Composition and Surface Optical Properties of GaSe:Eu Crystals before and after Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
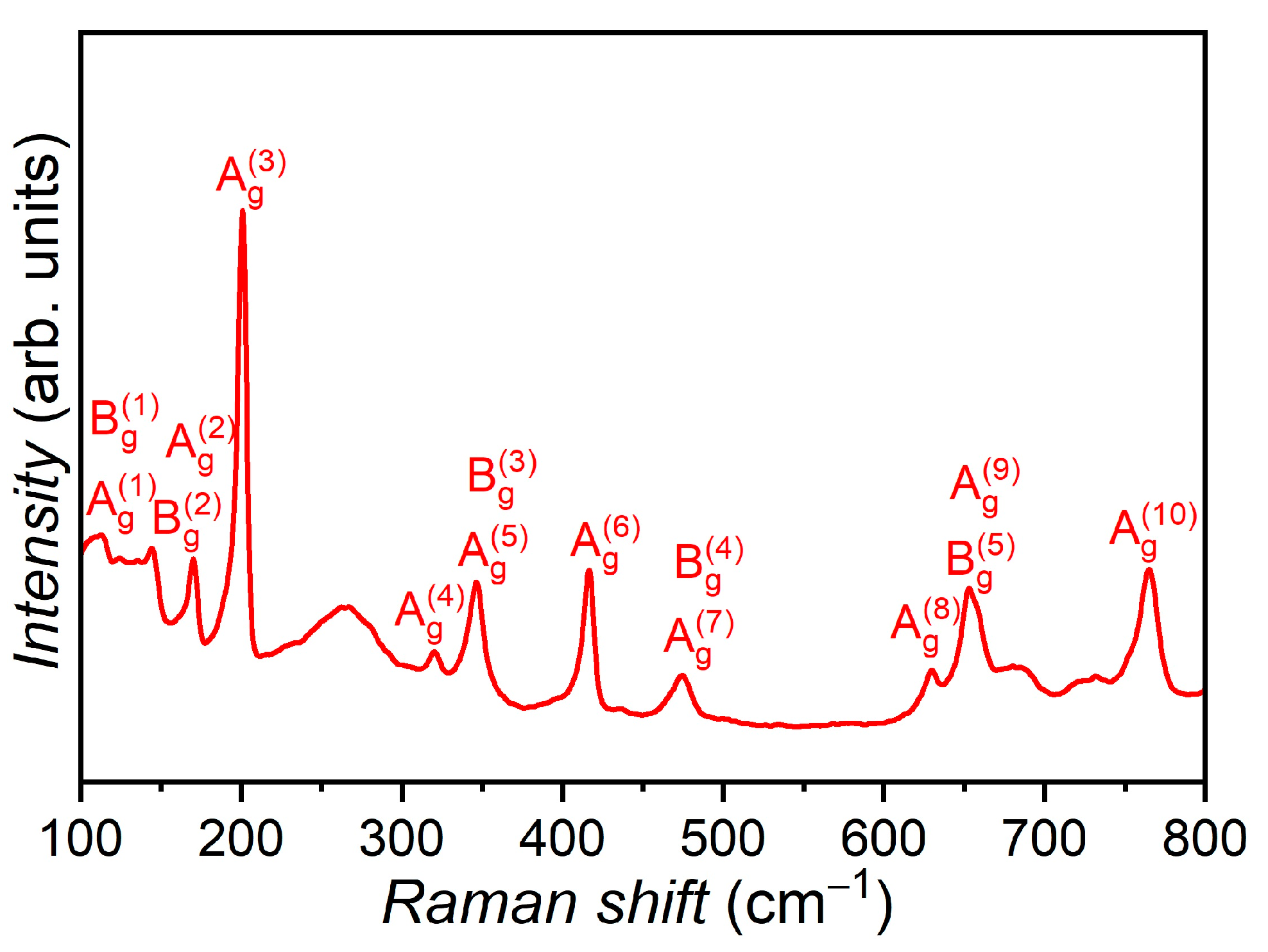
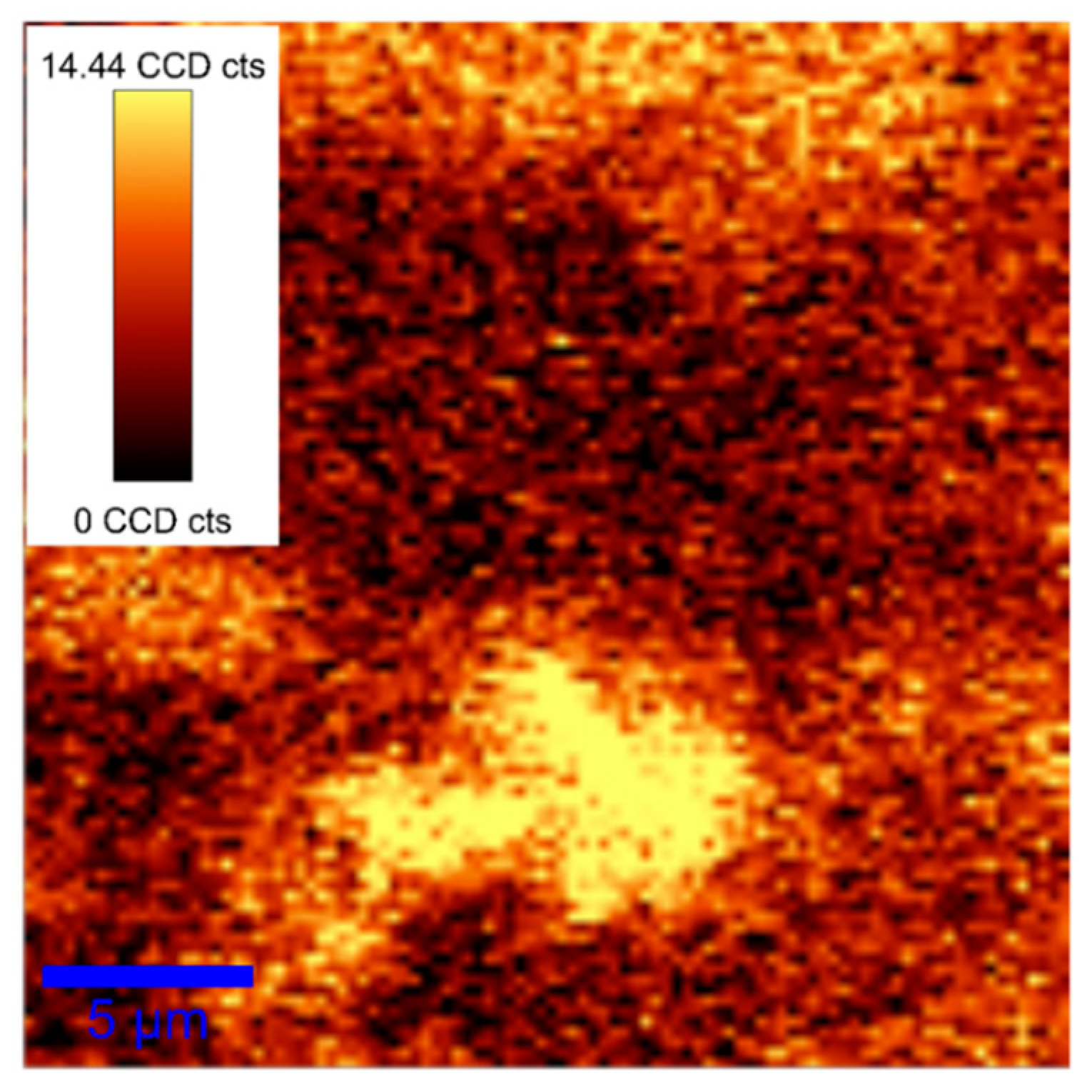
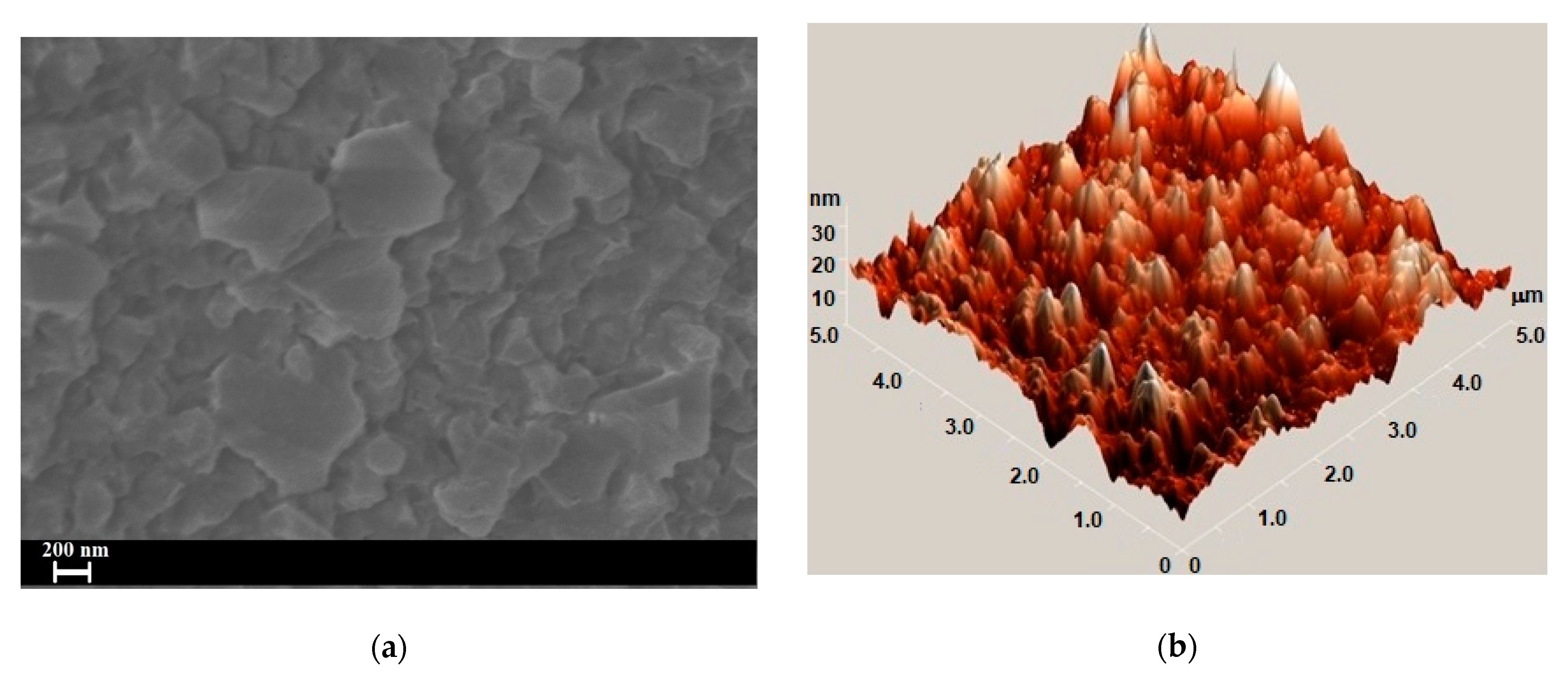
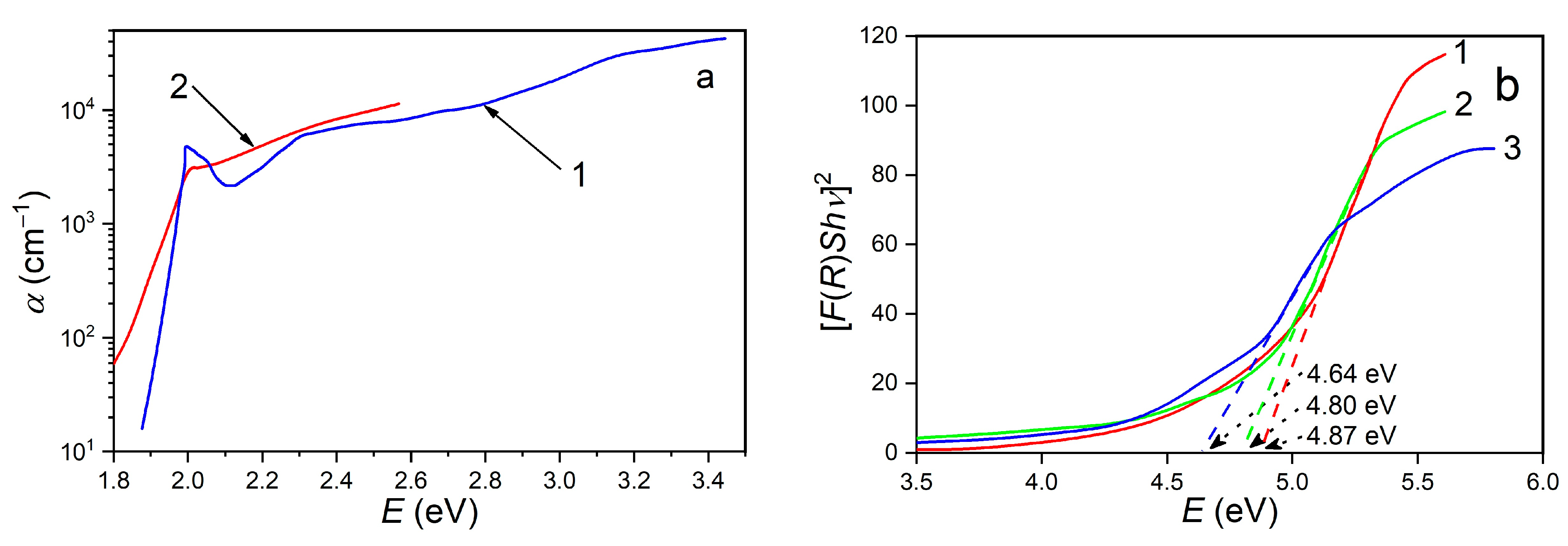
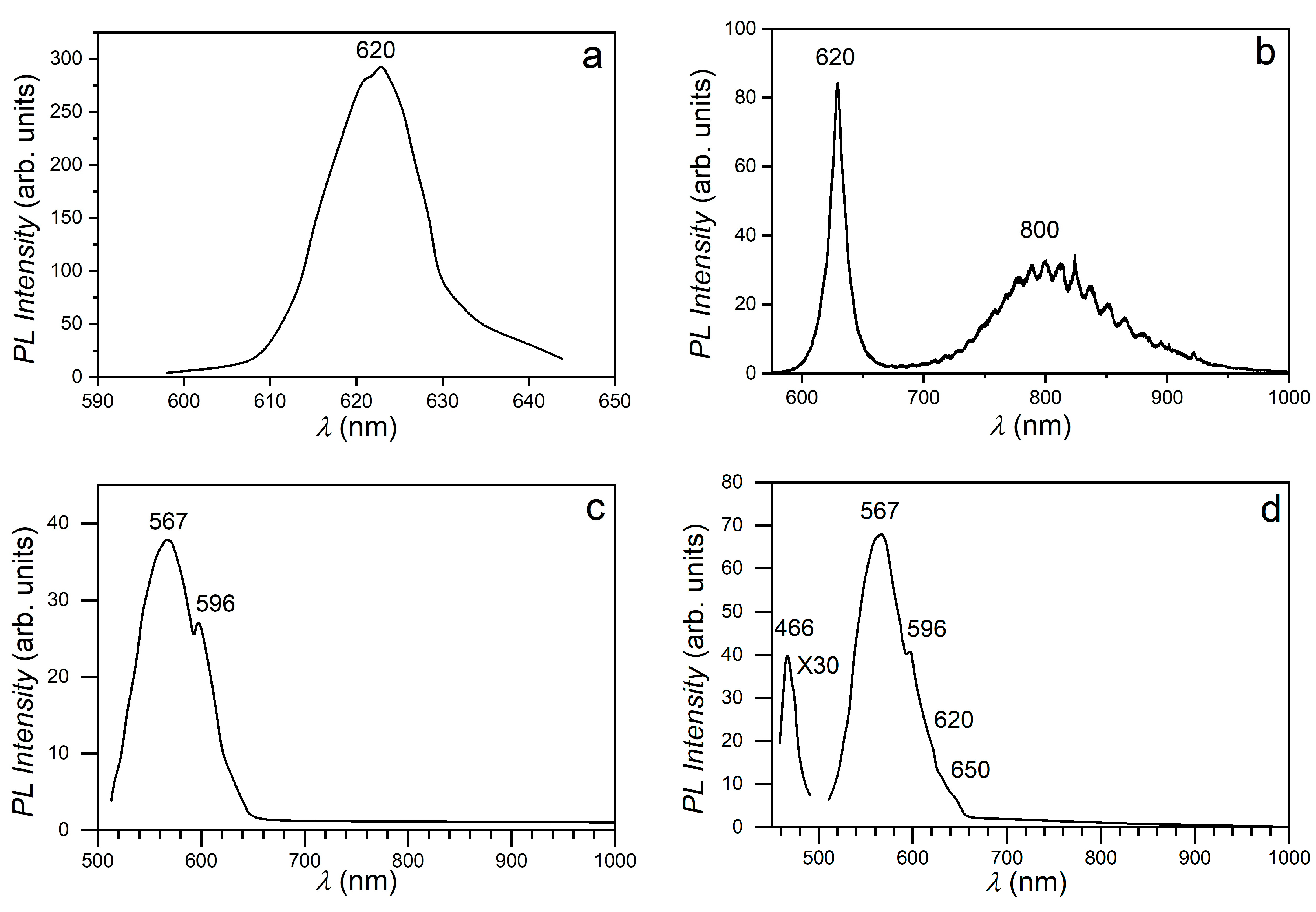
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuhn, A.; Chevy, A.; Chevalier, R. Crystal structure and interatomic distances in GaSe. Phys. Status Solidi A 1975, 31, 469–475. [Google Scholar] [CrossRef]
- Plucinski, L.; Johnson, R.L.; Kowalski, B.J.; Kopalko, K.; Orlowski, B.A.; Kovalyuk, Z.D.; Lashkarev, G.V. Electronic band structure of GaSe (0001): Angle-resolved photoemission and ab initio theory. Phys. Rev. B 2003, 68, 125304. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Steer, E.D.; Smith, E.F.; Kudrynskyi, Z.R.; Kovalyuk, Z.D.; Eaves, L.; Patanè, A.; Beton, P.H. Epitaxial growth of γ–InSe and α, β, and γ–In2Se3 on ε–GaSe. 2D Mater. 2018, 5, 035026. [Google Scholar] [CrossRef]
- Hopkinson, D.G.; Zólyomi, V.; Rooney, A.P.; Clark, N.; Terry, D.J.; Hamer, M.; Lewis, D.J.; Allen, C.S.; Kirkland, A.I.; Andreev, Y.; et al. Formation and healing of defects in atomically thin GaSe and InSe. ACS Nano 2019, 13, 5112–5123. [Google Scholar] [CrossRef] [PubMed]
- Mooser, E.; Schluter, M. The band-gap excitons in GaSe. Nuovo Cim. B 1973, 18, 164–208. [Google Scholar] [CrossRef]
- Schlüter, M. The electronic structure of GaSe. Nuovo Cim. B 1973, 13, 313–360. [Google Scholar] [CrossRef]
- Hu, P.; Wen, Z.; Wang, L.; Tan, P.; Xiao, K. Synthesis of few-layer GaSe nanosheets for high performance photodetectors. ACS Nano 2012, 6, 5988–5994. [Google Scholar] [CrossRef]
- Sorifi, S.; Moun, M.; Kaushik, S.; Singh, R. High-temperature performance of a GaSe nanosheet-based broadband photodetector. ACS Appl. Electron. Mater. 2020, 2, 670–676. [Google Scholar] [CrossRef]
- Guo, J.; Xie, J.-J.; Zhang, L.-M.; Kokh, K.; Andreev, Y.; Izaak, T.; Lanskii, G.; Shaĭduko, A.; Svetlichnyi, V. Characterization of optical quality of GaSe:Al crystals by exciton absorption peak parameters. J. Mater. Sci. Mater. Electron. 2014, 25, 1757–1760. [Google Scholar] [CrossRef]
- Cui, Y.; Dupere, R.; Burger, A.; Johnstone, D.; Mandal, K.C.; Payne, S.A. Acceptor levels in GaSe:In crystals investigated by deep-level transient spectroscopy and photoluminescence. J. Appl. Phys. 2008, 103, 013710. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Chang, C.-S.; Hsieh, W.-F. Photoluminescence study of GaSe doped with Er. Jpn. J. Appl. Phys. 2003, 42, 4222. [Google Scholar] [CrossRef]
- Kim, C.-D.; Jang, K.-W.; Lee, Y.-I. Optical properties of Tm-doped GaSe single crystals. Solid State Commun. 2004, 130, 701–704. [Google Scholar] [CrossRef]
- Drapak, S.I.; Gavrylyuk, S.V.; Kovalyuk, Z.D.; Lytvyn, O.S. Native oxide emerging of the cleavage surface of gallium selenide due to prolonged storage. Semiconductors 2008, 42, 414–421. [Google Scholar] [CrossRef]
- Balitskii, O.A.; Savchyn, V.P. Thermodynamic study of AIIIBVI compounds oxidation. Mater. Sci. Semicond. Process. 2004, 7, 55–58. [Google Scholar] [CrossRef]
- Filippo, E.; Siciliano, M.; Genga, A.; Micocci, G.; Tepore, A.; Siciliano, T. Single crystalline β-Ga2O3 nanowires synthesized by thermal oxidation of GaSe layer. Mater. Res. Bull. 2013, 48, 1741–1744. [Google Scholar] [CrossRef]
- Kowalski, B.M.; Manz, N.; Bethke, D.; Shaner, E.A.; Serov, A.; Kalugin, N.G. Role of humidity in oxidation of ultrathin GaSe. Mater. Res. Express 2019, 6, 085907. [Google Scholar] [CrossRef]
- Beechem, T.E.; Kowalski, B.M.; Brumbach, M.T.; McDonald, A.E.; Spataru, C.D.; Howell, S.W.; Ohta, T.; Pask, J.A.; Kalugin, N.G. Oxidation of ultrathin GaSe. Appl. Phys. Lett. 2015, 107, 173103. [Google Scholar] [CrossRef]
- Nogales, E.; Lopez, I.; Mendez, B.; Piqueras, J.; Lorenz, K.; Alves, E.; Garcia, J.A.; Teherani, F.H.; Look, D.; Rogers, D.J. Doped gallium oxide nanowires for photonics. In Proceedings of the International Society for Optics and Photonics (SPIE OPTO), San Francisco, CA, USA, 21–26 January 2012. [Google Scholar]
- Chen, Z.; Wang, X.; Zhang, F.; Noda, S.; Saito, K.; Tanaka, T.; Nishio, M.; Guo, Q. Temperature dependence of luminescence spectra in europium doped Ga2O3 film. J. Lumin. 2016, 177, 48–53. [Google Scholar] [CrossRef]
- Chen, Z.; Saito, K.; Tanaka, T.; Nishio, M.; Arita, M.; Guo, Q. Low temperature growth of europium doped Ga2O3 luminescent films. J. Cryst. Growth 2015, 430, 28–33. [Google Scholar] [CrossRef]
- Late, D.J.; Liu, B.; Luo, J.; Yan, A.; Matte, H.S.S.R.; Grayson, M.; Rao, C.N.R.; Dravid, V.P. GaS and GaSe ultrathin layer transistors. Adv. Mater. 2012, 24, 3549–3554. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R. Nanofunctional gallium oxide (Ga2O3) nanowires/nanostructures and their applications in nanodevices. Phys. Status Solidi Rapid Res. Lett. 2013, 7, 781–792. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Zhao, Q.; Yin, Z.; Zhang, Y.; Chen, B.; Xie, Y.; Jie, W. High-quality GaSe single crystal grown by the Bridgman method. Materials 2018, 11, 186. [Google Scholar] [CrossRef]
- Santos, N.F.; Rodrigues, J.; Fernandes, A.J.S.; Alves, L.C.; Alves, E.; Costa, F.M.; Monteiro, T. Optical properties of LFZ grown β-Ga2O3:Eu3+ fibres. Appl. Surf. Sci. 2012, 258, 9157–9161. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, R.; Choudhary, R.J.; Phase, D.M. Structural, XPS and magnetic studies of pulsed laser deposited Fe doped Eu2O3 thin film. Mater. Res. Bull. 2015, 70, 392–396. [Google Scholar] [CrossRef]
- Mesaros, A.; Toloman, D.; Nasui, M.; Mos, R.B.; Petrisor, T.; Vasile, B.S.; Surdu, V.A.; Perhaita, I.; Biris, A.; Pana, O. A valence states approach for luminescence enhancement by low dopant concentration in Eu-doped ZnO nanoparticles. J. Mater. Sci. 2015, 50, 6075–6086. [Google Scholar] [CrossRef]
- Du, H.; Tu, M.; Luo, S.; Liu, Y.; Qiu, X.; Lu, H.; Li, S.; Yuan, S.; Huang, W.; Jie, W.; et al. Reversible transition between bipolar resistive switching and threshold switching in 2D layered III–VI semiconductor GaSe. Appl. Phys. Lett. 2020, 116, 253102. [Google Scholar] [CrossRef]
- Zappia, M.I.; Bianca, G.; Bellani, S.; Serri, M.; Najafi, L.; Oropesa-Nuñez, R.; Martín-García, B.; Bouša, D.; Sedmidubský, D.; Pellegrini, V.; et al. Solution-Processed GaSe Nanoflake-Based Films for Photoelectrochemical Water Splitting and Photoelectrochemical-Type Photodetectors. Adv. Funct. Mater. 2020, 30, 1909572. [Google Scholar] [CrossRef]
- Afaneh, T.; Fryer, A.; Xin, Y.; Hyde, R.H.; Kapuruge, N.; Gutiérrez, H.R. Large-area growth and stability of monolayer gallium monochalcogenides for optoelectronic devices. ACS Appl. Nano Mater. 2020, 3, 7879–7887. [Google Scholar] [CrossRef]
- Hoff, R.M.; Irwin, J.C.; Lieth, R.M.A. Raman scattering in GaSe. Can. J. Phys. 1975, 53, 1606–1614. [Google Scholar] [CrossRef]
- Yu, J.; Cui, L.; He, H.; Yan, S.; Hu, Y.; Wu, H. Raman spectra of RE2O3 (RE = Eu, Gd, Dy, Ho, Er, Tm, Yb, Lu, Sc and Y): Laser-excited luminescence and trace impurity analysis. J. Rare Earths 2014, 32, 1–4. [Google Scholar] [CrossRef]
- Ousaka, Y.; Sakai, O.; Tachiki, M. Theory of Raman scattering in magnetically ordered phases of EuSe and EuTe. Solid State Commun. 1977, 23, 589–592. [Google Scholar] [CrossRef]
- Rau, R.C.; Glover, W.J., Jr. Thermal decomposition of europium hydroxide. J. Am. Ceram. Soc. 1964, 47, 382–387. [Google Scholar] [CrossRef]
- Silberstein, R.P.; Safran, S.A.; Dresselhaus, M.S. First-and second-order Raman scattering in EuSe near TN. J. Magn. Magn. Mater. 1979, 11, 408–411. [Google Scholar] [CrossRef]
- Dohy, D.; Lucazeau, G.; Revcolevschi, A. Raman spectra and valence force field of single-crystalline β-Ga2O3. J. Solid State Chem. 1982, 45, 180–192. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Saito, K.; Tanaka, T.; Nishio, M.; Guo, Q. The impact of growth temperature on the structural and optical properties of catalyst-free β-Ga2O3 nanostructures. Mater. Res. Express 2016, 3, 025003. [Google Scholar] [CrossRef]
- Filippo, E.; Tepore, M.; Baldassarre, F.; Siciliano, T.; Micocci, G.; Quarta, G.; Calcagnile, L.; Tepore, A. Synthesis of β-Ga2O3 microstructures with efficient photocatalytic activity by annealing of GaSe single crystal. Appl. Surf. Sci. 2015, 338, 69–74. [Google Scholar] [CrossRef]
- Pearton, S.J.; Yang, J.; Cary, P.H., IV; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. App. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Zalamai, V.V.; Syrbu, N.N.; Stamov, I.G.; Beril, S.I. Wannier-Mott excitons in GaSe single crystals. J. Opt.-UK 2020, 22, 085402. [Google Scholar] [CrossRef]
- Gnatenko, Y.P.; Skubenko, P.A. Exciton absorption of GaSe crystals in the indirect transition region. Phys. Status Solidi B 1981, 105, K9–K12. [Google Scholar] [CrossRef]
- Bassou, A. Optical properties of GaSe, characterization and simulation. Mater. Today Proc. 2021, 37, 3789–3792. [Google Scholar] [CrossRef]
- Pankove, J.I. Optical Processes in Semiconductors, 1st ed.; Prentice-Hall: Hoboken, NJ, USA, 1971. [Google Scholar]
- Boldish, S.I.; White, W.B. Optical band gaps of selected ternary sulfide minerals. Am. Mineral. 1998, 83, 865–871. [Google Scholar] [CrossRef]
- Forney, J.J.; Maschke, K.; Mooser, E. Influence of stacking disorder on Wannier excitons in layered semiconductors. J. Phys. C Solid State Phys. 1977, 10, 1887–1894. [Google Scholar] [CrossRef]
- Andreev, Y.M.; Vaitulevich, E.A.; Kokh, K.A.; Lanskii, G.V.; Losev, V.F.; Lubenko, D.M.; Svetlichnyi, V.A.; Soldatov, A.N.; Shaiduko, A.V. Optimal doping of GaSe crystals for nonlinear optics applications. Russ. Phys. J. 2014, 56, 1250–1257. [Google Scholar] [CrossRef]
- Feng, Z.-S.; Guo, J.; Xie, J.-J.; Zhang, L.-M.; Gao, J.-Y.; Andreev, Y.M.; Izaak, T.I.; Kokh, K.A.; Lanskii, G.V.; Shaiduko, A.V.; et al. GaSe: Er3+ crystals for SHG in the infrared spectral range. Opt. Commun. 2014, 318, 205–211. [Google Scholar] [CrossRef]
- Murphy, A.B. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, L.; Wang, X.; Ding, Z.; Li, Z.; Fu, X. Photocatalytic performance of α-, β-, and γ-Ga2O3 for the destruction of volatile aromatic pollutants in air. J. Catal. 2007, 250, 12–18. [Google Scholar] [CrossRef]
- Kumar, S.; Tessarek, C.; Christiansen, S.; Singh, R. A comparative study of β–Ga2O3 nanowires grown on different substrates using CVD technique. J. Alloys Compd. 2014, 587, 812–818. [Google Scholar] [CrossRef]
- Alema, F.; Hertog, B.; Osinsky, A.; Mukhopadhyay, P.; Toporkov, M.; Schoenfeld, W.V. Fast growth rate of epitaxial β–Ga2O3 by close coupled showerhead MOCVD. J. Cryst. Growth 2017, 475, 77–82. [Google Scholar] [CrossRef]
- Zhang, F.; Li, H.; Cui, Y.-T.; Li, G.-L.; Guo, Q. Evolution of optical properties and band structure from amorphous to crystalline Ga2O3 films. AIP Adv. 2018, 8, 045112. [Google Scholar] [CrossRef]
- Tan, L.; Liu, Q.; Ding, Y.; Lin, X.; Hu, W.; Cai, M.-Q.; Zhou, H. Effective shape-controlled synthesis of gallium selenide nanosheets by vapor phase deposition. Nano Res. 2020, 13, 557–563. [Google Scholar] [CrossRef]
- Shyama, P.S. Europium; Springer: Heidelberg, Germany, 1968; p. 30. [Google Scholar]
- Quan, S.; Wang, Y.; Liang, Y.; Jiang, J.; Zhong, B.; Yu, K.; Zhang, H.; Kan, G. Interference effect on photoluminescence intensity in GaSe up to 200 layers. J. Phys. Chem. C 2020, 124, 10185–10191. [Google Scholar] [CrossRef]
- Schmid, P.; Voitchovsky, J.P.; Mercier, A. Impurity effects on low temperature photoluminescence of GaSe. Phys. Status Solidi A 1974, 21, 443–450. [Google Scholar] [CrossRef]
- Bernier, G.; Jandl, S.; Provencher, R. Spontaneous and stimulated photoluminescence of GaSe in the energy range 2.075–2.125 eV. J. Lumin. 1986, 35, 289–300. [Google Scholar] [CrossRef]
- Capozzi, V.; Montagna, M. Optical spectroscopy of extrinsic recombinations in gallium selenide. Phys. Rev. B Condens. Matter 1989, 40, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-K.; Chang, C.-S.; Huang, W.-C. Electrical properties of GaSe doped with Er. J. Appl. Phys. 2004, 96, 1563–1567. [Google Scholar] [CrossRef]
- Gürbulak, B.; Yildirim, M.; Tüzemen, S.; Efeoǧlu, H.; Yoǧurtçu, Y.K. Temperature dependence of galvanomagnetic properties for Gd doped and undoped p-type GaSe. J. Appl. Phys. 1998, 83, 2030–2034. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, R.; Tang, D.; Zou, B. The effect of dopant and optical micro-cavity on the photoluminescence of Mn-doped ZnSe nanobelts. Nanoscale Res. Lett. 2013, 8, 314. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, R.; Tang, D.; Wang, X.; Fan, H.; Pan, A.; Zhang, Q.; Wan, Q.; Zou, B. Luminescence and local photonic confinement of single ZnSe: Mn nanostructure and the shape dependent lasing behavior. Nanotechnology 2013, 24, 055201. [Google Scholar] [CrossRef]
- Le Toullec, R.; Piccioli, N.; Mejatty, M.; Balkanski, M. Optical constants of ε-GaSe. Nuovo Cim. B 1977, 38, 159–167. [Google Scholar] [CrossRef]
- Natanovich, Z.A.; Vsevolodovna, O.G.; Isaevich, O.Y. Technique and Practice of Spectroscopy; Nauka: Moscow, Russia, 1972; p. 169. (In Russian) [Google Scholar]
- Sinha, S.P. Europium; Springer: New York, NY, USA, 1967. [Google Scholar]
- Peters, T.E.; Baglio, J.A. Luminescence and structural properties of thiogallate phosphors Ce+3 and Eu+2—Activated Phosphors. Part I. J. Electrochem. Soc. 1972, 119, 230. [Google Scholar] [CrossRef]
- Hidaka, C.; Yamagishi, E.; Takizawa, T. Preparation of Ca(1-x)EuxGa2S4 crystals and their photoluminescence, absorption and excitation spectra. J. Phys. Chem. Solids 2005, 66, 2058–2060. [Google Scholar] [CrossRef]
- Georgobiani, A.N.; Sturov, V.V.; Tyutyunnikov, V.I.; Tagiev, B.G.; Tagiev, O.B.; Djabborov, R.B. Radical-recombination luminescence, ion-luminescence and photoluminescence of CaGa2S4:Eu. J. Phys. Chem. Solids 2003, 64, 1519–1524. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Xie, E.; Ma, Z.; Zhao, A.; Liu, Z. Structure and photoluminescence of β–Ga2O3: Eu3+ nanofibers prepared by electrospinning. Appl. Surf. Sci. 2011, 257, 4968–4972. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprincean, V.; Qiu, H.; Tjardts, T.; Lupan, O.; Untilă, D.; Aktas, C.; Adelung, R.; Leontie, L.; Carlescu, A.; Gurlui, S.; et al. Composition and Surface Optical Properties of GaSe:Eu Crystals before and after Heat Treatment. Materials 2024, 17, 405. https://doi.org/10.3390/ma17020405
Sprincean V, Qiu H, Tjardts T, Lupan O, Untilă D, Aktas C, Adelung R, Leontie L, Carlescu A, Gurlui S, et al. Composition and Surface Optical Properties of GaSe:Eu Crystals before and after Heat Treatment. Materials. 2024; 17(2):405. https://doi.org/10.3390/ma17020405
Chicago/Turabian StyleSprincean, Veaceslav, Haoyi Qiu, Tim Tjardts, Oleg Lupan, Dumitru Untilă, Cenk Aktas, Rainer Adelung, Liviu Leontie, Aurelian Carlescu, Silviu Gurlui, and et al. 2024. "Composition and Surface Optical Properties of GaSe:Eu Crystals before and after Heat Treatment" Materials 17, no. 2: 405. https://doi.org/10.3390/ma17020405
APA StyleSprincean, V., Qiu, H., Tjardts, T., Lupan, O., Untilă, D., Aktas, C., Adelung, R., Leontie, L., Carlescu, A., Gurlui, S., & Caraman, M. (2024). Composition and Surface Optical Properties of GaSe:Eu Crystals before and after Heat Treatment. Materials, 17(2), 405. https://doi.org/10.3390/ma17020405







