Reactivity of Calcined Clays as SCM—A Review
Abstract
:1. Introduction
2. Clay—An Overview
2.1. Kaolinite
2.2. Montmorillonite
2.3. Illite/Muscovite
3. Methods to Investigate the Reactivity of Calcined Clays
4. Qualitative Literature Evaluation Regarding the Reactivity-Determining Factors
4.1. Characteristics of the Raw Material
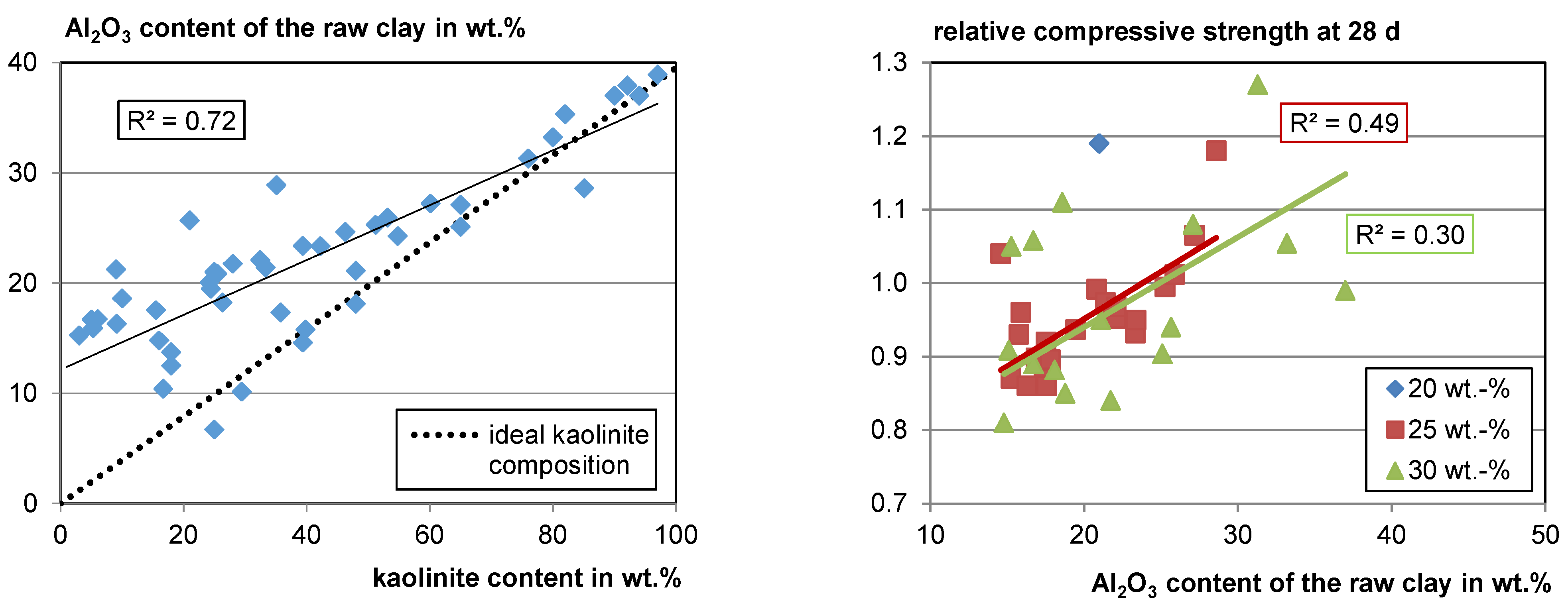
4.1.1. Mineral Phase Composition
General Remarks
Clay-Mineral Phases
Non-Clay Phases, including Limestone
4.1.2. Chemical Composition
4.2. Parameters of Calcination
4.2.1. Calciner Types, Grain Size, and Retention Time
4.2.2. Calcination Temperature
Kaolinite
Montmorillonite
Illite
4.2.3. Cooling
4.3. Characteristics of the Calcined Material
4.3.1. Physical Properties
4.3.2. Amorphous Phase
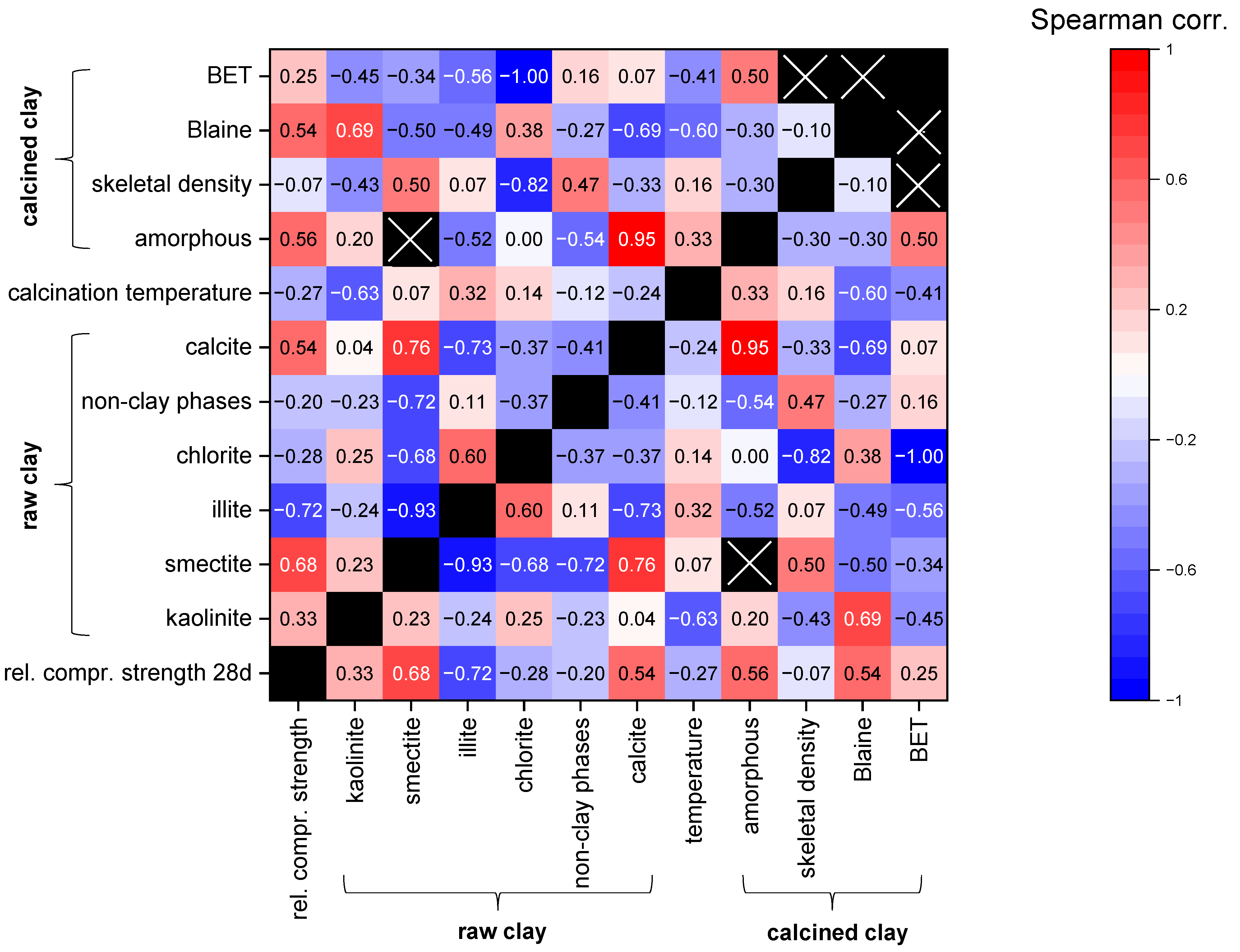
5. Quantitative Data Compilation and Evaluation
5.1. Approach
5.2. Evaluation
6. Conclusions and Outlook
- Phase composition of the raw material: The kaolinite content is the most important factor for the reactivity potential of the clays. However, montmorillonite is also known to have a significant reactivity potential. Illite has the lowest reactivity potential of the main clay phases. Calcite is known to react with alumina from the clays, forming carbo–aluminate hydrates that contribute to strength development. When calcining calcite and dolomite together with clay minerals, the reaction products can lower the melting point, build glassy phases and lower the recrystallization temperature. Other phases show minor effects on the reactivity.
- Calcination parameters: The furnace type due to different dispersion, the grain-size distribution prior to calcination due to different effectiveness of calcination progress depending on the surface-to-volume ratio, the calcination temperature due to the influence on the structural changes of the clay minerals, and the retention time are decisive for reactivity development. However, for the different types of clay-mineral phases, the parameters have different optima. In addition, for the same clay-mineral phase, the optima depend on their actual composition and crystallinity.
- Composition of the calcined material: The particle-size distribution/specific surface area prior to application are important as the surface area determines the reaction kinetics. The phase composition of the raw material and the calcination parameters determine the phase composition of the calcined material. Here, the reactivity of the amorphous phase is strongly dependent on the phase composition of the raw material.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beushausen, H. Performance-Based Specifications and Control of Concrete Durability: State-Of-the-Art Report RILEM TC 230-PSC; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Bundesministerium für Wirtschaft und Energie (Federal Ministry for Economic Affairs and Energy) (BMWi), Kommission “Wachstum, Strukturwandel und Beschäftigung”: Abschlussbericht, 2019. Available online: https://www.bmwk.de/Redaktion/DE/Downloads/A/abschlussbericht-kommission-wachstum-strukturwandel-und-beschaeftigung.pdf?__blob=publicationFile& (accessed on 9 November 2023).
- Jasmund, K.; Lagaly, G. Tonminerale und Tone: Struktur, Eigenschaften, Anwendungen und Einsatz in Industrie und Umwelt; Steinkopff: Heidelberg, Germany, 1993. [Google Scholar]
- Sabir, B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Bakera, A.T.; Alexander, M.G. Use of metakaolin as supplementary cementitious material in concrete, with focus on durability properties. RILEM Tech. Lett. 2019, 4, 89–102. [Google Scholar] [CrossRef]
- Thankam, G.L.; Renganathan, N.T. Ideal supplementary cementing material—Metakaolin: A review. Int. Rev. Appl. Sci. Eng. 2020, 11, 58–65. [Google Scholar] [CrossRef]
- Siddique, R.; Klaus, J. Influence of metakaolin on the properties of mortar and concrete: A review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Maier, M.; Forster, B.; Beuntner, N. Potential of Calcined Recycling Kaolin from Silica Sand Processing as Supplementary Cementitious Material. In Calcined Clays for Sustainable Concrete; Bishnoi, S., Ed.; RILEM Bookseries; Springer: Singapore, 2015; Volume 25, pp. 75–83. [Google Scholar]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Ambroise, J.; Gniewek, J.; Dejean, J.; Pera, J. Hydration of pozzolanic binders obtained by thermal activation of montmorillonite. Am. Ceram. Soc. Bull. 1987, 66, 1731–1733. [Google Scholar]
- Ambroise, J.; Murat, M.; Péra, J. Hydration reaction and hardening of calcined clays and related minerals V. Extension of the research and general conclusions. Cem. Concr. Res. 1985, 15, 261–268. [Google Scholar] [CrossRef]
- Sayanam, R.A.; Kalsotra, A.K.; Mehta, S.K.; Singh, R.S.; Mandal, G. Studies on thermal transformations and pozzolanic activities of clay from Jammu region (India). J. Therm. Anal. 1989, 35, 99–106. [Google Scholar] [CrossRef]
- Scrivener, K.; Favier, A. (Eds.) Calcined clays for sustainable concrete. In Proceedings of the 1st International Conference on Calcined Clays for Sustainable Concrete, Lausanne, Schwitzerland, 23–25 June 2015. [Google Scholar]
- Martirena, F.; Favier, A.; Scrivener, K. (Eds.) Calcined Clays for Sustainable Concrete; Springer: Dordrecht, The Netherlands, 2018. [Google Scholar]
- Bishnoi, S. (Ed.) Calcined Clays for Sustainable Concrete; Springer: Singapore, 2020. [Google Scholar]
- Luzu, B.; Duc, M.; Djerbi, A.; Gautron, L. High Performance Illitic Clay-Based Geopolymer: Influence of the Mechanochemical Activation Duration on the Strength Development. In Calcined Clays for Sustainable Concrete; Bishnoi, S., Ed.; RILEM Bookseries; Springer: Singapore, 2020; Volume 25. [Google Scholar]
- Garg, N.; Skibsted, J. Thermal Activation of a Pure Montmorillonite Clay and Its Reactivity in Cementitious Systems. J. Phys. Chem. C 2014, 118, 11464–11477. [Google Scholar] [CrossRef]
- Garg, N.; Skibsted, J. Pozzolanic reactivity of a calcined interstratified illite/smectite (70/30) clay. Cem. Concr. Res. 2016, 79, 101–111. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Trümer, A. Calcinierte Tone als Puzzolane der Zukunft—Von den Rohstoffen bis zur Wirkung im Beton. Ph.D. Thesis, Bauhaus-Universität, Weimar, Germany, 2019. [Google Scholar]
- Danner, T. Reactivity of Calcined Clays. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2013. [Google Scholar]
- Bullerjahn, F.; Zajac, M.; Pekarkova, J.; Nied, D. Novel SCM produced by the co-calcination of aluminosilicates with dolomite. Cem. Concr. Res. 2020, 134, 106083. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K.L. The Effect of Calcite and Gibbsite Impurities in Calcined Clay on Its Reactivity. In Calcined Clays for Sustainable Concrete; Bishnoi, S., Ed.; Springer: Singapore, 2020; pp. 357–362. [Google Scholar]
- Heim, D. Tone und Tonminerale: Grundlagen der Sedimentologie und Mineralogie; Enke: Stuttgart, Germany, 1990. [Google Scholar]
- Bergaya, F.; Lagaly, G. (Eds.) Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Grim, R.E. Applied Clay Mineralogy; McGraw-Hill Book Company, Inc.: New York, NY, USA; Toronto, ON, Canada; London, UK, 1962. [Google Scholar]
- Kumari, N.; Mohan, C. Basics of Clay Minerals and Their Characteristic Properties. In Clay and Clay Minerals [Working Title]; IntechOpen: London, UK, 2021. [Google Scholar]
- Emmerich, K.; Wolters, F.; Kahr, G.; Lagaly, G. Clay Profiling: The Classification of Montmorillonites. Clays Clay Miner. 2009, 57, 104–114. [Google Scholar] [CrossRef]
- Bleam, W.F. Clay Mineralogy and Clay Chemistry. In Soil and Environmental Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 85–116. [Google Scholar]
- Pinheiro, V.D.; Alexandre, J.; Xavier, G.D.C.; Marvila, M.T.; Monteiro, S.N.; de Azevedo, A.R.G. Methods for Evaluating Pozzolanic Reactivity in Calcined Clays: A Review. Materials 2023, 16, 4778. [Google Scholar] [CrossRef]
- Chapelle, J. Attaque sulfocalcique des laitiers et pouzzolanes. Rev. Matér. Constr. 1958, 512, 136–145. [Google Scholar]
- Li, X.; Snellings, R.; Antoni, M.; Alderete, N.M.; Ben Haha, M.; Bishnoi, S.; Cizer, Ö.; Cyr, M.; De Weerdt, K.; Dhandapani, Y.; et al. Reactivity tests for supplementary cementitious materials: RILEM TC 267-TRM phase 1. Mater. Struct. 2018, 51, 136. [Google Scholar] [CrossRef]
- NF P18-513; Addition for Concrete—Metakaolin—Specifications and Conformity Criteria. AFNOR: Paris, France, 2012.
- DIN EN 196-5; Methods of Testing Cement—Part 5: Pozzolanicity Test for Pozzolanic Cement. DIN EN: Berlin, Germany, 2011.
- IS 1727; Methods of Test for Pozzolanic Materials. BIS: New Delhi, India, 1967.
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Potential use of Argentine kaolinitic clays as pozzolanic material. Appl. Clay Sci. 2014, 101, 468–476. [Google Scholar] [CrossRef]
- Taylor-Lange, S.C.; Lamon, E.L.; Riding, K.A.; Juenger, M.C. Calcined kaolinite–bentonite clay blends as supplementary cementitious materials. Appl. Clay Sci. 2015, 108, 84–93. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Kaolinitic calcined clays: Factors affecting its performance as pozzolans. Constr. Build. Mater. 2012, 28, 276–281. [Google Scholar] [CrossRef]
- Walker, R.; Pavía, S. Physical properties and reactivity of pozzolans, and their influence on the properties of lime–pozzolan pastes. Mater. Struct. 2011, 44, 1139–1150. [Google Scholar] [CrossRef]
- DIN EN 196-2; Method of Testing Cement—Part 2: Chemical Analysis of Cement. DIN EN: Berlin, Germany, 2013.
- DIN EN 197-1; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. DIN EN: Berlin, Germany, 2011.
- Schulze, S.E.; Rickert, J. Suitability of natural calcined clays as supplementary cementitious material. Cem. Concr. Compos. 2019, 95, 92–97. [Google Scholar] [CrossRef]
- Surana, M.S.; Josh, S.N. Spectrophotometric method for estimating the reactivity of pozzolanic materials. Adv. Cem. Res. 1988, 1, 238–242. [Google Scholar] [CrossRef]
- Pierkes, R.; Schulze, S.E.; Rickert, J. Optimization of Cements with Calcined Clays as Supplementary Cementitious Materials. In Calcined Clays for Sustainable Concrete; Springer: Berlin/Heidelberg, Germany, 2015; pp. 59–66. [Google Scholar]
- He, C.; Makovicky, E.; Øsbæck, B. Thermal stability and pozzolanic activity of calcined kaolin. Appl. Clay Sci. 1994, 9, 165–187. [Google Scholar] [CrossRef]
- He, C.; Makovicky, E.; Øsbæck, B. Thermal stability and pozzolanic activity of calcined illite. Appl. Clay Sci. 1995, 9, 337–354. [Google Scholar] [CrossRef]
- Buchwald, A.; Hohmann, M.; Posern, K.; Brendler, E. The suitability of thermally activated illite/smectite clay as raw material for geopolymer binders. Appl. Clay Sci. 2009, 46, 300–304. [Google Scholar] [CrossRef]
- Liguori, B.; Capasso, I.; de Pertis, M.; Ferone, C.; Cioffi, R. Geopolymerization Ability of Natural and Secondary Raw Materials by Solubility Test in Alkaline Media. Environments 2017, 4, 56. [Google Scholar] [CrossRef]
- Garg, N.; Skibsted, J. Heated Montmorillonite: Structure, Reactivity, and Dissolution. In Calcined Clays for Sustainable Concrete; Scrivener, K., Favier, A., Eds.; RILEM Bookseries; Springer: Dordrecht, The Netherlands, 2015; Volume 10. [Google Scholar]
- ASTM C1897-20; Standard Test Methods for Measuring the Reactivity of Supplementary Cementitious Materials by Isothermal Calorimetry and Bound Water. ASTM Int: West Conshohocken, PA, USA, 2020; pp. 1–5.
- Londono-Zuluaga, D.; Gholizadeh-Vayghan, A.; Winnefeld, F.; Avet, F.; Haha, M.B.; Bernal, S.A.; Cizer, Ö.; Cyr, M.; Dolenec, S.; Durdzinski, P.; et al. Report of RILEM TC 267-TRM phase 3: Validation of the R3 reactivity test across a wide range of materials. Mater. Struct. 2022, 55, 142. [Google Scholar] [CrossRef]
- Escobar, K.D.; Díaz, A.A.; García, L.A.P. Pozzolanic Reactivity of the Calcination Products Obtained from Yaguajay Clay Deposit. In Proceedings of the International Conference of Sustainable Production and Use of Cement and Concrete, Villa Clara, Cuba, 23–30 June 2020; pp. 47–58. [Google Scholar]
- Marchetti, G.; Rahhal, V.; Pavlík, Z.; Pavlíková, M.; Irassar, E.F. Assessment of packing, flowability, hydration kinetics, and strength of blended cements with illitic calcined shale. Constr. Build. Mater. 2020, 254, 119042. [Google Scholar] [CrossRef]
- Cardinaud, G.; Rozière, E.; Martinage, O.; Loukili, A.; Barnes-Davin, L.; Paris, M.; Deneele, D. Calcined clay—Limestone cements: Hydration processes with high and low-grade kaolinite clays. Constr. Build. Mater. 2021, 277, 122271. [Google Scholar] [CrossRef]
- Argın, G.; Uzal, B. Enhancement of pozzolanic activity of calcined clays by limestone powder addition. Constr. Build. Mater. 2021, 284, 122789. [Google Scholar] [CrossRef]
- Huang, W.; Kazemi-Kamyab, H.; Sun, W.; Scrivener, K. Effect of replacement of silica fume with calcined clay on the hydration and microstructural development of eco-UHPFRC. Mater. Des. 2017, 121, 36–46. [Google Scholar] [CrossRef]
- Trezza, M.A.; Tironi, A.; Irassar, E.F. Thermal Activation of Two Complex Clays (Kaolinite-Pyrophillite-Illite) from Tandilia System, Buenos Aires, Argentina. In Calcined Clays for Sustainable Concrete; Martirena, F., Favier, A., Scrivener, K., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 469–474. [Google Scholar]
- Huang, Y.; Deng, J.; Wang, W.; Feng, Q.; Xu, Z. Preliminary Investigation of Pozzolanic Properties of Calcined Waste Kaolin. Mater. Sci. 2018, 24, 177–184. [Google Scholar] [CrossRef]
- Snellings, R.; Reyes, R.A.; Hanein, T.; Irassar, E.F.; Kanavaris, F.; Maier, M.; Marsh, A.T.; Valentini, L.; Zunino, F.; Diaz, A.A. Paper of RILEM TC 282-CCL: Mineralogical characterization methods for clay resources intended for use as supplementary cementitious material. Mater. Struct. 2022, 59, 145. [Google Scholar] [CrossRef]
- Lopez, R.F. Calcined Clayey Soils as a Potential Replacement for Cement in Developing Countries. Ph.D. Thesis, École polytechnique fédérale de Lausanne, Lausanne, Schweiz, 2009. [Google Scholar]
- Hollanders, S.; Adriaens, R.; Skibsted, J.; Cizer, Ö.; Elsen, J. Pozzolanic reactivity of pure calcined clays. Appl. Clay Sci. 2016, 132–133, 552–560. [Google Scholar] [CrossRef]
- He, C.; Osbaeck, B.; Makovicky, E. Pozzolanic reactions of six principal clay minerals: Activation, reactivity assessments and technological effects. Cem. Concr. Res. 1995, 25, 1691–1702. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Avet, F.; Snellings, R.; Diaz, A.A.; Haha, M.B.; Scrivener, K. Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Bratoev, B.; Doykov, I.; Ninov, J.; Lenchev, A. Pozzolanic activity assessment of calcined clays with complex minerals content. Adv. Cem. Res. 2018, 30, 103–112. [Google Scholar] [CrossRef]
- Kakali, G.; Perraki, T.; Tsivilis, S.; Badogiannis, E. Thermal treatment of kaolin: The effect of mineralogy on the pozzolanic activity. Appl. Clay Sci. 2001, 20, 73–80. [Google Scholar] [CrossRef]
- Tironi, A.; Cravero, F.; Scian, A.N.; Irassar, E.F. Hydration of Blended Cement with Halloysite Calcined Clay. In Calcined Clays for Sustainable Concrete; Martirena, F., Favier, A., Scrivener, K., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 455–460. [Google Scholar]
- Tironi, A.; Cravero, F.; Scian, A.N.; Irassar, E.F. Pozzolanic activity of calcined halloysite-rich kaolinitic clays. Appl. Clay Sci. 2017, 147, 11–18. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Sharma, M.; Bishnoi, S.; Martirena, F.; Scrivener, K. Limestone calcined clay cement and concrete: A state-of-the-art review. Cem. Concr. Res. 2021, 149, 106564. [Google Scholar] [CrossRef]
- Karunadasa, K.S.; Manoratne, C.H.; Pitawala, H.; Rajapakse, R. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Zunino, F.; Boehm-Courjault, E.; Scrivener, K. The impact of calcite impurities in clays containing kaolinite on their reactivity in cement after calcination. Mater. Struct. 2020, 53, 44. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. The Effect of Calcite in the Raw Clay on the Pozzolanic Activity of Calcined Illite and Smectite. In Calcined Clays for Sustainable Concrete; Bishnoi, S., Ed.; Springer: Singapore, 2020; pp. 131–138. [Google Scholar]
- Zunino, F.; Scrivener, K. Oxidation of pyrite (FeS2) and troilite (FeS) impurities in kaolinitic clays after calcination. Mater. Struct. 2021, 55, 9. [Google Scholar] [CrossRef]
- Ghorbel, H.; Samet, B. Effect of iron on pozzolanic activity of kaolin. Constr. Build. Mater. 2013, 44, 185–191. [Google Scholar] [CrossRef]
- Massazza, F. Pozzolana and Pozzolanic Cements. In Lea’s Chemistry of Cement and Concrete; Elsevier: Amsterdam, The Netherlands, 1998; pp. 471–635. [Google Scholar]
- van Aardt, J.; Visser, S. Reaction of Ca(OH)2 and of Ca(OH)2 + CaSO4.2H2O at various temperatures with feldspars in aggregates used for concrete making. Cem. Concr. Res. 1978, 8, 677–681. [Google Scholar] [CrossRef]
- Mechti, W.; Mnif, T.; Samet, B.; Rouis, M.J.R. Effects of the secondary minerals on the pozzolanic activity of calcined clay: Case of quartz. Int. J. Recent Res. Appl. Stud. 2012, 12, 61–71. [Google Scholar]
- Díaz, A.A.; Reyes, R.S.A.; Carratalá, F.A.; Hernández, J.F.M. Proposal of a Methodology for the Preliminary Assessment of Kaolinitic Clay Deposits as a Source of SCMs. In Calcined Clays for Sustainable Concrete; Martirena, F., Favier, A., Scrivener, K., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 29–34. [Google Scholar]
- Hanein, T.; Thienel, K.-C.; Zunino, F.; Marsh, A.T.M.; Maier, M.; Wang, B.; Canut, M.; Juenger, M.C.G.; Haha, M.B.; Avet, F.; et al. Clay calcination technology: State-of-the-art review by the RILEM TC 282-CCL. Mater. Struct. 2021, 55, 3. [Google Scholar] [CrossRef]
- Beuntner, N.; Thienel, C. Properties of Calcined Lias Delta Clay—Technological Effects, Physical Characteristics and Reactivity in Cement. In Calcined Clays for Sustainable Concrete: Proceedings of the 1st International Conference on Calcined Clays for Sustainable Concrete; Springer: Dordrecht, The Netherlands, 2015; pp. 43–50. [Google Scholar]
- Bridson, D. Properties of Flash-Calcined Kaolinite. Clays Clay Miner. 1985, 33, 258–260. [Google Scholar] [CrossRef]
- Chakchouk, A.; Trifi, L.; Samet, B.; Bouaziz, S. Formulation of blended cement: Effect of process variables on clay pozzolanic activity. Constr. Build. Mater. 2009, 23, 1365–1373. [Google Scholar] [CrossRef]
- Bich, C.; Ambroise, J.; Péra, J. Influence of degree of dehydroxylation on the pozzolanic activity of metakaolin. Appl. Clay Sci. 2009, 44, 194–200. [Google Scholar] [CrossRef]
- Beuntner, N. Zur Eignung und Wirkungsweise calcinierter Tone als reaktive Bindemittel-komponente im Zement. Ph.D. Thesis, Universität der Bundeswehr München, Munich, Germany, 2017. [Google Scholar]
- He, C.; Makovicky, E.; Osbaeck, B. Thermal treatment and pozzolanic activity of Na- and Ca-montmorillonite. Appl. Clay Sci. 1996, 10, 351–368. [Google Scholar] [CrossRef]
- Wang, M.R.; Guo, N.; He, P.G.; Yu, J.B.; Jia, D.C. Formation Mechanism and its Pozzolanic Activity of Metakaolin. KEM 2014, 602–603, 620–623. [Google Scholar] [CrossRef]
- Klinowski, J.; Rocha, J. 29Si and 27Al magic-angle-spinning NMR studies of the thermal transformation of kaolinite. Phys. Chem. Miner. 1990, 17, 179–186. [Google Scholar]
- Sánchez, R.T.; Basaldella, E.I.; Marco, J.F. The Effect of Thermal and Mechanical Treatments on Kaolinite: Characterization by XPS and IEP Measurements. J. Colloid Interface Sci. 1999, 215, 339–344. [Google Scholar] [CrossRef]
- Brindley, G.W.; Nakahira, M. A New Concept of the Transformation Sequence of Kaolinite to Mullite. Nature 1958, 181, 1333–1334. [Google Scholar] [CrossRef]
- De Gutiérrez, R.M.; Torres, J.; Vizcayno, C.; Castello, R. Influence of the calcination temperature of kaolin on the mechanical properties of mortars and concretes containing metakaolin. Clay Miner. 2008, 43, 177–183. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, T.; Sun, H.; Zeng, L.; Li, Y.; Zhou, C. Effect of montmorillonite layer charge on the thermal stability of bentonite. Clays Clay Miner. 2021, 69, 328–338. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.L.; Castellano, C.C.; Trezza, M.A.; Rahhal, V.F.; Cordoba, G.; Lemma, R. Calcined illite-chlorite shale as supplementary cementing material: Thermal treatment, grinding, color and pozzolanic activity. Appl. Clay Sci. 2019, 179, 105143. [Google Scholar] [CrossRef]
- O’farrell, M.; Sabir, B.; Wild, S. Strength and chemical resistance of mortars containing brick manufacturing clays subjected to different treatments. Cem. Concr. Compos. 2006, 28, 790–799. [Google Scholar] [CrossRef]
- Skibsted, J.; Snellings, R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem. Concr. Res. 2019, 124, 105799. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Y.; Wei, Q.; Cui, S.; Hao, L. Effect of the Formation of Amorphous Networks on the Structure and Hydration Characteristics of Granulated Blast Furnace Slag. Materials 2020, 13, 1462. [Google Scholar] [CrossRef] [PubMed]
- Zachariasen, W.H. Die Struktur der Gläser. Glastech. Berichte 1933, 11, 123. [Google Scholar]
- Warren, B. Summary of work on atomic arrangement in Glas. J. Am. Ceram. Soc. 1941, 24, 256–261. [Google Scholar] [CrossRef]
- Scholze, H. Glas: Natur, Struktur und Eigenschaften, 3rd ed.; Springer: Berlin, Heidelberg, 1988. [Google Scholar]
- Lee, S.K.; Stebbins, J.F. Disorder and the extent of polymerization in calcium silicate and aluminosilicate glasses: O-17 NMR results and quantum chemical molecular orbital calculations. Geochim. Cosmochim. Acta 2006, 70, 4275–4286. [Google Scholar] [CrossRef]
- Schöler, A.; Winnefeld, F.; Haha, M.B.; Lothenbach, B. The effect of glass composition on the reactivity of synthetic glasses. J. Am. Ceram. Soc. 2017, 100, 2553–2567. [Google Scholar] [CrossRef]
- Kuryaeva, R.G. The state of magnesium in silicate glasses and melts. Glass Phys. Chem. 2009, 35, 378–383. [Google Scholar] [CrossRef]
- Kucharczyk, S.; Zajac, M.; Stabler, C.; Thomsen, R.M.; Haha, M.B.; Skibsted, J.; Deja, J. Structure and reactivity of synthetic CaO-Al2O3-SiO2 glasses. Cem. Concr. Res. 2019, 120, 77–91. [Google Scholar] [CrossRef]
- Durdziński, P.T.; Snellings, R.; Dunant, C.F.; Haha, M.B.; Scrivener, K.L. Fly ash as an assemblage of model Ca–Mg–Na-aluminosilicate glasses. Cem. Concr. Res. 2015, 78, 263–272. [Google Scholar] [CrossRef]
- ASTM C311; Standard Test Methods for Sampling and Testing Fly Ash or Natural Pozzolans for Use in Portland-Cement Concrete. ASTM Int: West Conshohocken, PA, USA, 2022.
- Luo, Y.; Zhu, D.; Pan, J.; Zhou, X. Thermal decomposition behaviour and kinetics of Xinjiang siderite ore. Miner. Process. Extr. Met. 2016, 125, 17–25. [Google Scholar] [CrossRef]
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Hansen, J.P. Decomposition and oxidation of pyrite. Prog. Energy Combust. Sci. 2006, 32, 295–314. [Google Scholar] [CrossRef]
- Akasha, A.M. Using of Libyan Calcined Clay in Concrete. Calcined Clays Sustain. Concr. RILEM Bookser. 2015, 10, 555–561. [Google Scholar] [CrossRef]
- Balykov, A.S.; Nizina, T.A.; Volodin, V.V.; Kyashkin, V.M. Effects of Calcination Temperature and Time on the Physical-Chemical Efficiency of Thermally Activated Clays in Cement Systems. Mater. Sci. Forum 2021, 1017, 61–70. [Google Scholar] [CrossRef]
- Beuntner, N.; Rapp, K.; Thienel, K.-C. Efficiency of calcined clay in cementitious systems. In Proceedings of the 12th International Conference on Recent Advances in Concrete Technology and Sustainability Issues, Prag, Prague, Czech Republic, 30 October–2 November 2012. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. Characterisation of calcined raw clays suitable as supplementary cementitious materials. Appl. Clay Sci. 2018, 162, 391–402. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. Calcareous smectite clay as a pozzolanic alternative to kaolin. Eur. J. Environ. Civ. Eng. 2019, 25, 1–18. [Google Scholar] [CrossRef]
- Frías, M.; Rodríguez, O.; Vegas, I.; Vigil, R. Properties of calcined clay waste and its influence on blended cement behavior. J. Am. Ceram. Soc. 2008, 91, 1226–1230. [Google Scholar] [CrossRef]
- Frías, M.; Rodríguez, O.; de Rojas, M.I.S.; Ferreiro, S.; Nebreda, B.; Olmeda, J. New construction materials: Calcined paper sludges as active additions. Mater. Sci. Forum 2010, 636, 1222–1227. [Google Scholar] [CrossRef]
- JGonçalves, P.; Tavares, L.M.; Filho, R.D.T.; Fairbairn, E.M.R. Performance Evaluation of Cement Mortars Modified with Metakaolin or Ground Brick. Constr. Build. Mater. 2009, 23, 1971–1979. [Google Scholar] [CrossRef]
- Huenger, K.J.; Gerasch, R.; Sander, I.; Brigzinsky, M. On the reactivity of calcined clays from lower Lusatia for the production of durable concrete structures. Calcined Clays Sustain. Concr. RILEM Bookser. 2018, 16, 205–211. [Google Scholar] [CrossRef]
- Unpublished data of the Institute for Building Materials Research (ibac), RWTH Aachen University: Aachen, Germany, unpublished.
- Irassar, E.F.; Tironi, A.; Bonavetti, V.L.; Trezza, M.A.; Castellano, C.C.; Rahhal, V.F.; Donza, H.A.; Scian, A.N. Thermal Treatment and Pozzolanic Activity of Calcined Clay and Shale. ACI Mater. J. 2019, 116, 133–143. [Google Scholar] [CrossRef]
- Jafari, K.; Rajabipour, F. Performance of Impure Calcined Clay as a Pozzolan in Concrete. Transp. Res. Rec. J. Transp. Res. Board 2020, 2675, 98–107. [Google Scholar] [CrossRef]
- Lemma, R.; Castellano, C.C.; Bonavetti, V.L.; Trezza, M.A.; Rahhal, V.F.; Irassar, E.F. Thermal transformation of illitic-chlorite clay and its pozzolanic activity. Calcined Clays Sustain. Concr. RILEM Bookser. 2018, 16, 266–272. [Google Scholar] [CrossRef]
- Maier, M.; Beuntner, N.; Thienel, K.C. An approach for the evaluation of local raw material potential for calcined clay as SCM, based on geological and mineralogical data: Examples from German clay deposits. Calcined Clays Sustain. Concr. RILEM Bookser. 2020, 16, 37–47. [Google Scholar] [CrossRef]
- Maier, M.; Beuntner, N.; Thienel, K.C. Mineralogical characterization and reactivity test of common clays suitable as supplementary cementitious material. Appl. Clay Sci. 2021, 202, 105990. [Google Scholar] [CrossRef]
- Mohammed, S.; Elhem, G.; Mekki, B. Valorization of pozzolanicity of Algerian clay: Optimization of the heat treatment and mechanical characteristics of the involved cement mortars. Appl. Clay Sci. 2016, 132, 711–721. [Google Scholar] [CrossRef]
- Moulin, E.; Blanc, P. Properties of Artificial Pozzolan Blended Cements as a Function of Clay Precursor Mineralogy and Cement Chemical Parameters. Spec. Publ. 2001, 199, 361–378. [Google Scholar]
- Msinjili, N.S.; Sturm, P.; Kühne, H.C.; Gluth, G.J. Comparison of brick clays and a kaolinitic clay regarding calcination and performance in blended cement mortars. Calcined Clays Sustain. Concr. RILEM Bookser. 2020, 25, 85–93. [Google Scholar] [CrossRef]
- Filho, R.D.T.; Gonçalves, J.P.; Americano, B.B.; Fairbairn, E.M.R. Potential for use of crushed waste calcined-clay brick as a supplementary cementitious material in Brazil. Cem. Concr. Res. 2007, 37, 1357–1365. [Google Scholar] [CrossRef]






| Measured Parameter | Test Reference | Applied e.g., in | Comment |
|---|---|---|---|
| Portlandite consumption | Chapelle test [32] | [33] | No correlation to the 28 days relative compressive strength and a bad reproducibility |
| Modified Chapelle test (NF P18-513 [34]) | Good correlation to the 28 days relative compressive strength for pozzolanic SCMs and moderate reproducibility | ||
| The Frattini test (EN 196-5 [35]) | Good correlation to the 28 days relative compressive strength for pozzolanic SCMs and bad reproducibility caused by the use of different cements | ||
| The Indian standard lime reactivity test (IS 1727 [36]) | Moderate correlation to the 28 days relative compressive strength and good correlation for 90 days | ||
| Diverse other variations | [37,38,39,40] | - | |
| Ion solubility (Si) | Reactive silica test (EN 196-2 [41] and EN 197-1 [42]) | [33,43] | Bad correlation to relative strength |
| Method of Surana and Josh [44] | [43,44,45,46,47] | Good correlation of the dissolved Si with mortar compressive strength | |
| Ion solubility (Si and Al) | Method of Buchwald et al. [48] | [21,48,49] | - |
| Ion solubility | Diverse other variations | [22,50] | - |
| Reaction heat/bound water | R3 test (ASTM C1897-20) [51] | [33,52] | Good correlation to the 28 days relative compressive strength and moderate reproducibility |
| Reaction heat | Diverse variations | [53,54,55,56,57] | - |
| Electric conductivity | Diverse variations | [58,59] | - |
| Relative compressive strength | Diverse variations with cement paste, mortar, and concrete | Numerous studies | Often used as benchmark for reactivity; results strongly depending on the recipe |
| Process | Kaolinite | Montmorillonite | Illite |
|---|---|---|---|
| Temperature in °C | |||
| Dehydroxylation | ~450–700 | ~600–800 | ~450–700 |
| Amorphization | ~800–900 | ~900 | |
| Beginning sintering/melting | - | ~900 | |
| Beginning recrystallization | ~925 | ~1000 | |
| Parameter | n | |
|---|---|---|
| Relative mortar compressive strength at 28 d | 66 | |
| Content in the raw clay | Kaolinite | 55 |
| Smectite * | 24 | |
| Illite | 55 | |
| Chlorite | 16 | |
| Non-clay phases | 66 | |
| Calcite | 24 | |
| Applied/optimum calcination temperature | 64 | |
| Calcined clay | Amorphous content | 14 |
| Skeletal density | 13 | |
| Blaine | 20 | |
| BET | 11 | |
| Coefficient Range | Rating |
|---|---|
| 0.2–0.4 | Weak |
| 0.4–0.6 | Moderate |
| 0.6–0.8 | Strong |
| 0.8–1.0 | Very strong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Overmann, S.; Vollpracht, A.; Matschei, T. Reactivity of Calcined Clays as SCM—A Review. Materials 2024, 17, 312. https://doi.org/10.3390/ma17020312
Overmann S, Vollpracht A, Matschei T. Reactivity of Calcined Clays as SCM—A Review. Materials. 2024; 17(2):312. https://doi.org/10.3390/ma17020312
Chicago/Turabian StyleOvermann, Steffen, Anya Vollpracht, and Thomas Matschei. 2024. "Reactivity of Calcined Clays as SCM—A Review" Materials 17, no. 2: 312. https://doi.org/10.3390/ma17020312
APA StyleOvermann, S., Vollpracht, A., & Matschei, T. (2024). Reactivity of Calcined Clays as SCM—A Review. Materials, 17(2), 312. https://doi.org/10.3390/ma17020312








