Designing Composite Stimuli-Responsive Hydrogels for Wound Healing Applications: The State-of-the-Art and Recent Discoveries
Abstract
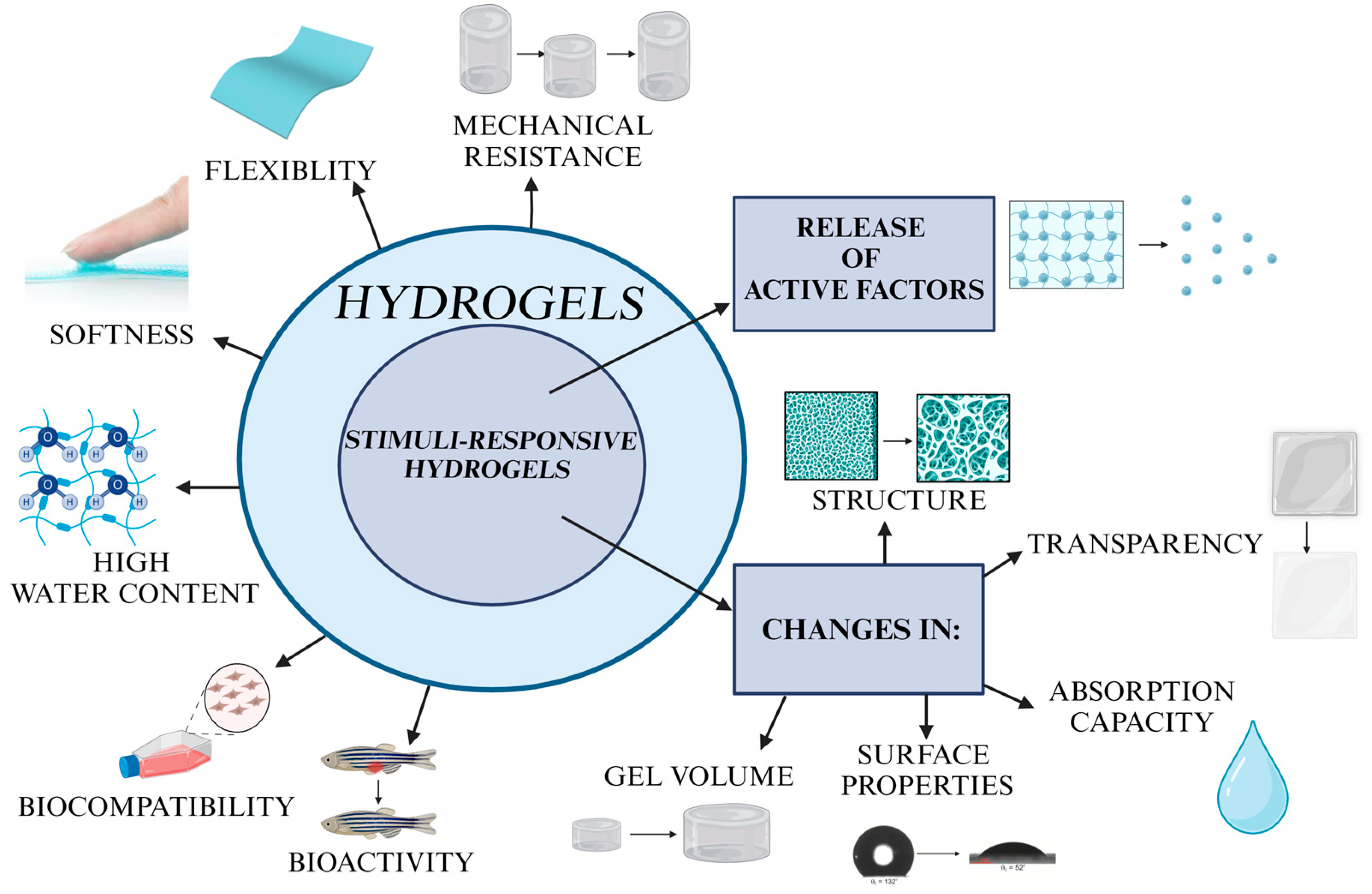
:1. Introduction
2. Challenges Related to Hydrogel-Based Wound Dressings
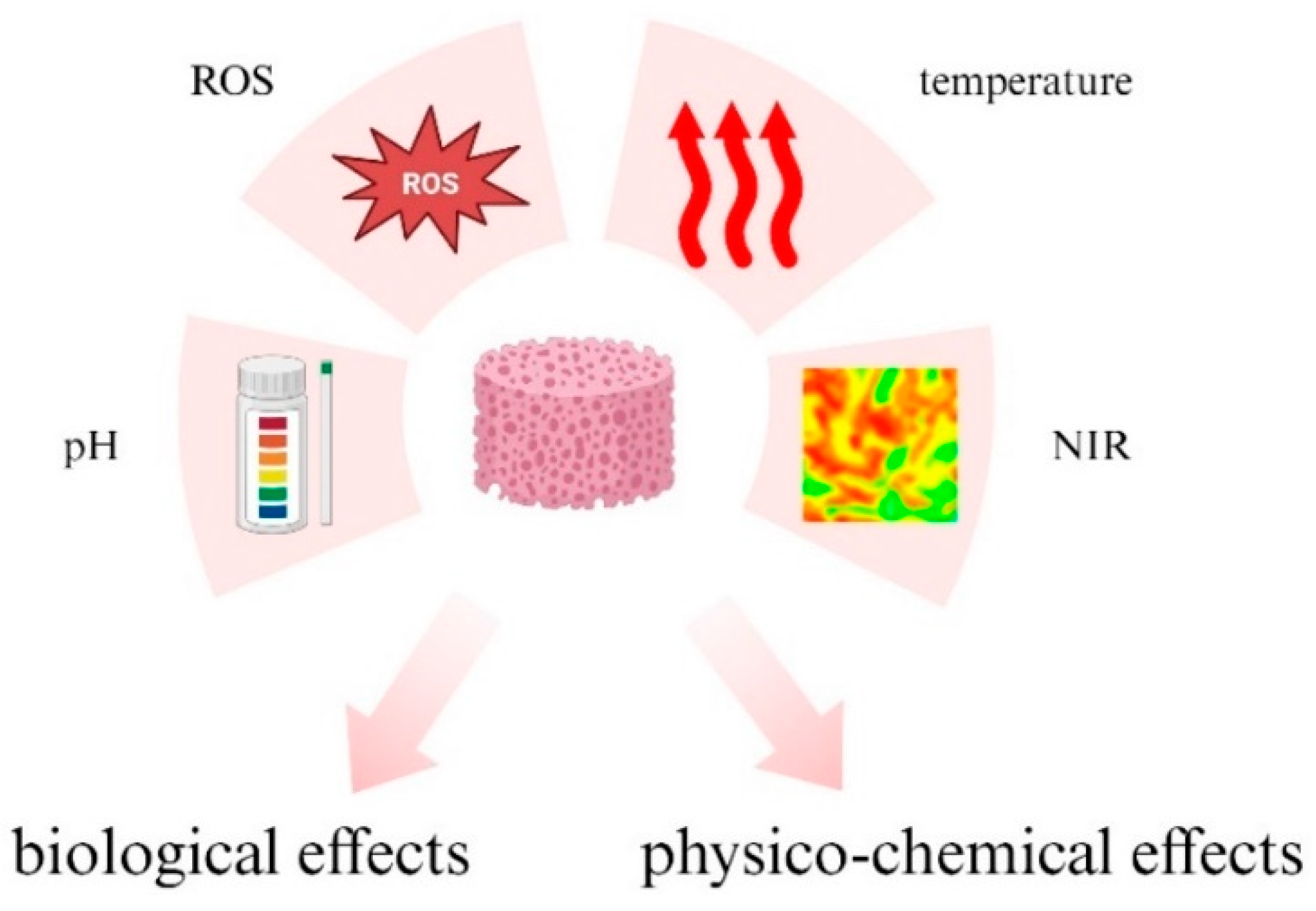
3. Various Stimuli Triggering the Hydrogels’ Response
- non-contact stimuli-responsive hydrogels (e.g., light-responsive, thermo-responsive, magnetic/electric field-responsive),
- contact stimuli-responsive hydrogels (e.g., pH-responsive, ion-responsive, chemically/biochemically responsive),
- multistimuli-responsive hydrogels (susceptible to the simultaneous or sequential action of two or more stimuli).
3.1. pH-Responsive Hydrogels
3.2. ROS-Responsive Hydrogels
3.3. Temperature-Responsive Hydrogels
3.4. NIR-Responsive Hydrogels
3.5. Examples of Existing Stimuli-Responsive Hydrogels
4. Loading Stimuli-Responsive Hydrogels with Active Substances
4.1. Hydrogels Loaded with Polyphenols
4.2. Hydrogels Loaded with Peptides, Polypeptides, and Proteins
- Interesting example of Thymosin β4
4.3. Hydrogels Loaded with Silver Nanoparticles
4.4. Hydrogels Loaded with Antibiotics and Drugs
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and innovations in treating chronic and acute wound infections: From basic science to clinical practice. Burn. Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Ye, J.; Rerkasem, K.; Mani, R. The venous ulcer continues to be a clinical challenge: An update. Burn. Trauma 2018, 6, 18. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef] [PubMed]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel Dressings for Advanced Wound Management. Curr. Med. Chem. 2019, 25, 5782–5797. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Özkahraman, B.; Özbaş, Z.; Yaşayan, G.; Tamahkar, E.; Alarçin, E. Advancements and future directions in the antibacterial wound dressings–A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Sharda, D.; Attri, K.; Choudhury, D. Future research directions of antimicrobial wound dressings. In Antimicrobial Dressings: The Wound Care Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 229–246. ISBN 9780323950749. [Google Scholar]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and biomarkers for wound healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, L.; Fiorati, A.; D’agostino, A.; Pelacani, C.M.; Chiesa, R.; Farè, S.; De Nardo, L. Smart Methylcellulose Hydrogels for pH-Triggered Delivery of Silver Nanoparticles. Gels 2022, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Yassin, M.; Phan, T. Vibrio mimicus wound infection in a burn patient. Radiol. Case Rep. 2021, 16, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Fabian, T.C. Damage Control in Trauma: Laparotomy Wound Management Acute to Chronic. Surg. Clin. N. Am. 2007, 87, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Furtado, K.A.X.; Infante, P.; Sobral, A.; Gaspar, P.; Eliseu, G.; Lopes, M. Prevalence of acute and chronic wounds–with emphasis on pressure ulcers–in integrated continuing care units in Alentejo, Portugal. Int. Wound J. 2020, 17, 1002–1010. [Google Scholar] [CrossRef]
- Boodhoo, K.; Vlok, M.; Tabb, D.L.; Myburgh, K.H.; van de Vyver, M. Dysregulated healing responses in diabetic wounds occur in the early stages postinjury. J. Mol. Endocrinol. 2021, 66, 141–155. [Google Scholar] [CrossRef]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef]
- Anderson, K.; Hamm, R.L. Factors that impair wound healing. J. Am. Coll. Clin. Wound Spec. 2012, 4, 84–91. [Google Scholar] [CrossRef]
- Mo, F.; Zhang, M.; Duan, X.; Lin, C.; Sun, D.; You, T. Recent Advances in Nanozymes for Bacteria-Infected Wound Therapy. Int. J. Nanomed. 2022, 17, 5947–5990. [Google Scholar] [CrossRef]
- Smith, F.; Sharp, A. Undertaking a person-centred assessment of patients with chronic wounds. Nurs. Stand. 2019, 34, 77–82. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous wound healing: An update from physiopathology to current therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Kearney, K.J.; Ariëns, R.A.S.; MacRae, F.L. The Role of Fibrin(ogen) in Wound Healing and Infection Control. Semin. Thromb. Hemost. 2022, 48, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraja, A.T. Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J. Med. Chem. 2017, 60, 3221–3240. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.K.; Chisty, A.H.; Khan, M.N.; Kabir, S.F.; Shahruzzaman; Rahman, M.M. Antibacterial Surface Modification to Prevent Biofilm Formation on Polymeric Biomaterials. In Nanoscale Engineering of Biomaterials: Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 425–455. ISBN 9789811636677. [Google Scholar]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Liu, X.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.W.K.; Li, C.; Cui, Z.; Liang, Y.; et al. Rapid bacteria trapping and killing of metal-organic frameworks strengthened photo-responsive hydrogel for rapid tissue repair of bacterial infected wounds. Chem. Eng. J. 2020, 396, 125194. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, W.; Xu, Z.; Jiang, C.; Han, S.; Ruan, J.; Wang, Y. Photothermal-assisted antibacterial application of graphene oxide-Ag nanocomposites against clinically isolated multi-drug resistant Escherichia coli. R. Soc. Open Sci. 2020, 7, 192019. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Boffito, M.; Ciardelli, G.; Chiono, V. Wound dressing products: A translational investigation from the bench to the market. Eng. Regen. 2022, 3, 182–200. [Google Scholar] [CrossRef]
- Serpico, L.; Dello Iacono, S.; Cammarano, A.; De Stefano, L. Recent Advances in Stimuli-Responsive Hydrogel-Based Wound Dressing. Gels 2023, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; You, Y.; Ma, Y.; Huang, W.; Liang, X.; Zhang, A.; Lin, Y. Bi-layer supramolecular polydimethylsiloxane elastomer film: Synthesis, characterization, and application in wound dressing on normal and diabetic rat. React. Funct. Polym. 2019, 141, 21–32. [Google Scholar] [CrossRef]
- Gwak, H.C.; Han, S.H.; Lee, J.; Park, S.; Sung, K.S.; Kim, H.J.; Chun, D.; Lee, K.; Ahn, J.H.; Kwak, K.; et al. Efficacy of a povidone-iodine foam dressing (Betafoam) on diabetic foot ulcer. Int. Wound J. 2020, 17, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.E.; Franken, C.P.M.; Gad, P.; Larsen, A.M.; Larsen, J.R.; van Neer, P.A.F.; Vuerstaek, J.; Wuite, J.; Neumann, H.A.M. A randomized, controlled study to compare the effectiveness of two foam dressings in the management of lower leg ulcers. Ostomy Wound Manag. 2002, 48, 34–41. [Google Scholar]
- Pele, K.G.; Amaveda, H.; Mora, M.; Marcuello, C.; Lostao, A.; Alamán-Díez, P.; Pérez-Huertas, S.; Ángeles Pérez, M.; García-Aznar, J.M.; García-Gareta, E. Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering. Gels 2023, 9, 505. [Google Scholar] [CrossRef]
- Truhan-Ortiz, R.; Moffatt, L.T.; Robson, M.C.; Jordan, M.H.; Shupp, J.W. In vivo and in vitro evaluation of the properties of Drawtex® LevafiberTM wound dressing in an infected burn wound model. Wound Repair Regen. 2012, 20, A42. [Google Scholar]
- Shen, Z.; Zhang, C.; Wang, T.; Xu, J. Advances in Functional Hydrogel Wound Dressings: A Review. Polymers 2023, 15, 2000. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. 2021, 146, 100641. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Z.; He, Y.; Lu, Q.; Chen, R.; Zhao, C.; Dong, D.; Sun, Y.; He, H. Dual light-responsive cellulose nanofibril-based in situ hydrogel for drug-resistant bacteria infected wound healing. Carbohydr. Polym. 2022, 297, 120042. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo de Santiago, G. Structural and Biological Engineering of 3D Hydrogels for Wound Healing; Elsevier: Amsterdam, The Netherlands, 2023; Volume 24, pp. 197–235. [Google Scholar]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhu, M.; Chen, L.; Zhang, Y.; Lu, T.; Deng, Y.; Ma, W.; Xu, J.; Huang, C.; Xiong, R. Design the molecule structures to achieve functional advantages of hydrogel wound dressings: Advances and strategies. Compos. Part B Eng. 2022, 247, 110313. [Google Scholar] [CrossRef]
- Sathyaraj, W.V.; Prabakaran, L.; Bhoopathy, J.; Dharmalingam, S.; Karthikeyan, R.; Atchudan, R. Therapeutic Efficacy of Polymeric Biomaterials in Treating Diabetic Wounds—An Upcoming Wound Healing Technology. Polymers 2023, 15, 1205. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, L.; Yang, Y.; Yin, Z.; Guo, B. Biodegradable gelatin/silver nanoparticle composite cryogel with excellent antibacterial and antibiofilm activity and hemostasis for Pseudomonas aeruginosa-infected burn wound healing. J. Colloid Interface Sci. 2022, 608, 2278–2289. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, M.M.; Alruwaili, N.K.; Alrowaili, Z.A.; Alomar, F.A.; Akhtar, S.; Alsaidan, O.A.; Alhakamy, N.A.; Zafar, A.; Elmowafy, M.; et al. Antibiotic-loaded psyllium husk hemicellulose and gelatin-based polymeric films for wound dressing application. Pharmaceutics 2021, 13, 236. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Jiang, Y.; Liu, Y.; Qu, G.; Chen, K.; Zhao, Y.; Wang, P.; Wu, X.; Ren, J. Novel Temperature-Sensitive Hydrogel Promotes Wound Healing Through YAP and MEK-Mediated Mechanosensitivity. Adv. Healthc. Mater. 2022, 11, e2201878. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Aderibigbe, B.A. Polymer-Based Hydrogels Enriched with Essential Oils: A Promising Approach for the Treatment of Infected Wounds. Polymers 2022, 14, 3772. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, S.; Liu, S.; Zhu, X.; Wang, P. Application of Nanomaterial in Hydrogels Related to Wound Healing. J. Nanomater. 2022, 2022, 4656037. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Xu, C.; Cui, L.; Meng, G.; Yang, S.; Wu, J.; Liu, Z.; Guo, X. PEG-α-CD/AM/liposome @amoxicillin double network hydrogel wound dressing—Multiple barriers for long-term drug release. J. Biomater. Appl. 2021, 35, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Zeng, Y.; Zaldivar-Silva, D.; Agüero, L.; Wang, S. Chitosan-Based Hemostatic Hydrogels: The Concept, Mechanism, Application, and Prospects. Molecules 2023, 28, 1473. [Google Scholar] [CrossRef]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable Self-Healing Adhesive Chitosan Hydrogel with Antioxidative, Antibacterial, and Hemostatic Activities for Rapid Hemostasis and Skin Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 34455–34469. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Z.; You, X.; Guo, Y.; Chen, P.; Li, H.; Tong, X. Tannic acid-loaded hydrogel coating endues polypropylene mesh with hemostatic and anti-inflammatory capacity for facilitating pelvic floor repair. Regen. Biomater. 2022, 9, rbac074. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiao, J.; Guan, S.; Geng, Z.; Zhao, R.; Gao, B. A hydrogen-bonded antibacterial curdlan-tannic acid hydrogel with an antioxidant and hemostatic function for wound healing. Carbohydr. Polym. 2022, 285, 119235. [Google Scholar] [CrossRef]
- Sun, X.; Tang, Z.; Pan, M.; Wang, Z.; Yang, H.; Liu, H. Chitosan/kaolin composite porous microspheres with high hemostatic efficacy. Carbohydr. Polym. 2017, 177, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, J.B.; Zazeri, G.; Povinelli, A.P.R.; de Godoy, M.F.; Braile, D.M.; da Rocha, T.R.F.; D’Amico, É.A.; Nery, J.G. Enhanced pro-coagulant hemostatic agents based on nanometric zeolites. Microporous Mesoporous Mater. 2017, 239, 263–271. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, C.; Li, G.; Liu, T.; Liang, J.F.; Wang, X. Graphene-kaolin composite sponge for rapid and riskless hemostasis. Colloids Surf. B Biointerfaces 2018, 169, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yao, W.; Tian, M.; Wei, J.; Song, Q.; Qiao, W. Mussel-inspired degradable antibacterial polydopamine/silica nanoparticle for rapid hemostasis. Biomaterials 2018, 179, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Michalicha, A.; Roguska, A.; Przekora, A.; Budzyńska, B.; Belcarz, A. Poly(levodopa)-modified β-glucan as a candidate for wound dressings. Carbohydr. Polym. 2021, 272, 118485. [Google Scholar] [CrossRef]
- Cheng, C.; Peng, X.; Xi, L.; Wan, C.; Shi, S.; Wang, Y.; Yu, X. An agar-polyvinyl alcohol hydrogel loaded with tannic acid with efficient hemostatic and antibacterial capacity for wound dressing. Food Funct. 2022, 13, 9622–9634. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, Z.; Correia, A.; Hasany, M.; Figueiredo, P.; Dobakhti, F.; Eskandari, M.R.; Hosseini, S.H.; Abiri, R.; Khorshid, S.; Hirvonen, J.; et al. A Hydrogen-Bonded Extracellular Matrix-Mimicking Bactericidal Hydrogel with Radical Scavenging and Hemostatic Function for pH-Responsive Wound Healing Acceleration. Adv. Healthc. Mater. 2021, 10, 2001122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Z.; Ke, Y.; Xia, Y.; Xu, X.; Liu, J.; Gong, Y.; Shi, Q.; Yin, J. An injectable serotonin-chondroitin sulfate hydrogel for bio-inspired hemostatic adhesives with high wound healing capability. Mater. Adv. 2021, 2, 5150–5159. [Google Scholar] [CrossRef]
- Michalicha, A.; Pałka, K.; Roguska, A.; Pisarek, M.; Belcarz, A. Polydopamine-coated curdlan hydrogel as a potential carrier of free amino group-containing molecules. Carbohydr. Polym. 2021, 256, 117524. [Google Scholar] [CrossRef]
- He, R.; Zhou, D.; Xiao, L.; Li, Y. Chlorella vulgaris Extract-Decorated Gold Nanoparticle Hybridized Antimicrobial Hydrogel as a Potential Dressing. Gels 2023, 9, 11. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Wang, Y.; Shi, Y.; Wang, F.; Lin, G. Research progress on mechanical properties and wear resistance of cartilage repair hydrogel. Mater. Des. 2022, 216, 110575. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Fabrication of hybrid nanofibers from biopolymers and poly (Vinyl alcohol)/poly (ε-caprolactone) for wound dressing applications. Polymers 2021, 13, 2104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; An, G.; Zhu, Y.; Liu, X.; Chen, Y.; Wu, H.; Wang, Y.; Shi, X.; Mao, C. 3D-printable self-healing and mechanically reinforced hydrogels with host-guest non-covalent interactions integrated into covalently linked networks. Mater. Horiz. 2019, 6, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, J.H.; Zhou, J.; Xu, F.; Zrínyi, M.; Dussault, P.H.; Osada, Y.; Chen, Y.M. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem. Soc. Rev. 2014, 43, 8114–8131. [Google Scholar] [CrossRef] [PubMed]
- Karvinen, J.; Kellomäki, M. Characterization of self-healing hydrogels for biomedical applications. Eur. Polym. J. 2022, 181, 111641. [Google Scholar] [CrossRef]
- Nasra, S.; Patel, M.; Shukla, H.; Bhatt, M.; Kumar, A. Functional hydrogel-based wound dressings: A review on biocompatibility and therapeutic efficacy. Life Sci. 2023, 334, 122232. [Google Scholar] [CrossRef]
- Zhang, S.; Ge, G.; Qin, Y.; Li, W.; Dong, J.; Mei, J.; Ma, R.; Zhang, X.; Bai, J.; Zhu, C.; et al. Recent advances in responsive hydrogels for diabetic wound healing. Mater. Today Bio 2023, 18, 100508. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, R.; Guo, B. Stimuli-responsive conductive hydrogels: Design, properties, and applications. Mater. Chem. Front. 2021, 5, 2092–2123. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple Stimuli-Responsive MXene-Based Hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing. Small 2022, 18, 2104368. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Nanomaterials-Based Wound Dressing for Advanced Management of Infected Wound. Antibiotics 2023, 12, 351. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.M.; Chang, Y.H. Water-induced shape memory behavior of poly (vinyl alcohol) and p-coumaric acid-modified water-soluble chitosan blended membrane. Carbohydr. Polym. 2021, 257, 117633. [Google Scholar] [CrossRef]
- Cui, Y.; Tan, M.; Zhu, A.; Guo, M. Mechanically strong and stretchable PEG-based supramolecular hydrogel with water-responsive shape-memory property. J. Mater. Chem. B 2014, 2, 2978–2982. [Google Scholar] [CrossRef]
- Gu, L.; Jiang, Y.; Hu, J. Bioinspired poly(vinyl alcohol)-silk hybrids: Two-way water-sensitive shape-memory materials. Mater. Today Commun. 2018, 17, 419–426. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Biswal, A.K.; Chang, Y.H.; Misra, P.K.; Yang, J.M. Synthesis and Characterization of Modified Poly(vinyl alcohol) Membrane and Study of Its Enhanced Water-Induced Shape-Memory Behavior. J. Polym. Environ. 2022, 30, 3409–3419. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Liu, K.; Ji, X.; Fatehi, P.; Chen, J. A cellulose nanofibril-reinforced hydrogel with robust mechanical, self-healing, pH-responsive and antibacterial characteristics for wound dressing applications. J. Nanobiotechnol. 2022, 20, 312. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Li, Q.; Wu, J. A novel hydrogel with glucose-responsive hyperglycemia regulation and antioxidant activity for enhanced diabetic wound repair. Nano Res. 2022, 15, 5305–5315. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Q.; Chen, Q.; Ma, Y.; Wang, Z.; Luo, L.; Ding, Q.; Li, H.; Tang, S. Carboxymethyl Chitosan/Tannic Acid Hydrogel with Antibacterial, Hemostasis, and Antioxidant Properties Promoting Skin Wound Repair. ACS Biomater. Sci. Eng. 2023, 9, 437–448. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Lopez, M.; Muras, A.; Silva, D.; Serro, A.P.; Otero, A.; Concheiro, A.; Alvarez-Lorenzo, C. Resveratrol-loaded hydrogel contact lenses with antioxidant and antibiofilm performance. Pharmaceutics 2021, 13, 532. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. View 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Bock, N.; Dargaville, B.L.; Hutmacher, D.W. Deciphering the molecular mechanism of water interaction with gelatin methacryloyl hydrogels: Role of ionic strength, ph, drug loading and hydrogel network characteristics. Biomedicines 2021, 9, 574. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Feng, Y.; Taraban, M.; Yu, Y.B. The effect of ionic strength on the mechanical, structural and transport properties of peptide hydrogels. Soft Matter 2012, 8, 11723–11731. [Google Scholar] [CrossRef]
- Cui, T.; Yu, J.; Wang, C.F.; Chen, S.; Li, Q.; Guo, K.; Qing, R.; Wang, G.; Ren, J. Micro-Gel Ensembles for Accelerated Healing of Chronic Wound via pH Regulation. Adv. Sci. 2022, 9, 2201254. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Jiang, H.; Ochoa, M.; Waimin, J.F.; Rahimi, R.; Ziaie, B. A pH-regulated drug delivery dermal patch for targeting infected regions in chronic wounds. Lab Chip 2019, 19, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Campo Dall′ Orto, V.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Yuan, M.; Liu, L.; Zhang, K.; Zhao, B.; He, B.; Liang, Y.; Li, F. pH-Responsive wound dressings: Advances and prospects. Nanoscale Horiz. 2023, 8, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Iqbal, I.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Jabeen, F.; Mohamad, M.R.; Jusoh, N. Development of antibacterial, degradable and ph-responsive chitosan/guar gum/polyvinyl alcohol blended hydrogels for wound dressing. Molecules 2021, 26, 5937. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elbarbary, A.M.; Hegazy, D.E.; Maziad, N.A. Radiation synthesis of pH-sensitive 2-(dimethylamino)ethyl methacrylate/ polyethylene oxide/ZnS nanocomposite hydrogel membrane for wound dressing application. J. Drug Deliv. Sci. Technol. 2022, 73, 103399. [Google Scholar] [CrossRef]
- Fan, X.; Yang, L.; Wang, T.; Sun, T.; Lu, S. pH-responsive cellulose-based dual drug-loaded hydrogel for wound dressing. Eur. Polym. J. 2019, 121, 109290. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Wu, J.; Wang, S.; Liu, X.; Huang, M.; Xu, P. Preparation, antibacterial activity and pH-responsive release behavior of silver sulfadiazine loaded bacterial cellulose for wound dressing applications. J. Taiwan Inst. Chem. Eng. 2016, 63, 404–410. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Khatoon, F. In Vitro Study of Temperature and pH-Responsive Gentamycin Sulphate-Loaded Chitosan-Based Hydrogel Films for Wound Dressing Applications. Polym.-Plast. Technol. Eng. 2015, 54, 573–580. [Google Scholar] [CrossRef]
- Ding, C.; Tian, M.; Feng, R.; Dang, Y.; Zhang, M. Novel Self-Healing Hydrogel with Injectable, pH-Responsive, Strain-Sensitive, Promoting Wound-Healing, and Hemostatic Properties Based on Collagen and Chitosan. ACS Biomater. Sci. Eng. 2020, 6, 3855–3867. [Google Scholar] [CrossRef]
- Morey, M.; Pandit, A. Responsive triggering systems for delivery in chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds. RSC Adv. 2014, 4, 51528–51536. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Z.; Yu, Q.; Ma, T. Preparation of reactive oxygen species-responsive antibacterial hydrogels for efficient anti-infection therapy. Mater. Lett. 2020, 263, 127254. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Han, L. Recent developments in stimuli-responsive hydrogels for biomedical applications. Biosurf. Biotribol. 2022, 8, 290–306. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Gu, Y.; Pinho, R.A. Exercise cuts both ways with ROS in remodifying innate and adaptive responses: Rewiring the redox mechanism of the immune system during exercise. Antioxidants 2021, 10, 1846. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual redox responsive assemblies formed from diselenide block copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. [Google Scholar] [CrossRef]
- Pan, W.; Qi, X.; Xiang, Y.; You, S.; Cai, E.; Gao, T.; Tong, X.; Hu, R.; Shen, J.; Deng, H. Facile formation of injectable quaternized chitosan/tannic acid hydrogels with antibacterial and ROS scavenging capabilities for diabetic wound healing. Int. J. Biol. Macromol. 2022, 195, 190–197. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Liu, X.; Chen, Y.; Duan, S.; Zhu, R.; Liu, Y.; Yin, L. Recent Advances on Reactive Oxygen Species-Responsive Delivery and Diagnosis System. Biomacromolecules 2019, 20, 2441–2463. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Esfahani, H.S.; Zandsalimi, K.; Shahidi, F.K.; Abrahamse, H. Accelerating skin regeneration and wound healing by controlled ROS from photodynamic treatment. Inflamm. Regen. 2022, 42, 40. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.M.; Barrias, C.C.; Gomes, P.; Martins, M.C.L. Smart biomaterial-based systems for intrinsic stimuli-responsive chronic wound management. Mater. Today Chem. 2021, 22, 100623. [Google Scholar] [CrossRef]
- Fierheller, M.; Sibbald, R.G. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv. Skin Wound Care 2010, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Said, S.S.; Campbell, S.; Hoare, T. Externally Addressable Smart Drug Delivery Vehicles: Current Technologies and Future Directions. Chem. Mater. 2019, 31, 4971–4989. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.Q.; Li, Z.B.; Wu, Y.L.; Li, D.W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Huang, H.Y.; Lu, Y.C.; Cheng, C.J.; Lee, T.M. Development of a flexible film made of polyvinyl alcohol with chitosan based thermosensitive hydrogel. J. Dent. Sci. 2023, 18, 822–832. [Google Scholar] [CrossRef]
- Lin, X.; Guan, X.; Wu, Y.; Zhuang, S.; Wu, Y.; Du, L.; Zhao, J.; Rong, J.; Zhao, J.; Tu, M. An alginate/poly(N-isopropylacrylamide)-based composite hydrogel dressing with stepwise delivery of drug and growth factor for wound repair. Mater. Sci. Eng. C 2020, 115, 111123. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L.; Kan, C. Thermoresponsive hydrogels and their biomedical applications: Special insight into their applications in textile based transdermal therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Juang, R.S.; Wang, K.S.; Cheng, Y.W.; Wu, W.E.; Lin, Y.H.; Jeng, R.J.; Huang, L.Y.; Yang, M.C.; Liu, S.H.; Liu, T.Y. Intelligent and thermo-responsive Au-pluronic® F127 nanocapsules for Raman-enhancing detection of biomolecules. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-responsive hydrogels: From recent progress to biomedical applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Anis, A.; Kim, D.; Pal, K. Environment sensitive hydrogels for drug delivery applications. Eur. Polym. J. 2019, 120, 109220. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, G. NIR light-responsive nanocarriers for controlled release. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100420. [Google Scholar] [CrossRef]
- Psarrou, M.; Mitraki, A.; Vamvakaki, M.; Kokotidou, C. Stimuli-Responsive Polysaccharide Hydrogels and Their Composites for Wound Healing Applications. Polymers 2023, 15, 986. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Lin, Q.; Cheng, J.; Lin, F.; Tang, L.; Huang, B.; Lu, B. Polydopamine-coated cellulose nanocrystal as functional filler to fabricate nanocomposite hydrogel with controllable performance in response to near-infrared light. Cellulose 2021, 28, 2255–2271. [Google Scholar] [CrossRef]
- Eells, J.T.; Gopalakrishnan, S.; Valter, K. Near-infrared photobiomodulation in retinal injury and disease. Adv. Exp. Med. Biol. 2016, 854, 437–441. [Google Scholar]
- Qiu, H.; Tan, M.; Ohulchanskyy, T.Y.; Lovell, J.F.; Chen, G. Recent progress in upconversion photodynamic therapy. Nanomaterials 2018, 8, 344. [Google Scholar] [CrossRef]
- Wu, S.; Butt, H.J. Near-Infrared-Sensitive Materials Based on Upconverting Nanoparticles. Adv. Mater. 2016, 28, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, J.; Deng, H.; Kan, H.; Xu, C.; Dong, K. Skin-permissible NIR-actuated hyperthermia using a photothermally responsive hydrogel membrane for the effective treatment of antibiotic-resistant bacterial infection. Biomater. Sci. 2022, 10, 960–969. [Google Scholar] [CrossRef]
- Han, Q.; Lau, J.W.; Do, T.C.; Zhang, Z.; Xing, B. Near-Infrared Light Brightens Bacterial Disinfection: Recent Progress and Perspectives. ACS Appl. Bio Mater. 2021, 4, 3937–3961. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Du, S.; Huang, Q.; Mo, M.; Gao, Y.; Li, M.; Tao, J.; Zhang, L.; Zhu, J. Photonic Hydrogels for Synergistic Visual Bacterial Detection and On-Site Photothermal Disinfection. ACS Appl. Mater. Interfaces 2022, 14, 5856–5866. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sheng, H.; Cao, D.; Zhang, F.; Zhang, W.; Yan, F.; Ding, D.; Cheng, N. S-nitrosoglutathione functionalized polydopamine nanoparticles incorporated into chitosan/gelatin hydrogel films with NIR-controlled photothermal/NO-releasing therapy for enhanced wound healing. Int. J. Biol. Macromol. 2022, 200, 77–86. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Q.; Cheng, H.; Yu, Q.; Zhao, W.; Zhao, C. Dually-Thermoresponsive Hydrogel with Shape Adaptability and Synergetic Bacterial Elimination in the Full Course of Wound Healing. Adv. Healthc. Mater. 2022, 11, e2201049. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, S.; Coy, E.; Li, S.; Załęski, K.; Zhang, Y.; Pan, H.; Wang, G. NIR-Responsive TiO2 Biometasurfaces: Toward In Situ Photodynamic Antibacterial Therapy for Biomedical Implants. Adv. Mater. 2022, 34, 2106314. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.D.; Miguel, S.P.; Mendonça, A.G.; Correia, I.J. Injectable in situ forming thermo-responsive graphene based hydrogels for cancer chemo-photothermal therapy and NIR light-enhanced antibacterial applications. Mater. Sci. Eng. C 2020, 117, 111294. [Google Scholar] [CrossRef]
- Ma, T.; Zhai, X.; Jin, M.; Huang, Y.; Zhang, M.; Pan, H.; Zhao, X.; Du, Y. Multifunctional wound dressing for highly efficient treatment of chronic diabetic wounds. View 2022, 3, 20220045. [Google Scholar] [CrossRef]
- Koyuncu, A.; Koç, S.; Akdere, Ö.E.; Çakmak, A.S.; Gümüşderelioğlu, M. Investigation of the synergistic effect of platelet-rich plasma and polychromatic light on human dermal fibroblasts seeded chitosan/gelatin scaffolds for wound healing. J. Photochem. Photobiol. B Biol. 2022, 232, 112476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, L.; Wei, C.; Guo, R. A bioactive dextran-based hydrogel promote the healing of infected wounds via antibacterial and immunomodulatory. Carbohydr. Polym. 2022, 291, 119558. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.J.; Li, L.F.; Hao, R.N.; Gong, M.; Wang, T.; Song, J.; Meng, Q.H.; Zhao, N.N.; Xu, F.J.; Lvov, Y.; et al. Phase-change composite filled natural nanotubes in hydrogel promote wound healing under photothermally triggered drug release. Bioact. Mater. 2023, 21, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Liu, X.; Wang, Y.; Li, X.; Shi, J. Photothermal-modulated drug release from a composite hydrogel based on silk fibroin and sodium alginate. Eur. Polym. J. 2021, 146, 110267. [Google Scholar] [CrossRef]
- Moorcroft, S.C.T.; Roach, L.; Jayne, D.G.; Ong, Z.Y.; Ong, Z.Y.; Evans, S.D. Nanoparticle-Loaded Hydrogel for the Light-Activated Release and Photothermal Enhancement of Antimicrobial Peptides. ACS Appl. Mater. Interfaces 2020, 12, 24544–24554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q.; et al. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2020, 382, 122990. [Google Scholar] [CrossRef]
- Huang, S.; Liu, H.; Liao, K.; Hu, Q.; Guo, R.; Deng, K. Functionalized GO Nanovehicles with Nitric Oxide Release and Photothermal Activity-Based Hydrogels for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 28952–28964. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, S.; Li, X.; Wang, X. Smart MXene/agarose hydrogel with photothermal property for controlled drug release. Int. J. Biol. Macromol. 2021, 190, 693–699. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B. Physical Double-Network Hydrogel Adhesives with Rapid Shape Adaptability, Fast Self-Healing, Antioxidant and NIR/pH Stimulus-Responsiveness for Multidrug-Resistant Bacterial Infection and Removable Wound Dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. J. Mater. Chem. B 2020, 8, 3908–3917. [Google Scholar] [CrossRef]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Dai, C.; Fan, L.; Jiang, Y.; Liu, C.; Zhou, Z.; Guan, P.; Tian, Y.; Xing, J.; Li, X.; et al. Injectable Self-Healing Natural Biopolymer-Based Hydrogel Adhesive with Thermoresponsive Reversible Adhesion for Minimally Invasive Surgery. Adv. Funct. Mater. 2021, 31, 2007457. [Google Scholar] [CrossRef]
- Zheng, Z.; Bian, S.; Li, Z.; Zhang, Z.; Liu, Y.; Zhai, X.; Pan, H.; Zhao, X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Guo, Q.; Ji, F.; Tian, X.; Cui, J.; Song, Y.; Sun, H.; Li, J.; Yao, F. Thermoresponsive polysaccharide-based composite hydrogel with antibacterial and healing-promoting activities for preventing recurrent adhesion after adhesiolysis. Acta Biomater. 2018, 74, 439–453. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, F.; Tang, L.; Wu, H.; Ni, Y.; Chen, L.; Huang, L.; Hu, X.; Lin, S.; Ding, C. Super-ductile, injectable, fast self-healing collagen-based hydrogels with multi-responsive and accelerated wound-repair properties. Chem. Eng. J. 2021, 405, 126756. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Pepelyshev, A.; Borodich, F.M.; Galanov, B.A.; Gorb, E.V.; Gorb, S.N. Adhesion of Soft Materials to Rough Surfaces: Experimental Studies, Statistical Analysis and Modelling. Coatings 2018, 8, 350. [Google Scholar] [CrossRef]
- Azeera, M.; Vaidevi, S.; Ruckmani, K. Characterization Techniques of Hydrogel and Its Applications. Polym. Polym. Compos. A Ref. Ser. 2019, 737–761. [Google Scholar] [CrossRef]
- Ni, Z.; Yu, H.; Wang, L.; Huang, Y.; Lu, H.; Zhou, H.; Liu, Q. Multistage ROS-Responsive and Natural Polyphenol-Driven Prodrug Hydrogels for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 52643–52658. [Google Scholar] [CrossRef]
- Guo, S.; Yao, M.; Zhang, D.; He, Y.; Chang, R.; Ren, Y.; Guan, F. One-Step Synthesis of Multifunctional Chitosan Hydrogel for Full-Thickness Wound Closure and Healing. Adv. Healthc. Mater. 2022, 11, 2101808. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Li, P.; Zhang, Y.; Lv, Y.; Wen, F.; Su, W. Polydopamine/tannic acid/chitosan/poloxamer 407/188 thermosensitive hydrogel for antibacterial and wound healing. Carbohydr. Polym. 2023, 302, 120349. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; She, W.; Luo, Y.; He, D.; Chen, J.; Ning, N.; Yu, Y.; De Beer, S.; Zhang, S. One-pot, self-catalyzed synthesis of self-adherent hydrogels for photo-thermal, antimicrobial wound treatment. J. Mater. Chem. B 2021, 9, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Torchio, A.; Ciardelli, G.; Boffito, M. In Situ Forming Bioartificial Hydrogels with ROS Scavenging Capability Induced by Gallic Acid Release with Potential in Chronic Skin Wound Treatment. Gels 2023, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Jongprasitkul, H.; Parihar, V.S.; Turunen, S.; Kellomäki, M. pH-Responsive Gallol-Functionalized Hyaluronic Acid-Based Tissue Adhesive Hydrogels for Injection and Three-Dimensional Bioprinting. ACS Appl. Mater. Interfaces 2023, 15, 33972–33984. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhong, Y.; Jiang, X. Thermosensitive and pH-responsive tannin-containing hydroxypropyl chitin hydrogel with long-lasting antibacterial activity for wound healing. Carbohydr. Polym. 2020, 236, 116096. [Google Scholar] [CrossRef]
- Al-Arjan, W.S.; Khan, M.U.A.; Almutairi, H.H.; Alharbi, S.M.; Razak, S.I.A. pH-Responsive PVA/BC-f-GO Dressing Materials for Burn and Chronic Wound Healing with Curcumin Release Kinetics. Polymers 2022, 14, 1949. [Google Scholar] [CrossRef]
- Lee, P.Y.; Li, Z.; Huang, L. Thermosensitive Hydrogel as a Tgf-β1 Gene Delivery Vehicle Enhances Diabetic Wound Healing. Pharm. Res. 2003, 20, 1995–2000. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Liu, H.; Ren, M.; Wang, Z.; Wang, X.; Liu, H.; Feng, Y.; Lin, Q.; Wang, C.; et al. pH-responsive hydrogel loaded with insulin as a bioactive dressing for enhancing diabetic wound healing. Mater. Des. 2021, 210, 110104. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Wang, S.; Dong, C.; Zhang, X.; Zhang, R.; Yang, L. Dextran and peptide-based pH-sensitive hydrogel boosts healing process in multidrug-resistant bacteria-infected wounds. Carbohydr. Polym. 2022, 278, 118994. [Google Scholar] [CrossRef]
- Kraehenbuehl, T.P.; Ferreira, L.S.; Zammaretti, P.; Hubbell, J.A.; Langer, R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials 2009, 30, 4318–4324. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, T.P.; Ferreira, L.S.; Hayward, A.M.; Nahrendorf, M.; van der Vlies, A.J.; Vasile, E.; Weissleder, R.; Langer, R.; Hubbell, J.A. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials 2011, 32, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, K.; He, Y.; Tao, B.; Li, K.; Lin, C.; Hu, J.; Wu, J.; Wu, Y.; Liu, S.; et al. ROS-responsive hydrogel coating modified titanium promotes vascularization and osteointegration of bone defects by orchestrating immunomodulation. Biomaterials 2022, 287, 121683. [Google Scholar] [CrossRef] [PubMed]
- Srikhao, N.; Theerakulpisut, S.; Chindaprasirt, P.; Okhawilai, M.; Narain, R.; Kasemsiri, P. Green synthesis of nano silver-embedded carboxymethyl starch waste/poly vinyl alcohol hydrogel with photothermal sterilization and pH-responsive behavior. Int. J. Biol. Macromol. 2023, 242, 125118. [Google Scholar] [CrossRef] [PubMed]
- Haidari, H.; Kopecki, Z.; Sutton, A.T.; Garg, S.; Cowin, A.J.; Vasilev, K. pH-Responsive “Smart” Hydrogel for Controlled Delivery of Silver Nanoparticles to Infected Wounds. Antibiotics 2021, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Haidari, H.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Bacteria-Activated Dual pH- and Temperature-Responsive Hydrogel for Targeted Elimination of Infection and Improved Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 51744–51762. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Xiao, Z.; Cao, J.; Wei, L.; Li, C.; Jiao, J.; Song, Z.; Liu, J.; Du, X.; Wang, S. NIR-activated multi-hit therapeutic Ag2S quantum dot-based hydrogel for healing of bacteria-infected wounds. Acta Biomater. 2022, 145, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhang, Z.; Xue, J.; Shang, J.; Ding, D.; Zhang, W.; Liu, Z.; Yan, F.; Cheng, N. Hybrid Ag nanoparticles/polyoxometalate-polydopamine nano-flowers loaded chitosan/gelatin hydrogel scaffolds with synergistic photothermal/chemodynamic/Ag+ anti-bacterial action for accelerated wound healing. Int. J. Biol. Macromol. 2022, 221, 135–148. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Chen, F.; Shi, J.; Song, P.; Feng, F.; Cui, J.; Zhang, J.; Ma, X.; Shen, J. Flexible wood-based pH-responsive hydrogel excipient for rapid recovery of infected wounds. React. Funct. Polym. 2023, 192, 105707. [Google Scholar] [CrossRef]
- Rezaei, F.; Damoogh, S.; Reis, R.L.; Kundu, S.C.; Mottaghitalab, F.; Farokhi, M. Dual drug delivery system based on pH-sensitive silk fibroin/alginate nanoparticles entrapped in PNIPAM hydrogel for treating severe infected burn wound. Biofabrication 2020, 13, 015005. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Niyompanich, J.; Chuysinuan, P.; Pavasant, P.; Supaphol, P. Development of thermoresponsive poloxamer in situ gel loaded with gentamicin sulfate for cavity wounds. J. Polym. Res. 2021, 28, 128. [Google Scholar] [CrossRef]
- Kang, W.; Liang, J.; Liu, T.; Long, H.; Huang, L.; Shi, Q.; Zhang, J.; Deng, S.; Tan, S. Preparation of silane-dispersed graphene crosslinked vinyl carboxymethyl chitosan temperature-responsive hydrogel with antibacterial properties. Int. J. Biol. Macromol. 2022, 200, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, W.; Liu, J.; Han, Y.; Yan, Q.; Dong, Y.; Liu, X.; Yang, D.; Ma, G.; Cao, H. A novel sprayable thermosensitive hydrogel coupled with zinc modified metformin promotes the healing of skin wound. Bioact. Mater. 2023, 20, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, H.; Li, S.; Huang, L.; Zhang, R.; Zhang, L.; Yu, A.; Duan, B. Polyphenol-driving assembly for constructing chitin-polyphenol-metal hydrogel as wound dressing. Carbohydr. Polym. 2022, 290, 119444. [Google Scholar] [CrossRef]
- Patil, N.; Jérôme, C.; Detrembleur, C. Recent advances in the synthesis of catechol-derived (bio)polymers for applications in energy storage and environment. Prog. Polym. Sci. 2018, 82, 34–91. [Google Scholar] [CrossRef]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Qiao, Z.; Lv, X.; He, S.; Bai, S.; Liu, X.; Hou, L.; He, J.; Tong, D.; Ruan, R.; Zhang, J.; et al. A mussel-inspired supramolecular hydrogel with robust tissue anchor for rapid hemostasis of arterial and visceral bleedings. Bioact. Mater. 2021, 6, 2829–2840. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef]
- Baldwin, A.; Booth, B.W. Biomedical applications of tannic acid. J. Biomater. Appl. 2022, 36, 1503–1523. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials-A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Abune, L.; Wang, Y. Affinity Hydrogels for Protein Delivery. Trends Pharmacol. Sci. 2021, 42, 300. [Google Scholar] [CrossRef] [PubMed]
- Malta, R.; Marques, A.C.; da Costa, P.C.; Amaral, M.H. Stimuli-Responsive Hydrogels for Protein Delivery. Gels 2023, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, J.; Zeng, P.; Chen, Y.; Xu, H.; Lu, J.R. High cell selectivity and low-level antibacterial resistance of designed amphiphilic peptide G(IIKK)3I-NH2. ACS Appl. Mater. Interfaces 2014, 6, 16529–16536. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible Thermoresponsive Peptide-PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Das, S.; Das, D. Rational Design of Peptide-based Smart Hydrogels for Therapeutic Applications. Front. Chem. 2021, 9, 770102. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Tian, Y.; Yin, Q.; Cheng, X.; Lian, M.; Zhou, B.; Zhang, X.; Yang, L. Efficacy of antimicrobial peptide DP7, designed by machine-learning method, against methicillin-resistant staphylococcus aureus. Front. Microbiol. 2019, 10, 452678. [Google Scholar] [CrossRef]
- Kumar, A.; Mahajan, M.; Awasthi, B.; Tandon, A.; Harioudh, M.K.; Shree, S.; Singh, P.; Shukla, P.K.; Ramachandran, R.; Mitra, K.; et al. Piscidin-1-analogs with double L- and D-lysine residues exhibited different conformations in lipopolysaccharide but comparable anti-endotoxin activities. Sci. Rep. 2017, 7, 39925. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, N.; Hamidabadi, H.G.; Khosravimelal, S.; Zahiri, M.; Ahovan, Z.A.; Bojnordi, M.N.; Eftekhari, B.S.; Hashemi, A.; Ganji, F.; Darabi, S.; et al. Antimicrobial peptides-loaded smart chitosan hydrogel: Release behavior and antibacterial potential against antibiotic resistant clinical isolates. Int. J. Biol. Macromol. 2020, 164, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 235805. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.; Müller, C.S.G.; Otto, A.M.; Netzker, R.; Hannappel, E. β-Thymosins, small acidic peptides with multiple functions. Int. J. Biochem. Cell Biol. 2001, 33, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Malinda, K.M.; Goldstein, A.L.; Kueinman, H.K. Thymosin β4 stimulates directional migration of human umbilical vein endothelial cells. FASEB J. 1997, 11, 474–481. [Google Scholar] [CrossRef]
- Malinda, K.M.; Sidhu, G.S.; Mani, H.; Banaudha, K.; Maheshwari, R.K.; Goldstein, A.L.; Kleinman, H.K. Thymosin β4 Accelerates Wound Healing. J. Investig. Dermatol. 1999, 113, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Reyes-Ordillo, K.; Cheng, Y.; Varatharajalu, R.; Ibrahim, J.; Lakshman, M.R. Thymosin β 4 prevents oxidative stress, inflammation, and fibrosis in ethanol- and lps-induced liver injury in mice. Oxid. Med. Cell. Longev. 2018, 2018, 9630175. [Google Scholar] [CrossRef]
- Sosne, G.; Szliter, E.A.; Barrett, R.; Kernacki, K.A.; Kleinman, H.; Hazlett, L.D. Thymosin Beta 4 Promotes Corneal Wound Healing and Decreases Inflammation in Vivo Following Alkali Injury. Exp. Eye Res. 2002, 74, 293–299. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Yeaman, M.R.; Selsted, M.E. Antimicrobial Peptides from Human Platelets. Infect. Immun. 2002, 70, 6524. [Google Scholar] [CrossRef]
- Xing, Y.; Ye, Y.; Zuo, H.; Li, Y. Progress on the Function and Application of Thymosin β4. Front. Endocrinol. 2021, 12, 767785. [Google Scholar] [CrossRef] [PubMed]
- Treadwell, T.; Kleinman, H.K.; Crockford, D.; Hardy, M.A.; Guarnera, G.T.; Goldstein, A.L. The regenerative peptide thymosin β4 accelerates the rate of dermal healing in preclinical animal models and in patients. Ann. N. Y. Acad. Sci. 2012, 1270, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, J. Thymosin β4 has a major role in dermal burn wound healing that involves actin cytoskeletal remodelling via heat-shock protein 70. J. Tissue Eng. Regen. Med. 2017, 11, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, H.K.; Kulik, V.; Goldstein, A.L. Thymosin β4 and the anti-fibrotic switch. Int. Immunopharmacol. 2023, 115, 109628. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Shi, Y.; Wang, H.; Zhao, X.; Sun, Q.; Huang, Y.; Qi, T.; Lin, G. Ethosomal Gel for Improving Transdermal Delivery of Thymosin β-4. Int. J. Nanomed. 2019, 14, 9275. [Google Scholar] [CrossRef] [PubMed]
- Bock-Marquette, I.; Maar, K.; Maar, S.; Lippai, B.; Faskerti, G.; Gallyas, F.; Olson, E.N.; Srivastava, D. Thymosin beta-4 denotes new directions towards developing prosperous anti-aging regenerative therapies. Int. Immunopharmacol. 2023, 116, 109741. [Google Scholar] [CrossRef] [PubMed]
- Shaghiera, A.D.; Widiyanti, P.; Yusuf, H. Synthesis and Characterization of Injectable Hydrogels with Varying Collagen–Chitosan–Thymosin β4 Composition for Myocardial Infarction Therapy. J. Funct. Biomater. 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.L.Y.; Reis, L.A.; Momen, A.; Radisic, M. Controlled release of thymosin β4 from injected collagen-chitosan hydrogels promotes angiogenesis and prevents tissue loss after myocardial infarction. Regen. Med. 2012, 7, 523–533. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Y.; Peng, H.; Whittaker, A.K.; Li, Y.; Zhao, Q.; Wang, Y.; Zhu, S.; Wang, Z. Cellulose nanocrystals reinforced highly stretchable thermal-sensitive hydrogel with ultra-high drug loading. Carbohydr. Polym. 2021, 266, 118122. [Google Scholar] [CrossRef]
- Peng, Y.; He, D.; Ge, X.; Lu, Y.; Chai, Y.; Zhang, Y.; Mao, Z.; Luo, G.; Deng, J.; Zhang, Y. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioact. Mater. 2021, 6, 3109–3124. [Google Scholar] [CrossRef]
- Chitra, G.; Franklin, D.S.; Sudarsan, S.; Sakthivel, M.; Guhanathan, S. Noncytotoxic silver and gold nanocomposite hydrogels with enhanced antibacterial and wound healing applications. Polym. Eng. Sci. 2018, 58, 2133–2142. [Google Scholar] [CrossRef]
- Janpetch, N.; Saito, N.; Rujiravanit, R. Fabrication of bacterial cellulose-ZnO composite via solution plasma process for antibacterial applications. Carbohydr. Polym. 2016, 148, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.; Wächter, J.; Windbergs, M. Therapy of infected wounds: Overcoming clinical challenges by advanced drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 1545–1567. [Google Scholar] [CrossRef] [PubMed]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Thirupathi, A.; Corrêa, M.E.A.B.; Gu, Y.; Silveira, P.C.L. The Use of Metallic Nanoparticles in Wound Healing: New Perspectives. Int. J. Mol. Sci. 2022, 23, 15376. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Karponis, D.; Mosahebi, A.; Seifalian, A.M. Nanoparticles in wound healing; from hope to promise, from promise to routine. Front. Biosci.-Landmark 2018, 23, 1038–1059. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. A facile method to prepare size-tunable silver nanoparticles and its antibacterial mechanism. Adv. Powder Technol. 2018, 29, 407–415. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Chattopadhyay, P.; Islam, J.; Ray, S.; Raju, P.S.; Mazumder, B. Aspects of Nanomaterials in Wound Healing. Curr. Drug Deliv. 2018, 16, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.B. Mechanisms of Antibiotic Resistance. Compend. Contin. Educ. Pract. Vet. 2001, 23, 464–472. [Google Scholar] [CrossRef]
- Salisbury, A.M.; Woo, K.; Sarkar, S.; Schultz, G.; Malone, M.; Mayer, D.O.; Percival, S.L. Tolerance of Biofilms to Antimicrobials and Significance to Antibiotic Resistance in Wounds. Surg. Technol. Int. 2018, 33, 59–66. [Google Scholar]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef]


| Composition | Stimuli Response Agent | Stimuli Mechanism | Material’s Properties | Ref. |
|---|---|---|---|---|
| Dodecyl, chitosan, WS2 nanosheet, ciprofloxacin | WS2 nanosheets | WS2 nanosheets generated heat upon exposure to near-infrared (NIR) light → triggering the release of the antibiotic at the wound site | Injectable, self-adapting, and rapidly molding hydrogels with good tissue adherence and antibacterial potential | [163] |
| AuNPs, Pluronic® F127, hydroxypropyl methylcellulose (HPMC) | Pluronic® F127 | Stiff gel formation when temperature increased from 4 °C to 32–37 °C | Improved bioavailability, skin permeation, antibacterial and anti-inflammatory activity of the prepared AuNPs’ thermoresponsive gels, burn wound treatment potential | [164] |
| Gelatin and chondroitin sulfate | Chondroitin sulfate | Tissue adherence at 37 °C, diminished at low temperatures (20 °C), enabling it to detach effortlessly from the tissue | Injectable self-healing bioadhesive, underwater adhesive properties, tissue adhesive and sealant for the closure of bleeding wounds | [165] |
| Catechol-modified quaternized chitosan, poly(d,l-lactide)-poly(ethylene glycol)-poly(d,lalactide) (PLEL) | PLEL | The temperature-dependent transition of PLEL solution from a reversible sol at 25 °C to a gel at 37 °C | Injectable, thermo-sensitive adhesive hydrogel with promoting wound healing ability, biocompatibility, and bioactivity through in vivo degradation, stimulated endothelial cell migration, and angiogenesis | [166] |
| Galactose-modified xyloglucan (MxG) and hydroxybutyl chitosan (HBC) | Galactose-modified xyloglucan | Gelation temperature and time can be modulated via adjusting the MxG/HBC ratio | The composite hydrogel could effectively prevent repeated adhesion after adhesiolysis, promote wound healing, and reduce scar formation | [167] |
| Pluronics, hyaluronic acid, corn silk extract, and nanosilver | Pluronics, | The viscoelastic parameters varied within the temperature range of 25 to 40 °C | Hydrogel with antibacterial activity toward Gram-positive and Gram-negative bacteria | [168] |
| Collagen (COL), guar gum (GG), poly(N-isopropylacrylamide) (PNIPAM), graphene oxide (GO) | PNIPAM and GO | Phase transition after human body temperature contact; thermosensitive, NIR responsive | Hydrogel with fast self-healing properties, super-ductile, injectable, remoldable, conductive, and skin wound-healing acceleration properties | [169] |
| Hydrogel-Modified Substance | Main Characteristics of Modified Matrices | Refs. |
|---|---|---|
| Polyphenols | mechanical strength, structural integrity, adhesion, high elasticity, self-healing properties, hemostatic properties, antibacterial properties, antioxidant properties, anti-inflammatory properties | [70,92,123,173,174,175,176,177,178,179,180] |
| Peptides, polypeptides, and proteins | Biocompatibility, regeneration processes, stimulation, antibacterial properties. | [181,182,183,184,185,186] |
| Silver nanoparticles | Antibacterial properties, anti-inflammatory properties, stability, durability. | [15,168,187,188,189,190,191] |
| Antibiotics | Antibacterial properties | [192,193,194,195,196,197,198] |
| Hydrogel Composition | Stimuli | Effects | Ref. |
|---|---|---|---|
| Gelatin (Gel), tannic acid (TA) Gel/TA | pH | pH-dependent release of TA. | [70] |
| Phenylboric acid-modified polyphosphazene (PPBA), tannic acid (TA), poly(vinyl alcohol) PPBA-TA-PVA | ROS | ROS-dependent release of TA (scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals and OH radicals in vitro) ROS-responsive degradation. | [173] |
| Quaternized chitosan (QCS), tannic acid (TA) QCS/TA | ROS | Self-healing properties, free radical-scavenging activity due to TA presence. | [123] |
| Physical crosslinked quaternized chitosan (QCS), tannic acid (TA), ferric iron Fe(III) QCS/TA/Fe | NIR | Antibacterial activity induced by NIR-stimulated modified hydrogels. | [174] |
| Polydopamine (P), tannic acid (T), chitosan (C), poloxamer 407/188 (PP) PTCPP hydrogel | Temp. NIR | Sol–gel transition of liquid hydrogel formulation at around 30 °C, significant enhancement of hydrogel’s antibacterial activity after NIR irradiation. | [175] |
| Poly(acrylamide) (PAM), naturally derived chitosan (CS), tannic acid/ferric ion chelates (TA@Fe3+) PAM/CS/TA@Fe3+ | NIR | In vivo and in vitro antibacterial activity to prevent microbial infection after NIR stimulation. | [176] |
| Hyaluronic acid (HA), poly(ether urethane), (D-DHP407), gallic acid (GA), HA/D-DHP407-GA | ROS Temp. | Reduction in intracellular ROS level due to ROS-induced GA release, sol–gel transition of liquid hydrogel precursor in response to temperature changes (37 °C). | [177] |
| Gallic-acid-functionalized hyaluronic acid (HAGA), hyaluronic acid methacrylate (HAMA) HAGA/HAMA hydrogel | pH Temp. | Swelling under acidic conditions and stability at neutral and basic pH. Self-healing ability at 37 °C and increased hydrogel-to-tissue adhesion due to gallic acid presence. | [178] |
| Resveratrol (RSV), polyethylene glycol (PEG)- cellulose nanofibrils (CNF) (RPC) Poly(vinyl alcohol) (PVA) RPC+PVA+BORAX→ RPC/PB hydrogel | pH | pH-dependent resveratrol release. | [92] |
| Hydroxypropyl chitin (HPCH), tannic acid (TA), ferric ion (Fe) HPCHC/TA/Fe | pH Temp. | pH-dependent TA release, temperature-dependent gelation. | [179] |
| polyvinyl alcohol (PVA), Bacterial cellulose (BC), graphene oxide (GO), curcumin, bacterial cellulose-functionalized-graphene oxide PVA/BC-f-GO Crosslinker: tetraethyl orthosilicate (TEOS) | pH | pH-dependent curcumin release. | [180] |
| Hydrogel Composition | Stimuli | Effects | Ref. |
|---|---|---|---|
| PEG–PLGA–PEG triblock copolymer loaded with TGF-β1 polypeptide | Temp. | Temperature-initiatied re-epithelialization and collagen synthesis | [181] |
| N-carboxyethyl chitosan, hyaluronic acid–aldehyde, adipic acid dihydrazide, insulin | pH | pH-responsive insulin release | [182] |
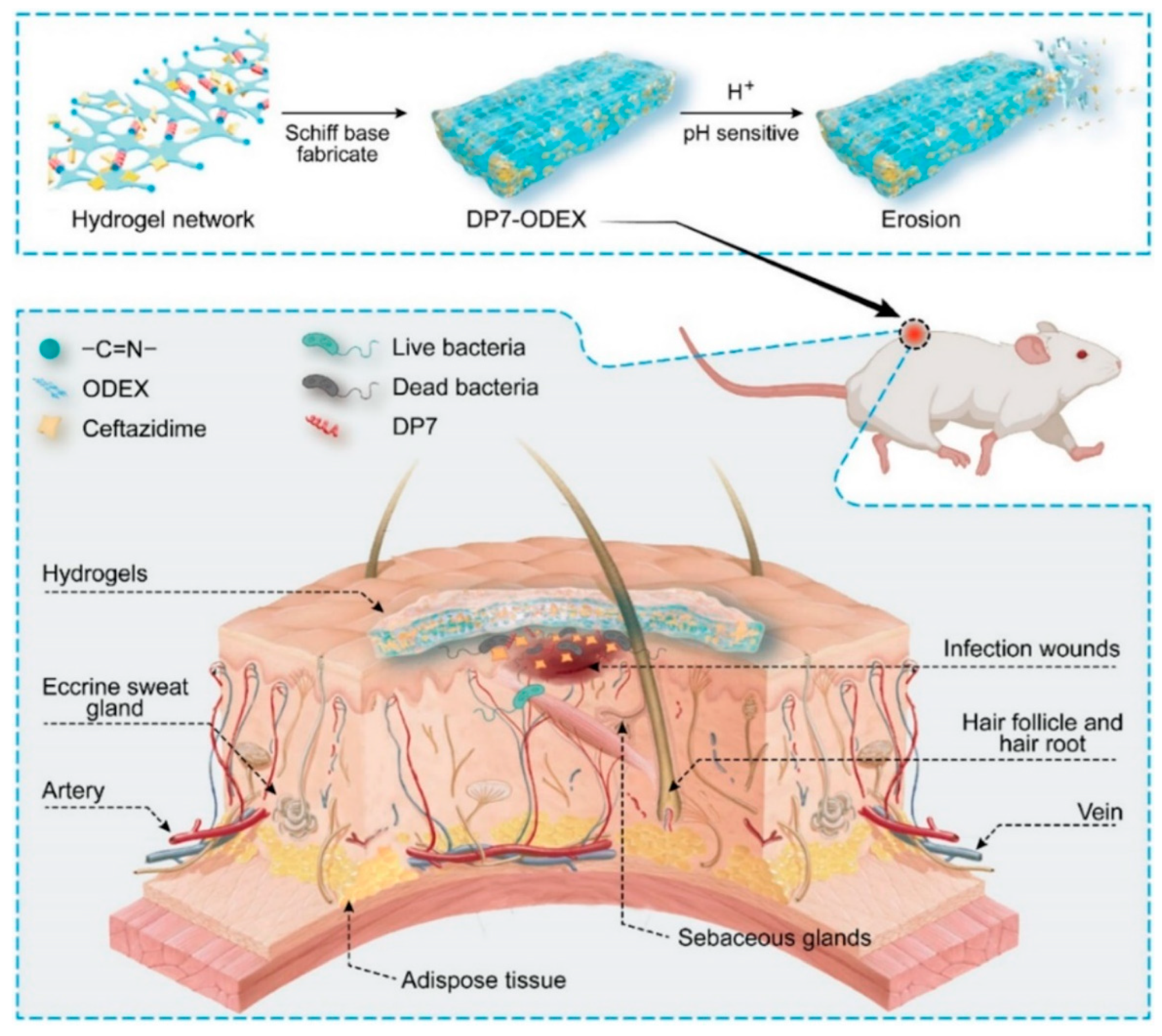
| oxidized dextran, antimicrobial peptide DP7, ceftazidime | pH | pH-sensitive hydrogel erosion accelerating the release rate of the drugs | [183] |
| PEG-based Tβ4-loaded hydrogels | MMPs | Enzymatic activity-dependent release of Tβ4 mediated by tissue metalloproteinases | [184] |
| PEG–vinylsulfone-based Tβ4-loded hydrogels | MMPs | Enzymatic activity-dependent release of Tβ4 mediated by tissue metalloproteinases | [185] |
| Tβ4@TNT–PDA/PVHA | ROS | ROS-dependent Tβ4 release by borate bonding cleavage | [186] |
| Hydrogel Composition | Stimuli | Effects | Ref. |
|---|---|---|---|
| Cassava starch modified by carboxymethylation (CMS), poly vinyl alcohol (PVA), CMS/PVA–H tannic acid (TA), Silver nanoparticles (AgNPs) H-AgNPs | NIR pH | NIR-stimulated antibacterial activity pH-responsive TA release | [187] |
| Methacrylic acid (mAA), acrylamide (AAm), N, N’-Methylenebisacrylamide (MBMa), poly(mAA-co-AAm) hydrogel Mercaptossucinic acid (MSA)-protected AgNPs (MSA–AgNPs) poly(mAA-co-AAm)–AgNPs | pH | pH-dependent AgNP release | [188] |
| N-isopropylacrylamide (Nipam)+acrylic acid (AAc)→ Pnipam AgNPs Pnipam–PAA–AgNPs | pH Temp. | pH-dependent AgNPs’ release Controlled release and delivery of AgNPs | [189] |
| N-isopropylacrylamide (NIPAAm), acrylamide (AAm), Ag2S quantum dots (Ag2SQDs) modified by mSiO2 mesoporous silica, (NP hydrogel), 3-(trimethoxylmethosilyl) propyl methacrylate (MPS), Ag2S QDs/mSiO2 NP–MPS | NIR | NIR laser-induced controlled release of silver ions (Ag+) | [190] |
| Pluronics F127 and F68, hyaluronic acid (HA), corn silk extract (CSE), AgNPs Pluronic/HA/CSE/Ag | Temp. | Temperature-dependent sol–gel transition | [168] |
| methylcellulose (MC), citric acid (CA), AgNPs MC/AgNPs nanocomposite hydrogels | Temp. pH | Temperature-induced changes in swelling rate and rheological properties pH-induced changes in swelling rate and rheological properties | [15] |
| Ag nanoparticles/phosphotungstic acid–polydopamine nano-flowers (AgNPs/POM–PDA), chitosan, gelatin, AgNPs/POM–PDA@ chitosan/gelatin | NIR | Ag+ release under NIR light irradiation | [191] |
| Hydrogel Composition | Stimuli | Effects | Ref. |
|---|---|---|---|
| N1-(4-boronobenzyl)-N3-(4-boronophenyl)-N1, N1, N3, N3-tetramethylpropane-1, 3-diaminium (TPA), poly(vinyl alcohol) (PVA) TPA + PVA = Hydrogel mupirocin (MP), granulocyte-macrophage colony-stimulating factor (GM-CSF), | ROS | ROS-responsive degradation | [192] |
| Triplochitin scleroxylon wood (TS), gentamicin (G), polyvinyl alcohol (PVA), chitosan (CS), FTS-G@PC Flexible wood-based hydrogel | pH | pH-responsive gentamicin release | [193] |
| Poly(N-isopropylacrylamide) (PNIPAM), epidermal growth factor (EGF), silk fibroin–sodium alginate, nanoparticles (SF–SANPs), Vancomycin (VANCO) PNIPAM and EGF/SF–SANPs | pH | pH-responsive vancomycin release | [194] |
| Hyaluronic acid (HA), boronic acid (BA), HA + BA = hydrogel micelle-loaded amikacin (AM), micelle-loaded naproxen (NAP), Hydrogel@AM&MIC Hydrogel@NAP&MIC | pH ROS | pH-dependent amikacin release ROS-responsive naproxen release | [195] |
| Poloxamer 188, solution of poloxamer 407, gentamicin | Temp. | Sol–gel transition at around 37 °C | [196] |
| Vinyl carboxymethyl chitosan (CG), graphene (GM), N-isopropylacrylamide (NIPAM), ciprofloxacin hydrochloride, NIPAM–CG/GM | Temp. | Temperature-dependent drug release | [197] |
| Pluronic F127 (PF127), complex of zinc and metformin, (ZnMet); ZnMet-PF127 | Temp. | Sol–gel transition at around 37 °C | [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalicha, A.; Belcarz, A.; Giannakoudakis, D.A.; Staniszewska, M.; Barczak, M. Designing Composite Stimuli-Responsive Hydrogels for Wound Healing Applications: The State-of-the-Art and Recent Discoveries. Materials 2024, 17, 278. https://doi.org/10.3390/ma17020278
Michalicha A, Belcarz A, Giannakoudakis DA, Staniszewska M, Barczak M. Designing Composite Stimuli-Responsive Hydrogels for Wound Healing Applications: The State-of-the-Art and Recent Discoveries. Materials. 2024; 17(2):278. https://doi.org/10.3390/ma17020278
Chicago/Turabian StyleMichalicha, Anna, Anna Belcarz, Dimitrios A. Giannakoudakis, Magdalena Staniszewska, and Mariusz Barczak. 2024. "Designing Composite Stimuli-Responsive Hydrogels for Wound Healing Applications: The State-of-the-Art and Recent Discoveries" Materials 17, no. 2: 278. https://doi.org/10.3390/ma17020278
APA StyleMichalicha, A., Belcarz, A., Giannakoudakis, D. A., Staniszewska, M., & Barczak, M. (2024). Designing Composite Stimuli-Responsive Hydrogels for Wound Healing Applications: The State-of-the-Art and Recent Discoveries. Materials, 17(2), 278. https://doi.org/10.3390/ma17020278









