Electrodeposited Heusler Alloys-Based Nanowires for Shape Memory and Magnetocaloric Applications
Abstract
:1. Introduction
1.1. Downsizing of Heusler Alloys
1.2. Heusler Alloy Nanoparticles
1.3. Heusler Alloy Nanowires
2. Materials and Methods
3. Results and Discussion
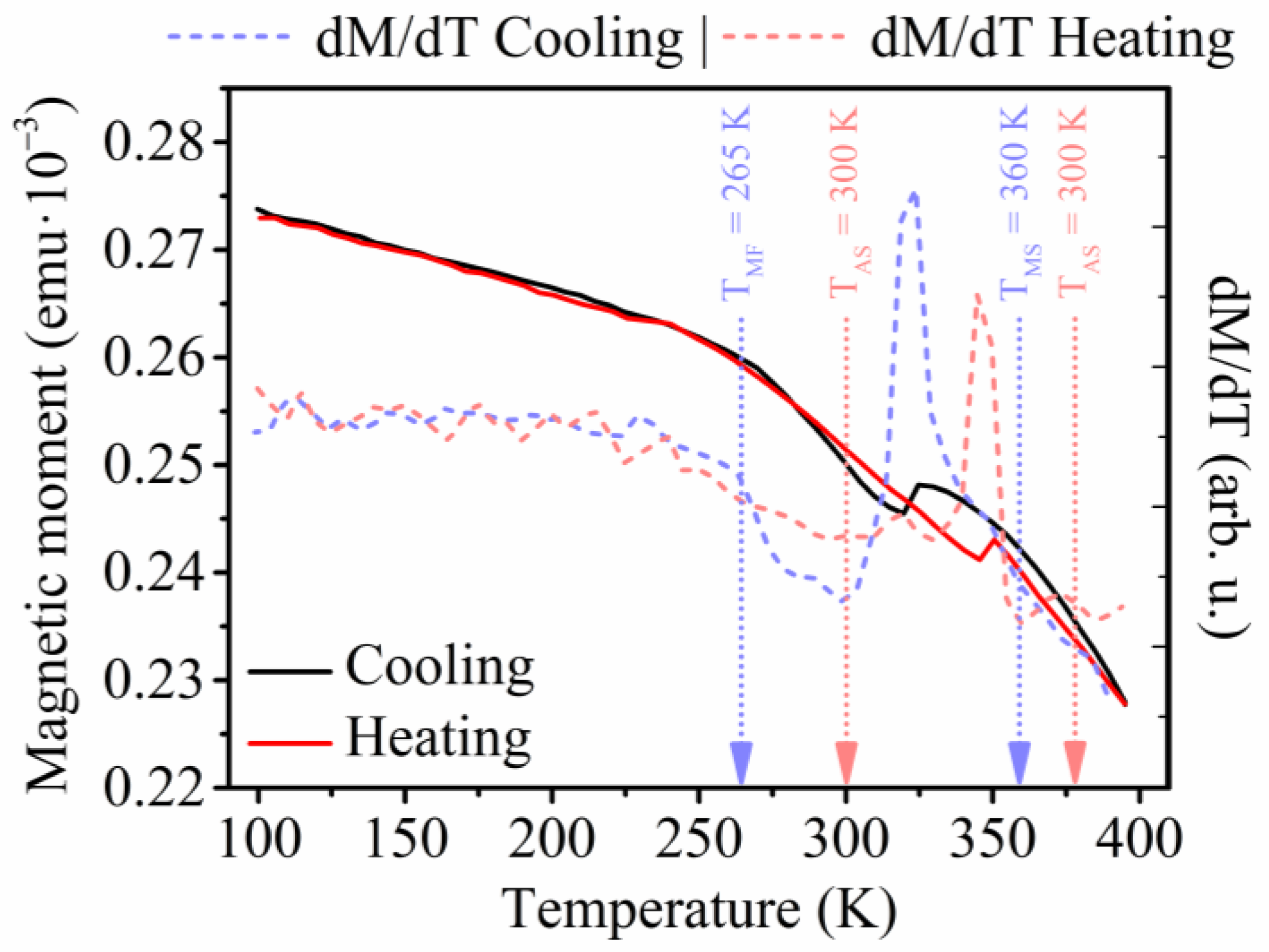
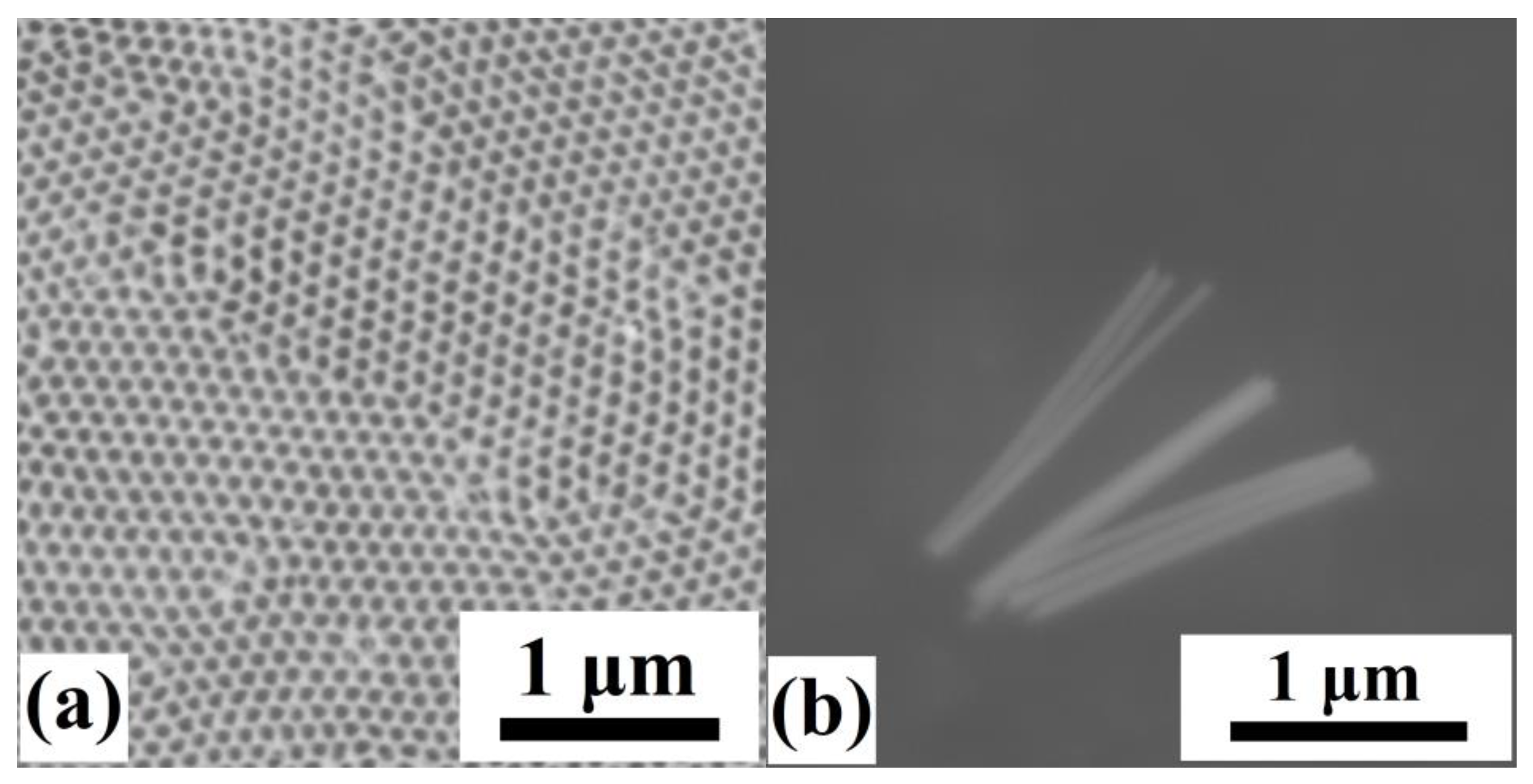
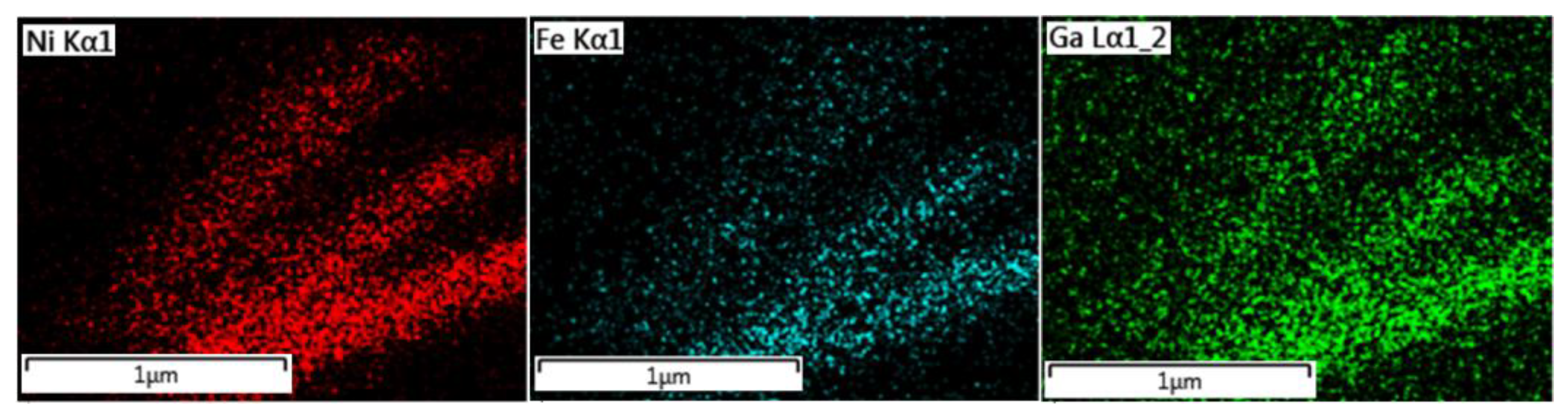
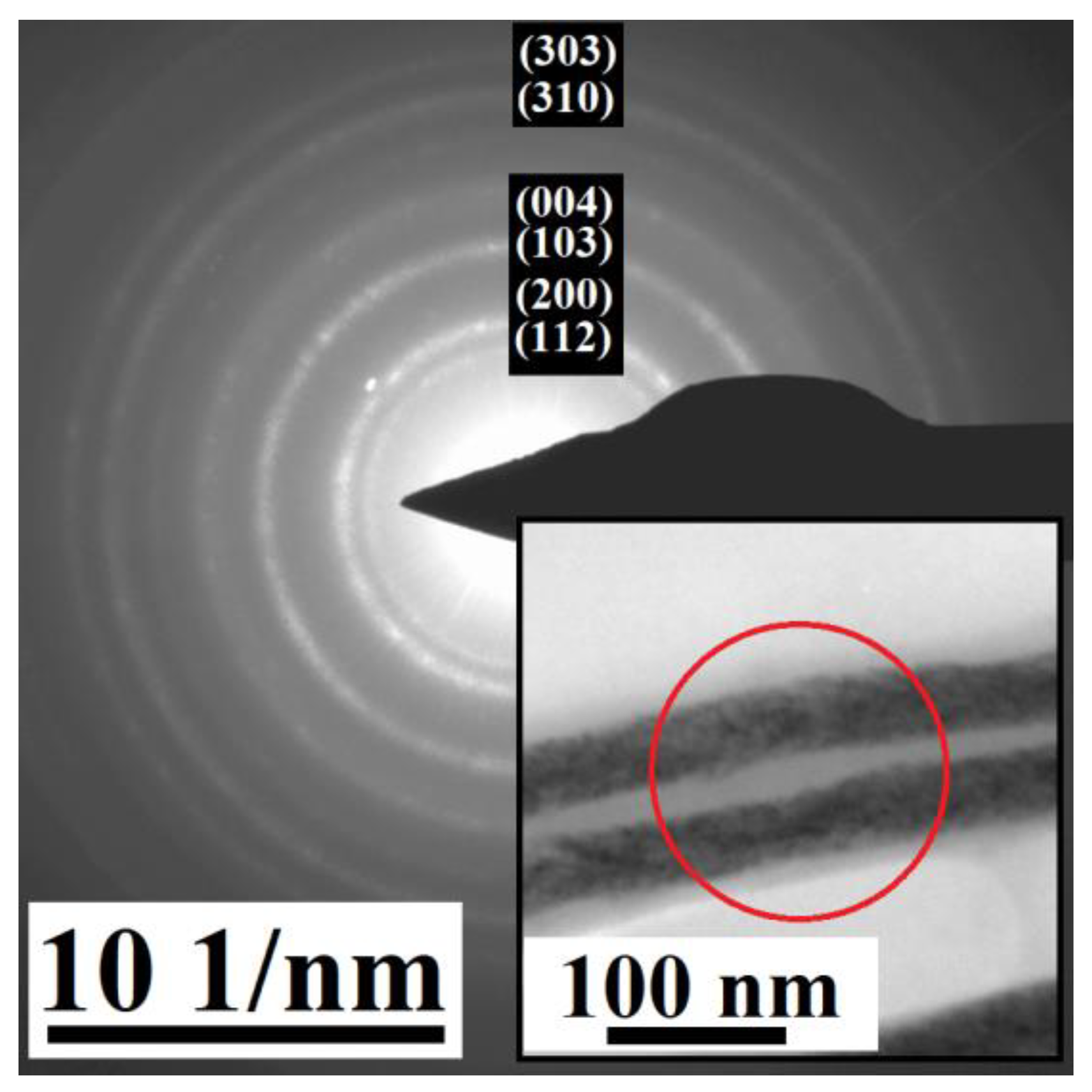
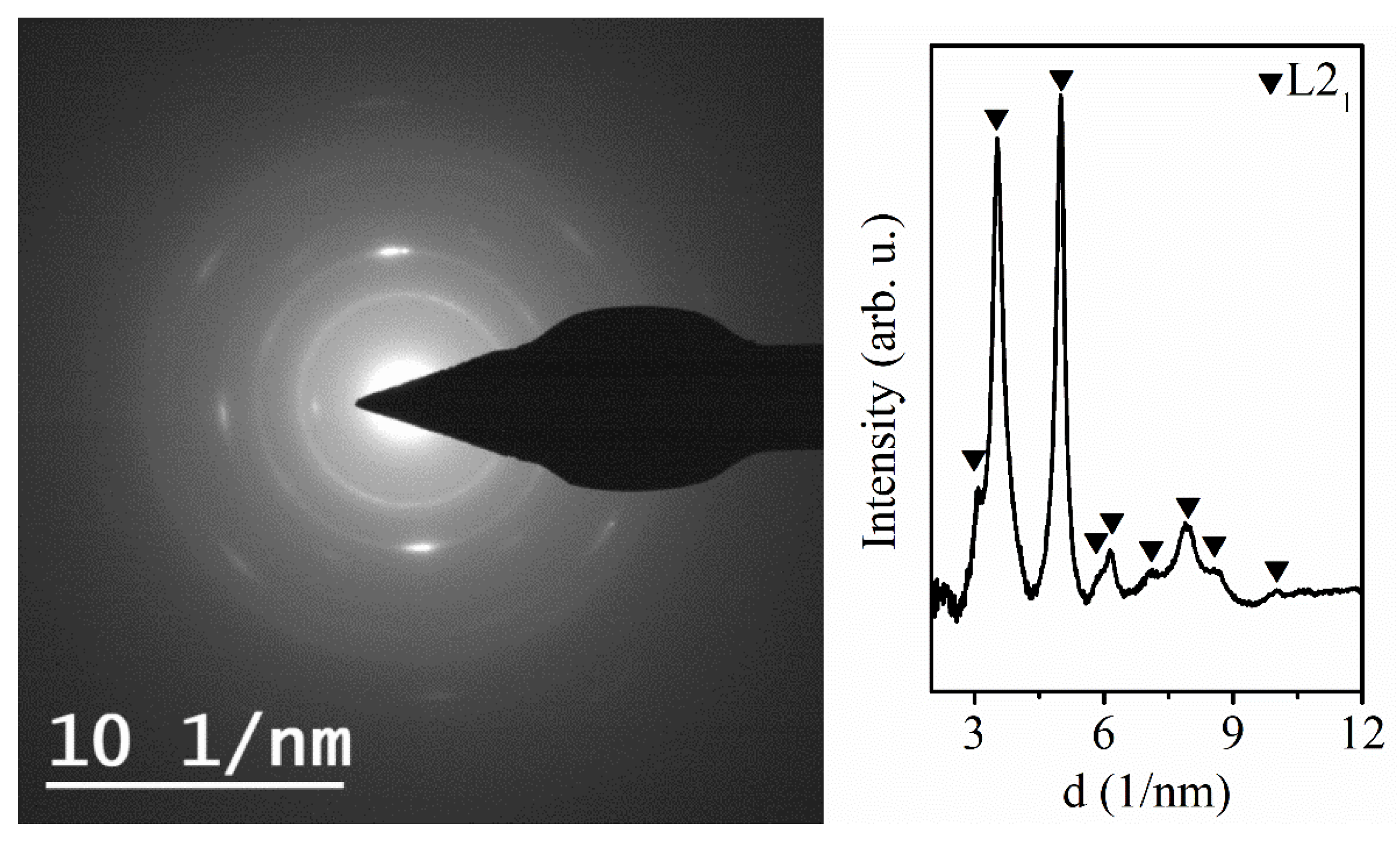
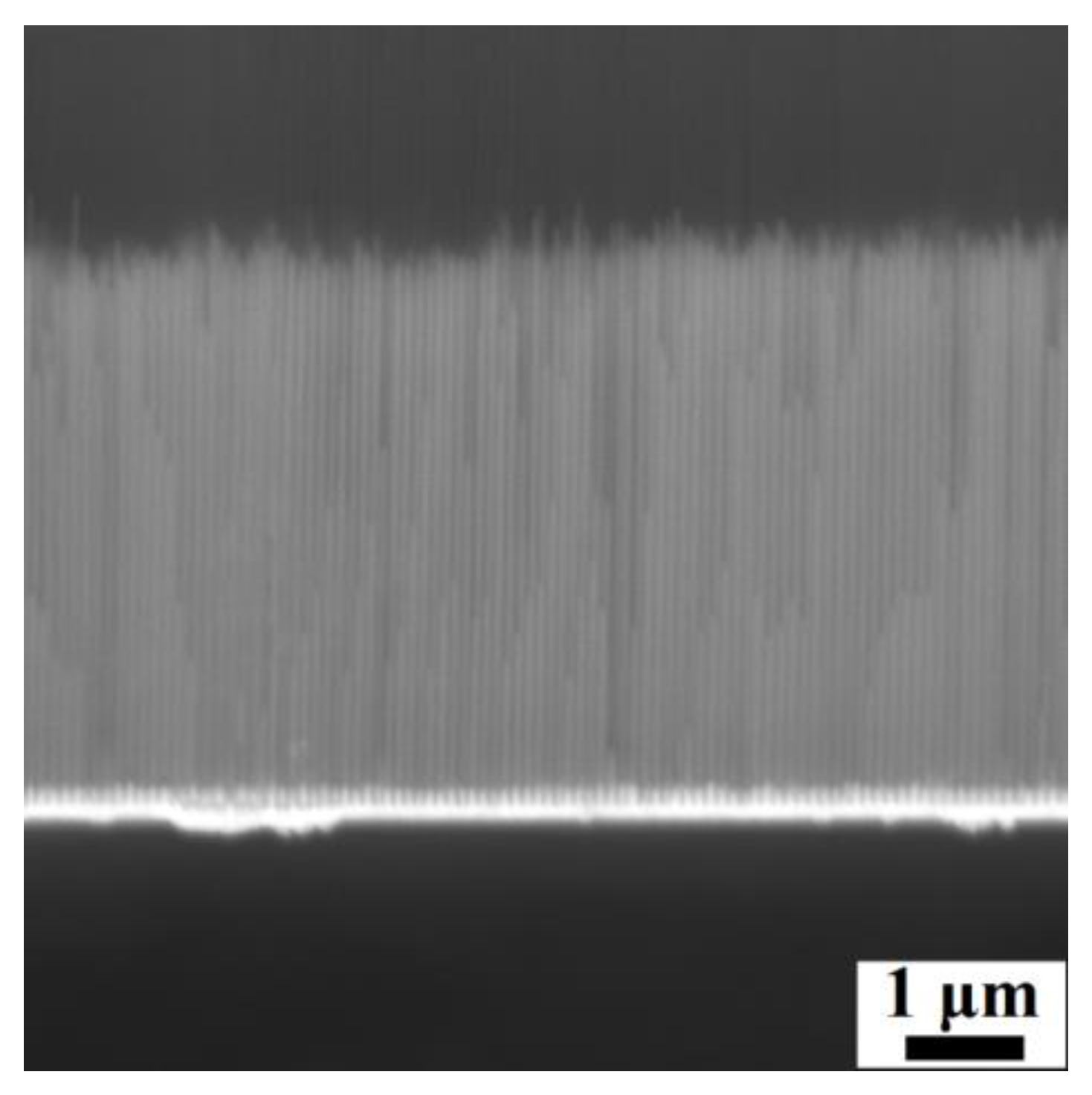
3.1. Electrodeposited Ni66Fe21Ga13 Nanowires
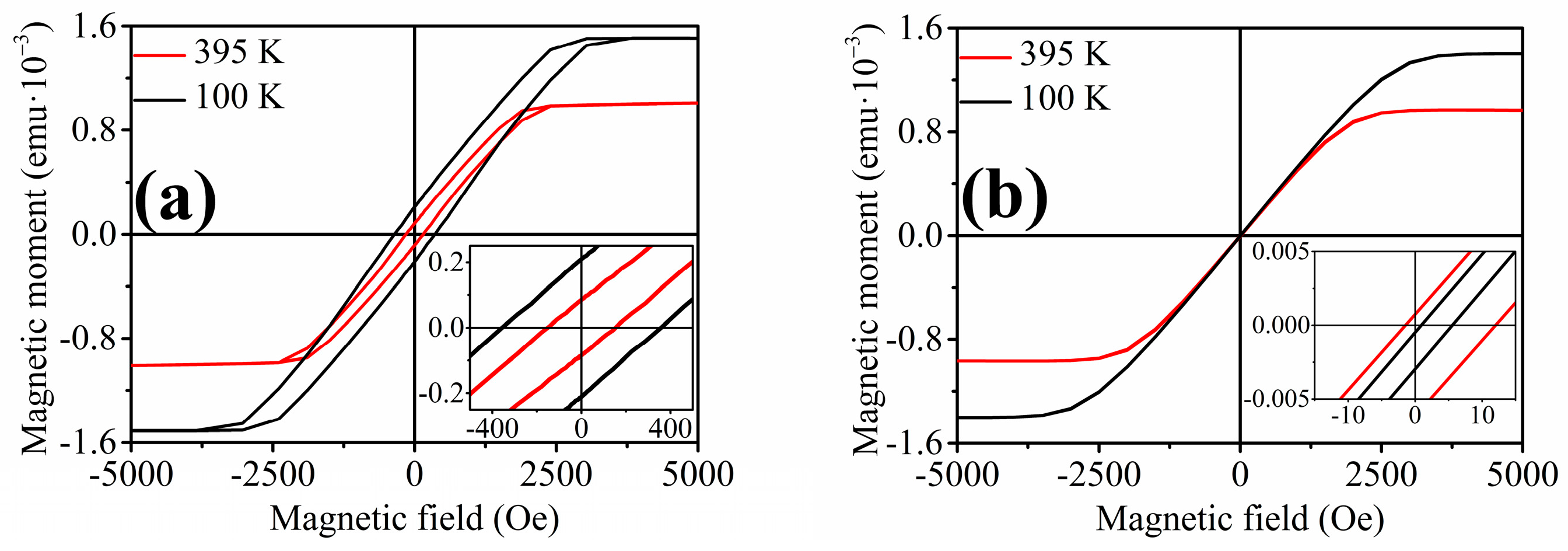
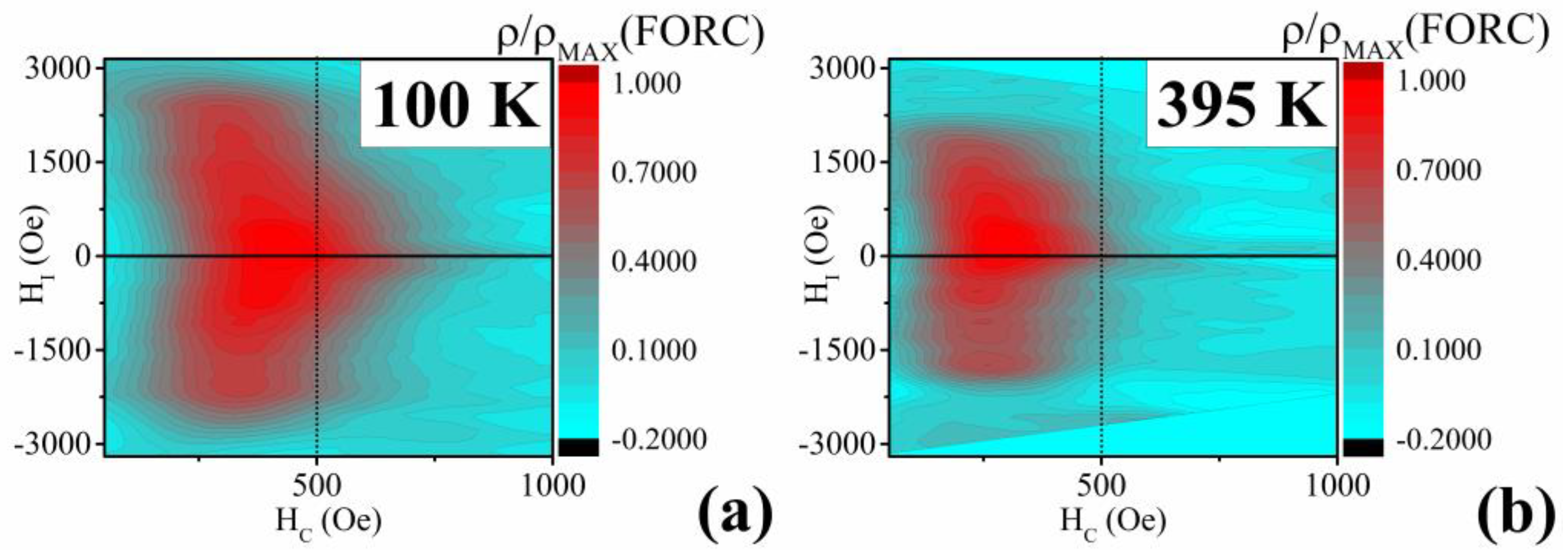
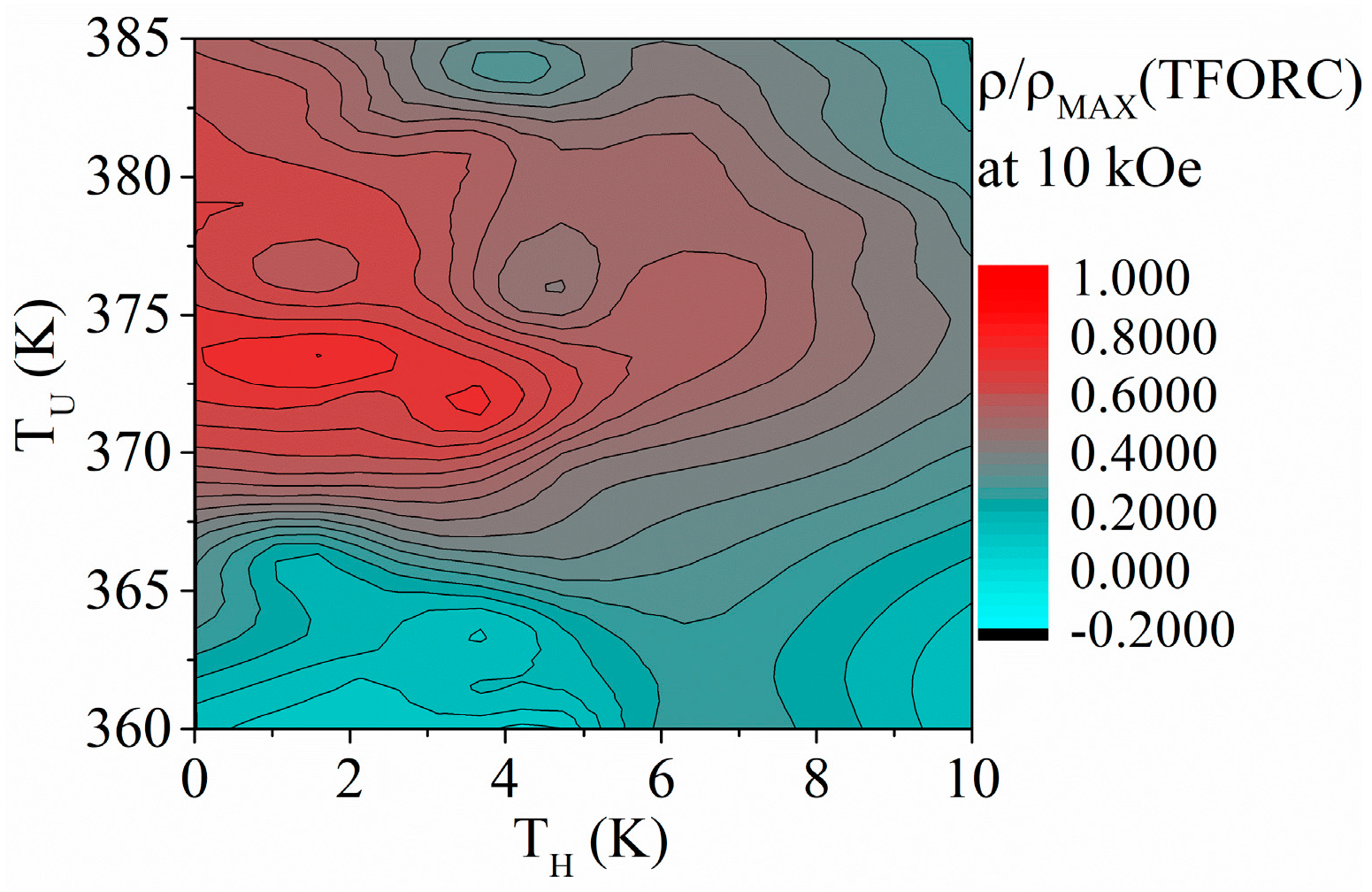
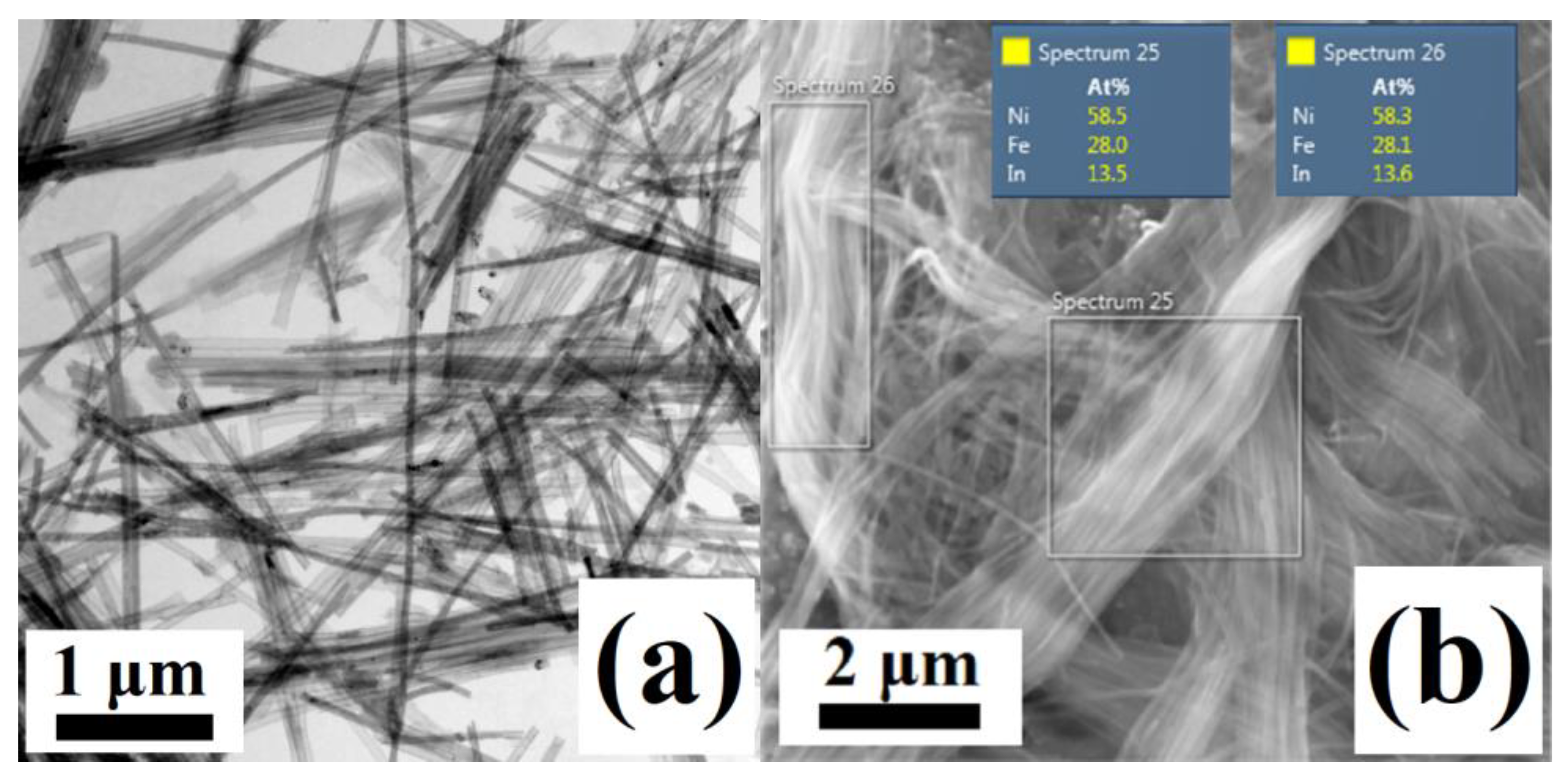
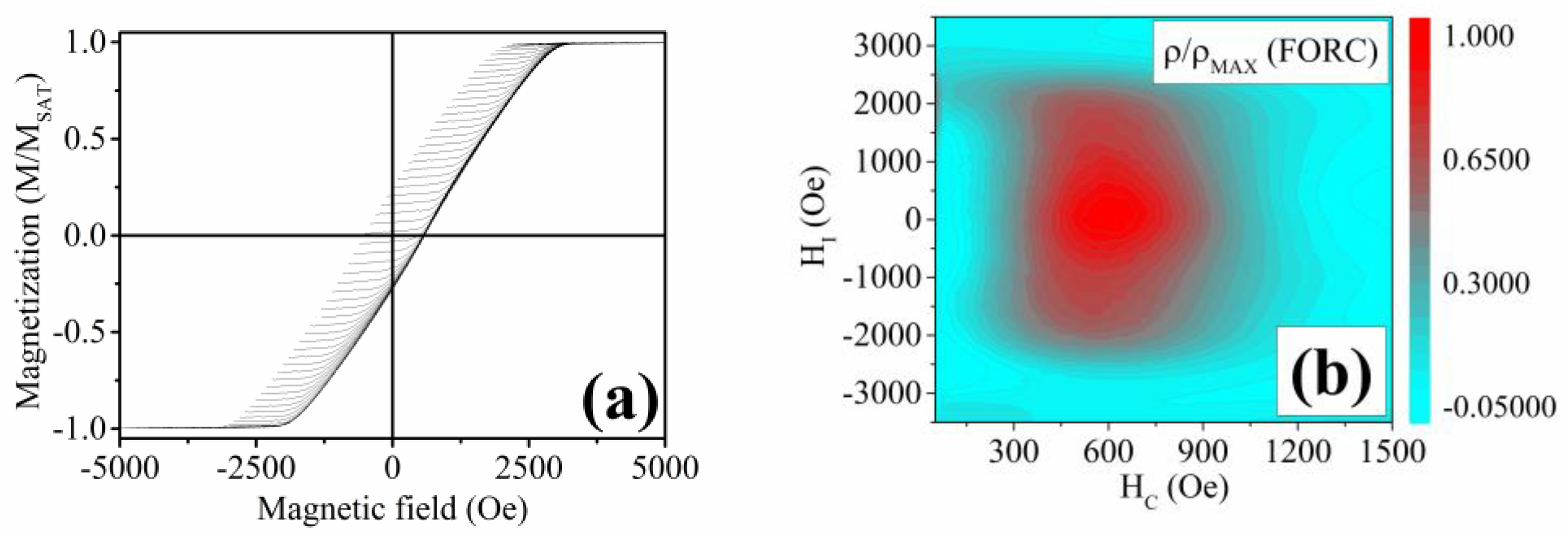
3.2. Ni58Fe28In14 Nanowires
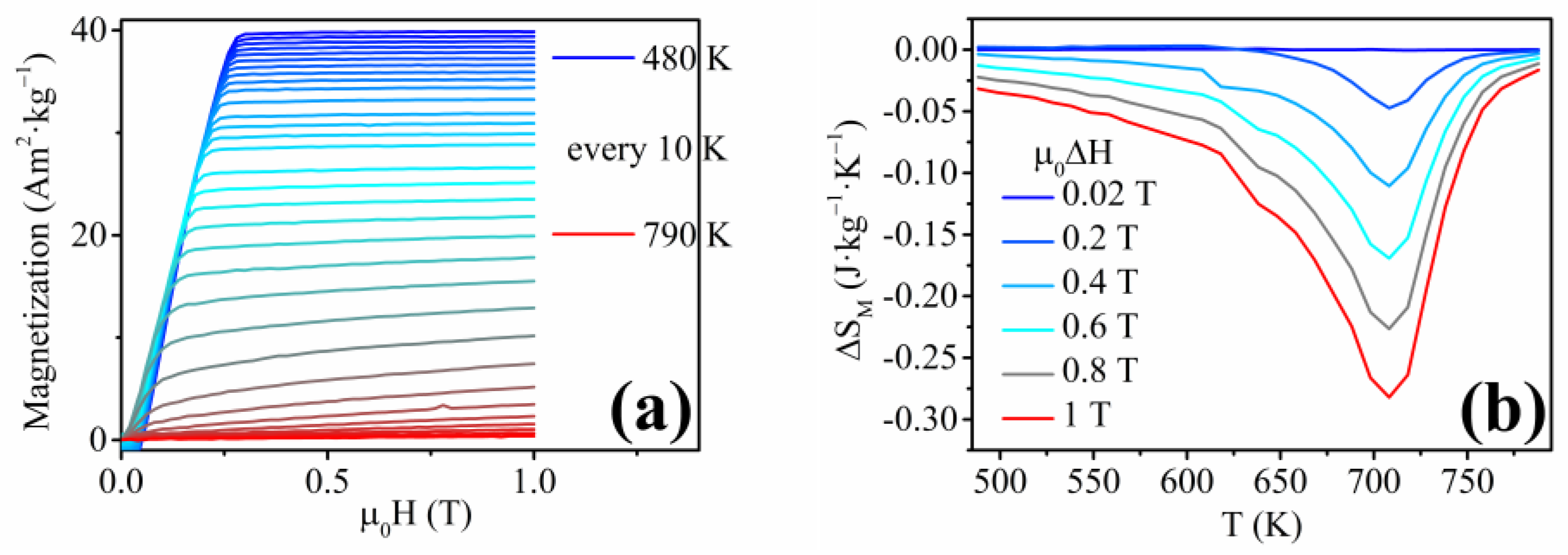
3.3. Ni50Fe31Sn19 Nanowires
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graf, T.; Felser, C.; Parkin, S.S.P. Simple Rules for the Understanding of Heusler Compounds. Prog. Solid State Chem. 2011, 39, 1–50. [Google Scholar] [CrossRef]
- Salazar-Mejía, C.; Devi, P.; Singh, S.; Felser, C.; Wosnitza, J. Influence of Cr Substitution on the Reversibility of the Magnetocaloric Effect in Ni-Cr-Mn-In Heusler Alloys. Phys. Rev. Mater. 2021, 5, 104406. [Google Scholar] [CrossRef]
- El-Khouly, A.; Novitskii, A.; Serhiienko, I.; Kalugina, A.; Sedegov, A.; Karpenkov, D.; Voronin, A.; Khovaylo, V.; Adam, A.M. Optimizing the Thermoelectric Performance of FeVSb Half-Heusler Compound via Hf–Ti Double Doping. J. Power Sources 2020, 477, 228768. [Google Scholar] [CrossRef]
- Pfeuffer, L.; Lemke, J.; Shayanfar, N.; Riegg, S.; Koch, D.; Taubel, A.; Scheibel, F.; Kani, N.A.; Adabifiroozjaei, E.; Molina-Luna, L.; et al. Microstructure Engineering of Metamagnetic Ni-Mn-Based Heusler Compounds by Fe-Doping: A Roadmap towards Excellent Cyclic Stability Combined with Large Elastocaloric and Magnetocaloric Effects. Acta Mater. 2021, 221, 117390. [Google Scholar] [CrossRef]
- Winiarski, M.J.; Kuderowicz, G.; Górnicka, K.; Litzbarski, L.S.; Stolecka, K.; Wiendlocha, B.; Cava, R.J.; Klimczuk, T. MgPd2Sb: A Mg-Based Heusler-Type Superconductor. Phys. Rev. B 2021, 103, 214501. [Google Scholar] [CrossRef]
- Górnicka, K.; Kuderowicz, G.; Winiarski, M.J.; Wiendlocha, B.; Klimczuk, T. Superconductivity in LiGa2Ir Heusler Type Compound with VEC = 16. Sci. Rep. 2021, 11, 16517. [Google Scholar] [CrossRef] [PubMed]
- Hennel, M.; Varga, M.; Frolova, L.; Nalevanko, S.; Ibarra-Gaytán, P.; Vidyasagar, R.; Sarkar, P.; Dzubinska, A.; Galdun, L.; Ryba, T.; et al. Heusler-Based Cylindrical Micro- and Nanowires. Phys. Status Solidi Appl. Mater. Sci. 2022, 219, 2100657. [Google Scholar] [CrossRef]
- Guillemard, C.; Petit-Watelot, S.; Rojas-Sánchez, J.C.; Hohlfeld, J.; Ghanbaja, J.; Bataille, A.; Le Fèvre, P.; Bertran, F.; Andrieu, S. Polycrystalline Co2Mn-Based Heusler Thin Films with High Spin Polarization and Low Magnetic Damping. Appl. Phys. Lett. 2019, 115, 172401. [Google Scholar] [CrossRef]
- Munir, J.; Jamil, M.; Jbara, A.S.; Fatima, K.; Ain, Q.; Ullah, H.; Yousaf, M. Spin-Polarized Electromagnetic and Optical Response of Full-Heusler Co2VZ (Z = Al, Be) Alloys for Spintronic Application. Eur. Phys. J. Plus 2021, 136, 1009. [Google Scholar] [CrossRef]
- Nawa, K.; Kurniawan, I.; Masuda, K.; Miura, Y.; Patrick, C.E.; Staunton, J.B. Temperature-Dependent Spin Polarization of Heusler Co2MnSi from the Disordered Local-Moment Approach: Effects of Atomic Disordering and Nonstoichiometry. Phys. Rev. B 2020, 102, 054424. [Google Scholar] [CrossRef]
- Hennel, M.; Galdun, L.; Ryba, T.; Varga, R. Study of Martensitic Transition Temperature on Ni54Fe19Ga23X4 Heusler Glass-Coated Microwires Doped by X = B, Al, Ga, In. J. Magn. Magn. Mater. 2022, 542, 168605. [Google Scholar] [CrossRef]
- Stern-Taulats, E.; Castillo-Villa, P.O.; Mañosa, L.; Frontera, C.; Pramanick, S.; Majumdar, S.; Planes, A. Magnetocaloric Effect in the Low Hysteresis Ni-Mn-In Metamagnetic Shape-Memory Heusler Alloy. J. Appl. Phys. 2014, 115, 173907. [Google Scholar] [CrossRef]
- De Groot, R.A.; Mueller, F.M.; Engen, P.G.; Buschow, K.H.J. New Class of Materials: Half-Metallic Ferromagnets. Phys. Rev. Lett. 1983, 50, 2024–2027. [Google Scholar] [CrossRef]
- Wolf, S.A.; Awschalom, D.D.; Buhrman, R.A.; Daughton, J.M.; Von Molnár, S.; Roukes, M.L.; Chtchelkanova, A.Y.; Treger, D.M. Spintronics: A Spin-Based Electronics Vision for the Future. Science 2001, 294, 1488. [Google Scholar] [CrossRef] [PubMed]
- Dieny, B.; Speriosu, V.S.; Metin, S.; Parkin, S.S.P.; Gurney, B.A.; Baumgart, P.; Wilhoit, D.R. Magnetotransport Properties of Magnetically Soft Spin-Valve Structures (Invited). J. Appl. Phys. 1991, 69, 4774. [Google Scholar] [CrossRef]
- Moodera, J.S.; Kinder, L.R.; Wong, T.M.; Meservey, R. Large Magnetoresistance at Room Temperature in Ferromagnetic Thin Film Tunnel Junctions. Phys. Rev. Lett. 1995, 74, 3273–3276. [Google Scholar] [CrossRef]
- Hirohata, A.; Sagar, J.; Lari, L.; Fleet, L.R.; Lazarov, V.K. Heusler-Alloy Films for Spintronic Devices. Appl. Phys. A Mater. Sci. Process. 2013, 111, 423–430. [Google Scholar] [CrossRef]
- Dunand, D.C.; Müllner, P. Size Effects on Magnetic Actuation in Ni-Mn-Ga Shape-Memory Alloys. Adv. Mater. 2011, 23, 216–232. [Google Scholar] [CrossRef]
- Ahmad, A.; Mitra, S.; Srivastava, S.K.; Das, A.K. Size-Dependent Structural and Magnetic Properties of Disordered Co2FeAl Heusler Alloy Nanoparticles. J. Magn. Magn. Mater. 2019, 474, 599–604. [Google Scholar] [CrossRef]
- Aslani, A.; Ghahremani, M.; Zhang, M.; Bennett, L.H.; Torre, E. Della Enhanced Magnetic Properties of Ni51Mn33.4In15.6 Heusler Alloy Nanoparticles. IEEE Trans. Magn. 2017, 53, 2504006. [Google Scholar] [CrossRef]
- Taylor, R.C.; Tsuei, C.C. Amorphous Heusler Alloys: The Effect of Annealing on Structure and Magnetic and Transport Properties. Solid State Commun. 1982, 41, 503–506. [Google Scholar] [CrossRef]
- Hayashi, N.; Morii, K.; Matsui, T.; Nakayama, Y. Formation of the PtMnSb Phase in Thin Multilayered Pt/Mn/Sb Films. Mater. Trans. JIM 1991, 32, 192–198. [Google Scholar] [CrossRef]
- Wang, C.; Meyer, J.; Teichert, N.; Auge, A.; Rausch, E.; Balke, B.; Hütten, A.; Fecher, G.H.; Felser, C. Heusler Nanoparticles for Spintronics and Ferromagnetic Shape Memory Alloys. J. Vac. Sci. Technol. B 2014, 32, 020802. [Google Scholar] [CrossRef]
- Wang, C.; Casper, F.; Guo, Y.; Gasi, T.; Ksenofontov, V.; Balke, B.; Fecher, G.H.; Felser, C.; Hwu, Y.K.; Lee, J.J. Resolving the Phase Structure of Nonstoichiometric Co2FeGa Heusler Nanoparticles. J. Appl. Phys. 2012, 112, 124314. [Google Scholar] [CrossRef]
- Nadutov, V.M.; Perekos, A.O.; Kokorin, V.V.; Trachevskii, V.V.; Konoplyuk, S.M.; Vashchuk, D.L. 27Al, 63Cu NMR Spectroscopy and Electrical Transport in Heusler Cu–Mn–Al Alloy Powders. Appl. Nanosci. 2018, 8, 965–971. [Google Scholar] [CrossRef]
- Du, J.H.; Zuo, Y.L.; Wang, Z.; Ma, J.H.; Xi, L. Properties of Co2FeAl Heusler Alloy Nano-Particles Synthesized by Coprecipitation and Thermal Deoxidization Method. J. Mater. Sci. Technol. 2013, 29, 245–248. [Google Scholar] [CrossRef]
- Andrade, V.M.; Checca, N.R.; de Paula, V.G.; Pirota, K.R.; Rossi, A.L.; Garcia, F.; Vovk, A.; Bunyaev, S.A.; Kakazei, G.N. Full Heusler Fe2CrAl Nanogranular Films Produced by Pulsed Laser Deposition for Magnonic Applications. J. Appl. Phys. 2023, 134, 023901. [Google Scholar] [CrossRef]
- Ghahremani, M.; Aslani, A.; Hosseinnia, M.; Bennett, L.H.; Della Torre, E. Direct and Indirect Measurement of the Magnetocaloric Effect in Bulk and Nanostructured Ni-Mn-In Heusler Alloy. AIP Adv. 2018, 8, 056426. [Google Scholar] [CrossRef]
- Aksoy, S. Synthesis and Characterization of NiMnIn Nanoparticles. J. Magn. Magn. Mater. 2015, 373, 236–239. [Google Scholar] [CrossRef]
- Vallal Peruman, K.; Mahendran, M.; Seenithurai, S.; Chokkalingam, R.; Singh, R.K.; Chandrasekaran, V. Internal Stress Dependent Structural Transition in Ferromagnetic Ni-Mn-Ga Nanoparticles Prepared by Ball Milling. J. Phys. Chem. Solids 2010, 71, 1540–1544. [Google Scholar] [CrossRef]
- Wang, C.; Levin, A.A.; Karel, J.; Fabbrici, S.; Qian, J.; ViolBarbosa, C.E.; Ouardi, S.; Albertini, F.; Schnelle, W.; Rohlicek, J.; et al. Size-Dependent Structural and Magnetic Properties of Chemically Synthesized Co-Ni-Ga Nanoparticles. Nano Res. 2017, 10, 3421–3433. [Google Scholar] [CrossRef]
- Seo, K.; Bagkar, N.; Kim, S.I.; In, J.; Yoon, H.; Jo, Y.; Kim, B. Diffusion-Driven Crystal Structure Transformation: Synthesis of Heusler Alloy Fe3Si Nanowires. Nano Lett. 2010, 10, 3643–3647. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, K.R.; Gyawali, P.; Forbes, A.; Pegg, I.L.; Philip, J. Synthesis and Characterization of Co2FeAl Nanowires. J. Appl. Phys. 2012, 111, 123906. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Qin, F.X.; Estevez, D.; Franco, V.; Peng, H.X. Structure, Magnetic and Magnetocaloric Properties of Ni2MnGa Heusler Alloy Nanowires. J. Magn. Magn. Mater. 2020, 513, 167100. [Google Scholar] [CrossRef]
- Simon, P.; Wolf, D.; Wang, C.; Levin, A.A.; Lubk, A.; Sturm, S.; Lichte, H.; Fecher, G.H.; Felser, C. Synthesis and Three-Dimensional Magnetic Field Mapping of Co2FeGa Heusler Nanowires at 5 Nm Resolution. Nano Lett. 2016, 16, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, D.; Luo, Z.; Wu, F.; Chen, C.; Liu, M.; Yi, L.; Piao, H.G.; Yu, G. Fabrication and Magnetic Properties of Structure-Tunable Co2FeGa-SiO2 Heusler Nanocompounds. AIP Adv. 2018, 8, 055107. [Google Scholar] [CrossRef]
- Prida, V.M.; Salaheldeen, M.; Pfitzer, G.; Hidalgo, A.; Vega, V.; González, S.; Teixeira, J.M.; Fernández, A.; Hernando, B. Template Assisted Deposition of Ferromagnetic Nanostructures: From Antidot Thin Films to Multisegmented Nanowires. Acta Phys. Pol. A 2017, 131, 822–827. [Google Scholar] [CrossRef]
- Ma, X.; Chen, G.; Zhang, X.; Jia, T.; Zhao, W.; Mo, Z.; Liu, H.; Dai, X.; Liu, G. Fabrication and Growth Mechanism of One-Dimensional Heusler Alloy Nanostructures with Different Morphologies on Anodic Aluminum Oxide Template by Magnetron Sputtering. Front. Mater. Sci. 2022, 16, 220615. [Google Scholar] [CrossRef]
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural Features of Oxide Coatings on Aluminum. J. Electrochem. Soc. 1953, 100, 411–419. [Google Scholar] [CrossRef]
- Despic, A.; Parkhutik, V. Electrochemistry of Aluminum in Aqueous Solutions and Physics of Its Anodic Oxide. In Modern Aspects of Electrochemistry; Bockris, J.O., White, R.E., Conway, B.E., Eds.; Springer: New York, NY, USA, 1989; pp. 401–504. ISBN 978-0-306-43127-2. [Google Scholar]
- Heber, K.V. Studies on Porous Al2O3 Growth-I. Physical Model. Electrochim. Acta 1978, 23, 127–133. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in Nano-Engineered Anodic Aluminum Oxide Membrane Development. Materials 2010, 4, 487–526. [Google Scholar] [CrossRef] [PubMed]
- Galdun, L.; Vega, V.; Vargová, Z.; Barriga-Castro, E.D.; Luna, C.; Varga, R.; Prida, V.M. Intermetallic Co2FeIn Heusler Alloy Nanowires for Spintronics Applications. ACS Appl. Nano Mater. 2018, 1, 7066–7074. [Google Scholar] [CrossRef]
- Penner, R.M.; Martin, C.R. Preparation and Electrochemical Characterization of Ultramicroelectrode Ensembles. Anal. Chem. 1987, 59, 2625–2630. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys; Academic Press Inc.: Cambridge, MA, USA, 1963; ISBN 978-1-4831-9807-1. [Google Scholar]
- Yang, R.; Sui, C.; Gong, J.; Qu, L. Silver Nanowires Prepared by Modified AAO Template Method. Mater. Lett. 2007, 61, 900–903. [Google Scholar] [CrossRef]
- Foss, C.A.; Hornyak, G.L.; Stockert, J.A.; Martin, C.R. Optical Properties of Composite Membranes Containing Arrays of Nanoscopic Gold Cylinders. J. Phys. Chem. 1992, 96, 7497–7499. [Google Scholar] [CrossRef]
- Han, N.; Sun, M.; Zhou, Y.; Xu, J.; Cheng, C.; Zhou, R.; Zhang, L.; Luo, J.; Huang, B.; Li, Y. Alloyed Palladium–Silver Nanowires Enabling Ultrastable Carbon Dioxide Reduction to Formate. Adv. Mater. 2021, 33, 2005821. [Google Scholar] [CrossRef]
- Pardavi-Horvath, M.; Si, P.E.; Vazquez, M.; Rosa, W.O.; Badini, G. Interaction Effects in Permalloy Nanowire Systems. J. Appl. Phys. 2008, 103, 07D517. [Google Scholar] [CrossRef]
- McGary, P.D.; Tan, L.; Zou, J.; Stadler, B.J.H.; Downey, P.R.; Flatau, A.B. Magnetic Nanowires for Acoustic Sensors. J. Appl. Phys. 2006, 99, 08B310. [Google Scholar] [CrossRef]
- Maleki, K.; Sanjabi, S.; Alemipour, Z. DC Electrodeposition of NiGa Alloy Nanowires in AAO Template. J. Magn. Magn. Mater. 2015, 395, 289–293. [Google Scholar] [CrossRef]
- Li, W.J.; Khan, U.; Irfan, M.; Javed, K.; Liu, P.; Ban, S.L.; Han, X.F. Fabrication and Magnetic Investigations of Highly Uniform CoNiGa Alloy Nanowires. J. Magn. Magn. Mater. 2017, 432, 124–128. [Google Scholar] [CrossRef]
- Khan, S.; Ahmad, N.; Ahmed, N.; Safeer, A.; Iqbal, J.; Han, X.F. Structural, Magnetic and Transport Properties of Fe-Based Full Heusler Alloy Fe2CoSn Nanowires Prepared by Template-Based Electrodeposition. J. Magn. Magn. Mater. 2018, 465, 462–470. [Google Scholar] [CrossRef]
- Khan, S.; Ahmad, N.; Ahmed, N.; Han, X.F. Analysis of Electronic, Magnetic and Half-Metallic Properties of L21-Type (Co2Mn0.5Fe0.5Sn) Heusler Alloy Nanowires Synthesized by AC-Electrodeposition in AAO Templates. J. Magn. Magn. Mater. 2018, 460, 120–127. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Kou, X. Communication—Electrodeposition, Microstructure and Magnetic Properties of Co2FeSn Heusler Alloy Nanowires. J. Electrochem. Soc. 2018, 165, D813. [Google Scholar] [CrossRef]
- Galdun, L.; Szabo, P.; Vega, V.; Barriga-Castro, E.D.; Mendoza-Reséndez, R.; Luna, C.; Kovac, J.; Milkovic, O.; Varga, R.; Prida, V.M. High Spin Polarization in Co2FeSn Heusler Nanowires for Spintronics. ACS Appl. Nano Mater. 2020, 3, 7438–7445. [Google Scholar] [CrossRef]
- Safeer, A.; Ahmad, N.; Khan, S.; Azam, L.A.; Bashir, D. Magnetization Behavior of Electrochemically Synthesized Co2MnSn Full Heusler Alloy Nanowire Arrays. J. Appl. Phys. 2019, 125, 034302. [Google Scholar] [CrossRef]
- Sharma, M.; Das, A.; Kuanr, B.K. Co-Based Full Heusler Alloy Nanowires: Modulation of Static and Dynamic Properties through Deposition Parameters. AIP Adv. 2019, 9, 125054. [Google Scholar] [CrossRef]
- Javed, K.; Zhang, X.M.; Parajuli, S.; Ali, S.S.; Ahmad, N.; Shah, S.A.; Irfan, M.; Feng, J.F.; Han, X.F. Magnetization Behavior of NiMnGa Alloy Nanowires Prepared by DC Electrodeposition. J. Magn. Magn. Mater. 2020, 498, 166232. [Google Scholar] [CrossRef]
- Wei, H.; Kou, X. Size-Dependent Magnetic and Magnetoresistance Properties of Co2FeGa Nanowires. Funct. Mater. Lett. 2022, 15, 07n08. [Google Scholar] [CrossRef]
- Parajuli, S.; Cheng, C.; Wei, J.W.; Martuza, K.G.; Irfan, M.; Zhang, X.M.; Javed, K.; Feng, J.F.; Han, X.F. Heusler Compound Ni2FeGa Nanoarchitectures. J. Magn. Magn. Mater. 2021, 539, 168355. [Google Scholar] [CrossRef]
- Hilse, M.; Jenichen, B.; Herfort, J. GaAs-Fe3Si Semiconductor–Ferromagnet Core–Shell Nanowires for Spintronics. In Novel Compound Semiconductor Nanowires: Materials, Devices, and Applications; Ishikawa, F., Buyanova, I.A., Eds.; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2017; pp. 221–253. ISBN 9781315364407. [Google Scholar]
- Ali, S.S.; Cheng, C.; Parajuli, S.; Zhang, X.; Feng, J.; Han, X.F. Fabrication, Structural and Magnetic Behavior of Novel One-Dimensional Fe2MnGa Nanostructures. J. Magn. Magn. Mater. 2022, 549, 169022. [Google Scholar] [CrossRef]
- Varga, M.; Galdun, L.; Kunca, B.; Vega, V.; García, J.; Prida, V.M.; Barriga-Castro, E.D.; Luna, C.; Diko, P.; Saksl, K.; et al. FORC and TFORC Analysis of Electrodeposited Magnetic Shape Memory Nanowires Array. J. Alloys Compd. 2022, 897, 163211. [Google Scholar] [CrossRef]
- Varga, M.; Galdun, L.; Diko, P.; Saksl, K.; Varga, R. Analysis of Magnetocaloric Effect in Parallel Ni-Mn-Ga Heusler Alloy Nanowires. J. Alloys Compd. 2023, 944, 169196. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Gschneidner, K.A. Giant Magnetocaloric Effect in Gd5(Si2Ge2). Phys. Rev. Lett. 1997, 78, 4494–4497. [Google Scholar] [CrossRef]
- Hennel, M.; Galdun, L.; Ryba, T.; Varga, R. Transition Temperature Tuning of Ni2FeGa Based Heusler Alloys in Form of Glass-Coated Microwires. J. Magn. Magn. Mater. 2020, 511, 166973. [Google Scholar] [CrossRef]
- Santhi, K.; Kumarsan, D.; Vengidusamy, N.; Arumainathan, S. Electrochemical Alloying of Immiscible Ag and Co for Their Structural and Magnetic Analyses. J. Magn. Magn. Mater. 2017, 433, 202–208. [Google Scholar] [CrossRef]
- Roy, M.K.; Subrahmanyam, V.S.; Verma, H.C. Defect Studies in Fe-Cu Alloys Prepared by Electrodeposition. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2004, 328, 375–379. [Google Scholar] [CrossRef]
- Roberts, A.P.; Pike, C.R.; Verosub, K.L. First-Order Reversal Curve Diagrams: A New Tool for Characterizing the Magnetic Properties of Natural Samples. J. Geophys. Res. Solid Earth 2000, 105, 28461–28475. [Google Scholar] [CrossRef]
- Pike, C.; Fernandez, A. An Investigation of Magnetic Reversal in Submicron-Scale Co Dots Using First Order Reversal Curve Diagrams. J. Appl. Phys. 1999, 85, 6668–6676. [Google Scholar] [CrossRef]
- Franco, V.; Gottschall, T.; Skokov, K.P.; Gutfleisch, O. First-Order Reversal Curve (FORC) Analysis of Magnetocaloric Heusler-Type Alloys. IEEE Magn. Lett. 2016, 7, 6602904. [Google Scholar] [CrossRef]
- Díaz-García, A.; Moreno-Ramírez, L.M.; Law, J.Y.; Albertini, F.; Fabbrici, S.; Franco, V. Characterization of Thermal Hysteresis in Magnetocaloric NiMnIn Heusler Alloys by Temperature First Order Reversal Curves (TFORC). J. Alloys Compd. 2021, 867, 159184. [Google Scholar] [CrossRef]
- Moreno-Ramírez, L.M.; Franco, V. Setting the Basis for the Interpretation of Temperature First Order Reversal Curve (TFORC) Distributions of Magnetocaloric Materials. Metals 2020, 10, 1039. [Google Scholar] [CrossRef]
- Hennel, M.; Galdun, L.; Varga, R. Analysis of Magnetocaloric Effect in Ni2FeGa-Based Glass-Coated Microwires. J. Magn. Magn. Mater. 2022, 560, 169646. [Google Scholar] [CrossRef]
- Brown, P.J.; Gandy, A.P.; Ishida, K.; Kainuma, R.; Kanomata, T.; Morito, H.; Neumann, K.U.; Oikawa, K.; Ziebeck, K.R.A. Crystal Structures and Magnetization Distributions in the Field Dependent Ferromagnetic Shape Memory Alloy Ni54Fe19Ga27. J. Phys. Condens. Matter 2007, 19, 016201. [Google Scholar] [CrossRef]
- Muxworthy, A.R.; Roberts, A.P. First-Order Reversal Curve (FORC) Diagrams. In Encyclopedia of Geomagnetism and Paleomagnetism; Gubbins, D., Herrero-Bervera, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 266–272. ISBN 978-1-4020-3992-8. [Google Scholar]
- Chandra, S.; Biswas, A.; Datta, S.; Ghosh, B.; Raychaudhuri, A.K.; Srikanth, H. Inverse Magnetocaloric and Exchange Bias Effects in Single Crystalline La0.5Sr0.5MnO3 Nanowires. Nanotechnology 2013, 24, 505712. [Google Scholar] [CrossRef]
- Staňo, M.; Fruchart, O. Magnetic Nanowires and Nanotubes. In Handbook of Magnetic Materials; Bruck, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 27, pp. 155–267. ISBN 9780444641618. [Google Scholar]
- Frol’ová, L.; Ryba, T.; Vargová, Z.; Komanický, V.; Kovác, J.; Gyepes, R.; Varga, R. Magnetic and Structural Characterization of Nickel and Iron Based Heusler Ribbons Ni2FeZ (Z = In, Sn, Sb). Acta Phys. Pol. A 2017, 131, 735–737. [Google Scholar] [CrossRef]







| Composition | Research Aim | Fabrication | Ref. |
|---|---|---|---|
| Fe3Si | Binary HA nanowires | crystal transformation | 2010 [32] |
| Co2FeAl | High TC, diameter < 500 nm | electrospinning | 2012 [33] |
| Co2FeGa | Electron tomography on HA nanowires | Annealing in SBA-15 template | 2014 [35] |
| Co2FeGa | Diameter below 7 nm | Annealing in SBA-15 template | 2016 [36] |
| GaAs-Fe3Si | Semiconductor–Ferromagnet Core–Shell Nanowires | Molecular beam epitaxy | 2017 [62] |
| Co2NiGa | Composition tuning through deposition potential | DC Electrodeposition | 2017 [52] |
| Co2FeSn | Easy axis perpendicular to the nanowire axis | DC Electrodeposition | 2018 [55] |
| Co2FeGa-SiO2 | Diameter 4–7 nm, length below 200 nm | Chemical approach in SBA-15 | 2018 [36] |
| Co2Mn0.5Fe0.5Sn | 100% spin-polarization, L21 structure | AC Electrodeposition | 2018 [54] |
| Fe2CoSn | Spintronic material due to suitable transport properties | AC Electrodeposition | 2018 [53] |
| Co2FeIn | High spin polarization, suitable for spintronics | DC electrodeposition | 2018 [43] |
| Co2MnSn | Easy axis parallel to the nanowire axis | AC Electrodeposition | 2019 [57] |
| Co2MnAl | Composition tuning through deposition parameters | DC electrodeposition | 2019 [58] |
| Ni-Mn-Ga | Diameter of ≈200 nm | DC electrodeposition | 2020 [59] |
| Co2FeSn | 100% spin-polarization | DC electrodeposition | 2020 [56] |
| Ni2MnGa | 2nd order phase transformation MCE | electrospinning | 2020 [34] |
| Ni2FeGa | Electrodeposited nanowires and nanotubes | DC electrodeposition | 2021 [61] |
| Co2FeGa | Magnetoresistance properties of HA nanowires | Template assisted electrodeposition | 2022 [60] |
| Fe2MnGa | Magnetic properties of novel HA nanowires | DC electrodeposition | 2022 [63] |
| Ni-Fe-Ga | FORC + TFORC analysis of functional nanowires | DC electrodeposition | 2022 [64] |
| Co2MnAl, Co2CrAl and Co2TiAl | Growth of nanowires and nanotubes | Magnetron Sputtering in AAO template | 2022 [38] |
| Ni2MnGa | Magnetocaloric effect due to first-order phase transformation | DC electrodeposition | 2023 [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, M.; Galdun, L.; Vronka, M.; Diko, P.; Heczko, O.; Varga, R. Electrodeposited Heusler Alloys-Based Nanowires for Shape Memory and Magnetocaloric Applications. Materials 2024, 17, 407. https://doi.org/10.3390/ma17020407
Varga M, Galdun L, Vronka M, Diko P, Heczko O, Varga R. Electrodeposited Heusler Alloys-Based Nanowires for Shape Memory and Magnetocaloric Applications. Materials. 2024; 17(2):407. https://doi.org/10.3390/ma17020407
Chicago/Turabian StyleVarga, Michal, Ladislav Galdun, Marek Vronka, Pavel Diko, Oleg Heczko, and Rastislav Varga. 2024. "Electrodeposited Heusler Alloys-Based Nanowires for Shape Memory and Magnetocaloric Applications" Materials 17, no. 2: 407. https://doi.org/10.3390/ma17020407





