Effect of N on the Microstructure and Wear Resistance of 4Cr13 Corrosion-Resistant Plastic Mold Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results
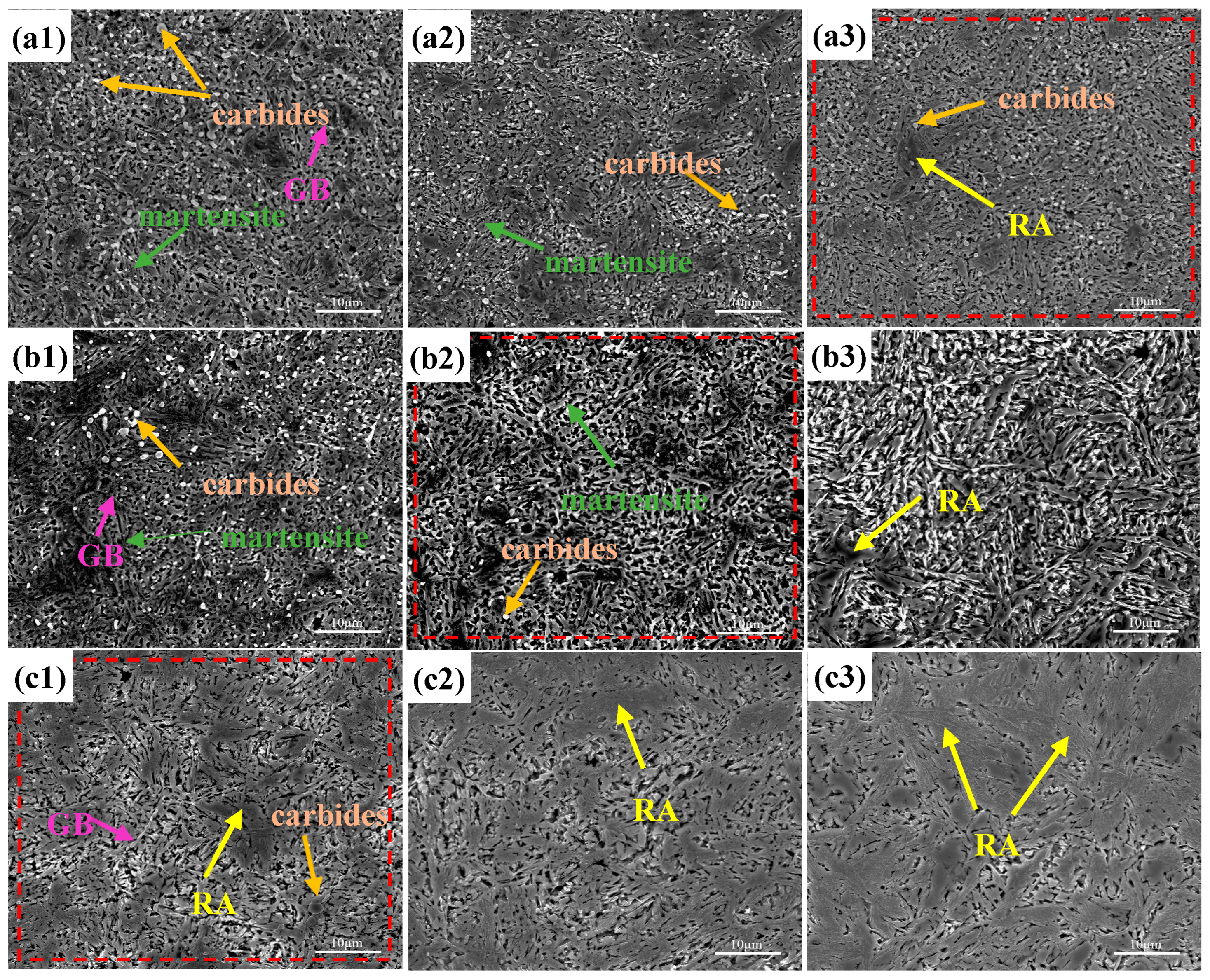
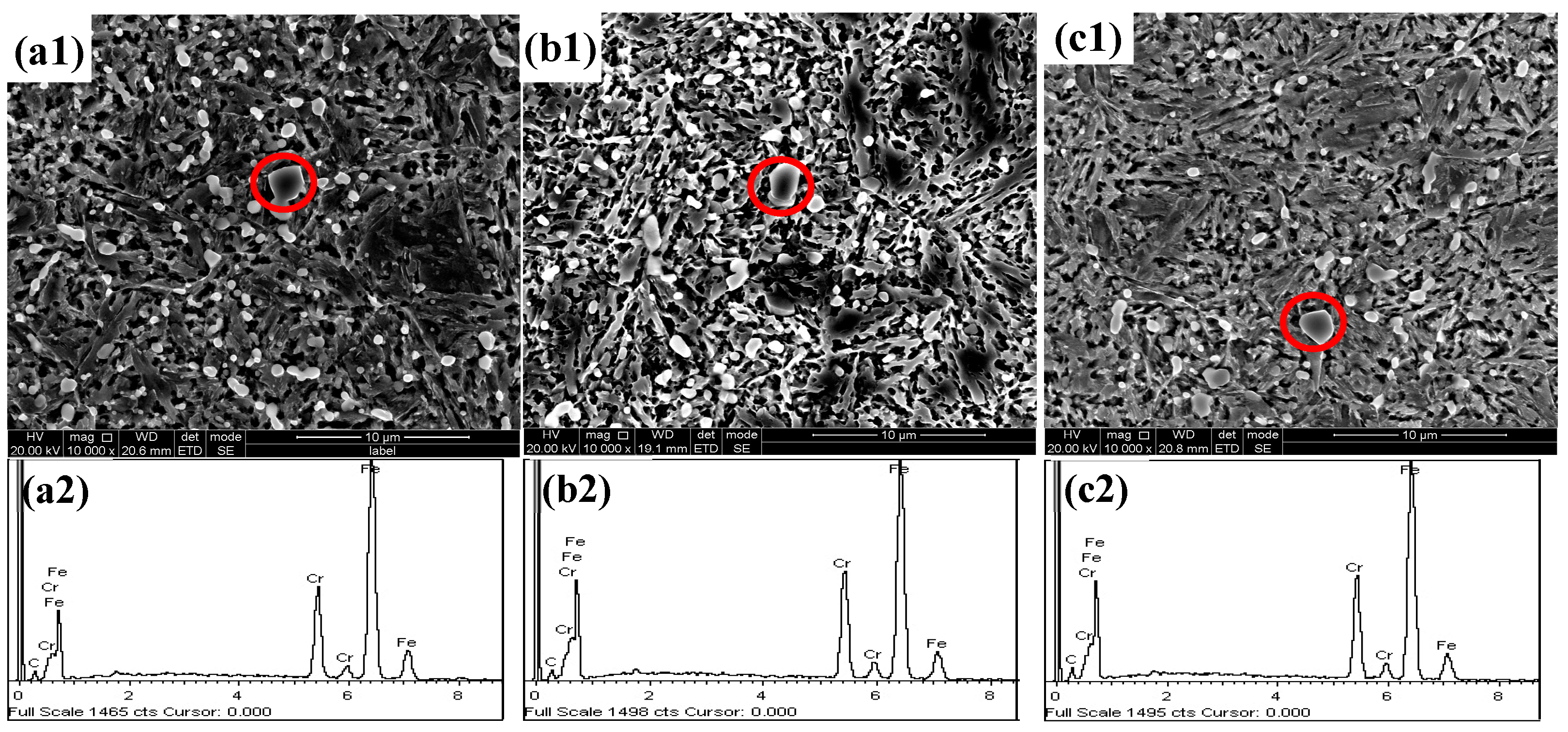
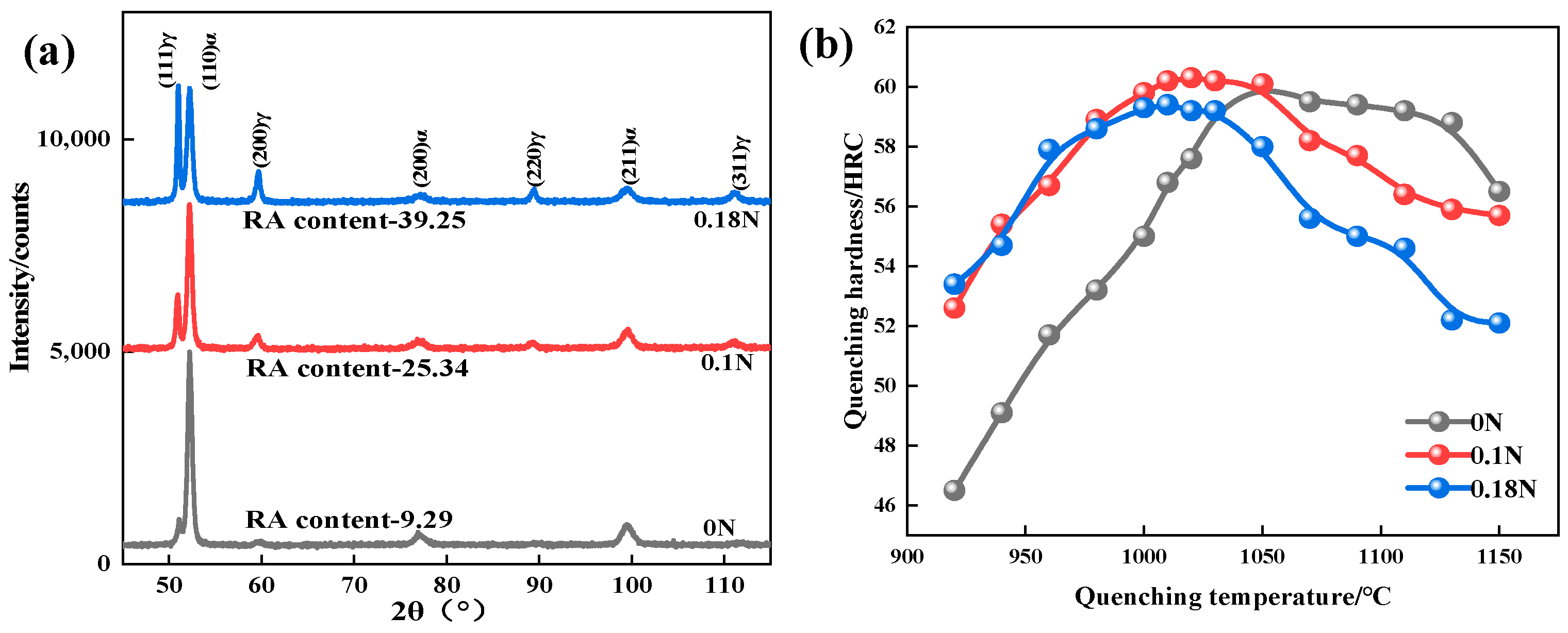
3.1. Quenching Microstructure and Hardness
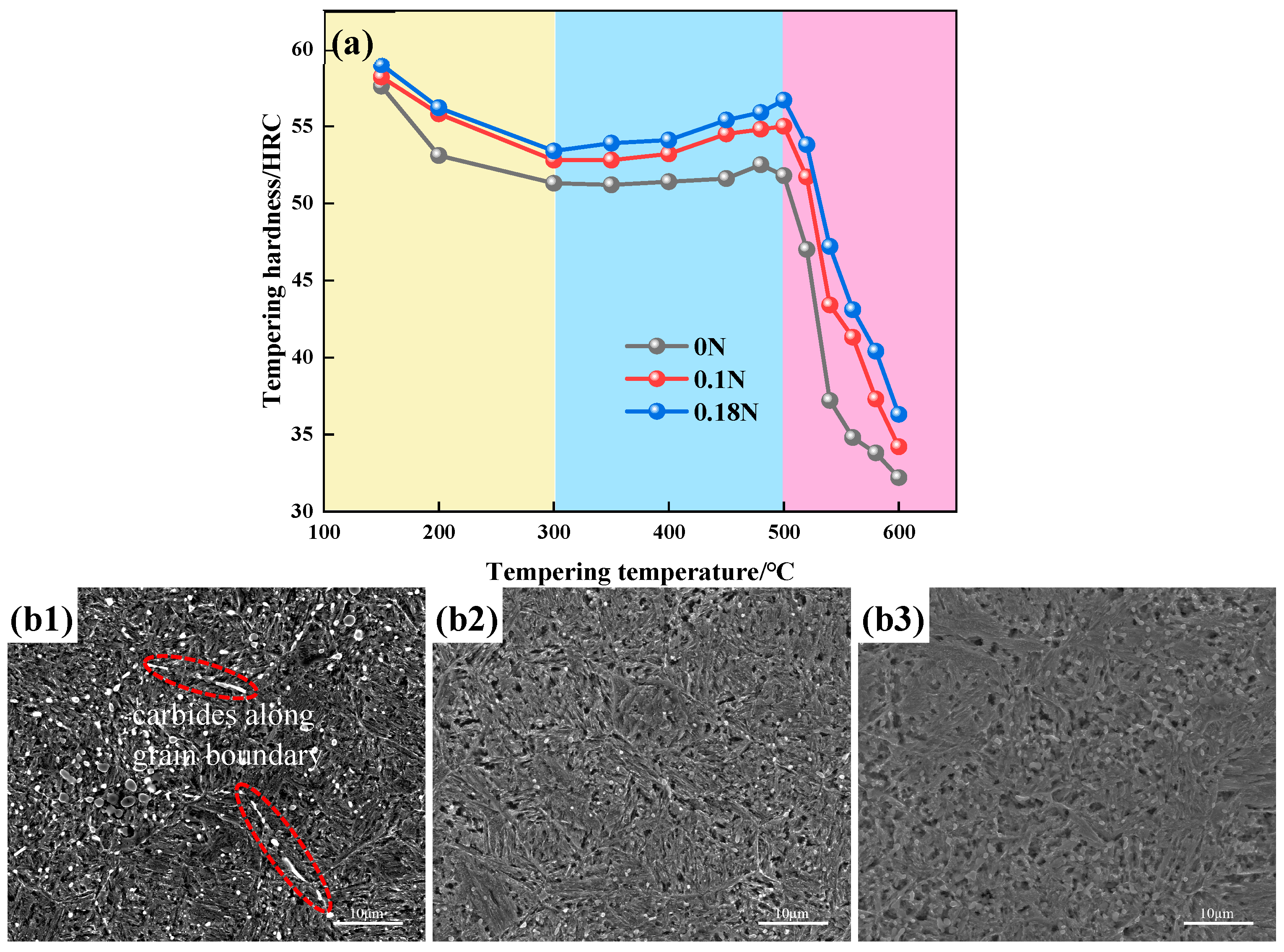
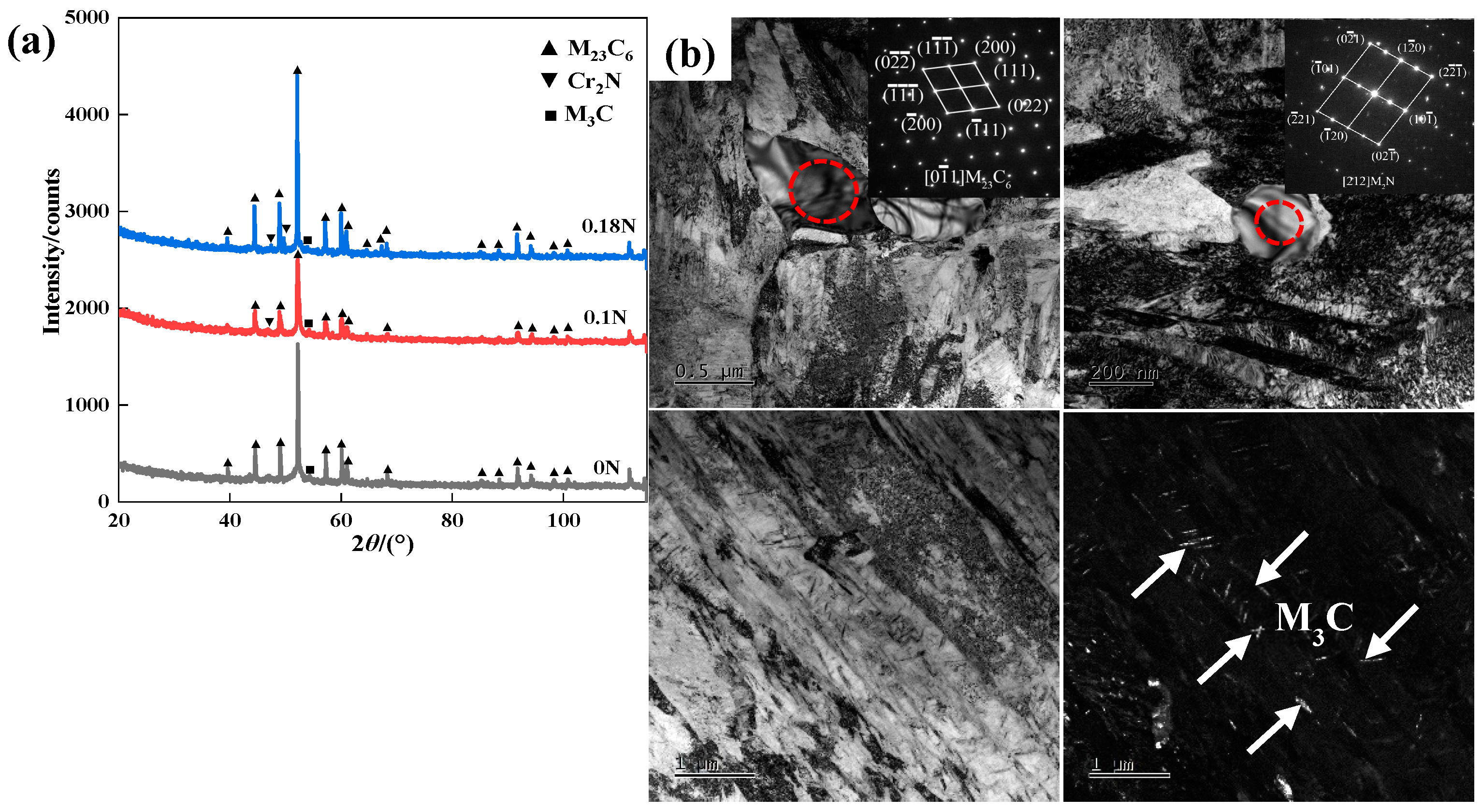
3.2. Tempering Microstructure and Hardness
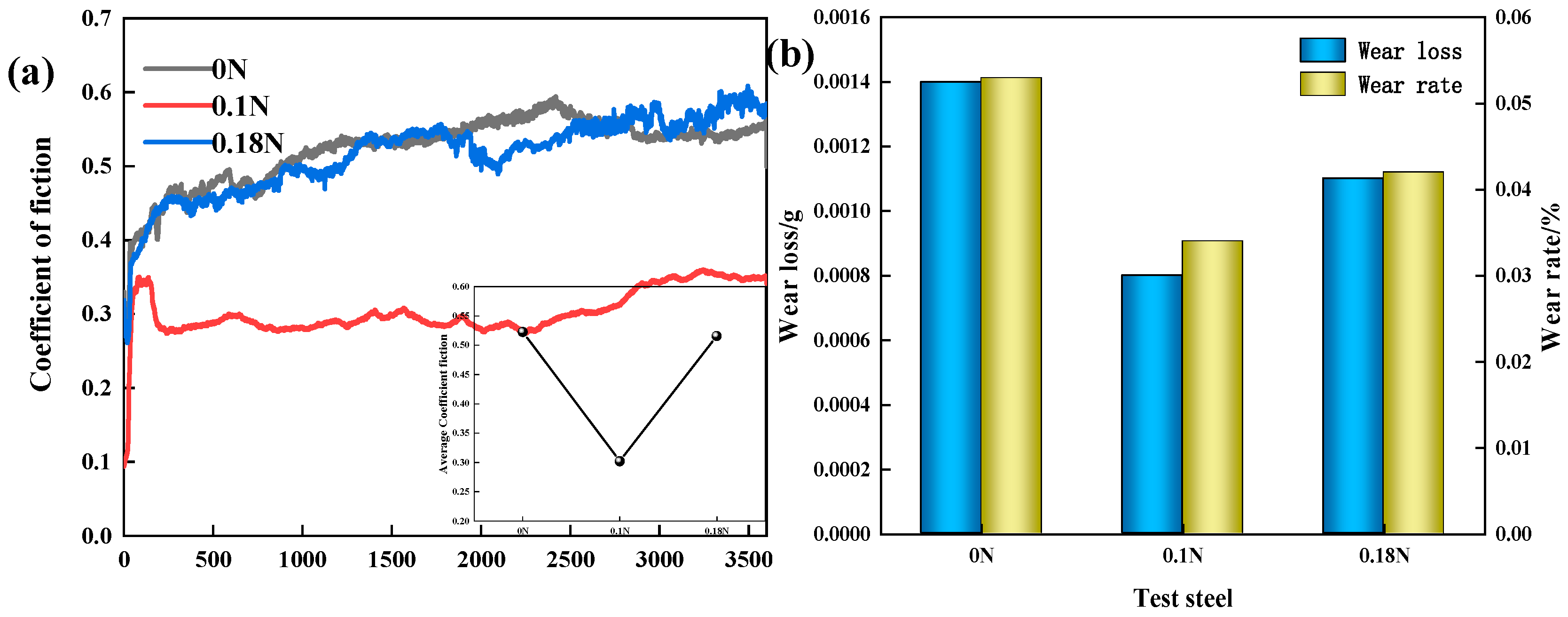
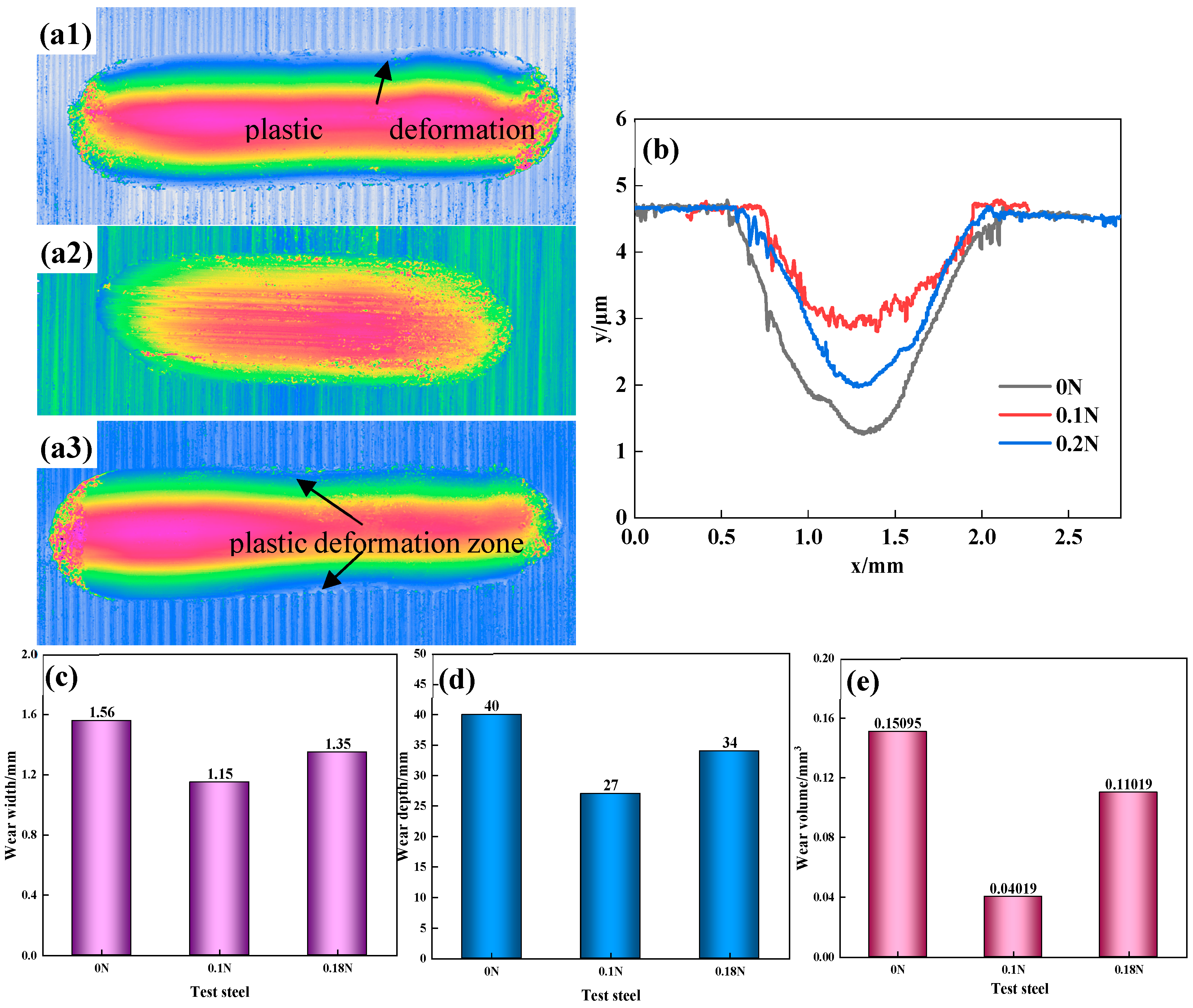
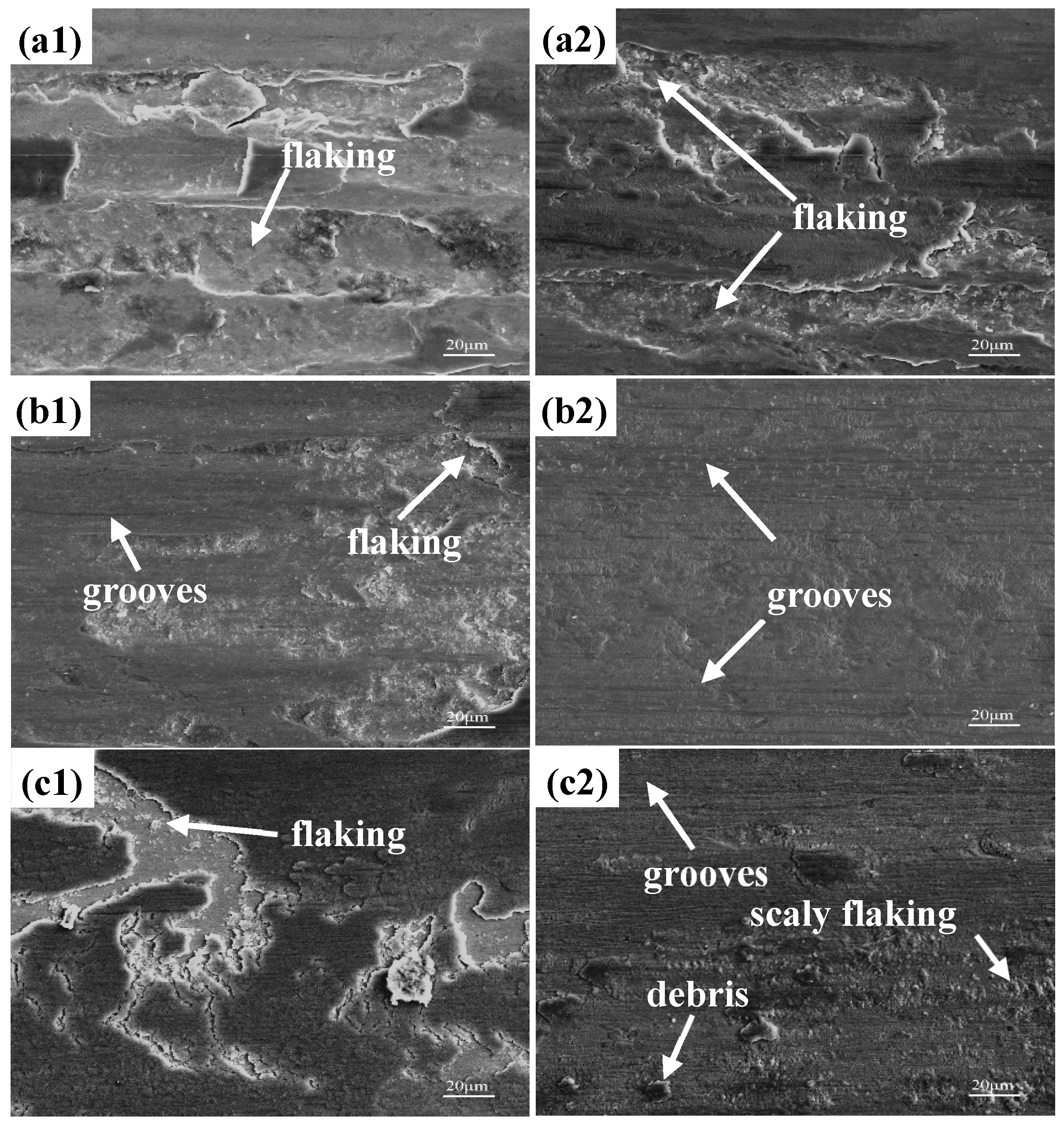
3.3. Wear Resistance
4. Discussion
5. Conclusions
- (1)
- The quenching microstructure of the test steel was martensite, carbide, and residual austenite. When the quenching temperature was below 1050 °C, due to the solid-solution-strengthening effect of N, the hardness of the nitrogen-containing steel was higher than that of the nitrogen-free steel. When the quenching temperature was higher than 1050 °C, the content of the residual austenite in the nitrogen-containing steel increased, and the hardness rapidly decreased, showing that the higher the N content, the more rapidly the hardness decreased.
- (2)
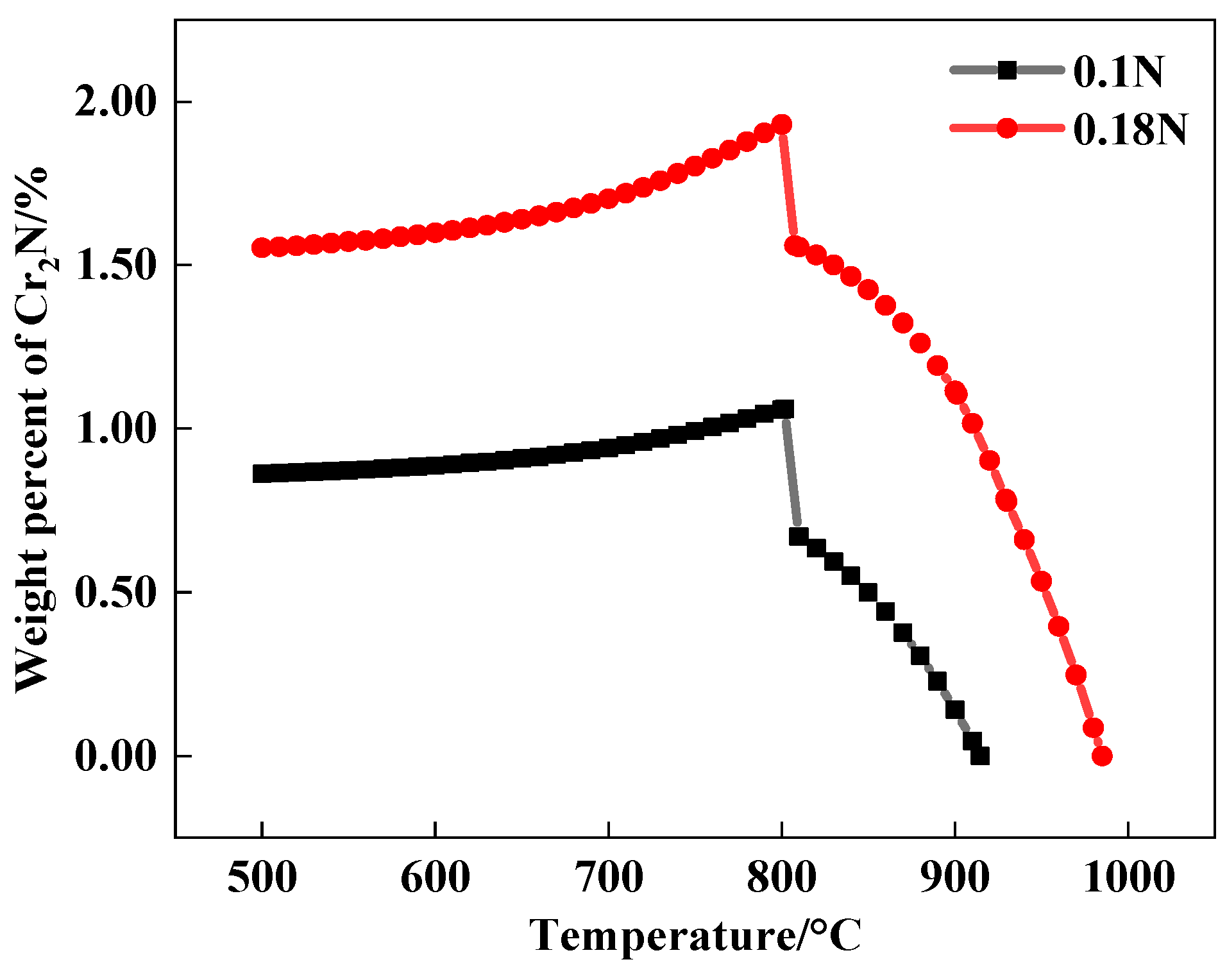
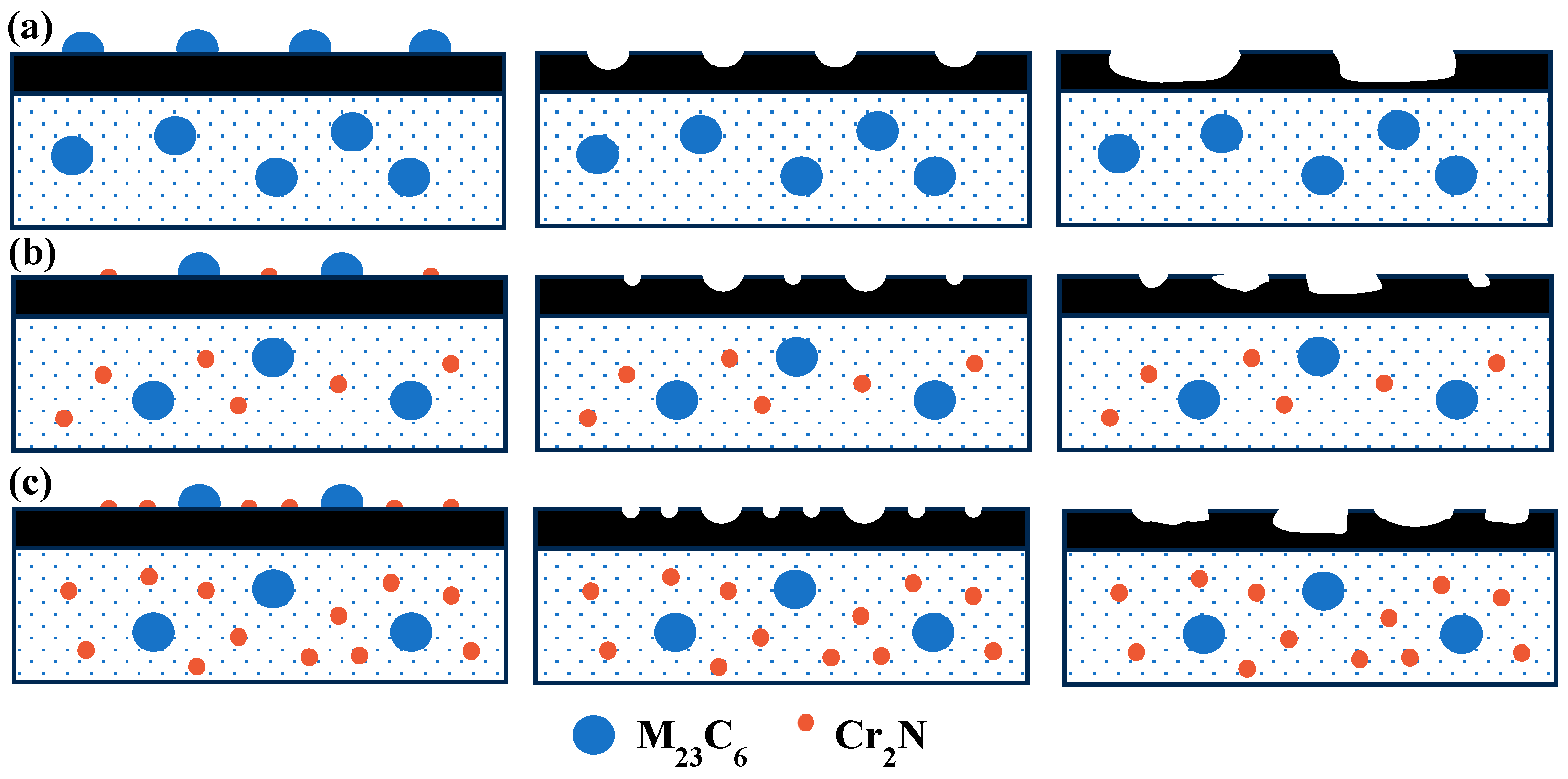
- With the addition of the N element, the type of second-phase material precipitated during tempering changed from M23C6 to M23C6 and Cr2N. The addition of the N element delayed the precipitation of large carbides, and the steel tended to precipitate fine Cr2N. The strengthening effect of the second phase resulted in greater hardness of the nitrogen-containing test steel.
- (3)
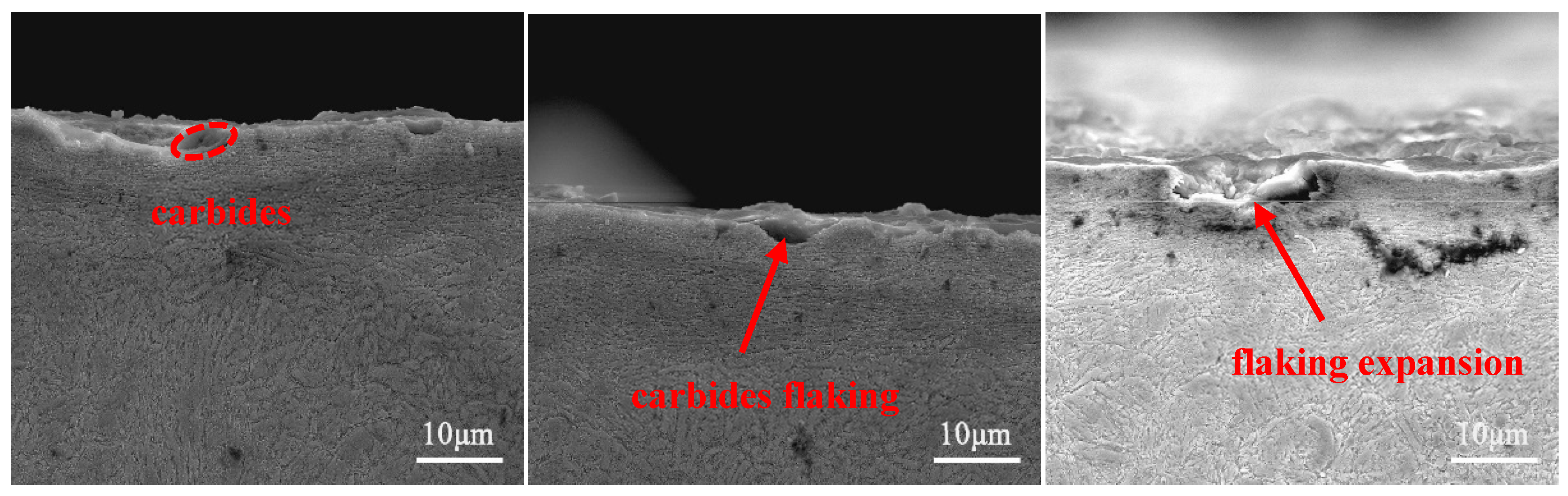
- The wear mechanism of the three test steels was predominantly abrasive wear, with some adhesive and flaking wear, and among the test steels, 0.1 N steel had the best wear resistance. The proper addition of N could not only increase the hardness of the steel but also delay the precipitation of the large-sized second phases, reducing the degree of wear. However, an excessive addition of N led to excessive precipitation of the second-phase particles, and the second-phase particles gradually flaked during the wear process before continuing to participate in the wear process, as third-body wear particles, resulting in internal notches and reducing wear resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perko, J.; Redl, C.; Leiner, H. Influence of heat treatment on the microstructure and toughness of bohler M333 isoplast steel. La Metall. Ital. 2009, 101, 1–5. [Google Scholar]
- Aquino, J.M.; Rovere, C.A.D.; Kuri, S.E. Localized corrosion susceptibility of supermartensitic stainless steel in welded joints. Corrosion 2008, 64, 35–39. [Google Scholar] [CrossRef]
- Park, E.S.; Yoo, D.Y.; Sung, J.H. Formation of reversed austenite during tempering of 14Cr-7Ni-0.3Nb-0.7Mo-0.03C super martensitic stainless steel. Metals Mater. Int. 2004, 10, 521. [Google Scholar] [CrossRef]
- Ngo, H.K.L.; Nakashima, K.; Tsuchiyama, T. Effect of solution nitriding on microstructure and hardness in 12% Cr martensitic stainless steels. J. Iron Steel Inst. Jpn. 2012, 98, 25. [Google Scholar] [CrossRef]
- Chalaya, N.M. Injection molding of plastic (review of scientific and practical seminar). Plast. Massy Sint. Svojstva Pererab. Primen. 2005, 4, 3–9. [Google Scholar]
- Vlcak, P.; Drahokoupil, J.; Vertat, P.; Sepitka, J.; Duchon, J. Hardness response to the stability of a Ti(+N) solid solution in an annealed TiN/Ti(+N)/Ti mixture layer formed by nitrogen ion implantation into titanium. Alloys Compd. 2018, 746, 490–495. [Google Scholar] [CrossRef]
- Cobb, H.M. The History of Stainless Steel; ASM International: Materials Park, OH, USA, 2010. [Google Scholar]
- Hill, H.; Huth, S.; Weber, S.; Theisen, W. Corrosion properties of a plastic mould steel with special focus on processiong route. Mater. Corros. 2010, 65, 436–443. [Google Scholar] [CrossRef]
- Stein, G.; Hucklenbroich, I. Manufacturing and applications of high nitrogen steels. Mater. Manuf. Process. 2004, 19, 7. [Google Scholar] [CrossRef]
- López, D.; Falleiros, N.A.; Tschiptschin, A.P. Corrosion-erosion behaviour of austenitic and martensitic high nitrogen stainless steels. Wear 2007, 263, 347. [Google Scholar] [CrossRef]
- Seifert, M.; Siebert, S.; Huth, S.; Theisen, W.; Berns, H. New developments in martensitic stainless steels containing C+N. Steel Res. 2015, 12, 1508–1516. [Google Scholar] [CrossRef]
- Hanninen, H.; Romu, J.; Ilola, R. Effect of processing and manufacturing of high nitrogen-containing stainless steels on their mechanical, corrosion and wear properties. J. Mater. Process. Technol. 2011, 117, 424–430. [Google Scholar] [CrossRef]
- Newman, R.C.; Shahrabi, T. The effect of alloyed nitrogen or dissolved nitrate ions on the anodic behaviour of austenitic stainless steel in hydrochloric acid. Corros. Sci. 1987, 27, 827–838. [Google Scholar] [CrossRef]
- Zhan, L.C. Study on Microstructure and Properties of High Nitrogen Corrosion Resistant Plastic Mold Steel. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2013. [Google Scholar]
- Wang, L.C. Research on the Effect of Nitrogen and Molybdenum on the Microstructures and Behaviours of the Corrosion-Resistant Steel for Plastic Mould. Master’s Thesis, Northeastern University, Liaoning, China, 2018. [Google Scholar]
- Xie, G.L. Effect of C-N Content on Microstructure and Properties of Cr13 Type Corrosion-Resistant Plastic Mold Steel. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2022. [Google Scholar]
- Kopf, A. Better wear reduction and corrosion resistance are just a few of the benefits of using miniature and small-size ball bearings made of new nitrogen alloy steels. Allaying Fail. Nitrogen Alloy Bear. 2005, 8, 48. [Google Scholar]
- Xu, H.F.; Zhao, J.; Cao, W.Q.; Shi, J.; Wang, C.Y.; Wang, C.; Li, J.; Dong, H. Heat treatment effects on the microstructure and mechanical properties of a medium manganese steel (0.2C–5Mn). Mater. Sci. Eng. A 2012, 532, 435–442. [Google Scholar] [CrossRef]
- Cui, K. Steel and Non-Ferrous Metal Materials; China Machine Press: Beijing, China, 1981. [Google Scholar]
- Gavriljuk, V.G.; Berns, H. High Nitrogen Steels; Springer: Berlin/Heildelberg, Germany; New York, NY, USA, 1999. [Google Scholar]
- Raj, B.; Mudali, U.K. High Nitrogen Steels and Stainless Steel; Alpha Science: Pangbourne, UK, 2004. [Google Scholar]
- Ma, X.P.; Wang, L.J.; Qin, B.; Liu, C.M.; Subramanian, S.V. Effect of N on microstructure and mechanical properties of 16Cr5Ni1Mo martensitic stainless steel. Mater. Des. 2012, 34, 74–81. [Google Scholar] [CrossRef]
- Toro, A.; Misiolek, W.Z.; Tschiptschin, A.P. Correlations between microstructure and surface properties in a high nitrogen martensitic stainless steel. Acta Mater. 2003, 51, 3363–3374. [Google Scholar] [CrossRef]
- Berns, H. Manufacture and application of high nitrogen steels. ISIJ Int. 1996, 36, 909–914. [Google Scholar] [CrossRef]
- Joseph, J.; Haghdadi, N.; Shamlaye, K.; Hodgson, P.; Barnett, M. The sliding wear behaviour of CoCrFeMnNi and AlxCoCrFeNi high entropy alloys at elevated temperatures. Wear 2019, 428–429, 32–44. [Google Scholar] [CrossRef]
- Beninca, F.P.; Romero, M.C.; Camporez, R.M.; Neto, C.A.; Strey, N.F.; Scandian, C. Evolution of morphology, microstructure and hardness of bodies and debris during sliding wear of carbon steels in a closed tribosystem. Wear 2023, 523, 204809. [Google Scholar] [CrossRef]
- Kaiko, O.; Pallaspuro, S.; Javaheri, V.; Kaikkonen, P.; Ghosh, S.; Valtonen, K.; Kaijalainen, A.; Kömi, J. High-stress abrasive wear performance of medium-carbon direct-quenched and partitioned, carbide-free bainitic, and martensitic steels. Wear 2023, 526–527, 204925. [Google Scholar]
- Liu, M.J.; Xu, L.J.; Zhu, C.H.; Li, Z.; Wei, S.Z. Research progress of high entropy alloy: Surface treatment improves friction and wear properties. J. Mater. Res. Technol. 2023, 28, 752–773. [Google Scholar] [CrossRef]
- Fu, J.; Liu, H.; Feng, L.S.; Wu, D.; Tang, H.; Xia, Y. Wear performance of modified H-13 and H418E steels for TBM disc cutter ring. Eng. Fail. Analy. 2024, 156, 107783. [Google Scholar] [CrossRef]
- Bhushan, R.K. Effect of tool wear on surface roughness in machining of AA7075/10 wt.% SiC composite. Compos. Part C Open Access 2022, 8, 100254. [Google Scholar] [CrossRef]
- Liu, Z.C.; Kong, D.J. Effects of TiC mass fraction on microstructure, corrosive–wear and electrochemical properties of laser cladded CoCrFeNiMo high–entropy alloy coatings. Tribol. Int. 2023, 186, 108640. [Google Scholar] [CrossRef]
- Shao, H.S. Friction and Wear; Coal Industry Press: Beijing, China, 1992. [Google Scholar]
- Amini, K.; Dayaghi, S.; Yazdanian, S.M.; Seiyedbeigi, M.; Gharavi, F. The effect of tempering temperature on microstructure and the mechanical properties of forged steel containing chrome, manganese and molybdenum. Mechanika 2018, 24, 852–856. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, C.O.; Joon, W.P.; Lee, H.C.; Lee, S. Effects of tempering temperature on wear resistance and surface roughness of a high speed steel roll. ISIJ Int. 2001, 41, 859–865. [Google Scholar] [CrossRef]
- Rahman, N.U.; Rooij, M.B.; Matthews, D.T.A.; Walmag, G.; Sinnaeve, M.; Romer, G.R.B.E. Wear characterization of multilayer laser cladded high speed steels. Tribol. Int. 2019, 130, 52–62. [Google Scholar] [CrossRef]
- Yong, Q.L. The Second Phase in Steel; Metallurgical Industry Press: Beijing, China, 2006. [Google Scholar]
- Mehtedi, M.E.; Ricci, P.; Drudi, L.; Mohtadi, S.E.; Cabibbo, M.; Spigarelli, S. Analysis of the effect of Deep Cryogenic Treatment on the hardness and microstructure of X30 CrMoN15 1 steel. Mater. Des. 2012, 33, 136–144. [Google Scholar] [CrossRef]
- Toppo, R.; Roy, M. Influence of various counter-bodies on the sliding friction and wear response of M50 steel. Ironmak. Steelmak. 2023, 50, 470–484. [Google Scholar] [CrossRef]
- Shah, M.; Bakshi, S.D. Three-body abrasive wear of carbide-free bainite, martensite and bainite-martensite structure of similar hardness. Wear 2018, 402–403, 207–215. [Google Scholar] [CrossRef]
- Wei, W.T.; Ke, J.Z.; Liu, Z.; Liu, G.C.; Liu, Q.L. Understanding the microstructural evolution and fretting wear behaviors of M50 bearing steel heat treated at different temperatures. Mater. Res. Technol. 2023, 27, 6661–6671. [Google Scholar] [CrossRef]
| Test Steel | C | Si | Mn | Cr | N |
|---|---|---|---|---|---|
| 0 N | 0.35 | 0.34 | 0.34 | 13.2 | - |
| 0.1 N | 0.42 | 0.35 | 0.34 | 13.4 | 0.1 |
| 0.18 N | 0.4 | 0.3 | 0.33 | 13.3 | 0.18 |
| Test Steel | Mass Fraction of Precipitated-Phase Elements, wt% | ||||
|---|---|---|---|---|---|
| Cr | Fe | Mn | N | ∑ | |
| 0 N | 1.604 | 1.539 | 0.019 | / | 3.162 |
| 0.1 N | 1.934 | 1.625 | 0.021 | 0.020 | 3.600 |
| 0.18 N | 1.997 | 1.534 | 0.020 | 0.028 | 3.579 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Zhou, J.; Gu, J.; Chi, H.; Ma, D.; Xie, G. Effect of N on the Microstructure and Wear Resistance of 4Cr13 Corrosion-Resistant Plastic Mold Steel. Materials 2024, 17, 308. https://doi.org/10.3390/ma17020308
Fan Y, Zhou J, Gu J, Chi H, Ma D, Xie G. Effect of N on the Microstructure and Wear Resistance of 4Cr13 Corrosion-Resistant Plastic Mold Steel. Materials. 2024; 17(2):308. https://doi.org/10.3390/ma17020308
Chicago/Turabian StyleFan, Yi, Jian Zhou, Jinbo Gu, Hongxiao Chi, Dangshen Ma, and Guanli Xie. 2024. "Effect of N on the Microstructure and Wear Resistance of 4Cr13 Corrosion-Resistant Plastic Mold Steel" Materials 17, no. 2: 308. https://doi.org/10.3390/ma17020308






