Preparation and Compression Behavior of High Porosity, Microporous Open-Cell Al Foam Using Supergravity Infiltration Method
Abstract
1. Introduction
2. Experimental
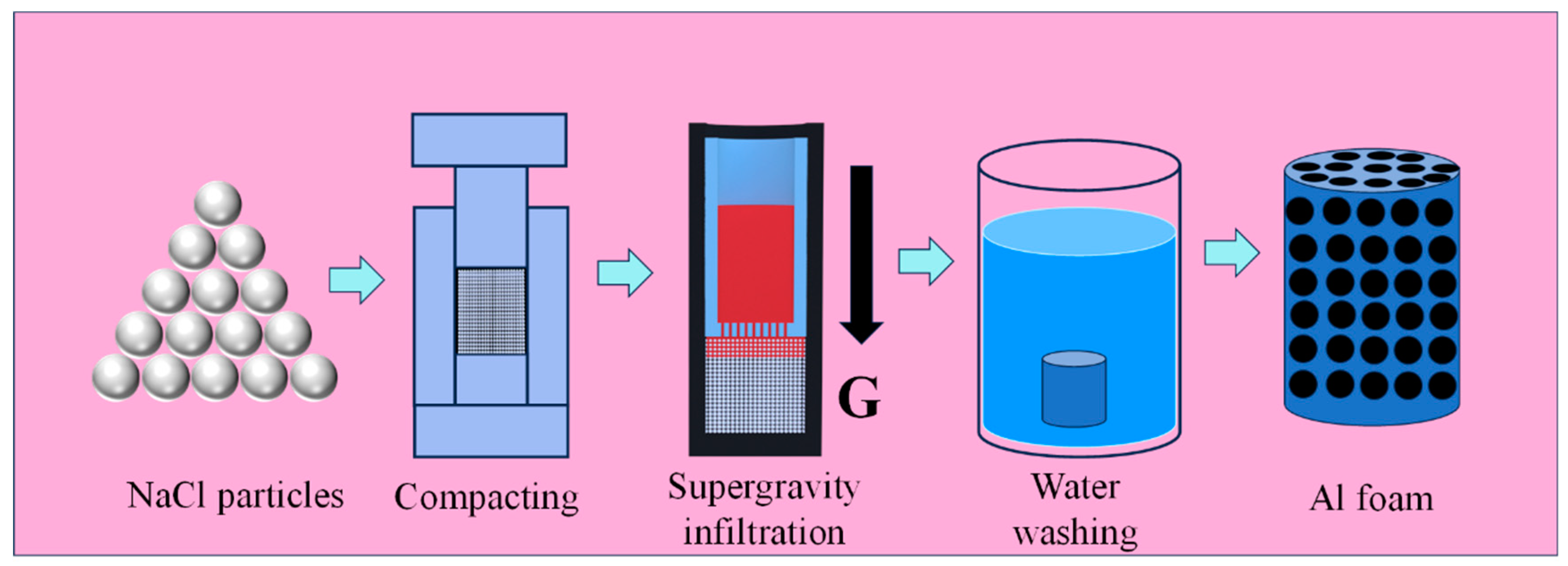
2.1. Materials
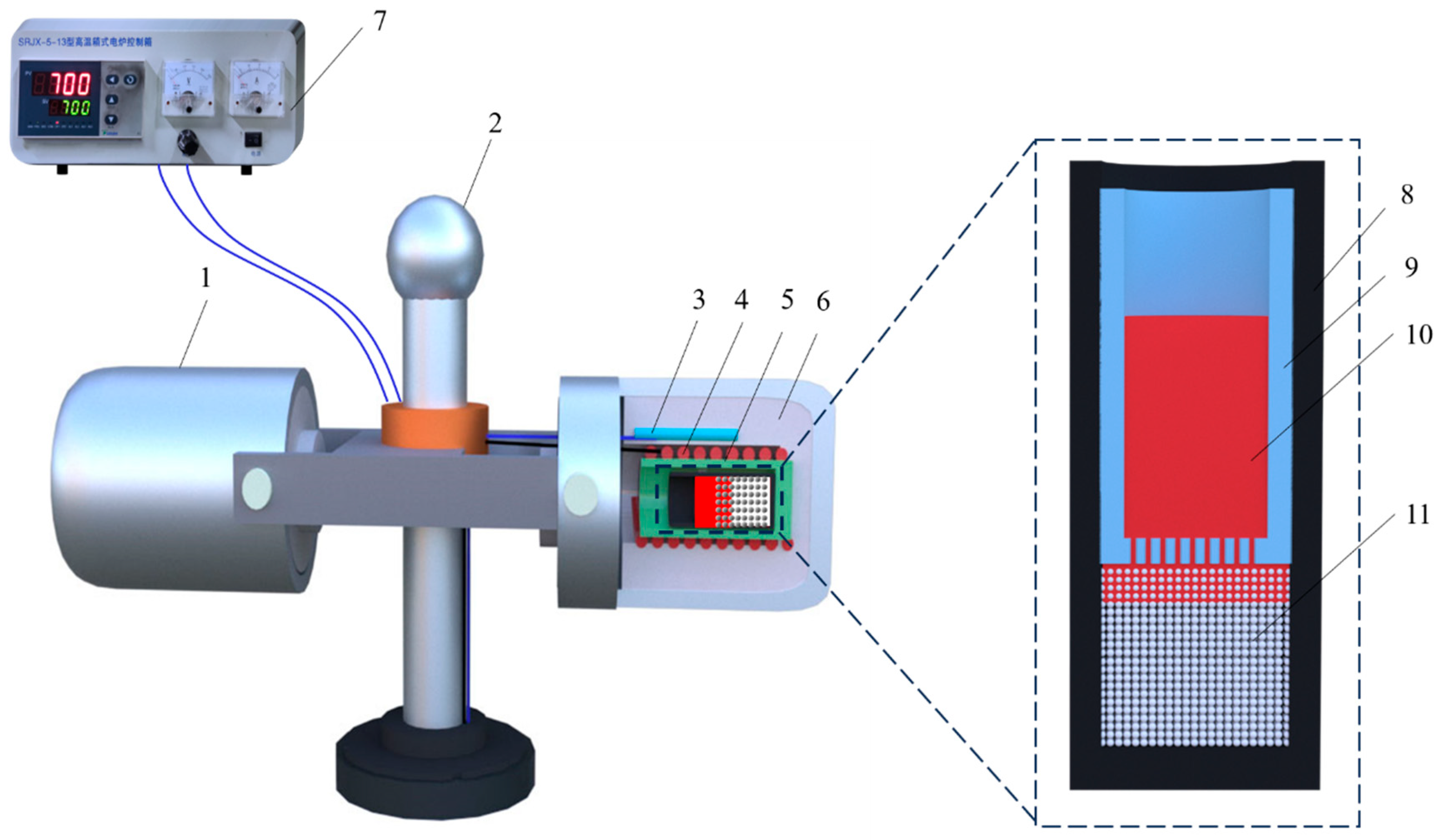
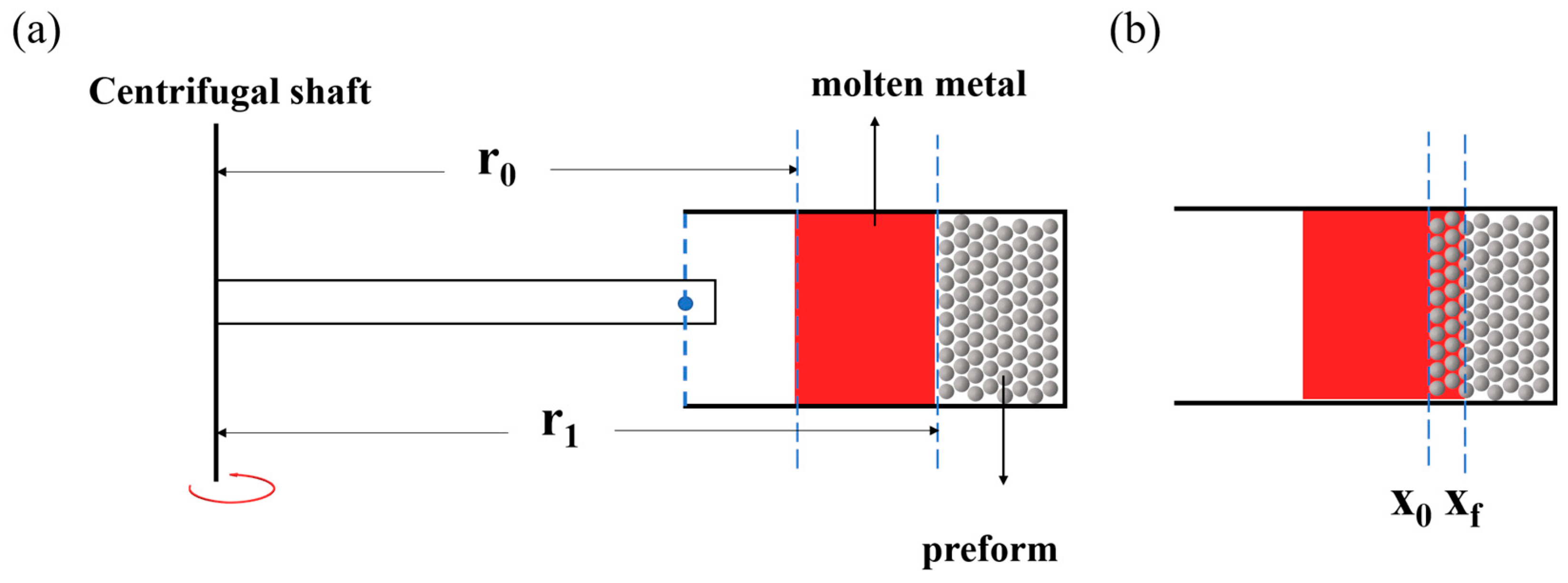
2.2. Centrifugal Infiltration
2.3. Characterization
3. Results
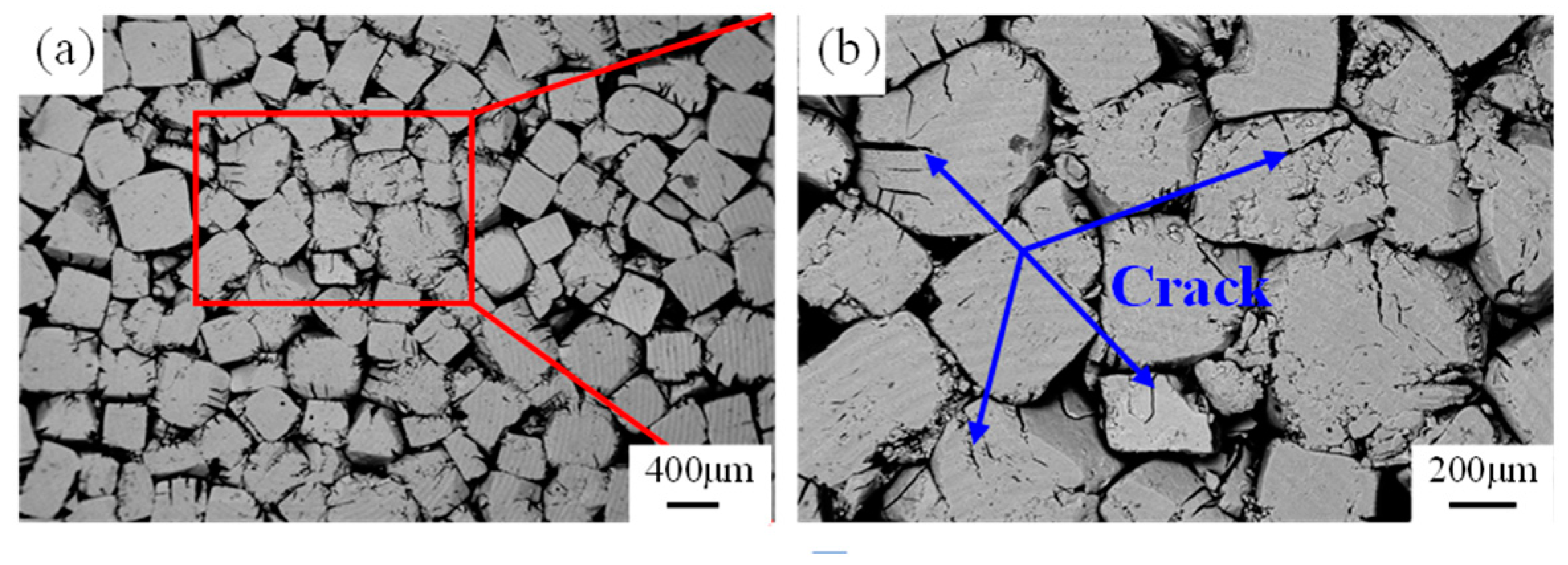
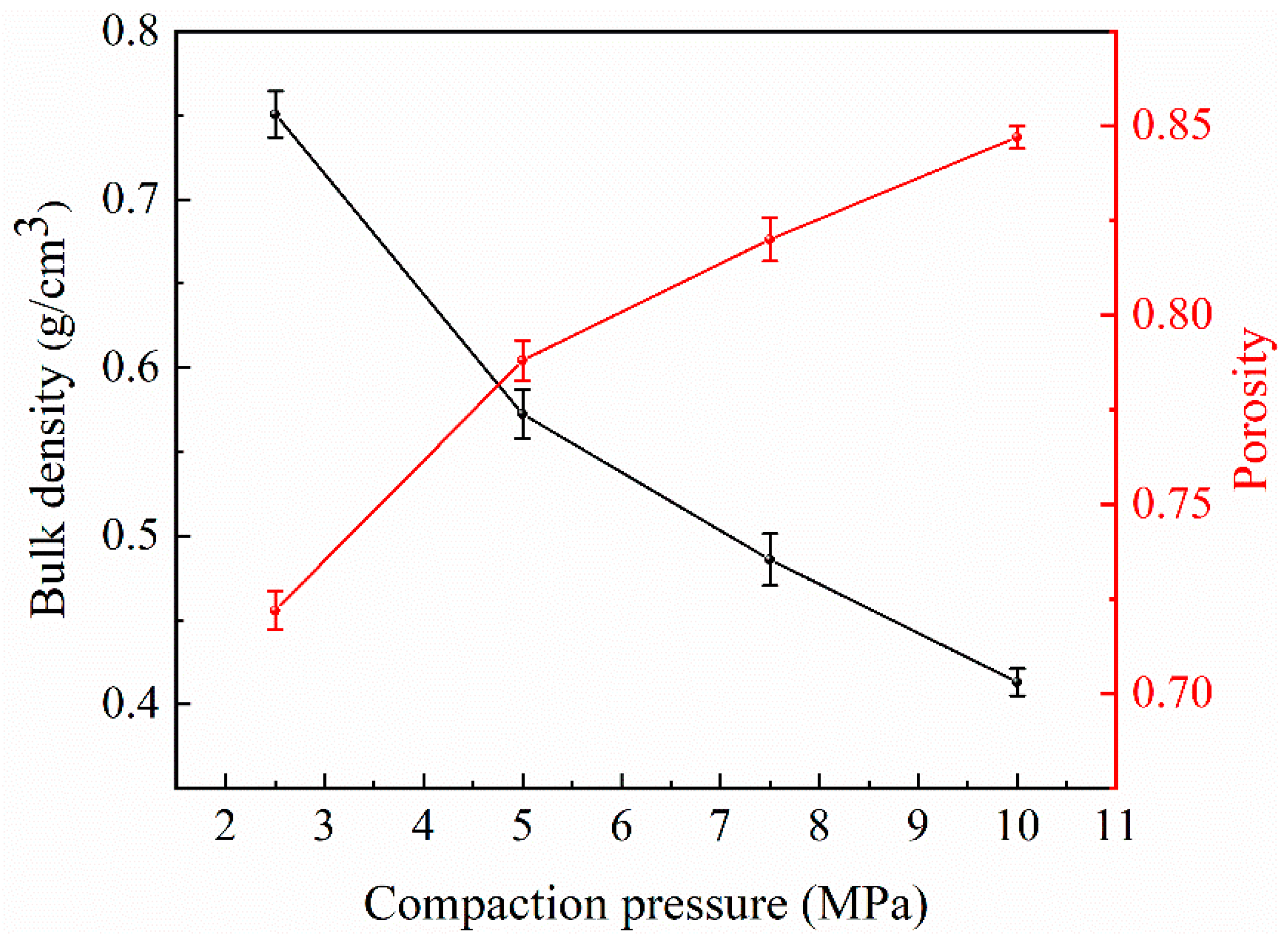
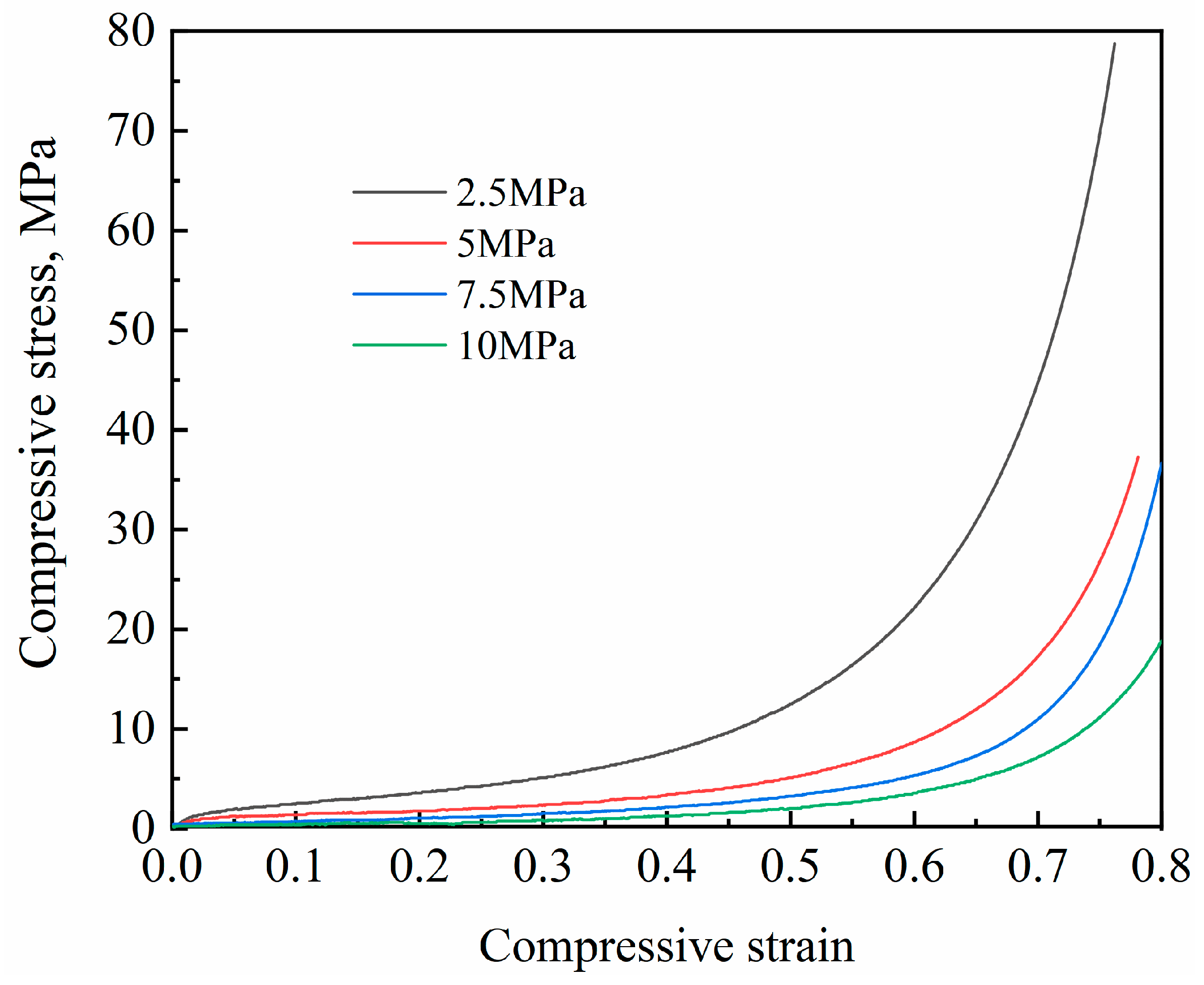
3.1. Effect of Compaction Pressure on NaCl Preform
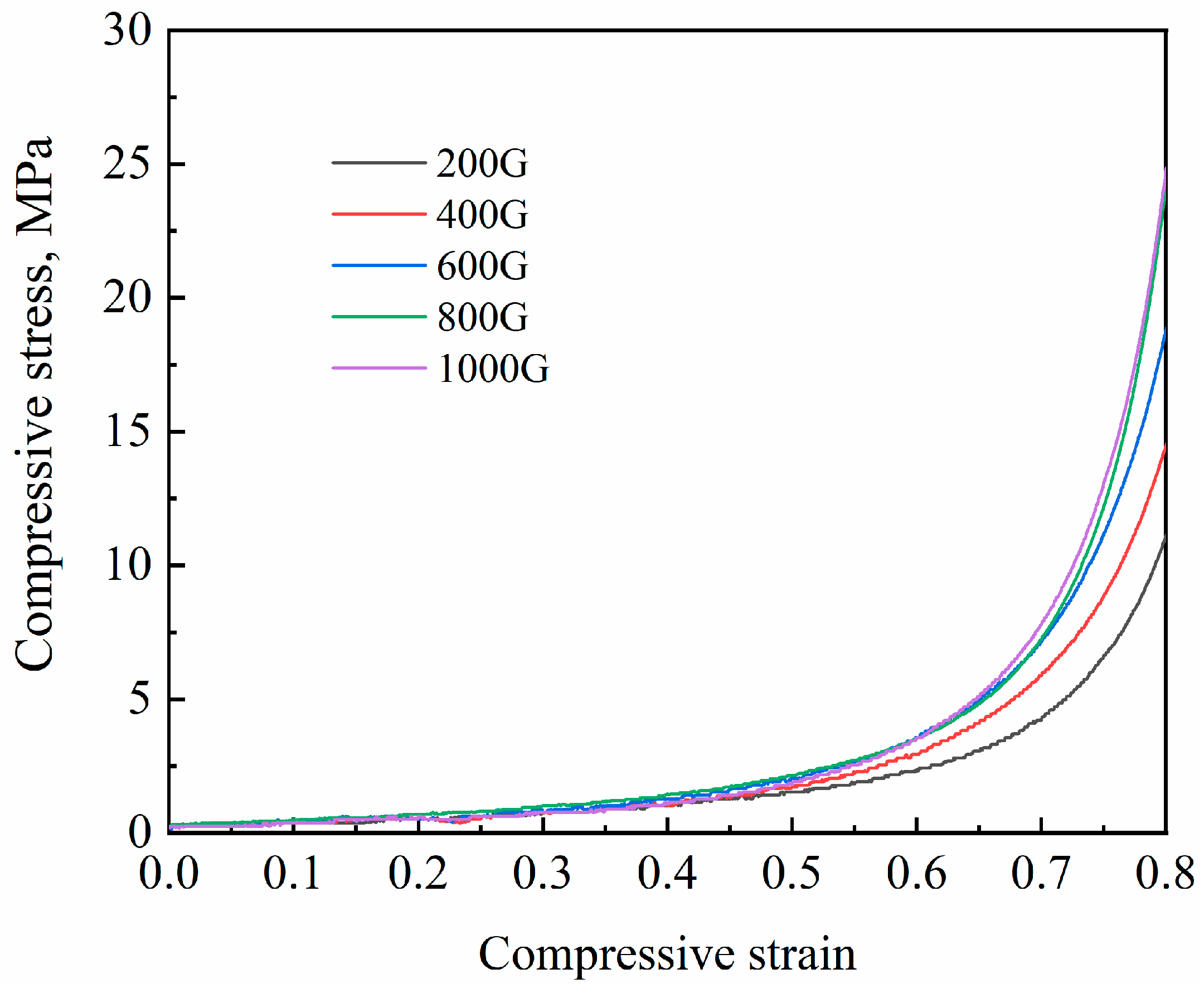
3.2. Effect of Gravity Coefficient
4. Discussion
4.1. Compressive Behavior Sensitivity
4.2. Supergravity Infiltration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oriňaková, R.; Gorejová, R.; Králová, Z.O.; Petráková, M.; Oriňak, A. Novel trends and recent progress on preparation methods of biodegradable metallic foams for biomedicine: A review. J. Mater. Sci. 2021, 56, 13925–13963. [Google Scholar] [CrossRef]
- du Plessis, A.; Razavi, N.; Benedetti, M.; Murchio, S.; Leary, M.; Watson, M.; Bhate, D.; Berto, F. Properties and applications of additively manufactured metallic cellular materials: A review. Prog. Mater. Sci. 2022, 125, 100918. [Google Scholar] [CrossRef]
- Mohsenizadeh, M.; Gasbarri, F.; Munther, M.; Beheshti, A.; Davami, K. Additively-manufactured lightweight Metamaterials for energy absorption. Mater. Des. 2018, 139, 521–530. [Google Scholar] [CrossRef]
- Parveez, B.; Jamal, N.A.; Anuar, H.; Ahmad, Y.; Aabid, A.; Baig, M. Microstructure and Mechanical Properties of Metal Foams Fabricated via Melt Foaming and Powder Metallurgy Technique: A Review. Materials 2022, 15, 5302. [Google Scholar] [CrossRef] [PubMed]
- Peroni, L.; Avalle, M.; Peroni, M. The mechanical behaviour of aluminium foam structures in different loading conditions. Int. J. Impact Eng. 2008, 35, 644–658. [Google Scholar] [CrossRef]
- Vinay, B.U.; Rao, K.S. Development of Aluminum Foams by Different Methods and Evaluation of its Density by Archimedes Principle. Bonfring Int. J. Ind. Eng. Manag. Sci. 2012, 2, 148–152. [Google Scholar]
- Thiyagarajan, R.; Senthil Kumar, M. A Review on Closed Cell Metal Matrix Syntactic Foams: A Green Initiative towards Eco-Sustainability. Mater. Manuf. Process. 2021, 36, 1333–1351. [Google Scholar] [CrossRef]
- Harussani, M.M.; Sapuan, S.M.; Nadeem, G.; Rafin, T.; Kirubaanand, W. Recent applications of carbon-based composites in defence industry: A review. Def. Technol. 2022, 18, 1281–1300. [Google Scholar] [CrossRef]
- Afolabi, L.O.; Ariff, Z.M.; Hashim, S.F.S.; Alomayri, T.; Mahzan, S.; Kamarudin, K.-A.; Muhammad, I.D. Syntactic foams formulations, production techniques, and industry applications: A review. J. Mater. Res. Technol. 2020, 9, 10698–10718. [Google Scholar]
- Broxtermann, S.; Su, M.M.; Hao, H.; Fiedler, T. Comparative study of stir casting and infiltration casting of expanded glass-aluminium syntactic foams. J. Alloys Compd. 2020, 845, 155415. [Google Scholar] [CrossRef]
- Zou, Y.; He, D.; Jiang, J. New type of spherical pore Al alloy foam with low porosity and high strength. Sci. China Ser. B Chem. 2004, 47, 407–413. [Google Scholar] [CrossRef]
- Casas, B.Y.; Carranza, J.C.; Béjar, L.; Aguilar, C.; Figueroa, I.A.; Alfonso, I. Production of aluminum foams with hierarchical porosity by a combination of two different manufacturing methods. J. Alloys Compd. 2020, 831, 154780. [Google Scholar] [CrossRef]
- Soni, B.; Biswas, S. Evaluation of mechanical properties under quasi-static compression of open-cell foams of 6061-T6 Al alloy fabricated by pressurized salt infiltration casting method. Mater. Charact. 2017, 130, 198–203. [Google Scholar] [CrossRef]
- Xia, Y.; Shi, J.; Mu, Y. Compressive behaviour of open-cell Al–Si alloy foam produced by infiltration casting. Mater. Sci. Technol. 2023, 39, 2433–2445. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, J.; Chang, K.; Meng, L.; Zhang, N.; Guo, Z. Manufacturing of open-cell aluminum foams via infiltration casting in super-gravity fields and mechanical properties. RSC Adv. 2018, 8, 15933–15939. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Z.; Guo, L.; Meng, L.; Guo, Z. Rapid fabrication of W–Cu composites via low-temperature infiltration in supergravity fields. J. Alloys Compd. 2019, 809, 151782. [Google Scholar] [CrossRef]
- Shunmugasamy, V.C.; Mansoor, B. Compressive behavior of a rolled open-cell aluminum foam. Mater. Sci. Eng. A 2018, 715, 281–294. [Google Scholar] [CrossRef]
- Bo, Z.; Tianning, C. Calculation of sound absorption characteristics of porous sintered fiber metal. Appl. Acoust. 2009, 70, 337–346. [Google Scholar] [CrossRef]
- Avalle, M.; Belingardi, G.; Ibba, A. Mechanical models of cellular solids: Parameters identification from experimental tests. Int. J. Impact Eng. 2007, 34, 3–27. [Google Scholar] [CrossRef]
- Maconachie, T.; Leary, M.; Lozanovski, B.; Zhang, X.; Qian, M.; Faruque, O.; Brandt, M. SLM lattice structures: Properties, performance, applications and challenges. Mater. Des. 2019, 183, 108137. [Google Scholar] [CrossRef]
- Sánchez-Martínez, A.; Cruz, A.; González-Nava, M.; Suárez, M.A. Main process parameters for manufacturing open-cell Zn-22Al-2Cu foams by the centrifugal infiltration route and mechanical properties. Mater. Des. 2016, 108, 494–500. [Google Scholar] [CrossRef]
- El Sayed, Z.; Abd-Alrazzaq, M.; Ahmed, M.H. Experimental investigation of infiltration casting process parameters to produce open-cell Al-A356 alloy foams for functional and mechanical applications. Int. J. Adv. Manuf. Technol. 2022, 119, 6761–6774. [Google Scholar] [CrossRef]
- Wan, T.; Liang, G.-Q.; Wang, Z.-M.; Zhou, C.-X.; Liu, Y. Fabrication and compressive behavior of open-cell aluminum foams via infiltration casting using spherical CaCl2 space-holders. China Foundry 2022, 19, 89–98. [Google Scholar] [CrossRef]
- Kim, J.; Kordijazi, A.; Rohatgi, P. Pressure Infiltration of Aluminum Melts into a Loose Bed of Hollow Cenosphere Particles. JOM 2022, 74, 2071–2082. [Google Scholar] [CrossRef]
- Garcia-Cordovilla, C.; Louis, E.; Narciso, J. Pressure infiltration of packed ceramic particulates by liquid metals. Acta Mater. 1999, 47, 4461–4479. [Google Scholar] [CrossRef]
- Goodall, R.; Despois, J.-F.; Marmottant, A.; Salvo, L.; Mortensen, A. The effect of preform processing on replicated aluminium foam structure and mechanical properties. Scr. Mater. 2006, 54, 2069–2073. [Google Scholar] [CrossRef]
- Njoku, R.E.; Boniface, O.O.; Aigbodion, V.S. Influence of infiltration pressure on the window size and relative density of open-cell porous aluminium manufactured by replication casting. Int. J. Adv. Manuf. Technol. 2023, 126, 3015–3021. [Google Scholar] [CrossRef]
- Wannasin, J.; Flemings, M.C. Fabrication of metal matrix composites by a high-pressure centrifugal infiltration process. J. Mater. Process. Technol. 2005, 169, 143–149. [Google Scholar] [CrossRef]
- Siebold, A.; Nardin, M.; Schultz, J.; Walliser, A.; Oppliger, M. Effect of dynamic contact angle on capillary rise phenomena. Colloids Surf. A Physicochem. Eng. Asp. 2000, 161, 81–87. [Google Scholar] [CrossRef]
- Etemadi, R.; Wang, B.; Pillai, K.M.; Niroumand, B.; Omrani, E.; Rohatgi, P. Pressure infiltration processes to synthesize metal matrix composites—A review of metal matrix composites, the technology and process simulation. Mater. Manuf. Process. 2018, 33, 1261–1290. [Google Scholar] [CrossRef]
- Nishida, Y.; Ohira, G. Modelling of infiltration of molten metal in fibrous preform by centrifugal force. Acta Mater. 1999, 47, 841–852. [Google Scholar] [CrossRef]
- Otomo, R.; Harada, S. End Effect on Permeability of Loose Particulate Bed Having Different Internal Structures. Part. Sci. Technol. 2011, 29, 2–13. [Google Scholar] [CrossRef]
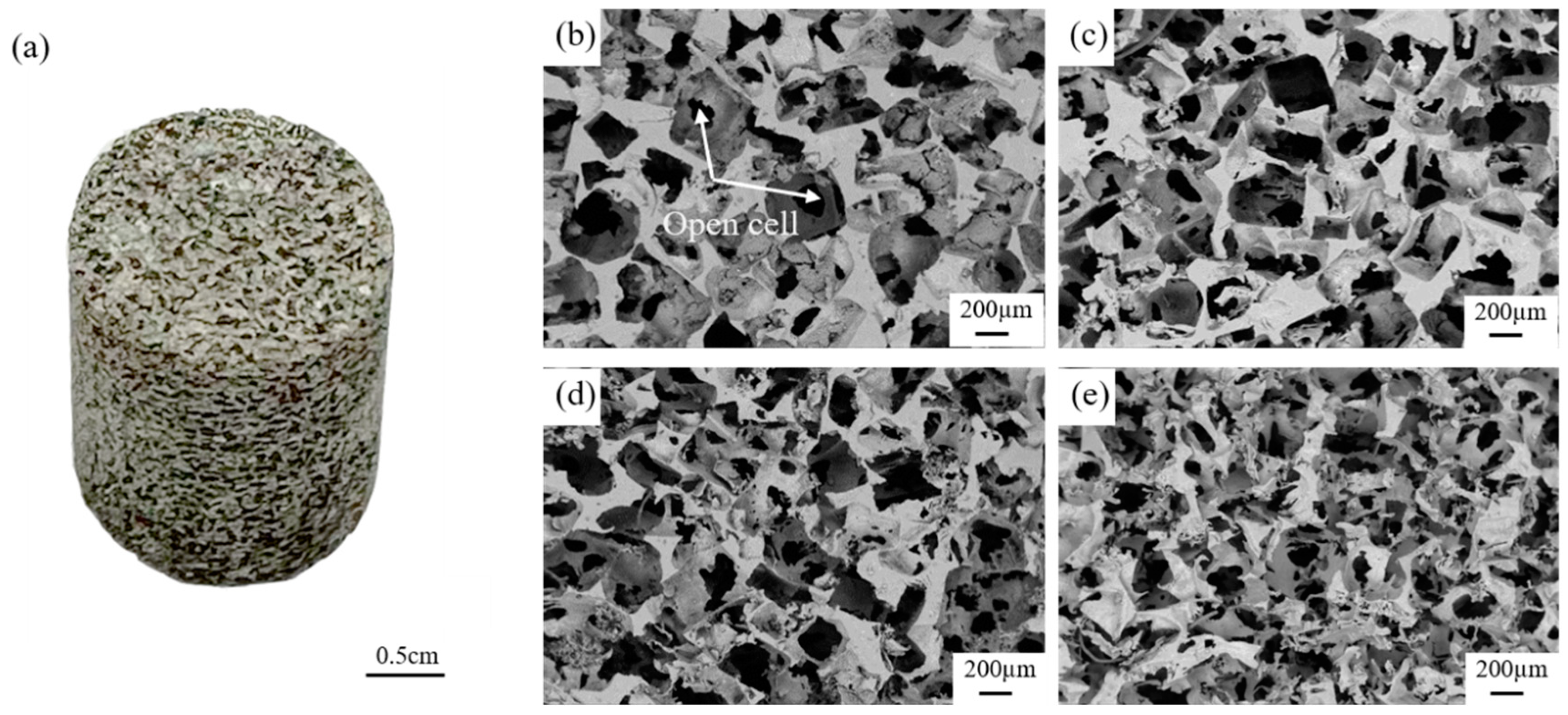

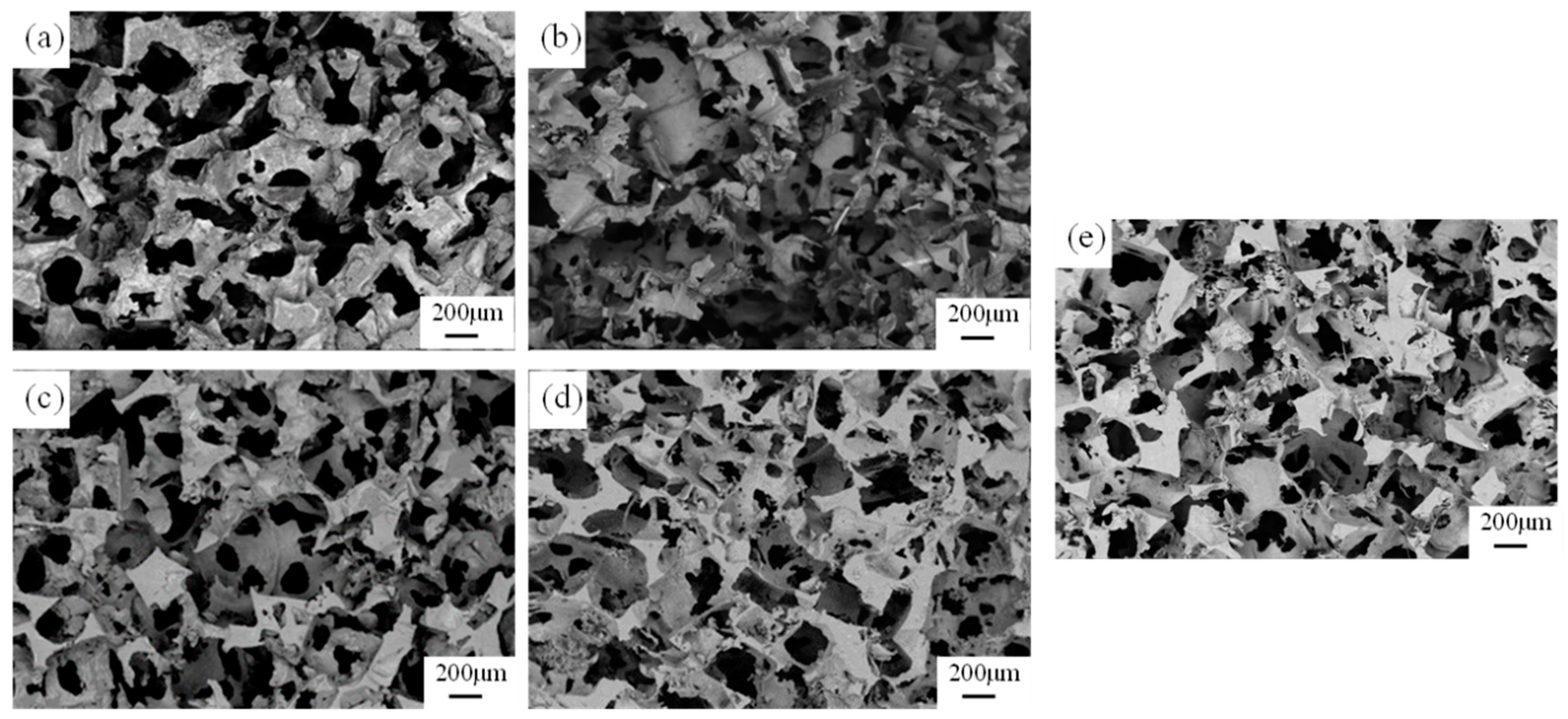

| Group | Compaction Pressure, MPa | Gravity Coefficient |
|---|---|---|
| 1 | 2.5 | 1000 |
| 2 | 5 | 1000 |
| 3 | 7.5 | 1000 |
| 4 | 10 | 1000 |
| 5 | 10 | 200 |
| 6 | 10 | 400 |
| 7 | 10 | 600 |
| 8 | 10 | 800 |
| Compaction Pressure, MPa | Bulk Density (ρc), g/cm3 | Porosity (ϕ) | Yield Strength (σs), MPa | Plateau Stress (σpl), MPa | Densification Strain (εD) |
|---|---|---|---|---|---|
| 2.5 | 0.751 ± 0.014 | 0.722 ± 0.005 | 1.68 ± 0.008 | 7.88 ± 0.07 | 0.64 ± 0.003 |
| 5 | 0.572 ± 0.014 | 0.788 ± 0.005 | 1.02 ± 0.007 | 3.89 ± 0.06 | 0.67 ± 0.003 |
| 7.5 | 0.486 ± 0.015 | 0.820 ± 0.006 | 0.57 ± 0.006 | 3.11 ± 0.09 | 0.73 ± 0.004 |
| 10 | 0.413 ± 0.008 | 0.847 ± 0.003 | 0.38 ± 0.005 | 2.08 ± 0.02 | 0.74 ± 0.002 |
| Graity Coefficient | Bulk Density (ρc), g/cm3 | Porosity (ϕ) | Yield Strength (σs), MPa | Plateau Stress (σpl), MPa | Densification Strain (εD) |
|---|---|---|---|---|---|
| 200 | 0.343 ± 0.008 | 0.873 ± 0.003 | 0.26 ± 0.006 | 1.57 ± 0.03 | 0.76 ± 0.002 |
| 400 | 0.381 ± 0.011 | 0.859 ± 0.004 | 0.31 ± 0.002 | 1.84 ± 0.02 | 0.75 ± 0.003 |
| 600 | 0.402 ± 0.008 | 0.851 ± 0.003 | 0.36 ± 0.007 | 2.07 ± 0.04 | 0.74 ± 0.001 |
| 800 | 0.413 ± 0.010 | 0.847 ± 0.004 | 0.37 ± 0.008 | 2.16 ± 0.02 | 0.74 ± 0.003 |
| 1000 | 0.413 ± 0.008 | 0.847 ± 0.003 | 0.38 ± 0.005 | 2.08 ± 0.02 | 0.74 ± 0.002 |
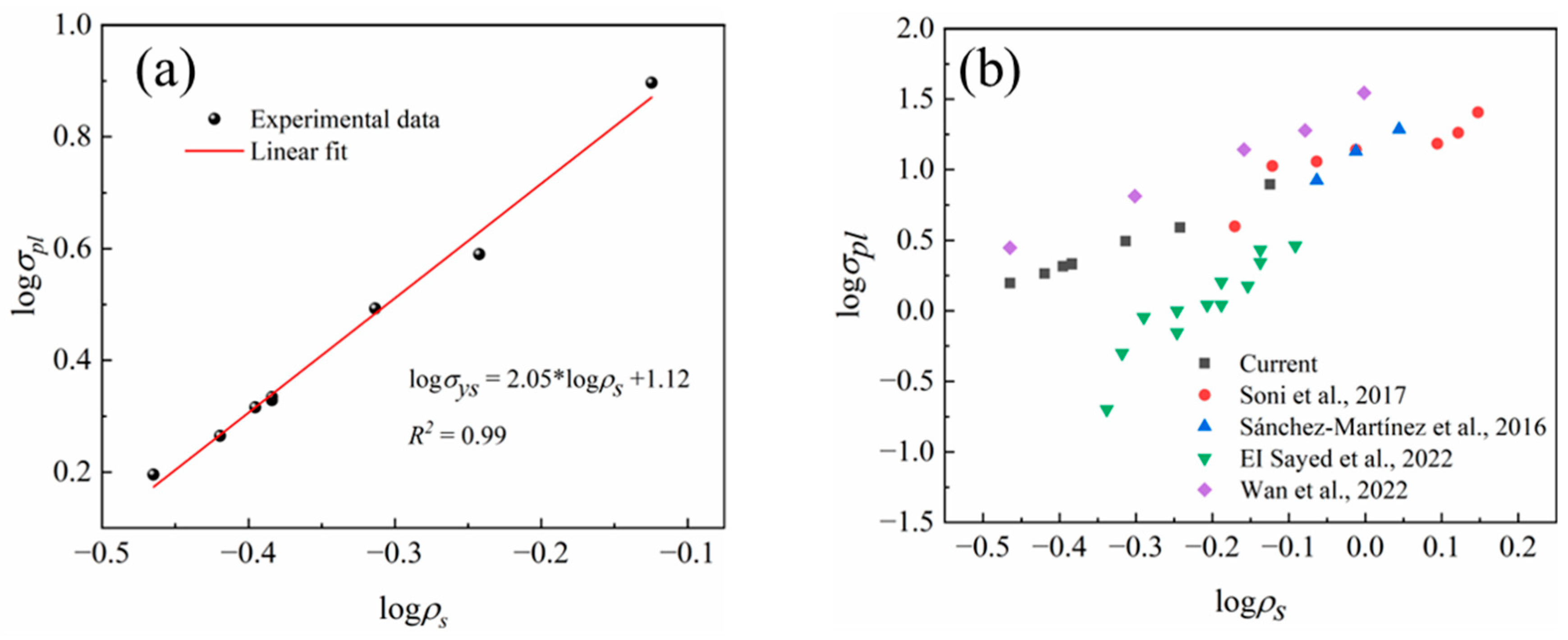
| References | Materials | Particle | Pore Size, mm | Relative Density | n in Equation (5) | Preparation Method |
|---|---|---|---|---|---|---|
| [13] | 6061-T6 Al alloy | NaCl | 0.75–2.5 | 0.25–0.52 | 1.91 | Pressure infiltration |
| [21] | Zn-22Al-2Cu | NaCl | 0.42–0.85 | 0.32–0.41 | 3.34 | Centrifugal infiltration |
| [22] | A356 Al alloy | NaCl | 2 and 4 | 0.19–0.3 | 3.92 | Pressure infiltration |
| [23] | ZL102 Al alloy | Ca2Cl | 3 | 0.13–0.39 | 2.3 | Pressure infiltration |
| Current | Pure Al | NaCl | 0.3–0.5 | 0.12–0.28 | 2.05 | Supergravity infiltration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Z.; Guo, Z. Preparation and Compression Behavior of High Porosity, Microporous Open-Cell Al Foam Using Supergravity Infiltration Method. Materials 2024, 17, 337. https://doi.org/10.3390/ma17020337
Li Y, Wang Z, Guo Z. Preparation and Compression Behavior of High Porosity, Microporous Open-Cell Al Foam Using Supergravity Infiltration Method. Materials. 2024; 17(2):337. https://doi.org/10.3390/ma17020337
Chicago/Turabian StyleLi, Yuan, Zhe Wang, and Zhancheng Guo. 2024. "Preparation and Compression Behavior of High Porosity, Microporous Open-Cell Al Foam Using Supergravity Infiltration Method" Materials 17, no. 2: 337. https://doi.org/10.3390/ma17020337
APA StyleLi, Y., Wang, Z., & Guo, Z. (2024). Preparation and Compression Behavior of High Porosity, Microporous Open-Cell Al Foam Using Supergravity Infiltration Method. Materials, 17(2), 337. https://doi.org/10.3390/ma17020337





