|---|
| Red | Ox | Red | Ox | |
| BSNa-1 | 645(17) | 237 * | 263(3) | 236(2) |
| BSNa-2 | 708(30) | 265(15) | 290(14) | 263(15) |
| BSNa-3 | 755(62) | 278(10) | 300(11) | 276(10) |
| C/10 | C/5 | C/2 | C | |||||
|---|---|---|---|---|---|---|---|---|
| Red | Ox | Red | Ox | Red | Ox | Red | Ox | |
| BSNa-1 | 247(0) | 233(3) | 233(1) | 227(3) | 225(4) | 223(4) | 211(5) | 217 * |
| BSNa-2 | 271(13) | 260(16) | 259(13) | 257(19) | 250(15) | 247(15) | 245(16) | 245(16) |
| BSNa-3 | 285(9) | 273(9) | 270(9) | 266(9) | 261(9) | 254(9) | 251(10) | 248(10) |
| Cycle 1 | Cycle 2 | |||
|---|---|---|---|---|
| Red | Ox | Red | Ox | |
| DMK | 755(62) | 278(10) | 300 (11) | 276(10) |
| MIPK | 660(35) | 261(3) | 280(5) | 261(3) |
| DMK-NMP | 857(18) | 273(2) | 300(1) | 272(2) |
| NMP | 804(1) | 261(4) | 284(5) | 259(5) |
| C/10 | C/5 | C/2 | C | |||||
|---|---|---|---|---|---|---|---|---|
| Red | Ox | Red | Ox | Red | Ox | Red | Ox | |
| DMK | 285(9) | 273(9) | 270(9) | 266(9) | 261(9) | 254(9) | 251(10) | 248(10) |
| MIPK | 271(3) | 258(3) | 258(2) | 252(3) | 245(3) | 243(3) | 238(3) | 241(10) |
| DMK-NMP | 283(1) | 269(3) | 267(2) | 262(3) | 255(3) | 253(4) | 247(5) | 246(5) |
| NMP | 262(10) | 257(2) | 245(9) | 241(8) | 232(8) | 235(1) | 220(6) | 229(3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Berenguer, I.; Herradón, B.; Amarilla, J.M.; Castillo-Martínez, E. Towards More Sustainable Schiff Base Carboxylate Anodes for Sodium-Ion Batteries. Materials 2024, 17, 4918. https://doi.org/10.3390/ma17194918
Gómez-Berenguer I, Herradón B, Amarilla JM, Castillo-Martínez E. Towards More Sustainable Schiff Base Carboxylate Anodes for Sodium-Ion Batteries. Materials. 2024; 17(19):4918. https://doi.org/10.3390/ma17194918
Chicago/Turabian StyleGómez-Berenguer, Irene, Bernardo Herradón, José Manuel Amarilla, and Elizabeth Castillo-Martínez. 2024. "Towards More Sustainable Schiff Base Carboxylate Anodes for Sodium-Ion Batteries" Materials 17, no. 19: 4918. https://doi.org/10.3390/ma17194918
APA StyleGómez-Berenguer, I., Herradón, B., Amarilla, J. M., & Castillo-Martínez, E. (2024). Towards More Sustainable Schiff Base Carboxylate Anodes for Sodium-Ion Batteries. Materials, 17(19), 4918. https://doi.org/10.3390/ma17194918







