Synthesis of Amphiphilic Block Copolymer and Its Application in Pigment-Based Ink
Abstract
1. Introduction
2. Experiment
2.1. Materials
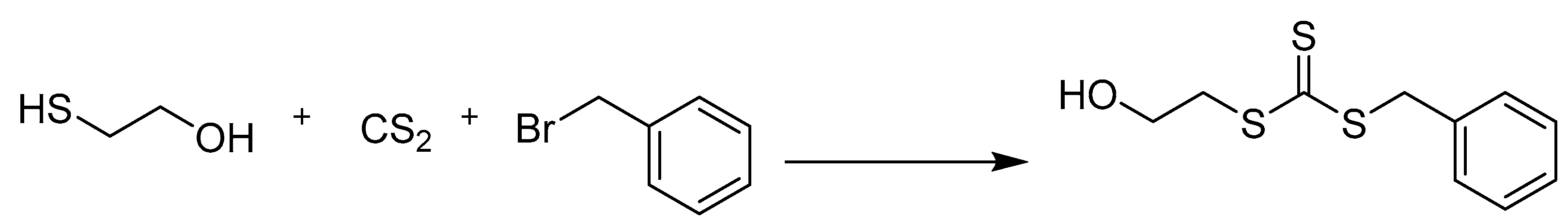
2.2. Synthesis of Benzyl 2-Hydroxyethyl Carbonotrithioate
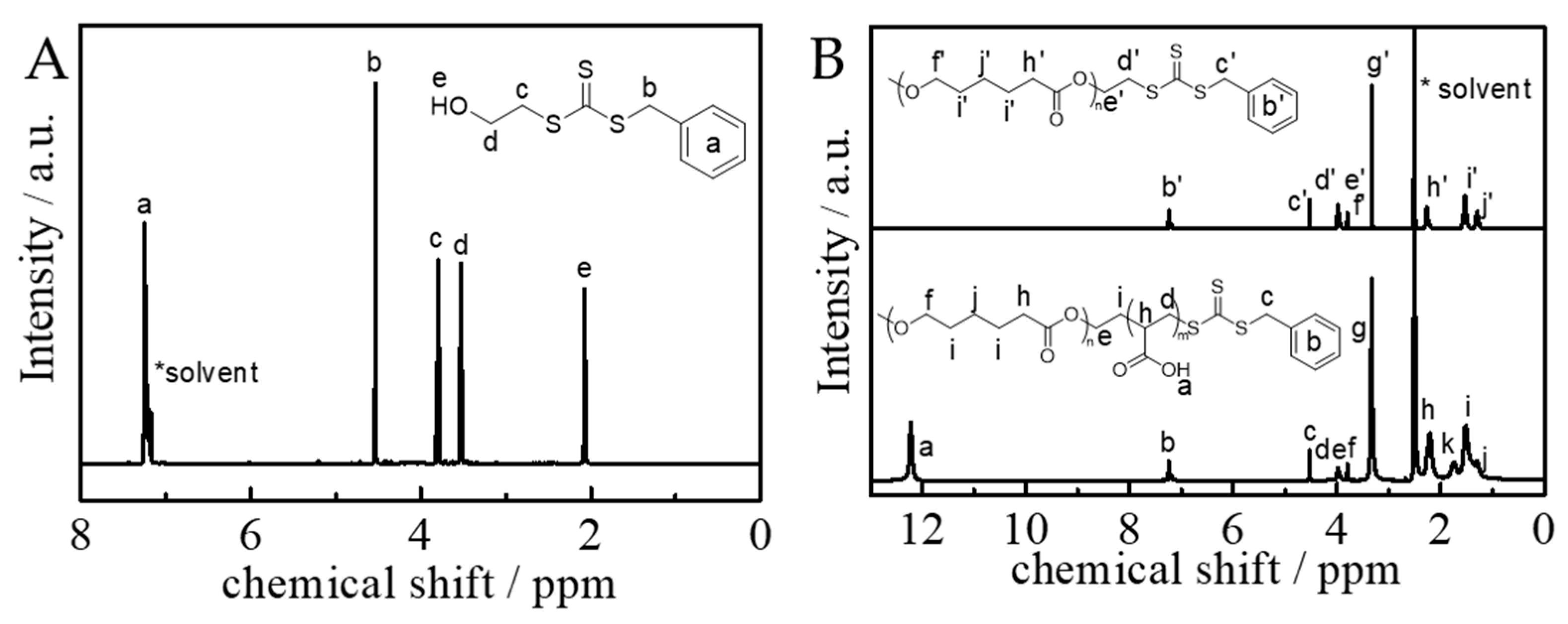
2.3. Synthesis of Amphiphilic AB Di-Block Copolymer of PCL-b-PAA
2.4. Preparation of Ink Dispersion
2.5. Characterizations
3. Results and Discussion
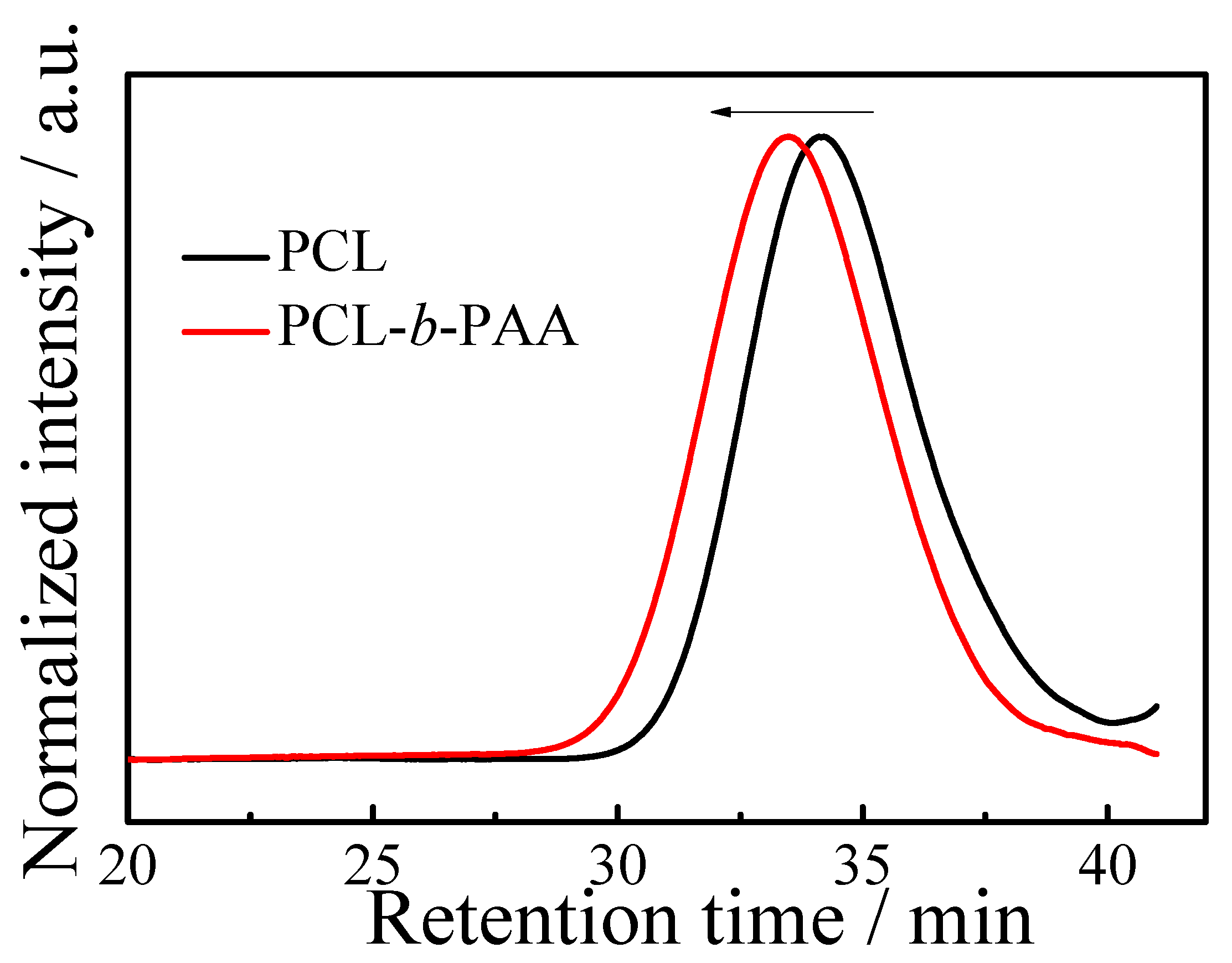
3.1. Synthesis and Structural Characterization of BSTSE and Amphiphilic Block Copolymer
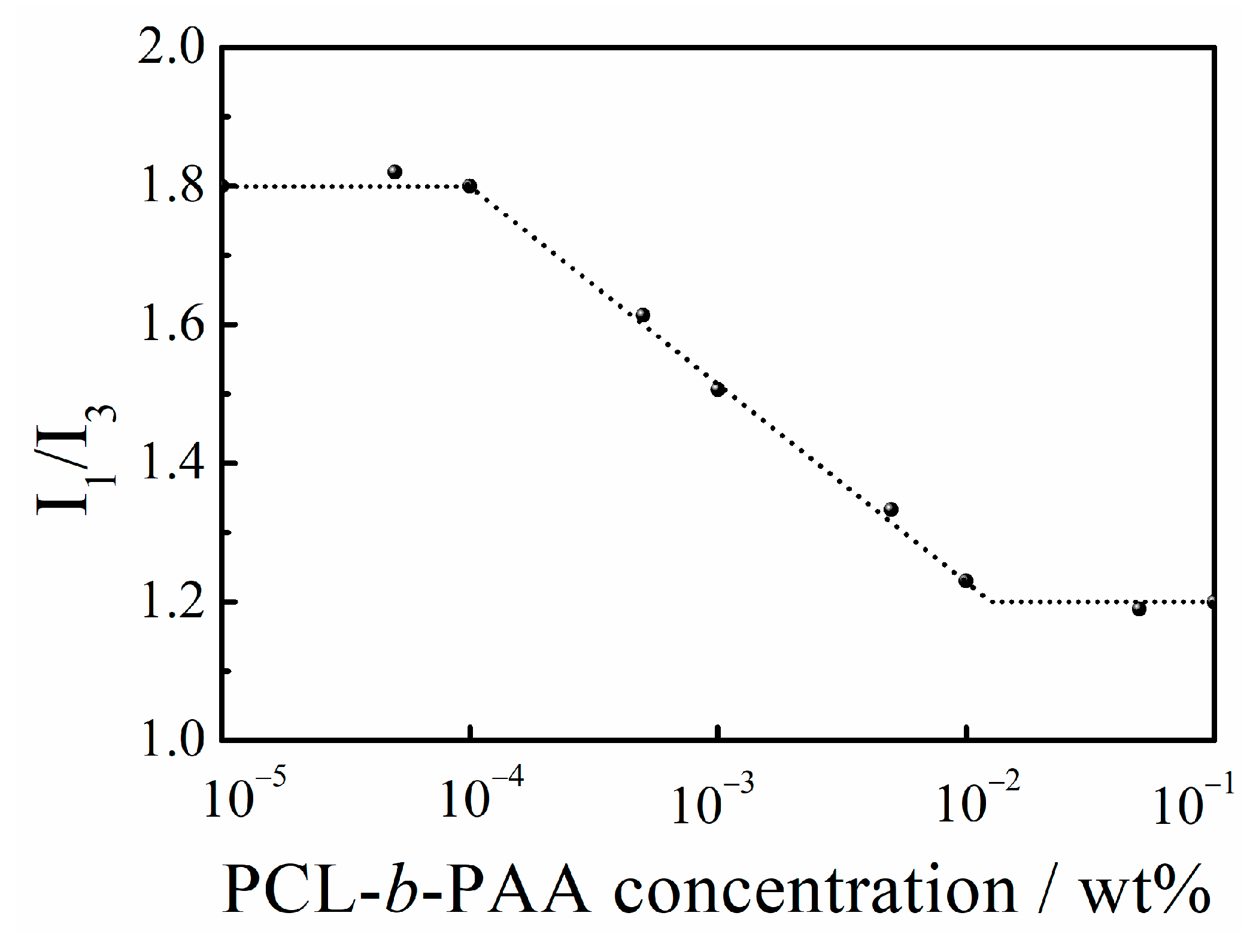
3.2. Aqueous Self-Assembly and Solution Behavior
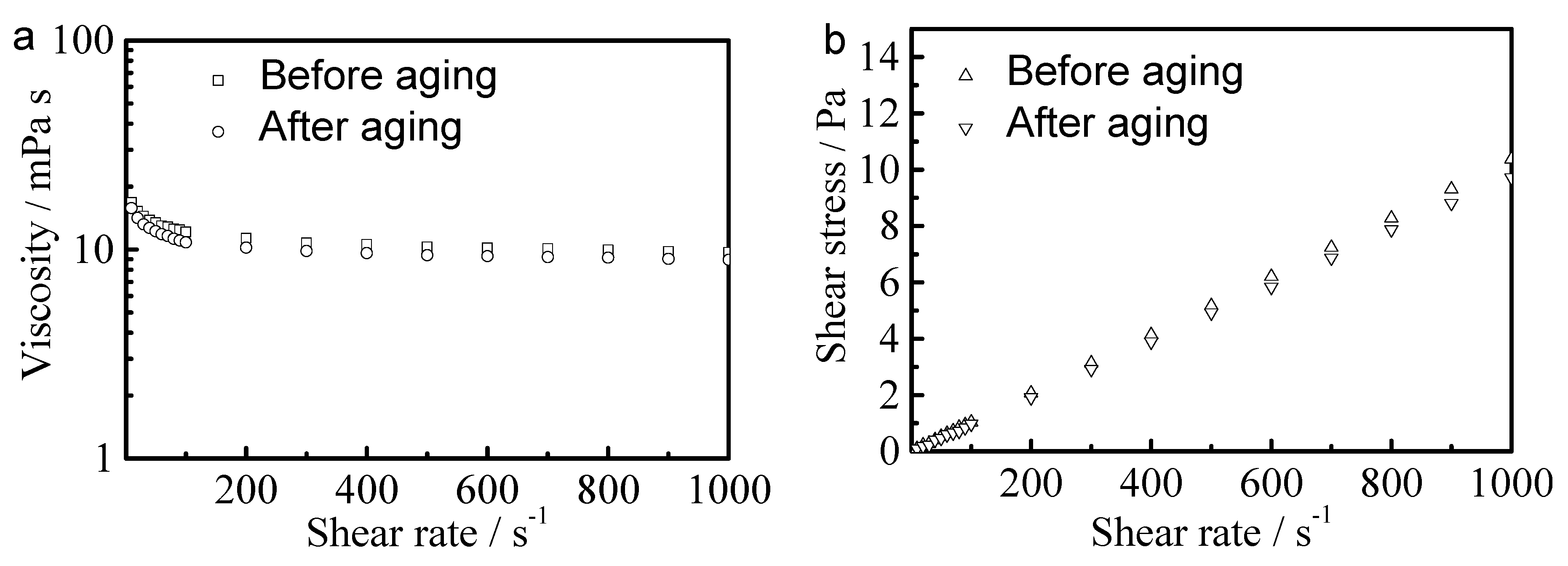
3.3. Rheology of Dye Dispersion with PCL-b-PAA
3.4. Zeta Potential and Interfacial Parameter
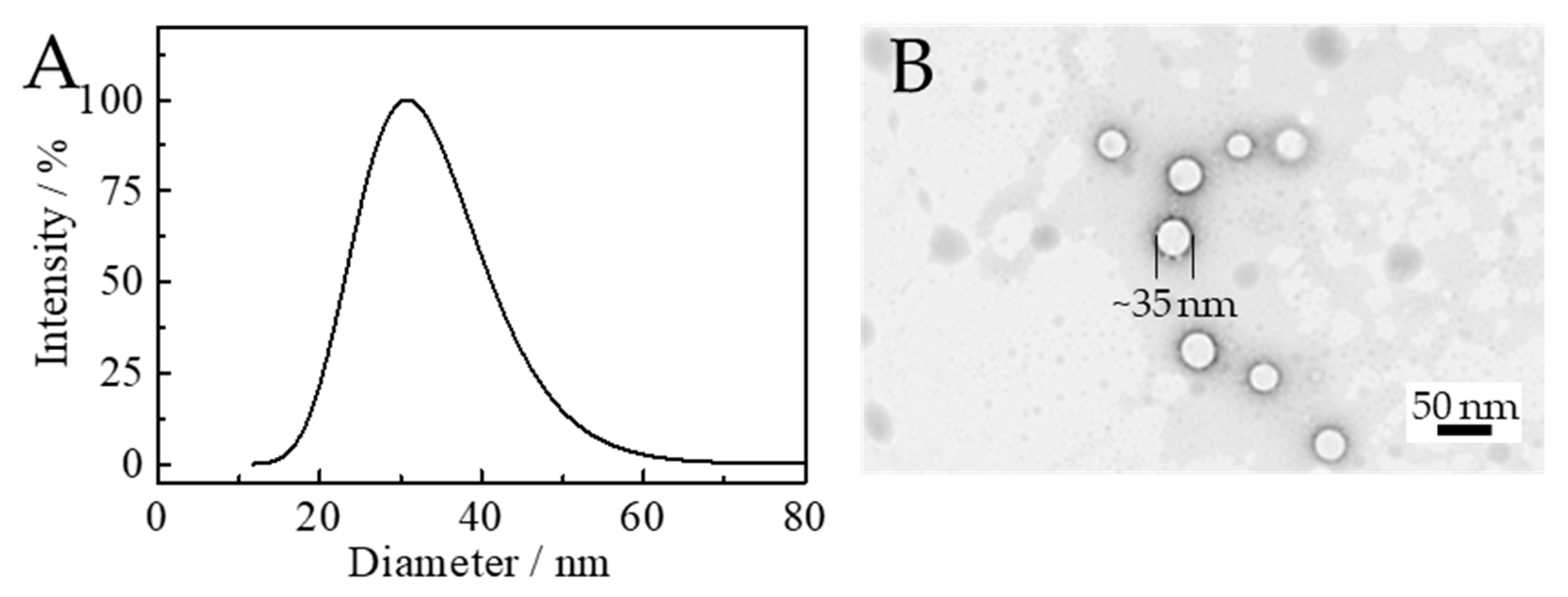
3.5. Particle Size Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panganiban, B.; Qiao, B.; Jiang, T.; DelRe, C.; Obadia, M.M.; Nguyen, T.D.; Smith, A.A.A.; Hall, A.; Sit, I.; Crosby, M.G.; et al. Random heteropolymers preserve protein function in foreign environments. Science 2018, 359, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Sugioka, Y.; Nakamura, J.; Ohtsuki, C.; Sugawara-Narutaki, A. Thixotropic Hydrogels Composed of Self-Assembled Nanofibers of Double-Hydrophobic Elastin-like Block Polypeptides. Int. J. Mol. Sci. 2021, 22, 4104. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.; Sonmez, S.; Tutak, D. Effect of coating pigment type on paper printability with water-based inks. J. Coat. Technol. Res. 2022, 19, 1149–1157. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, K.; Fang, K.; Shi, F.; Pan, Y.; Sun, F.; Wang, D.; Xie, R.; Chen, W. Insights into coloration enhancement of mercerized cotton fabric on reactive dye digital inkjet printing. RSC Adv. 2022, 12, 10386–10394. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Chen, R.; Wang, X.; Zhao, C.-X.; Chen, Q.; Hai, M.; Chen, D.; Yang, Z.; Weitz, D.A. Controlled co-precipitation of biocompatible colorant-loaded nanoparticles by microfluidics for natural color drinks. Lab A Chip 2019, 19, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.D.; Zheng, Y. The Formulation, Development and Application of Oil Dispersants. J. Mar. Sci. Eng. 2022, 10, 425. [Google Scholar] [CrossRef]
- Kim, G.Y.; Perumal, S.; Kim, S.C.; Lee, S.-H.; Noh, S.M.; Park, Y.I.; Cheong, I.W.; Kim, J.C. Design and prediction of dye dispersibility stabilized by polymeric dispersants using a Dye–Monomer interaction force measurement. Dye. Pigment. 2020, 172, 107791. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Tu, Z.; Hua, W.; He, P.; Li, H.; Zhang, B.; Ren, T. Influence of the hydrophilic moiety of polymeric surfactant on their surface activity and physical stability of pesticide suspension concentrate. J. Mol. Liq. 2020, 317, 114136. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020, 53, 2713–2723. [Google Scholar] [CrossRef]
- Yang, P.; Gao, W.; Zhang, T.; Pursch, M.; Luong, J.; Sattler, W.; Singh, A.; Backer, S. Two-dimensional liquid chromatography with active solvent modulation for studying monomer incorporation in copolymer dispersants. J. Sep. Sci. 2019, 42, 2805–2815. [Google Scholar] [CrossRef]
- Qi, L.; Ou, K.; Hou, Y.; Yuan, P.; Yu, W.; Li, X.; Wang, B.; He, J.; Cui, S.; Chen, X. Unidirectional water-transport antibacterial trilayered nanofiber-based wound dressings induced by hydrophilic-hydrophobic gradient and self-pumping effects. Mater. Des. 2021, 201, 109461. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Kaneti, Y.V.; Hou, D.; Yamauchi, Y.; Mai, Y. Self-assembly of block copolymers towards mesoporous materials for energy storage and conversion systems. Chem. Soc. Rev. 2020, 49, 4681–4736. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Mehrkhodavandi, P. Strategies for the synthesis of block copolymers with biodegradable polyester segments. Polym. Chem. 2021, 12, 783–806. [Google Scholar] [CrossRef]
- Cummins, C.; Lundy, R.; Walsh, J.J.; Ponsinet, V.; Fleury, G.; Morris, M.A. Enabling future nanomanufacturing through block copolymer self-assembly: A review. Nano Today 2020, 35, 100936. [Google Scholar] [CrossRef]
- Luan, M.; Shen, D.; Zhou, P.; Li, D.; Li, P.; Shi, B.; Wang, G. One-pot synthesis of block copolymer dispersant by ICAR ATRP with ppm copper catalyst and the dispersibility on pigment. Prog. Org. Coat. 2022, 169, 106914. [Google Scholar] [CrossRef]
- Chan, D.H.H.; Kynaston, E.L.; Lindsay, C.; Taylor, P.; Armes, S.P. Block Copolymer Nanoparticles are Effective Dispersants for Micrometer-Sized Organic Crystalline Particles. ACS Appl. Mater. Interfaces 2021, 13, 30235–30243. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Tanaka, H.; Suwa, Y.; Irifune, S.; Osawa, S.; Otsuka, H. Tuning Pre-Solution of an Amphiphilic Polymeric Dispersant with Low Acid-Value toward Colored-Ink Preparation. Appl. Sci. 2023, 13, 1834. [Google Scholar] [CrossRef]
- Varlas, S.; Lawrenson, S.B.; Arkinstall, L.A.; O’Reilly, R.K.; Foster, J.C. Self-assembled nanostructures from amphiphilic block copolymers prepared via ring-opening metathesis polymerization (ROMP). Prog. Polym. Sci. 2020, 107, 101278. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Ma, J.; Yang, X.; Deng, Y. Recent advances in amphiphilic block copolymer templated mesoporous metal-based materials: Assembly engineering and applications. Chem. Soc. Rev. 2020, 49, 1173–1208. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, S.; Li, Y.; Shi, J. Cooperative organizations of small molecular surfactants and amphiphilic block copolymers: Roles of surfactants in the formation of binary co-assemblies. Aggregate 2021, 2, e49. [Google Scholar] [CrossRef]
- Song, Y.; Fang, K.; Bukhari, M.N.; Zhang, K.; Tang, Z. Improved inkjet printability of dye-based inks through enhancing the interaction of dye molecules and polymer nanospheres. J. Mol. Liq. 2021, 324, 114702. [Google Scholar] [CrossRef]
- Grime, R.L.; Goulding, J.; Uddin, R.; Stoddart, L.A.; Hill, S.J.; Poyner, D.R.; Briddon, S.J.; Wheatley, M. Single molecule binding of a ligand to a G-protein-coupled receptor in real time using fluorescence correlation spectroscopy, rendered possible by nano-encapsulation in styrene maleic acid lipid particles. Nanoscale 2020, 12, 11518–11525. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, H.J. Polymeric Dispersants in Ink Jet Technology. Adv. Mater. 1998, 10, 1215–1218. [Google Scholar] [CrossRef]
- Ohtake, T.; Ito, H.; Toyoda, N. Amphiphilic block copolymer surfactant-containing quaternized pyridinium salt segments for color dispersion. Polym. J. 2022, 54, 1203–1211. [Google Scholar] [CrossRef]
- Colombani, O.; Lejeune, E.; Charbonneau, C.; Chassenieux, C.; Nicolai, T. Ionization of Amphiphilic Acidic Block Copolymers. J. Phys. Chem. B 2012, 116, 7560–7565. [Google Scholar] [CrossRef] [PubMed]
- Charleux, B.; Delaittre, G.; Rieger, J.; D’Agosto, F. Polymerization-Induced Self-Assembly: From Soluble Macromolecules to Block Copolymer Nano-Objects in One Step. Macromolecules 2012, 45, 6753–6765. [Google Scholar] [CrossRef]
- Loiseau, J.; Doërr, N.; Suau, J.M.; Egraz, J.B.; Llauro, M.F.; Ladavière, C.; Claverie, J. Synthesis and Characterization of Poly(acrylic acid) Produced by RAFT Polymerization. Application as a Very Efficient Dispersant of CaCO3, Kaolin, and TiO2. Macromolecules 2003, 36, 3066–3077. [Google Scholar] [CrossRef]
- Kuila, D.; Blay, G.A.; Borjas, R.E.; Hughes, S.; Maddox, P.; Rice, K.; Stansbury, W.; Laurel, N. Polyacrylic acid (poly-A) as a chelant and dispersant. J. Appl. Polym. Sci. 1999, 73, 1097–1115. [Google Scholar] [CrossRef]
- Chen, X.; Huang, W.; He, B.; Zhang, Y. Synthesis and Application of Tackifying Dispersant Poly (Vinyl Alcohol-Acrylic Acid-Triallyl Cyanate). Polymers 2022, 14, 557. [Google Scholar] [CrossRef]
- Ayyangar, N.R.; Srinivasan, K.V.; Daniel, T. Polycyclic compounds part VI. structural features of C.I. disperse yellow 232. Dye. Pigment. 1990, 13, 301–310. [Google Scholar] [CrossRef]
- Skey, J.; O’Reilly, R.K. Facile one pot synthesis of a range of reversible addition–fragmentation chain transfer (RAFT) agents. Chem. Commun. 2008, 35, 4183–4185. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Lund, R.; Willner, L.; Richter, D.; Lindner, P.; Narayanan, T. Kinetic Pathway of the Cylinder-to-Sphere Transition in Block Copolymer Micelles Observed in Situ by Time-Resolved Neutron and Synchrotron Scattering. ACS Macro Lett. 2013, 2, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A. Solvent Effect on the Vibrational Structures of the Fluorescence and Absorption Spectra of Pyrene. Bull. Chem. Soc. Jpn. 1971, 44, 3272–3277. [Google Scholar] [CrossRef]
- Poozesh, S.; Akafuah, N.; Saito, K. Effects of automotive paint spray technology on the paint transfer efficiency—A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2017, 232, 282–301. [Google Scholar] [CrossRef]
- Shin, P.; Sung, J.; Lee, M.H. Control of droplet formation for low viscosity fluid by double waveforms applied to a piezoelectric inkjet nozzle. Microelectron. Reliab. 2011, 51, 797–804. [Google Scholar] [CrossRef]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym. Sci. 2008, 286, 1173–1180. [Google Scholar] [CrossRef]
- Jin, S.; Liu, M.; Zhang, F.; Chen, S.; Niu, A. Synthesis and characterization of pH-sensitivity semi-IPN hydrogel based on hydrogen bond between poly(N-vinylpyrrolidone) and poly(acrylic acid). Polymer 2006, 47, 1526–1532. [Google Scholar] [CrossRef]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of Ionic Strength, pH, and Cation Valence on Aggregation Kinetics of Titanium Dioxide Nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef]
- He, B.; Yang, S.; Qin, Z.; Wen, B.; Zhang, C. The roles of wettability and surface tension in droplet formation during inkjet printing. Sci. Rep. 2017, 7, 11841. [Google Scholar] [CrossRef]
- Zhang, L.; Eisenberg, A. Morphogenic Effect of Added Ions on Crew-Cut Aggregates of Polystyrene-b-poly(acrylic acid) Block Copolymers in Solutions. Macromolecules 1996, 29, 8805–8815. [Google Scholar] [CrossRef]
- Dong, Y.; Mosquera-Giraldo, L.I.; Troutman, J.; Skogstad, B.; Taylor, L.S.; Edgar, K.J. Amphiphilic hydroxyalkyl cellulose derivatives for amorphous solid dispersion prepared by olefin cross-metathesis. Polym. Chem. 2016, 7, 4953–4963. [Google Scholar] [CrossRef]
- Yu, J.; Grossiord, N.; Koning, C.E.; Loos, J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon 2007, 45, 618–623. [Google Scholar] [CrossRef]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and Sedimentation of Aqueous Nanoscale Zerovalent Iron Dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and Deposition of Engineered Nanomaterials in Aquatic Environments: Role of Physicochemical Interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef]
- Phenrat, T.; Saleh, N.; Sirk, K.; Kim, H.-J.; Tilton, R.D.; Lowry, G.V. Stabilization of aqueous nanoscale zerovalent iron dispersions by anionic polyelectrolytes: Adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation. J. Nanoparticle Res. 2008, 10, 795–814. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]



| Sample | ζ Potential (mV) a | γ (mN m−2) b | Γmax × 106 (mol m−2) | Amin (nm2 molecule−1) |
|---|---|---|---|---|
| PCL-b-PAA | −14 | 48 | 2.05 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Xu, J. Synthesis of Amphiphilic Block Copolymer and Its Application in Pigment-Based Ink. Materials 2024, 17, 330. https://doi.org/10.3390/ma17020330
Yuan J, Xu J. Synthesis of Amphiphilic Block Copolymer and Its Application in Pigment-Based Ink. Materials. 2024; 17(2):330. https://doi.org/10.3390/ma17020330
Chicago/Turabian StyleYuan, Jingjing, and Jinbao Xu. 2024. "Synthesis of Amphiphilic Block Copolymer and Its Application in Pigment-Based Ink" Materials 17, no. 2: 330. https://doi.org/10.3390/ma17020330
APA StyleYuan, J., & Xu, J. (2024). Synthesis of Amphiphilic Block Copolymer and Its Application in Pigment-Based Ink. Materials, 17(2), 330. https://doi.org/10.3390/ma17020330






