A Critical Review Examining the Characteristics of Modified Concretes with Different Nanomaterials
Abstract
:1. Introduction
2. Research Significance
3. Methodology
4. Construction Industry: Prospects and Challenges
5. Nanotechnology
6. Nanomaterials in Construction: Opportunity or Threat?
7. Nanomaterials in Concrete
7.1. Nano-CaCO3
| N.O. | n-CaCO3 (%) | CS | TS | FS | Other Reviews | Results | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 0, 1, 2, 3, 4 | Y | N | N | Slump, Water sorptivity, Volume of permeable voids, RCPT, Chloride diffusion, SEM, XRD, MIP, DTA/TG | Improving compressive strength (the best dosage is 1%): Early ages = about 148–146% improvement, Age 90 days = about 40% recovery, Reduction of water absorption (the best dosage is 1%): 28 days = 17% reduction, 90 days = 30% reduction, Reduction of permeable voids (by 46%), Reducing the chloride ion permeability (the best dosage is 1%): 28 days = 20%, 90 days = 50%, Reducing the chloride diffusion coefficient (by 73%), Effectively reduces capillary porosities and refines pores | [82] |
| 2 | 0, 5, 10, 15 | Y | N | N | Setting time, Heat of hydration | Improve flowability, Significant effect on the setting and hardening | [83] |
| 3 | 0, 1, 2, 3, 4 | Y | Y | Y | Slump, Water absorption | Improving compressive strength (best dose = 4%), Improving tensile strength (best dose = 4%), Improvement of flexural strength (best dose = 4%), The reduction of flowability and the increase of the heat of hydration | [84] |
| 4 | 0, 2 | Y | Y | Y | Impact test (by SHPB), SEM, MIP | Improving compressive, tensile, and flexural strength, Improving the dynamic compressive strength, peak strain, impact toughness, energy dissipation | [85] |
| 5 | 0, 1, 2, 3 | Y | Y | N | Slump, J-ring, V-funnel, L-box, Electrical resistivity, Freeze and thaw cycle, Permeability, UPV, SEM, XRD | Improving compressive strength (best dose = 3%), Improvement of tensile strength (best dose = 3%), Decreased permeability (best dose = 3%), Improving resistance to freeze and thaw cycle (best dose = 2%) | [86] |
| 6 | 0, 1, 2, 3 | Y | Y | N | Modulus of elasticity, Free Shrinkage, Restrained Ring Test | Improving compressive and tensile strength (best dose = 1%), Improving modulus of elasticity (best dose = 1%), Reduction of free and restrained shrinkage (best dose = 1%), Reducing residual stress, Reduction of relaxed stress and tensile creep (dose 1%) | [77] |
| 7 | 0, 1 | Y | Y | N | Rheological, Raman, FTIR, SEM, XRD, DTA/TG | Preventing weight loss of cement at 800 °C, Increased the shear stress limit and the yield stress, Improved compressive strength | [87] |
| 8 | 0, 1, 2, 3 | N | N | N | Carbonation resistance, Chloride resistance, TG/DTG, SEM, XRD | Improved resistance to carbonation, Improved resistance to chloride, Improved the microstructure of concrete | [90] |
7.2. Nano-Clay
| N.O. | n-Clay (%) | CS | TS | FS | Other Reviews | Results | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | 0, 0.1, 0.3, 0.5 | Y | N | N | Thermal conductivity coefficients | Improve compressive strength, Improvement of thermal conductivity coefficients, The best dosage: 0.3% and 0.5% | [91] |
| 2 | 0, 5, 7.5, 10 | Y | Y | Y | Slipping bond strength, Split bond strength, SEM, XRD, AFM | The best dosage for optimal mechanical properties = 7.5%, Compressive, tensile, flexural, and bond strengths: Sonicated technique mixtures about 1.42–3.47 times as-received technique, Improvement of concrete microstructure, Better reactivity of nanomaterials in the Sonicated technique compared to the as-received technique | [95] |
| 3 | 0, 1, 3, 5 | Y | N | N | Freezing-thaw cycle, Accelerated corrosion, Pull-out | Maintaining the good condition of concrete after 125 freeze–thaw cycles (sample containing 3% and 5% nano), Improving bond behavior (preventing rebar corrosion), Improve compressive strength | [101,102] |
| 4 | 0, 1, 3, 5, 7, 9 | Y | N | Y | Workability (Flow ability, water requirement, Setting time, Soundness), Chloride diffusivity, MIP, SEM, XRD, RCM | Decrease Flow ability, Increase water demand of normal consistency, Slight reduction of initial and final setting time, Improving the compressive and flexural strength of mortar, Significant increase in chloride penetration resistance | [103] |
| 5 | 0, 1, 3, 5 | Y | N | N | Simulated acid rain environment, TG/DS, BSEM, XRD | Improving the microstructure (increasing C-S-H and decreasing CH), Increasing resistance to acid attack (best dose = 3%) | [104] |
| 6 | 0, 3 | Y | N | Y | Elastic modulus, Shrinkage | Significant improvement in Shrinkage Cracking, Improving compressive strength, flexural strength, and elastic modulus | [105] |
| 7 | 0, 0.25, 0.5, 0.75, 1 | Y | Y | N | Slump, V-funnel, L-box, Electrical resistivity, Water penetration, SEM, XRD | Reducing slump flow and increasing flow time, Improving compressive strength (best dose = 0.5%), Improvement of tensile strength (best dose = 0.75%), Increase in electrical resistivity (best dose = 1%), Microstructure improvement | [107] |
7.3. Nano-Titanium Dioxide (Nano-TiO2)
| N.O. | n-TiO2 (%) | CS | TS | FS | Other Reviews | Results | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | 0, 3, 5 | Y | N | N | Weight loss, Ultrasonic pulse velocity, Radiation transmission, SEM | V-Funnel and L-box: Improving the workability properties (use up to 3%), Improve compressive strength, Improved formation of C-S-H, Reduction of water penetration depth, Reducing the penetration of chloride ions | [121] |
| 2 | 0, 2, 4, 6, 8 | Y | N | N | Impact test, Ultrasonic pulse velocity, Radiation attenuation, SEM | Improving compressive strength (best performance 6% nano), Improved impact strength (best performance 6% nano), Reduction of inner pores, Increasing protective effects against gamma rays | [119] |
| 3 | 0, 2, 4, 6 | Y | N | N | Ultrasonic pulse velocity, Gamma-ray shielding, SEM | With increasing temperature = compressive strength increases first then decreases, With increasing temperature = UPV first increases and then decreases, The better performance of mixtures containing nano compared to the control mixture in terms of the linear attenuation coefficient and compressive strength, Best performance = mixture containing 6% nano | [120] |
| 4 | 0, 1, 1.5, 3, 5 | Y | N | N | X-ray diffraction (XRD), SEM | Improvement of 28-day compressive strength (improvement by 9%), Filling pores, Improvement of the paste-aggregate interface | [122] |
| 5 | 0, 3, 6, 10 | Y | N | N | Modulus of elasticity, MIP test, SEM | Improvement of 28-day compressive strength, A better distribution and refinement of the pores, 10% anatase I mixture and control mixture = highest porosity, Anatase mixture I = the poorest mechanical performance, Best performance = anatase II or rutile, Reduction of concrete rigidity | [123] |
| 6 | 0, 1, 2, 3 | Y | N | N | MIP test, Chloride diffusion | Effective improvement of durability, Increasing the resistance to chloride (the best dosage = 2%). | [124] |
| 7 | 0, 1 | Y | N | N | Chloride diffusion | Increase scouring abrasion resistance, Reducing the chloride ion diffusion coefficient | [125] |
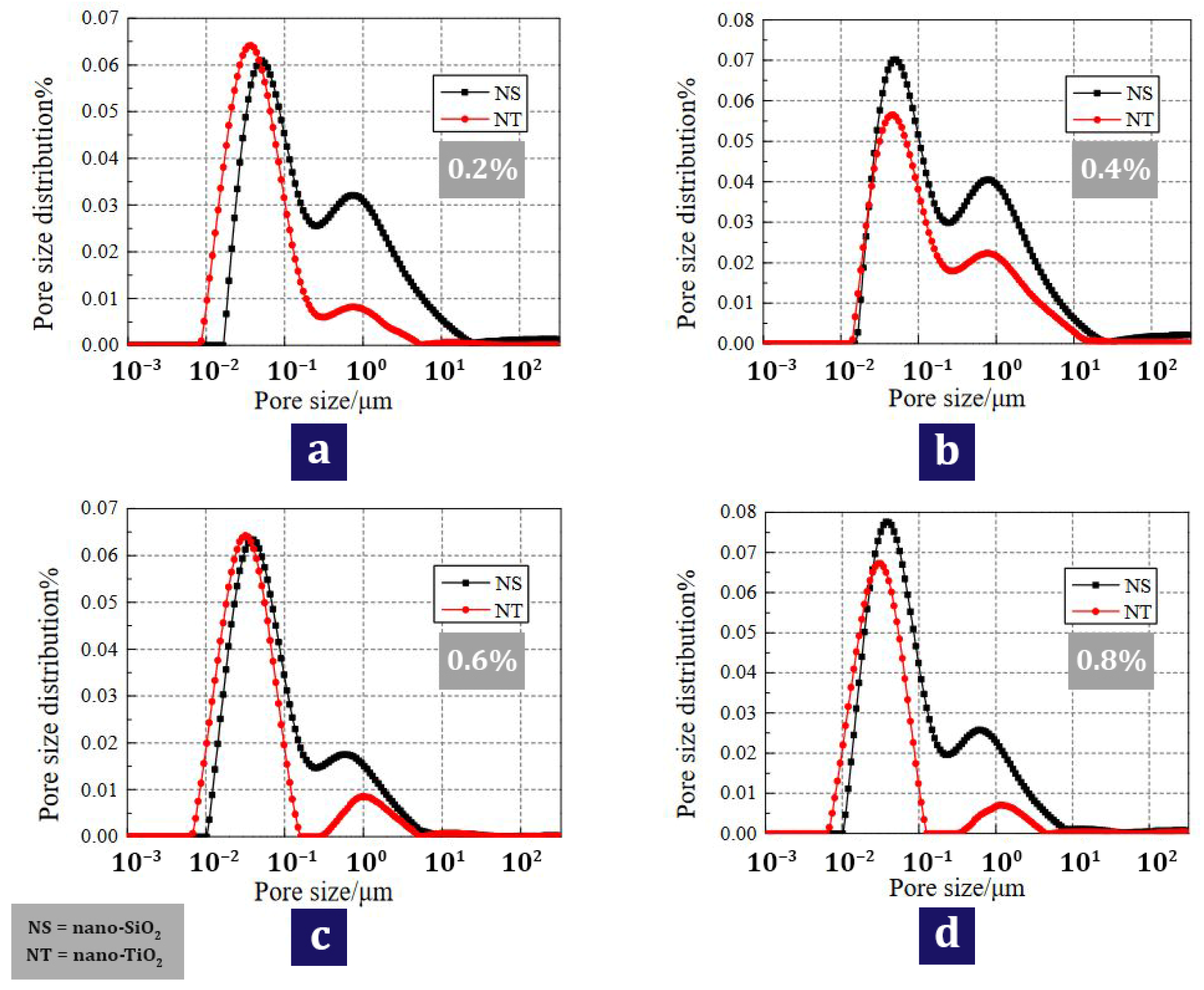
| 8 | 0.2, 0.4, 0.6, 0.8 | N | N | N | Freeze–thaw durability, Nuclear Magnetic Resonance, Industrial CT Scanning, Imaging Test | Preventing pore and crack expansion, Improving freezing and thawing resistance | [126] |
| 9 | 0, 1, 3, 5 | Y | N | Y | Chloride permeability, Pore structure | Improving compressive and flexural strength, Improving and purifying the structure of the pore structure, Improving resistance to chloride penetration | [128] |
| 10 | 0, 2 | Y | N | N | Water absorption, Frost resistance | Improve compressive strength, Decreased water absorption, Improve frost resistance | [129] |
7.4. Nano-Silica (Nano-SiO2)
| N.O. | n-SiO2 (%) | CS | TS | FS | Other Reviews | Results | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | 0, 0.75, 1.5, 3 | Y | Y | Y | Slump, Modulus of elasticity, Ultrasonic pulse velocity, Water absoration, SEM | Slump reduction, Improved compressive and tensile strength, Slight increase in the modulus of elasticity, Significant improvement in non-destructive test results, Improved density, Decreasing water absorption, Reducing the volume of voids | [149] |
| 2 | 0, 0.75, 1.5, 3 | Y | Y | Y | Slump, Ultrasonic Pulse Velocity | Decreasing slump, Improved compressive, tensile, and flexural strength, Improvement of the ITZ region, Significant improvement in non-destructive test results | [150] |
| 3 | 1, 2, 4 | Y | N | Y | Oven-dry density, Thermal conductivity, Specific heat, Water Porosity, Water Absorption, air-void characteristics, MIP test, SEM | Increased superplasticizer required, Significant improvement of resistance and reduction of transport properties, Modification of the air-void system | [151] |
| 4 | 0, 0.4, 0.8, 1.2 | Y | Y | N | Water absorption, SEM | Improving compressive strength: The mixture of 50% recycled aggregate → 0.4% nano (10%↑), 0.8% nano (18%↑), 1.2% nano (22%↑), The mixture of 100% recycled aggregate → 0.4% nano (6%↑), 0.8% nano (13%↑), 1.2% nano (16%↑), Improved tensile strength, Improve water absorption, Improvement of the ITZ region | [165] |
| 5 | 0, 0.3, 0.9 | Y | N | N | Water permeability, Water sorptivity, Water absorption, Chloride migration coefficient, SEM | Improvement of ITZ, Correction of the pore size distribution, Reduction of water penetration depth = 45%, Reduction of chloride migration coefficient = 28.7%, Reduction of diffusion coefficient = 31% | [153] |
| 6 | 0, 2.5, 5, 7.5, 10 | Y | Y | N | Density, Modulus of Elasticity | Increased density, Improved tensile strength Improvement of elasticity modulus and improved compressive strength: 3 days → 3.82–11.84% 7 days → 3.87–17.24% 28 days → 4.93–24.59% | [166] |
| 7 | 0, 1, 2, 3 | Y | N | N | Bulk density, Water absorption, Din permeability, Carbonation, Acid attack, Rapid chloride penetration, SEM, EDS, XRD, TG/DTG | Decreased permeability, Reduce water absorption, Improving resistance in acid attack | [154] |
| 8 | 0, 2, 4, 6, 8 | Y | N | N | Sulfate attack, SEM | Improving compressive strength (especially at early ages), Improving the resistance of concrete against sulfate attack, Improving the microstructure of concrete | [155] |
| 9 | 0, 2, 4 | Y | N | N | Isothermal Calorimetry, Autogenous shrinkage, XRD | Improve compressive strength Improvement of autogenous shrinkage, The increase in the total accumulated heat | [156] |
| 10 | 0, 1, 2, 3 | Y | N | N | Slump, V-funnel, T50, L-box, Drying shrinkage, SEM | Reduction of drying shrinkage, Improve compressive strength, Microstructure improvement | [157] |
7.5. The Combined Use of Nanomaterials in Concrete
7.6. Nanomaterial Performance in Microstructure
7.7. Environmental and Economic Performance of Nanomaterials
8. Bio-Inspired Materials
9. Discussion
10. Conclusions
- Using 1–2% nano-CaCO3 can reduce slump by 3.5–14.28%.
- In the early ages of concrete, the presence of nano-CaCO3 can significantly increase the compressive strength of concrete. At older ages, there is an improvement in compressive strength, but to a lesser extent than at early ages. Nano-CaCO3 can improve tensile strength (19–36%) and flexural strength (17–35%) for concrete.
- The use of 2% nano-CaCO3 also improved the behavior of concrete against dynamic load.
- The durability of concrete was improved by using nano-CaCO3. Reduction of water absorption (by 17–30%), reduction of chloride penetration (by 20–50%), increased resistance to carbonation (by 66.8%), improvement against acid attacks (reduction of mass loss by 4.2%), improvement against freeze and thaw cycles (at 28 days, 3.6% decrease in compressive strength loss), and improvement in electrical resistance (48.14% at 28 days) are among the things that can be mentioned.
- Using 1% nano-CaCO3 can prevent a 76% reduction of mass loss at 800 °C temperature (cement paste).
- The use of nano-CaCO3 can effectively reduce capillary porosities and purify pores.
- The use of nano-clay can lead to a decrease in workability (1–3% nano-clay reduces slump by 3.5–14.5%.)
- Because nano-clay is a rich source of aluminosilicates, it can produce C-A-H and C-S-H gels, which are very effective in improving the mechanical resistance of concrete. The use of 7.5% nano-clay can increase the compressive, tensile, and flexural strength by 24.52%, 9.76%, and 18%, respectively (the use of the Sonicated technique increases the strength by about 1.42–3.47 times).
- Nano-clay can have a positive effect on the durability of concrete. Improving resistance to acid attack (3% nano, prevents 1.6% mass loss), improvement against sulfate attack (9% nano, 41.5% prevention of compressive strength loss), improving resistance to freeze and thaw cycles (improvement by 34%), improving electrical resistance (by 31–38.5%) are among the things that nano-clay brings to concrete.
- When the tempering temperature does not exceed 300 °C, the mixture containing nano-clay shows more resistance compared to the control mixture. The temperature in the range of 440 °C to 450 °C leads to a significant decrease in the compressive strength of concrete containing nano-clay. Reaching a temperature of 800–1000 °C shows a decrease in the compressive strength of mixtures containing clay, but it results in a better situation compared to the control mixture.
- Nano-clay in the structure of concrete provides improvement of the microstructure. Four factors can be mentioned among the improvement features of this type of material: (1) pozzolanic reactivity, (2) filling effect, (3) nucleation effect, and (4) needle effect.
- Adding nano-TiO2 to concrete can improve protective effects against gamma rays. This capability is very important in nuclear facilities, radioactive waste products transportation, and radiotherapy rooms, which are more exposed to radiation.
- Adding 0.5% of nano-TiO2 to the mixture can reduce the slump by 16.34%. Increasing the content of nano-TiO2 can result in a decrease in workability.
- The improvement of compressive strength of concrete through nano-TiO2 is more obvious at older ages. In the field of tensile and flexural strength, nano-TiO2 can improve the strength of concrete. In terms of resistance, nano-TiO2 shows a weaker performance compared to nano-SiO2. Both nano-TiO2 and nano-SiO2 CH use cement paste, but it should be noted that the reaction products are different. In this regard, C-S-H produced by nano-SiO2 is of high quality and useful.
- The use of nano-TiO2 seems to increase the impact resistance of concrete by up to 35%.
- Nano-TiO2 can improve the durability properties of concrete acceptably. In this regard, the use of 0.9% of this type of nano improves the resistance against chloride penetration by about 33%. Also, reducing water absorption (1.5% nano, reduction by 6.65%), improving against sulfate attack (3% nano, by 3.87% preventing compressive strength loss), increasing resistance to different freeze and thaw cycles (2% nano improvement in equal to 300 cycles), improvement of electrical resistance (32.86%), improvement of shrinkage (15.23%) was obtained.
- Under high temperatures, nano-TiO2 shows acceptable performance. The use of 0.5% to 1.5% of nano-TiO2 in a concrete mixture under a high temperature of 800 °C can prevent compressive strength loss by 1% to 7.5%.
- Nano-TiO2 covers the pores in C-S-H gel and due to its particle size, it causes adhesive cement to thicken. Nano-TiO2 does not have a favorable nucleation effect and this issue can be related to its tendency to consume C2S and C3S. The presence of nano-TiO2 can slow down the precipitation of CH, which causes the concrete structure to increase in porosity at the age of 1 day (this process improves with the passage of time and the deposition of nano-TiO2).
- Due to the high surface area, nano-SiO2 reduces the workability of the fresh mixture (11.55–41.35%).
- At early ages, and more obviously at older ages, nano-SiO2 improves the bond between mortar and aggregates and results in an increase in compressive, tensile, and flexural strength.
- Acid causes the destruction and deterioration of the calcium hydroxide and also C-S-H entities. By modifying the pore size distribution, nano-SiO2 creates a favorable structure and a good defensive barrier against various ion attacks and permeability. In this regard, 6% nano-SiO2 succeeded in increasing the compressive strength of concrete by 12.18% of the compressive strength at pH = 2.5. Also, improving resistance to 300 freeze and thaw cycles, increasing electrical resistance (40%), reducing water absorption (3.21%), reducing chloride ion penetration (29%), and reducing carbonation (23%) are other positive results of nano-SiO2.
- Under high temperatures, nano-SiO2 shows acceptable performance. The use of 1.5% and 3% of nano-SiO2 in concrete mixture under high temperatures (400–600 °C) prevented the reduction of compressive strength by 22% and 12%, respectively.
- Nano-SiO2 can homogenize the paste morphology at ITZ and refine the pore size distribution. The pozzolanic property of nano-SiO2 leads to a reaction with Ca(OH)2 and denser and better C-S-H gel is produced. The formation of C-S-H gel in the early ages is completed by nano-SiO2 with smaller particles better than larger particles. In the case of concretes containing recycled aggregates, the ITZ zone is almost destroyed, but the addition of nano-SiO2 restores and strengthens this zone.
11. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| BC | Before Christ |
| CAC | Cement Association of Canada |
| CNT | Carbon nanotube |
| CS | Compressive Strength |
| CH crystals | Calcium Hydroxide crystals |
| CO2 | Carbon dioxide |
| CO | Carbon monoxide |
| Ca2+ | Calcium ions |
| C2S | Dicalcium silicate |
| C3S | Tricalcium silicate |
| C4AF | Tetracalcium aluminoferrite |
| C3AH6 | Tricalcium aluminate hexahydrate |
| C-S-H | Calcium Silicate Hydrate |
| C-A-S-H | Calcium Aluminate Sulfate Hydrate |
| CT | Computed Tomography scanning |
| Ca(OH)2 | Calcium hydroxide |
| DNA | Deoxyribonucleic acid |
| DTA/TG | Differential Thermal Analysis/Thermogravimetry |
| EDS | Energy Dispersive X-ray Spectroscopy |
| FS | Flexural Strength |
| FGC | Functionally Graded Concretes |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GtCO2 | Gigatonnes of carbon dioxide |
| GHG | Greenhouse gas |
| UHPC | Ultra-High-Performance Concrete |
| HPC | High-Performance Concrete |
| HC | Heavy-Weight Concrete |
| HSC | High-Strength Concrete |
| ITZ | Interfacial Transition Zone |
| LCA | Life Cycle Assessment |
| MPa | Megapascal |
| MIP | Mercury Intrusion Porosimetry |
| NNI | National Nanotechnology Initiative |
| nm | Nanometer (1 m = 109 nm) |
| Ni-Cd | Nickel–Cadmium |
| NS | Nano-SiO2 |
| NT | Nano-TiO2 |
| NOx | Nitrogen Oxides |
| NCC | Nanocrystalline cellulose |
| N | NO (The test was not conducted) |
| nano-SiO2 or n-SiO2 | nano-sized silicon dioxide |
| nano-TiO2 or n-TiO2 | nano-sized titanium dioxide |
| nano-CaCO3 or n-CaCO3 | nano-sized calcium carbonate |
| nano-Fe2O3 | nano-sized iron oxide |
| nano-Al2O3 | nano-sized aluminum oxide |
| OPC | Ordinary Portland Cement |
| pH | Potential Hydrogen (a measure of the acidity or alkalinity of a solution) |
| QDs | Quantum dots |
| RCM | Rapid Chloride Migration |
| RCPT | Rapid Chloride Permeability Test |
| RA | Recycled Aggregates |
| SEM | Scanning Electron Microscopy |
| SCC | Self-Compacting Concrete |
| SHPB | Split Hopkinson Pressure Bar test |
| SiO2-TiO2 | A composite material composed of silicon dioxide (SiO2) and titanium dioxide (TiO2) nanoparticles |
| T50 | T50 test (to measure the flow rate of SCC) |
| TS | Tensile Strength |
| UV | Ultraviolet |
| UHSC | Ultra-High Strength Concrete |
| USA | The United States of America |
| UK | The United Kingdom |
| UPV | Ultrasonic Pulse Velocity |
| VOCs | Volatile Organic Compounds |
| W/C | Water-Cement ratio |
| XRD | X-ray Diffraction |
| Y | YES (The test was conducted) |
| °C | Degree Celsius |
| μm | Micrometer (1 m = 106 µm) |
| 28d, 56d, 90d | 28 days, 56 days, 90 days |
Appendix A
| N.O. | Nano | Replacement (%) | CS | TS | FS | Other Reviews | Results | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | CaCO3 | 0, 1, 2, 3, 4, 5 + (45 kg/m3) fly ash | Y | N | N | Modulus of elasticity, SEM, Sulfate attack | Increasing resistance to sulfate attack (best dose = 1%), Improving the microstructure of concrete | [88] |
| 2 | CaCO3 | 0, 1, 2 + (20%) Slag | Y | N | Y | Normal consistency, Setting time, Volume stability, Resonance frequency, Thermal conductivity, Acid attack and mass loss, XRD | Improve compressive strength, Improved flexural strength, Resonance frequency improvement, Increasing resistance to acid attack (best dose = 1%) | [89] |
| 3 | Clay | 0, 1, 3, 6, 9 | Y | N | N | Capillary absorption, Total porosity, Expansion strain, Weight loss, UPV, SEM | Reduction of water capillary absorption and total porosity, Improve compressive strength, Improving the resistance of concrete against MgSO4 attack, Improving the resistance of concrete against Nitric acid attack and Sulfuric acid attack (at 1.0 pH value) | [108] |
| 4 | Clay | 0, 1, 2, 3 | Y | Y | Y | Water penetration, Water absorption | Improving compressive, tensile, flexural strength (best dose = 1%), Water penetration reduction (best dose = 1%), Water absorption reduction (best dose = 1%) | [109] |
| 5 | TiO2 | 0, 1, 2, 3, 4, 5 + (356.12 kg/m3) fly ash | Y | Y | Y | Water absorption, Volume of permeable pore space, Sorptivity, Sulfate attack, Chloride attack, SEM, XRD | Improving compressive, tensile, and flexural strength, Reduction of water absorption and volume of permeable pore space, Improving the resistance of concrete exposed to sulfate attack, Improving the resistance of concrete exposed to chloride attack, Improving the microstructure of concrete | [127] |
| 6 | TiO2 | 0, 1, 3, 5 + fly ash | Y | N | N | Pore structure, Fluidity, Drying shrinkage, Carbonation, SEM | Carbonation depth reduction, Reduction of drying shrinkage, Improved compressive strength | [132] |
| 7 | TiO2 | 0, 2, 4, 6, 8, 10 | Y | N | N | Workability, Setting time, Porosity, Water absorption and sorptivity, Electrical resistivity, Rapid chloride permeability, SEM, EDX | Improving the strength of mortar, Improvement of microstructure, Increase in electrical resistance, Porosity reduction | [135] |
| 8 | TiO2 | 0, 1, 2, 3 + (5%) silica fume | Y | N | N | UPV, Energy absorption and brittleness, Modulus of elasticity, Mass loss, Gas permeability, SEM, XRD | Improve compressive strength, Positive effect on modulus of elasticity, Reduced permeability, Improvement of mortar structure, Preventing the reduction of mass loss (up to 300 °C) | [136] |
| 9 | TiO2 | 0, 0.5, 1, 2, 3, 5 + fly ash + steel fiber | Y | N | Y | Dry shrinkage, Chloride diffusion, Freeze–thaw, Carbonation, MIP | Improving the strength of concrete, Reduction of dry shrinkage, Increased resistance to chloride ion, Improved resistance to freeze–thaw cycles | [250] |
| 10 | TiO2 | 0, 3, 5 | Y | Y | Y | Slump, Water absorption, Freeze and thaw, Abrasion resistance, Bulk electrical resistivity, Modulus of elasticity, Pore size distribution, MIP | Improving the strength of concrete, Reduce water absorption, Reducing the height of capillary absorption, Reduce abrasion weight loss, Increase in electrical resistivity, Reduction of shrinkage, Reduction of harmful pores | [139] |
| 11 | TiO2 | 0.4, 0.8, 1.2 | N | N | N | Capillary-water-absorption test after freeze–thaw cycles, Pore Test, SEM | Reduce the cumulative water absorption and porosity of recycled aggregate, Filling the gap between the aggregate, Enhancing the frost resistance, Enhance the interfacial bonding force between aggregate and cement | [253] |
| 12 | TiO2 | 0, 0.5, 1, 1.5, 2, 2.5, 3 | Y | Y | Y | Slump, Sorptivity, Water absorption, UPV, Dynamic modulus of elasticity, Chloride penetration, SEM | Improving compressive, tensile, and flexural strength, Reducing water absorption and apparent porosity, Reduction of chloride penetration, Improvement of concrete microstructure | [130] |
| 13 | TiO2 | 0, 3 | Y | N | N | Ultrasonic Wave Velocity, Mass loss, Sulfate attack, XRD | Increasing the resistance of concrete against sulfate attack, Reduce mass loss, Reduce compressive loss, Improving the microstructure of concrete | [131] |
| 14 | TiO2 | 0, 1, 3, 5 | Y | N | N | Setting time, Potentiodynamic polarization, SEM, XRD, EDS | Improve compressive strength, Improving the resistance of composite exposed to the acidic environment, Reducing the corrosion rate, Reduction of pores and production of C-S-H gel, Increasing particle-packing density | [133] |
| 15 | TiO2 | 0, 0.5, 1, 1.5 | Y | Y | Y | SEM, XRD | Improving compressive, tensile, and flexural strength, Preventing the reduction of residual compressive strength at high temperatures, Improvement of concrete microstructure | [137] |
| 16 | SiO2 | 0, 2, 4, 6 | Y | N | N | Weight loss, Electrical resistance, Water absorption | Obtaining a higher compressive strength than the control mixture (even under the influence of acid), Better performance in reducing water absorption than the control mixture (even under the influence of acid), Increase in electrical resistivity by increasing nano-SiO2 content | [159] |
| 17 | SiO2 | 0, 3, 5, 7 | Y | N | N | Water absorption, Frost resistance (freeze and thaw cycle) | Improve compressive strength, Decreased water absorption, Improving frost resistance (in all 50, 150, and 300 cycles) | [160] |
| 18 | SiO2 | 0, 1.5, 3, 4.5 + (0–22.5 kg/m3) Silica fume | Y | Y | N | Compressive strength, Tensile strength, Mass loss | Improved tensile strength (at high temperature), Improved compressive strength (at high temperature), Preventing weight loss of concrete at high temperatures, Better performance of nano-SiO2 than silica fume | [162] |
| 19 | SiO2 | 0, 1, 2, 3 | Y | N | N | Resistance to chloride ion penetration, Nuclear magnetic resonance, SEM, XRD | Under curing conditions −3 °C: Reducing compressive strength, Decreased RCP ability, Weakening of ITZ, and increasing the coarsening degree of pore structure | [164] |
| 20 | SiO2 | 0, 1.5, 3, 4.5 | Y | N | N | Pull out, Water permeability, SEM, XRD | Improved compressive strength (at high temperature), Improved bond strength (at high temperatures), Improving the microstructure of concrete (at high temperatures) | [163] |
References
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human Domination of Earth’s Ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Moein, M.M.; Saradar, A.; Rahmati, K.; Rezakhani, Y.; Ashkan, S.A.; Karakouzian, M. Reliability Analysis and Experimental Investigation of Impact Resistance of Concrete Reinforced with Polyolefin Fiber in Different Shapes, Lengths, and Doses. J. Build. Eng. 2023, 69, 106262. [Google Scholar] [CrossRef]
- Cooke, S.J.; Bergman, J.N.; Nyboer, E.A.; Reid, A.J.; Gallagher, A.J.; Hammerschlag, N.; Van de Riet, K.; Vermaire, J.C. Overcoming the Concrete Conquest of Aquatic Ecosystems. Biol. Conserv. 2020, 247, 108589. [Google Scholar] [CrossRef]
- Meyer, W.B.; Turner, B.L. Human Population Growth and Global Land-Use/Cover Change. Annu. Rev. Ecol. Syst. 1992, 23, 39–61. [Google Scholar] [CrossRef]
- United Nations. UN DESA World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Statista. Construction Industry Spending Globally 2025; Statista: New York, NY, USA, 2019. [Google Scholar]
- Maraveas, C. Production of Sustainable Construction Materials Using Agro-Wastes. Materials 2020, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Morse, N.B.; Pellissier, P.A.; Cianciola, E.N.; Brereton, R.L.; Sullivan, M.M.; Shonka, N.K.; Wheeler, T.B.; McDowell, W.H. Novel Ecosystems in the Anthropocene: A Revision of the Novel Ecosystem Concept for Pragmatic Applications. Ecol. Soc. 2014, 19, 12. [Google Scholar] [CrossRef]
- Moein, M.M.; Soliman, A. Predicting the Compressive Strength of Alkali-Activated Concrete Using Various Data Mining Methods. In Proceedings of the Canadian Society of Civil Engineering Annual Conference, Lecture Notes in Civil Engineering, Moncton, NB, Canada, 24–27 May 2023; Volume 248. [Google Scholar]
- Saradar, A.; Nemati, P.; Paskiabi, A.S.; Moein, M.M.; Moez, H.; Vishki, E.H. Prediction of Mechanical Properties of Lightweight Basalt Fiber Reinforced Concrete Containing Silica Fume and Fly Ash: Experimental and Numerical Assessment. J. Build. Eng. 2020, 32, 101732. [Google Scholar] [CrossRef]
- Watts, J. Concrete: The Most Destructive Material on Earth|Cities|The Guardian. The Guardian, 15 March 2019. [Google Scholar]
- Mousavinejad, S.H.G.; Saradar, A.; Jabbari, M.; Moein, M.M. Evaluation of Fresh and Hardened Properties of Self-Compacting Concrete Containing Different Percentages of Waste Tiles. J. Build. Pathol. Rehabil. 2023, 8, 81. [Google Scholar] [CrossRef]
- Sadrmomtazi, A.; Sobhani, J.; Mirgozar, M.A.; Najimi, M. Properties of Multi-Strength Grade EPS Concrete Containing Silica Fume and Rice Husk Ash. Constr. Build Mater. 2012, 35, 211–219. [Google Scholar] [CrossRef]
- Oxford Economics. Future of Construction: A Global Forecast for Construction to 2030; Oxford Economics: Oxford, UK, 2021. [Google Scholar]
- Gil, L.; Bernardo, J. An Approach to Energy and Climate Issues Aiming at Carbon Neutrality. Renew. Energy Focus 2020, 33, 37–42. [Google Scholar] [CrossRef]
- Sikora, A. European Green Deal—Legal and Financial Challenges of the Climate Change. ERA Forum 2021, 21, 681–697. [Google Scholar] [CrossRef]
- Agency, E.E. Scientific Advice for the Determination of an EU-Wide 2040 Climate Target and a Greenhouse Gas Budget for 2030–2050; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- Eurostat Greenhouse Gases-Construction, Data from: Eurostat Database (15/11/2023). Available online: https://ec.europa.eu/eurostat/databrowser/view/ENV_AC_AIGG_Q__custom_2691128/default/table?lang=en&bookmarkId=4bb9ab20-296b-4119-88e9-580ea7741c0a (accessed on 18 December 2023).
- Johnson, C.N.; Balmford, A.; Brook, B.W.; Buettel, J.C.; Galetti, M.; Guangchun, L.; Wilmshurst, J.M. Biodiversity Losses and Conservation Responses in the Anthropocene. Science 2017, 356, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, K.; Saradar, A.; Mohtasham Moein, M.; Sardrinejad, I.; Bristow, J.; Yavari, A.; Karakouzian, M. Evaluation of Engineered Cementitious Composites (ECC) Containing Polyvinyl Alcohol (PVA) Fibers under Compressive, Direct Tensile, and Drop-Weight Test. Multiscale Multidiscip. Model. Exp. Des. 2022, 6, 147–164. [Google Scholar] [CrossRef]
- Tajasosi, S.; Saradar, A.; Barandoust, J.; Mohtasham Moein, M.; Zeinali, R.; Karakouzian, M. Multi-Criteria Risk Analysis of Ultra-High Performance Concrete Application in Structures. CivilEng 2023, 4, 1016–1035. [Google Scholar] [CrossRef]
- Pourahmadi Sefat Arabani, H.; SadrMomtazi, A.; Mirgozar Langaroudi, M.A.; Kohani Khoshkbijari, R.; Amooie, M. Durability of Self-Compacting Lightweight Aggregate Concretes (LWSCC) as Repair Overlays. J. Rehabil. Civ. Eng. 2017, 5, 101–113. [Google Scholar] [CrossRef]
- Gagg, C.R. Cement and Concrete as an Engineering Material: An Historic Appraisal and Case Study Analysis. Eng. Fail. Anal. 2014, 40, 114–140. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made—Supplementary Information. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Mohtasham Moein, M.; Saradar, A.; Rahmati, K.; Ghasemzadeh Mousavinejad, S.H.; Bristow, J.; Aramali, V.; Karakouzian, M. Predictive Models for Concrete Properties Using Machine Learning and Deep Learning Approaches: A Review. J. Build. Eng. 2023, 63, 105444. [Google Scholar] [CrossRef]
- Wangler, T.; Lloret, E.; Reiter, L.; Hack, N.; Gramazio, F.; Kohler, M.; Bernhard, M.; Dillenburger, B.; Buchli, J.; Roussel, N.; et al. Digital Concrete: Opportunities and Challenges. RILEM Tech. Lett. 2016, 1, 67–75. [Google Scholar] [CrossRef]
- Baker, I. Fifty Materials That Make the World; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Aitcin, P.-C. Cements of Yesterday and Today, Concretes of Tomorrow. In Binders for Durable and Sustainable Concrete; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Waters, C.N.; Zalasiewicz, J. Concrete: The Most Abundant Novel Rock Type of the Anthropocene. In Encyclopedia of the Anthropocene; Elsevier: Amsterdam, The Netherlands, 2017; Volumes 1–5. [Google Scholar]
- Mohtasham Moein, M.; Saradar, A.; Rahmati, K.; Hatami Shirkouh, A.; Sadrinejad, I.; Aramali, V.; Karakouzian, M. Investigation of Impact Resistance of High-Strength Portland Cement Concrete Containing Steel Fibers. Cem. Mater. High Perform. Concr. 2022, 15, 7157. [Google Scholar] [CrossRef] [PubMed]
- Koushkbaghi, M.; Alipour, P.; Tahmouresi, B.; Mohseni, E.; Saradar, A.; Sarker, P.K. Influence of Different Monomer Ratios and Recycled Concrete Aggregate on Mechanical Properties and Durability of Geopolymer Concretes. Constr. Build. Mater. 2019, 205, 519–528. [Google Scholar] [CrossRef]
- Sadrmomtazi, A.; Tahmouresi, B.; Saradar, A. Effects of Silica Fume on Mechanical Strength and Microstructure of Basalt Fiber Reinforced Cementitious Composites (BFRCC). Constr. Build. Mater. 2018, 162, 321–333. [Google Scholar] [CrossRef]
- Mansoori, A.; Mohtasham Moein, M.; Mohseni, E. Effect of Micro Silica on Fiber-Reinforced Self-Compacting Composites Containing Ceramic Waste. J. Compos. Mater. 2020, 1, 95–107. [Google Scholar] [CrossRef]
- Weerheijm, J.; Van Breugel, K. Introduction to Concrete: A Resilient Material System. In Understanding the Tensile Properties of Concrete; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Sadrmomtazi, A.; Noorollahi, Z.; Tahmouresi, B.; Saradar, A. Effects of Hauling Time on Self-Consolidating Mortars Containing Metakaolin and Natural Zeolite. Constr. Build. Mater. 2019, 221, 283–291. [Google Scholar] [CrossRef]
- Trout, E.A.R. The History of Calcareous Cements. In Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- Hunter, L.C. Engineering in History. By Richard Shelton Kirby, Sidney Withington, Arthur Burr Darling, and Frederick Gridley Kilgour. New York, Toronto, London: McGraw-Hill Book Company, Inc., 1956. Pp. Vii, 530. $8.50. J. Econ. Hist. 1957, 17, 458–459. [Google Scholar] [CrossRef]
- Ganiron, T.U.J. Effect of Sawdust as Fine Aggregate in Concrete Mixture for Building Construction. Int. J. Adv. Sci. Technol. 2014, 63, 73–82. [Google Scholar] [CrossRef]
- Ghasemzadeh Mosavinejad, S.H.; Langaroudi, M.A.M.; Barandoust, J.; Ghanizadeh, A. Electrical and Microstructural Analysis of UHPC Containing Short PVA Fibers. Constr. Build. Mater. 2020, 235, 117448. [Google Scholar] [CrossRef]
- Ge, Z.; Gao, Z.; Sun, R.; Zheng, L. Mix Design of Concrete with Recycled Clay-Brick-Powder Using the Orthogonal Design Method. Constr. Build. Mater. 2012, 31, 289–293. [Google Scholar] [CrossRef]
- Safiuddin, M.; Gonzalez, M.; Cao, J.; Tighe, S.L. State-of-the-Art Report on Use of Nano-Materials in Concrete. Int. J. Pavement Eng. 2014, 15, 940–949. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Eatmon, T.D. A Life-Cycle Assessment of Portland Cement Manufacturing: Comparing the Traditional Process with Alternative Technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). WBCSD Cement Technology Roadmap 2009—Carbon Emissions Reductions up to 2050; World Business Council for Sustainable Development [WBCSD]: Paris, France, 2009; Volume 1. [Google Scholar]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Impacts of Booming Concrete Production on Water Resources Worldwide. Nat. Sustain. 2018, 1, 69–76. [Google Scholar] [CrossRef]
- Gates, B. Have You Hugged a Concrete Pillar Today? 2018. Available online: https://www.gatesnotes.com (accessed on 18 December 2023).
- Swanson, A. How China Used More Cement in 3 Years than the U.S. Did in the Entire 20th Century. Washington Post, 24 March 2015. [Google Scholar]
- Lazaro, A.; Yu, Q.L.; Brouwers, H.J.H. Nanotechnologies for Sustainable Construction. In Sustainability of Construction Materials; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Roco, M.C.; Williams, R.S. Nanotechnology Research Directions: IWGN Workshop Report Vision for Nanotechnology R & D in the Next Decade; Springer Science & Business Media: Berlin, Germany, 1999; Volume 15. [Google Scholar]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, S.; Liu, Y.S. Nanoparticles in the Diagnosis and Treatment of Vascular Aging and Related Diseases. Signal Transduct. Target. Ther. 2022, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Junk, A.; Riess, F. From an Idea to a Vision: There’s Plenty of Room at the Bottom. Am. J. Phys. 2006, 74, 825–830. [Google Scholar] [CrossRef]
- Feynman, R.P. There’s Plenty of Room at the Bottom. J. Microelectromechanical Syst. 1992, 1, 60–66. [Google Scholar] [CrossRef]
- Badri Narayanan, K.; Sakthivel, N. Extracellular Biological Synthesis of Gold Nanoparticles. In Colloids in Biotechnology; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Drexler, K.E. The Engines of Creation; Anchor Books: New York, NY, USA, 1986; Volume 3. [Google Scholar]
- Lee, J.; Mahendra, S.; Alvarez, P.J.J. Nanomaterials in the Construction Industry: A Review of Their Applications and Environmental Health and Safety Considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef]
- Ge, Z.; Gao, Z. Applications of Nanotechnology and Nanomaterials in Construction. First Inter. Confer. Construc. Develop. Countries 2008, 2008, 235–240. [Google Scholar]
- Strategic Development Council. Roadmap 2030: The U.S. Concrete Industry Technology Roadmap; Strategic Development Council: Detroit, MI, USA, 2002. [Google Scholar]
- Kourmpanis, B.; Papadopoulos, A.; Moustakas, K.; Stylianou, M.; Haralambous, K.J.; Loizidou, M. Preliminary Study for the Management of Construction and Demolition Waste. Waste Manag. Res. 2008, 26, 267–275. [Google Scholar] [CrossRef]
- Yuan, H.; Shen, L. Trend of the Research on Construction and Demolition Waste Management. Waste Manag. 2011, 31, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Hoch, M. Organotin Compounds in the Environment—An Overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Turner, A. Marine Pollution from Antifouling Paint Particles. Mar. Pollut. Bull. 2010, 60, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.A.; Hise, S.; Kiedrowski, B.; Krouse, C. Urban Battery Litter. J. Environ. Eng. 2009, 135, 46–57. [Google Scholar] [CrossRef]
- Carwile, J.L.; Luu, H.T.; Bassett, L.S.; Driscoll, D.A.; Yuan, C.; Chang, J.Y.; Ye, X.; Calafat, A.M.; Michels, K.B. Polycarbonate Bottle Use and Urinary Bisphenol A Concentrations. Environ. Health Perspect. 2009, 117, 1368–1372. [Google Scholar] [CrossRef]
- Abelmann, A.; Glynn, M.E.; Pierce, J.S.; Scott, P.K.; Serrano, S.; Paustenbach, D.J. Historical Ambient Airborne Asbestos Concentrations in the United States-An Analysis of Published and Unpublished Literature (1960s–2000s). Inhal. Toxicol. 2015, 27, 754–766. [Google Scholar] [CrossRef]
- Rincón, A.G.; Pulgarin, C. Bactericidal Action of Illuminated TiO2 on Pure Escherichia Coli and Natural Bacterial Consortia: Post-Irradiation Events in the Dark and Assessment of the Effective Disinfection Time. Appl. Catal. B 2004, 49, 99–112. [Google Scholar] [CrossRef]
- Wolfrum, E.J.; Huang, J.; Blake, D.M.; Maness, P.C.; Huang, Z.; Fiest, J.; Jacoby, W.A. Photocatalytic Oxidation of Bacteria, Bacterial and Fungal Spores, and Model Biofilm Components to Carbon Dioxide on Titanium Dioxide-Coated Surfaces. Environ. Sci. Technol. 2002, 36, 3412–3419. [Google Scholar] [CrossRef]
- Attik, G.; Brown, R.; Jackson, P.; Creutzenberg, O.; Aboukhamis, I.; Rihn, B.H. Internalization, Cytotoxicity, Apoptosis, and Tumor Necrosis Factor-α Expression in Rat Alveolar Macrophages Exposed to Various Dusts Occurring in the Ceramics Industry. Inhal. Toxicol. 2008, 20, 1101–1112. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative Eco-Toxicity of Nanoscale TiO2, SiO2, and ZnO Water Suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Dutta, D.; Sundaram, S.K.; Teeguarden, J.G.; Riley, B.J.; Fifield, L.S.; Jacobs, J.M.; Addleman, S.R.; Kaysen, G.A.; Moudgil, B.M.; Weber, T.J. Adsorbed Proteins Influence the Biological Activity and Molecular Targeting of Nanomaterials. Toxicol. Sci. 2007, 100, 303–315. [Google Scholar] [CrossRef]
- Herzog, E.; Byrne, H.J.; Casey, A.; Davoren, M.; Lenz, A.G.; Maier, K.L.; Duschl, A.; Oostingh, G.J. SWCNT Suppress Inflammatory Mediator Responses in Human Lung Epithelium in Vitro. Toxicol. Appl. Pharmacol. 2009, 234, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Lovrić, J.; Cho, S.J.; Winnik, F.M.; Maysinger, D. Unmodified Cadmium Telluride Quantum Dots Induce Reactive Oxygen Species Formation Leading to Multiple Organelle Damage and Cell Death. Chem. Biol. 2005, 12, 1227–1234. [Google Scholar] [CrossRef]
- Anastas, P.T.; Zimmerman, J.B. Design through the 12 Principles of Green Engineering. Environ. Sci. Technol. 2003, 37, 79A–1056. [Google Scholar] [CrossRef]
- Camiletti, J.; Soliman, A.M.; Nehdi, M.L. Effects of Nano- and Micro-Limestone Addition on Early-Age Properties of Ultra-High-Performance Concrete. Mater. Struct. 2013, 46, 881–898. [Google Scholar] [CrossRef]
- Sharma, H.; Majeed, B.; Sharma, S. Influence of Nano-Modification on Strength Parameters of Concrete. In Lecture Notes in Civil Engineering; Springer: Singapore, 2020; Volume 35. [Google Scholar]
- Feng, Z.; Shen, D.; Xu, S.; Shao, H.; Jiang, G. Effect of Nano-CaCO3 on Early-Age Properties and Cracking Potential of High-Strength Concrete. J. Mater. Civ. Eng. 2023, 35, 04023013. [Google Scholar] [CrossRef]
- Li, W.; Huang, Z.; Cao, F.; Sun, Z.; Shah, S.P. Effects of Nano-Silica and Nano-Limestone on Flowability and Mechanical Properties of Ultra-High-Performance Concrete Matrix. Constr. Build. Mater. 2015, 95, 366–374. [Google Scholar] [CrossRef]
- Sá e Sant’Anna, S.; de Souza, D.A.; de Araujo, D.M.; de Freitas Carvalho, C.; Yoshida, M.I. Physico-Chemical Analysis of Flexible Polyurethane Foams Containing Commercial Calcium Carbonate. Mater. Res. 2008, 11, 433–438. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, H.; Chan, C.M.; Wu, J. High Impact Toughness Polypropylene/CaCO3 Nanocomposites and the Toughening Mechanism. Macromolecules 2008, 41, 9204–9213. [Google Scholar] [CrossRef]
- Péra, J.; Husson, S.; Guilhot, B. Influence of Finely Ground Limestone on Cement Hydration. Cem. Concr. Compos. 1999, 21, 99–105. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Supit, S.W.M. Mechanical and Durability Properties of High Volume Fly Ash (HVFA) Concrete Containing Calcium Carbonate (CaCO3) Nanoparticles. Constr. Build. Mater. 2014, 70, 309–321. [Google Scholar] [CrossRef]
- Camiletti, J.; Soliman, A.M.; Nehdi, M.L. Effect of Nano-Calcium Carbonate on Early-Age Properties of Ultrahigh-Performance Concrete. Mag. Concr. Res. 2013, 65, 297–307. [Google Scholar] [CrossRef]
- Al Ghabban, A.; Al Zubaidi, A.B.; Jafar, M.; Fakhri, Z. Effect of Nano SiO2 and Nano CaCO3 on the Mechanical Properties, Durability and Flowability of Concrete. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 454. [Google Scholar]
- Wang, Z.-H.; Bai, E.-L.; Xu, J.-Y.; Du, Y.-H.; Zhu, J.-S. Effect of Nano-SiO2 and Nano-CaCO3 on the Static and Dynamic Properties of Concrete. Sci. Rep. 2022, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, R.; Divandari, H. The Effect of Nano-CaCO3 and Nano-SiO2 on Properties of Self-Compacting Concrete. J. Struct. Constr. Eng. 2021, 7, 187–201. [Google Scholar] [CrossRef]
- Salih, A.; Rafiq, S.; Mahmood, W.; AL-Darkazali, H.; Noaman, R.; Ghafor, K.; Qadir, W. Systemic Multi-Scale Approaches to Predict the Flowability at Various Temperature and Mechanical Properties of Cement Paste Modified with Nano-Calcium Carbonate. Constr. Build. Mater. 2020, 262, 120777. [Google Scholar] [CrossRef]
- Qiao, H.; Hakuzweyezu, T.; Yang, B.; Li, K. Experimental Study on Sulfate Erosion Resistance of Nano-CaCO3 Modified Concrete. Can. J. Civ. Eng. 2022, 49, 590–596. [Google Scholar] [CrossRef]
- Balcikanli Bankir, M.; Ozturk, M.; Sevim, U.K.; Depci, T. Effect of N-CaCO3 on Fresh, Hardened Properties and Acid Resistance of Granulated Blast Furnace Slag Added Mortar. J. Build. Eng. 2020, 29, 101209. [Google Scholar] [CrossRef]
- Li, G.; Zhuang, Z.; Lv, Y.; Wang, K.; Hui, D. Enhancing Carbonation and Chloride Resistance of Autoclaved Concrete by Incorporating Nano-CaCO3. Nanotechnol. Rev. 2020, 9, 998–1008. [Google Scholar] [CrossRef]
- Wang, W.C. Compressive Strength and Thermal Conductivity of Concrete with Nanoclay under Various High-Temperatures. Constr. Build. Mater. 2017, 147, 305–311. [Google Scholar] [CrossRef]
- Mirgozar Langaroudi, M.A.; Mohammadi, Y. Effect of Nano-Clay on Workability, Mechanical, and Durability Properties of Self-Consolidating Concrete Containing Mineral Admixtures. Constr. Build. Mater. 2018, 191, 619–634. [Google Scholar] [CrossRef]
- Guggenheim, S.; Martin, R.T.; Alietti, A.; Drits, V.A.; Formoso, M.L.L.; Galán, E.; Köster, H.M.; Morgan, D.J.; Paquet, H.; Watanabe, T.; et al. Definition of Clay and Clay Mineral: Joint Report of the AIPEA Nomenclature and CMS Nomenclature Committees. Clays Clay Miner. 1995, 43, 255–256. [Google Scholar] [CrossRef]
- Abdalla, J.A.; Thomas, B.S.; Hawileh, R.A.; Yang, J.; Jindal, B.B.; Ariyachandra, E. Influence of Nano-TiO2, Nano-Fe2O3, Nanoclay and Nano-CaCO3 on the Properties of Cement/Geopolymer Concrete. Clean. Mater. 2022, 4, 100061. [Google Scholar] [CrossRef]
- Hamed, N.; El-Feky, M.S.; Kohail, M.; Nasr, E.S.A.R. Effect of Nano-Clay de-Agglomeration on Mechanical Properties of Concrete. Constr. Build. Mater. 2019, 205, 245–256. [Google Scholar] [CrossRef]
- Farzadnia, N.; Abang Ali, A.A.; Demirboga, R.; Anwar, M.P. Effect of Halloysite Nanoclay on Mechanical Properties, Thermal Behavior and Microstructure of Cement Mortars. Cem. Concr. Res. 2013, 48, 97–104. [Google Scholar] [CrossRef]
- Ghodke, S.; Bhanvase, B.; Sonawane, S.; Mishra, S.; Joshi, K. Nanoencapsulation and Nanocontainer Based Delivery Systems for Drugs, Flavors, and Aromas. In Encapsulations: Nanotechnology in the Agri-Food Industry; Academic Press: Cambridge, MA, USA, 2016; Volume 2. [Google Scholar]
- Varga, G. The Structure of Kaolinite and Metakaolinite. Epa. J. Silic. Based Compos. Mater. 2007, 59, 6–9. [Google Scholar] [CrossRef]
- Huang, W. Chapter 3—Clay Nanopapers. In Micro and Nano Technologies; William Andrew Publishing: New York, NY, USA, 2018. [Google Scholar]
- Giannelis, E.P.; Krishnamoorti, R.; Manias, E. Polymer-Silicate Nanocomposites: Model Systems for Confined Polymers and Polymer Brushes. Adv. Polym. Sci. 1999, 138, 107–147. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Wang, Q.; Shah, S.P. Effects of Nano-Kaolinite Clay on the Freeze-Thaw Resistance of Concrete. Cem. Concr. Compos. 2015, 62, 1–12. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Shah, S.P. Influence of Nanoclay on Concrete Subjected to Freeze-Thaw Cycles and Bond Behavior between Rebar and Concrete. Key Eng. Mater. 2016, 711, 256–262. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Kawashima, S.; Shah, S.P. Influence of Kaolinite Clay on the Chloride Diffusion Property of Cement-Based Materials. Cem. Concr. Compos. 2014, 45, 117–124. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Wang, Q.; Shah, S.P. The Effects of Nano-Calcined Kaolinite Clay on Cement Mortar Exposed to Acid Deposits. Constr. Build. Mater. 2016, 102, 486–495. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, S.; Fan, Y.; Shah, S.P. Study on Shrinkage Cracking Morphology of Cement Mortar with Different Nanoclay Particles under Restraint. Buildings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Polat, R.; Demirboğa, R.; Khushefati, W.H. Effects of Nano and Micro Size of CaO and MgO, Nano-Clay and Expanded Perlite Aggregate on the Autogenous Shrinkage of Mortar. Constr. Build. Mater. 2015, 81, 268–275. [Google Scholar] [CrossRef]
- Hosseini, P.; Afshar, A.; Vafaei, B.; Booshehrian, A.; Molaei Raisi, E.; Esrafili, A. Effects of Nano-Clay Particles on the Short-Term Properties of Self-Compacting Concrete. Eur. J. Environ. Civ. Eng. 2017, 21, 127–147. [Google Scholar] [CrossRef]
- Diab, A.M.; Elyamany, H.E.; Abd Elmoaty, A.E.M.; Sreh, M.M. Effect of Nanomaterials Additives on Performance of Concrete Resistance against Magnesium Sulfate and Acids. Constr. Build. Mater. 2019, 210, 210–231. [Google Scholar] [CrossRef]
- Shafabakhsh, G.; Janaki, A.M.; Ani, O.J. Laboratory Investigation on Durability of Nano Clay Modified Concrete Pavement. Eng. J. 2020, 24, 35–44. [Google Scholar] [CrossRef]
- Constantinides, G. Nanoscience and Nanoengineering of Cement-Based Materials. In Nanotechnology in Eco-Efficient Construction: Materials, Processes and Applications; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Bautista-Gutierrez, K.P.; Herrera-May, A.L.; Santamaría-López, J.M.; Honorato-Moreno, A.; Zamora-Castro, S.A. Recent Progress in Nanomaterials for Modern Concrete Infrastructure: Advantages and Challenges. Materials 2019, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.K.; Giri, P.K. Plasmonic Metal and Semiconductor Nanoparticle Decorated TiO2-Based Photocatalysts for Solar Light Driven Photocatalysis. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ambikakumari Sanalkumar, K.U.; Yang, E.H. Self-Cleaning Performance of Nano-TiO2 Modified Metakaolin-Based Geopolymers. Cem. Concr. Compos. 2021, 115, 103847. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, X.; Hou, P.; Ye, Z. Influences of Nano-TiO2 on the Properties of Cement-Based Materials: Hydration and Drying Shrinkage. Constr. Build. Mater. 2015, 81, 35–41. [Google Scholar] [CrossRef]
- Chen, Y. A Review on the Effects of Nanoparticles on Properties of Self-Compacting Concrete. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 452. [Google Scholar]
- Tajasosi, S.; Shirzad-Siboni, M.; Vagheei, R.; Barandoust, J. Cement Matrix Composition Impact on the Photocatalytic Performance of Immobilized TiO2 Particles over the Fixed Bed Photoreactor for Denitrification of Water. Pollution 2023, 9, 1295–1308. [Google Scholar] [CrossRef]
- Guerrini, G.L. Photocatalytic Performances in a City Tunnel in Rome: NOx Monitoring Results. Constr. Build. Mater. 2012, 27, 165–175. [Google Scholar] [CrossRef]
- De Souza Rastelli, A.N.; Carreira, E.T.; Dias, H.B.; Hamblin, M.R. Nanobiomaterials in Dentistry. In Nanobiomaterials in Dentistry: Applications of Nanobiomaterials; William Andrew Publishing: New York, NY, USA, 2016; Volume 11. [Google Scholar]
- Nikbin, I.M.; Mohebbi, R.; Dezhampanah, S.; Mehdipour, S.; Mohammadi, R.; Nejat, T. Gamma Ray Shielding Properties of Heavy-Weight Concrete Containing Nano-TiO2. Radiat. Phys. Chem. 2019, 162, 157–167. [Google Scholar] [CrossRef]
- Nikbin, I.M.; Mehdipour, S.; Dezhampanah, S.; Mohammadi, R.; Mohebbi, R.; Moghadam, H.H.; Sadrmomtazi, A. Effect of High Temperature on Mechanical and Gamma Ray Shielding Properties of Concrete Containing Nano-TiO2. Radiat. Phys. Chem. 2020, 174, 108967. [Google Scholar] [CrossRef]
- Joshaghani, A.; Balapour, M.; Mashhadian, M.; Ozbakkaloglu, T. Effects of Nano-TiO2, Nano-Al2O3, and Nano-Fe2O3 on Rheology, Mechanical and Durability Properties of Self-Consolidating Concrete (SCC): An Experimental Study. Constr. Build. Mater. 2020, 245, 118444. [Google Scholar] [CrossRef]
- Ren, J.; Lai, Y.; Gao, J. Exploring the Influence of SiO2 and TiO2 Nanoparticles on the Mechanical Properties of Concrete. Constr. Build. Mater. 2018, 175, 277–285. [Google Scholar] [CrossRef]
- Staub de Melo, J.V.; Trichês, G. Study of the Influence of Nano-TiO2 on the Properties of Portland Cement Concrete for Application on Road Surfaces. Road Mater. Pavement Des. 2018, 19, 1011–1026. [Google Scholar] [CrossRef]
- Ying, J.; Zhou, B.; Xiao, J. Pore Structure and Chloride Diffusivity of Recycled Aggregate Concrete with Nano-SiO2 and Nano-TiO2. Constr. Build. Mater. 2017, 150, 49–55. [Google Scholar] [CrossRef]
- Li, H.; Xiao, H.; Guan, X.; Wang, Z.; Yu, L. Chloride Diffusion in Concrete Containing Nano-TiO2 under Coupled Effect of Scouring. Compos. B Eng. 2014, 56, 698–704. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, T.; Luo, T.; Zhou, M.; Ma, W.; Zhang, K. The Effects of Nano-SiO2 and Nano-TiO2 Addition on the Durability and Deterioration of Concrete Subject to Freezing and Thawing Cycles. Materials 2019, 12, 3608. [Google Scholar] [CrossRef]
- Sastry, K.V.S.G.K.; Sahitya, P.; Ravitheja, A. Influence of Nano TiO2 on Strength and Durability Properties of Geopolymer Concrete. Mater. Today Proc. 2021, 45, 1017–1025. [Google Scholar] [CrossRef]
- Zhang, M.H.; Li, H. Pore Structure and Chloride Permeability of Concrete Containing Nano-Particles for Pavement. Constr. Build. Mater. 2011, 25, 608–616. [Google Scholar] [CrossRef]
- Salemi, N.; Behfarnia, K.; Zaree, S.A. Effect of Nanoparticles on Frost Durability of Concrete. Asian J. Civ. Eng. 2014, 15, 411–420. [Google Scholar]
- Rawat, G.; Gandhi, S.; Murthy, Y.I. Durability Aspects of Concrete Containing Nano-Titanium Dioxide. ACI Mater. J. 2023, 120, 25–36. [Google Scholar] [CrossRef]
- Xu, C.; Liao, H.H.; Chen, Y.L.; Du, X.; Peng, B.; Fernandez-Steeger, T.M. Corrosion Performance of Nano-TiO2-Modified Concrete under a Dry–Wet Sulfate Environment. Materials 2021, 14, 5900. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Yan, C.; Luo, W.; Zhou, W. Effects of Adding Nano-TiO2 on Compressive Strength, Drying Shrinkage, Carbonation and Microstructure of Fluidized Bed Fly Ash Based Geopolymer Paste. Constr. Build. Mater. 2016, 106, 115–125. [Google Scholar] [CrossRef]
- Daniyal, M.; Akhtar, S.; Azam, A. Effect of Nano-TiO2 on the Properties of Cementitious Composites under Different Exposure Environments. J. Mater. Res. Technol. 2019, 8, 6158–6172. [Google Scholar] [CrossRef]
- Fattah, K.; Tamimi, A.; Alkadi, A.; Afaneh, M.; Awada, M.; Khalaf, A. Self-Cleansing Cement Matrix Using Nano Titanium Dioxide. Dep. Civ. Eng. Am. Univ. Sharjah 2019, 6, 37–40. [Google Scholar]
- Gopalakrishnan, R.; Vignesh, B.; Jeyalakshmi, R. Mechanical, Electrical and Microstructural Studies on Nano-TiO2 Admixtured Cement Mortar Cured with Industrial Wastewater. Eng. Res. Express 2020, 2, 025010. [Google Scholar] [CrossRef]
- Farzadnia, N.; Abang Ali, A.A.; Demirboga, R.; Anwar, M.P. Characterization of High Strength Mortars with Nano Titania at Elevated Temperatures. Constr. Build. Mater. 2013, 43, 469–479. [Google Scholar] [CrossRef]
- Guler, S.; Türkmenoğlu, Z.F.; Ashour, A. Performance of Single and Hybrid Nanoparticles Added Concrete at Ambient and Elevated Temperatures. Constr. Build. Mater. 2020, 250, 118847. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Gao, Y. Effect of TiO2 Nanoparticles on Physical and Mechanical Properties of Cement at Low Temperatures. Adv. Mater. Sci. Eng. 2018, 2018, 8934689. [Google Scholar] [CrossRef]
- Joshaghani, A. Evaluating the Effects of Titanium Dioxide (TiO2) and Carbon-Nanofibers (CNF) as Cement Partial Replacement on Concrete Properties. MOJ Civ. Eng. 2018, 4, 29–38. [Google Scholar] [CrossRef]
- Chithra, S.; Senthil Kumar, S.R.R.; Chinnaraju, K. The Effect of Colloidal Nano-Silica on Workability, Mechanical and Durability Properties of High Performance Concrete with Copper Slag as Partial Fine Aggregate. Constr. Build. Mater. 2016, 113, 794–804. [Google Scholar] [CrossRef]
- Fallah, S.; Nematzadeh, M. Mechanical Properties and Durability of High-Strength Concrete Containing Macro-Polymeric and Polypropylene Fibers with Nano-Silica and Silica Fume. Constr. Build. Mater. 2017, 132, 170–187. [Google Scholar] [CrossRef]
- Rashad, A.M. Effect of Nanoparticles on the Properties of Geopolymer Materials. Mag. Concr. Res. 2019, 71, 1283–1301. [Google Scholar] [CrossRef]
- Singh, L.P.; Karade, S.R.; Bhattacharyya, S.K.; Yousuf, M.M.; Ahalawat, S. Beneficial Role of Nanosilica in Cement Based Materials—A Review. Constr. Build.Mater. 2013, 47, 1069–1077. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, S.; Singh, S.K. Nanosilica: Recent Progress in Synthesis, Functionalization, Biocompatibility, and Biomedical Applications. ACS Biomater. Sci. Eng. 2019, 5, 4882–4898. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Mamaeva, V.; Sahlgren, C.; Lindén, M. Nanoparticles in Targeted Cancer Therapy: Mesoporous Silica Nanoparticles Entering Preclinical Development Stage. Nanomedicine 2012, 7, 111–120. [Google Scholar] [CrossRef]
- Galagudza, M.M.; Korolev, D.V.; Sonin, D.L.; Postnov, V.N.; Papayan, G.V.; Uskov, I.S.; Belozertseva, A.V.; Shlyakhto, E.V. Targeted Drug Delivery into Reversibly Injured Myocardium with Silica Nanoparticles: Surface Functionalization, Natural Biodistribution, and Acute Toxicity. Int. J. Nanomed. 2010, 5, 231–237. [Google Scholar] [CrossRef]
- Flikkema, E.; Bromley, S.T. A New Interatomic Potential for Nanoscale Silica. Chem. Phys. Lett. 2003, 378, 622–629. [Google Scholar] [CrossRef]
- Rahman, I.A.; Vejayakumaran, P.; Sipaut, C.S.; Ismail, J.; Chee, C.K. Size-Dependent Physicochemical and Optical Properties of Silica Nanoparticles. Mater. Chem. Phys. 2009, 114, 328–332. [Google Scholar] [CrossRef]
- Mukharjee, B.B.; Barai, S.V. Influence of Incorporation of Colloidal Nano-Silica on Behaviour of Concrete. Iran. J. Sci. Technol. Trans. Civ. Eng. 2020, 44, 657–668. [Google Scholar] [CrossRef]
- Mukharjee, B.B.; Barai, S.V. Influence of Nano-Silica on the Properties of Recycled Aggregate Concrete. Constr. Build. Mater. 2014, 55, 29–37. [Google Scholar] [CrossRef]
- Elrahman, M.A.; Chung, S.Y.; Sikora, P.; Rucinska, T.; Stephan, D. Influence of Nanosilica on Mechanical Properties, Sorptivity, and Microstructure of Lightweight Concrete. Materials 2019, 12, 3078. [Google Scholar] [CrossRef] [PubMed]
- Naji Givi, A.; Abdul Rashid, S.; Aziz, F.N.A.; Salleh, M.A.M. Experimental Investigation of the Size Effects of SiO2 Nano-Particles on the Mechanical Properties of Binary Blended Concrete. Compos. B Eng. 2010, 41, 673–677. [Google Scholar] [CrossRef]
- Du, H.; Du, S.; Liu, X. Durability Performances of Concrete with Nano-Silica. Constr. Build. Mater. 2014, 73, 705–712. [Google Scholar] [CrossRef]
- Kashyap, V.S.; Sancheti, G.; Yadav, J.S. Durability and Microstructural Behavior of Nano Silica-Marble Dust Concrete. Clean. Mater. 2023, 7, 100165. [Google Scholar] [CrossRef]
- Moslemi, A.M.; Khosravi, A.; Izadinia, M.; Heydari, M. Application of Nano Silica in Concrete for Enhanced Resistance against Sulfate Attack. Adv. Mater. Res. 2014, 829, 874–878. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Cho, H.K.; Wang, X.Y. Effect of Nano-Silica on the Autogenous Shrinkage, Strength, and Hydration Heat of Ultra-High Strength Concrete. Appl. Sci. 2020, 10, 5202. [Google Scholar] [CrossRef]
- Almohammad-albakkar, M.; Behfarnia, K. Effects of the Combined Usage of Micro and Nano-Silica on the Drying Shrinkage and Compressive Strength of the Self-Compacting Concrete. J. Sustain. Cem. Based Mater. 2021, 10, 92–110. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H.J.H. Effect of Nano-Silica on the Hydration and Microstructure Development of Ultra-High Performance Concrete (UHPC) with a Low Binder Amount. Constr. Build. Mater. 2014, 65, 140–150. [Google Scholar] [CrossRef]
- Mahdikhani, M.; Bamshad, O.; Fallah Shirvani, M. Mechanical Properties and Durability of Concrete Specimens Containing Nano Silica in Sulfuric Acid Rain Condition. Constr. Build. Mater. 2018, 167, 929–935. [Google Scholar] [CrossRef]
- Behfarnia, K.; Salemi, N. The Effects of Nano-Silica and Nano-Alumina on Frost Resistance of Normal Concrete. Constr. Build. Mater. 2013, 48, 580–584. [Google Scholar] [CrossRef]
- Tarangini, D.; Sravana, P.; Srinivasa Rao, P. Effect of Nano Silica on Frost Resistance of Pervious Concrete. Mater. Today Proc. 2022, 51, 2185–2189. [Google Scholar] [CrossRef]
- Bastami, M.; Baghbadrani, M.; Aslani, F. Performance of Nano-Silica Modified High Strength Concrete at Elevated Temperatures. Constr. Build. Mater. 2014, 68, 402–408. [Google Scholar] [CrossRef]
- Elkady, H.M.; Yasien, A.M.; Elfeky, M.S.; Serag, M.E. Assessment of Mechanical Strength of Nano Silica Concrete (NSC) Subjected to Elevated Temperatures. J. Struct. Fire Eng. 2019, 10, 90–109. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Wang, Q.; Zhang, R.; Pei, W.; Zhou, Y. Influence of Nano-Silica on the Performances of Concrete under the Negative-Temperature Curing Condition. Cold Reg. Sci. Technol. 2021, 191, 103357. [Google Scholar] [CrossRef]
- Younis, K.H.; Mustafa, S.M. Feasibility of Using Nanoparticles of SiO2 to Improve the Performance of Recycled Aggregate Concrete. Adv. Mater. Sci. Eng. 2018, 2018, 1512830. [Google Scholar] [CrossRef]
- Saloma; Nasution, A.; Imran, I.; Abdullah, M. Experimental Investigation on Nanomaterial Concrete. Int. J. Civ. Environ. Eng. 2013, 13, 15–20. [Google Scholar]
- Ren, Z.; Liu, Y.; Yuan, L.; Luan, C.; Wang, J.; Cheng, X.; Zhou, Z. Optimizing the Content of Nano-SiO2, Nano-TiO2 and Nano-CaCO3 in Portland Cement Paste by Response Surface Methodology. J. Build. Eng. 2021, 35, 102073. [Google Scholar] [CrossRef]
- Mohamed, A.M. Influence of nano materials on flexural behavior and compressive strength of concrete. HBRC J. 2016, 12, 212–225. [Google Scholar] [CrossRef]
- Shchelokova, E.A.; Tyukavkina, V.V.; Tsyryatyeva, A.V.; Kasikov, A.G. Synthesis and Characterization of SiO2-TiO2 Nanoparticles and Their Effect on the Strength of Self-Cleaning Cement Composites. Constr. Build. Mater. 2021, 283, 122769. [Google Scholar] [CrossRef]
- Sun, J.; Xu, K.; Shi, C.; Ma, J.; Li, W.; Shen, X. Influence of Core/Shell TiO2@SiO2 Nanoparticles on Cement Hydration. Constr. Build. Mater. 2017, 156, 114–122. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The Effects of Silica/Titania Nanocomposite on the Mechanical and Bactericidal Properties of Cement Mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- Han, B.; Ding, S.; Wang, J.; Ou, J. Nano-Engineered Cementitious Composites: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Guo, S.Y.; Luo, H.H.; Tan, Z.; Chen, J.Z.; Zhang, L.; Ren, J. Impermeability and Interfacial Bonding Strength of TiO2-Graphene Modified Epoxy Resin Coated OPC Concrete. Prog. Org. Coat. 2021, 151, 106029. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, J. Interfacial Heat Transfer Behavior of Graphene-Based Filler and Calcium-Silicate-Hydrate in Cement Composites. Int. J. Heat Mass Transf. 2021, 176, 121165. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeon, S.E.; Kim, J.K.; Yang, S. An Experimental Study on Thermal Conductivity of Concrete. Cem. Concr. Res. 2003, 33, 363–371. [Google Scholar] [CrossRef]
- Ha, J.H.; Jung, Y.S.; Cho, Y.G. Thermal Crack Control in Mass Concrete Structure Using an Automated Curing System. Autom. Constr. 2014, 45, 16–24. [Google Scholar] [CrossRef]
- Tahmouresi, B.; Nemati, P.; Asadi, M.A.; Saradar, A.; Mohtasham Moein, M. Mechanical Strength and Microstructure of Engineered Cementitious Composites: A New Configuration for Direct Tensile Strength, Experimental and Numerical Analysis. Constr. Build. Mater. 2021, 269, 121361. [Google Scholar] [CrossRef]
- Concrete, H. Restrained Shrinkage Cracking of Fiber-Reinforced High-Strength Concrete. Fibers 2018, 6, 12. [Google Scholar] [CrossRef]
- Nabighods, K.; Saradar, A.; Mohtasham Moein, M.; Mirgozar Langaroudi, M.A.; Byzyka, J.; Karakouzian, M. Evaluation of Self-Compacting Concrete Containing Pozzolan (Zeolite, Metakaolin & Silica Fume) and Polypropylene Fiber against Sulfate Attacks with Different PH: An Experimental Study. Innov. Infrastruct. Solut. 2023, 9, 1. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Detwiler, R.J.; Gjørv, O.E. Development of Microstructures in Plain Cement Pastes Hydrated at Different Temperatures. Cem. Concr. Res. 1991, 21, 179–189. [Google Scholar] [CrossRef]
- Lothenbach, B.; Matschei, T.; Möschner, G.; Glasser, F.P. Thermodynamic Modelling of the Effect of Temperature on the Hydration and Porosity of Portland Cement. Cem. Concr. Res. 2008, 38, 1–18. [Google Scholar] [CrossRef]
- Ayuela, A.; Dolado, J.S.; Campillo, I.; De Miguel, Y.R.; Erklzia, E.; Sánchez-Portal, D.; Rubio, A.; Porro, A.; Echenique, P.M. Silicate Chain Formation in the Nanostructure of Cement-Based Materials. J. Chem. Phys. 2007, 127, 164710. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.; Bensted, J. Structure and Performance of Cements; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Marchon, D.; Flatt, R.J. Mechanisms of Cement Hydration. In Science and Technology of Concrete Admixtures; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Richardson, I.G. The Calcium Silicate Hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Skinner, L.B.; Chae, S.R.; Benmore, C.J.; Wenk, H.R.; Monteiro, P.J.M. Nanostructure of Calcium Silicate Hydrates in Cements. Phys. Rev. Lett. 2010, 104, 195502. [Google Scholar] [CrossRef]
- Jin, M.; Jiang, L.; Lu, M.; Bai, S. Monitoring Chloride Ion Penetration in Concrete Structure Based on the Conductivity of Graphene/Cement Composite. Constr. Build. Mater. 2017, 136, 394–404. [Google Scholar] [CrossRef]
- Selvam, R.P.; Subramani, V.J.; Murray, S. Potential Application of Nanotechnology on Cement Based Materials. Structure. 2009. Available online: https://mack-blackwell.uark.edu/Research/mbtc2095-3004.pdf (accessed on 8 January 2024).
- Meng, T.; Ying, K.; Yang, X.; Hong, Y. Comparative Study on Mechanisms for Improving Mechanical Properties and Microstructure of Cement Paste Modified by Different Types of Nanomaterials. Nanotechnol. Rev. 2021, 10, 370–384. [Google Scholar] [CrossRef]
- Singh, L.P.; Ali, D.; Sharma, U. Studies on Optimization of Silica Nanoparticles Dosage in Cementitious System. Cem. Concr. Compos. 2016, 70, 60–68. [Google Scholar] [CrossRef]
- Chen, J.; Kou, S.; Poon, C. Hydration and Properties of Nano-TiO2 Blended Cement Composites. Cem. Concr. Compos. 2012, 34, 642–649. [Google Scholar] [CrossRef]
- Kurihara, R.; Maruyama, I. Influences of Nano-TiO2 Particles on Alteration of Microstructure of Hardened Cement. Proc. Jpn. Concr. Inst. 2016, 38, 219–224. [Google Scholar]
- Venkata Rao, M.; Sivagamasundari, R.; Vamsi Nagaraju, T. Achieving Strength and Sustainability in Ternary Blended Concrete: Leveraging Industrial and Agricultural By-Products with Controlled Nano-SiO2 Content. Clean. Mater. 2023, 9, 100198. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Fakoorpoor, S.M.; Khaloo, A.; Hosseini, P. Improving the Performance of Cement-Based Composites Containing Superabsorbent Polymers by Utilization of Nano-SiO2 Particles. Mater. Des. 2012, 42, 94–101. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Justnes, H. Revisiting the Microstructure of Hydrated Tricalcium Silicate—A Comparison to Portland Cement. Cem. Concr. Compos. 2004, 26, 947–956. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, Í.; Fernández, J.M.; Alvarez, J.I. The Effect of TiO2 Doped Photocatalytic Nano-Additives on the Hydration and Microstructure of Portland and High Alumina Cements. Nanomaterials 2017, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Nijland, T.G.; Larbi, J.A. Microscopic Examination of Deteriorated Concrete. In Non-Destructive Evaluation of Reinforced Concrete Structures: Deterioration Processes and Standard Test Methods; Woodhead Publishing: Sawston, UK, 2010. [Google Scholar]
- Çopuroğlu, O. Revealing the Dark Side of Portlandite Clusters in Cement Paste by Circular Polarization Microscopy. Materials 2016, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Yu, Y.; Qian, X.; Zhan, S.; Qian, K. Effect of Nano-TiO2 on the Mechanical Properties of Cement Mortar. Constr. Build. Mater. 2012, 29, 241–245. [Google Scholar] [CrossRef]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver Nanoparticles in the Environment: Sources, Detection and Ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.R.; Pool, E.J.; Somerset, V.S. Ecotoxicity of Silver Nanomaterials in the Aquatic Environment: A Review of Literature and Gaps in Nano-Toxicological Research. J. Environ. Sci. Health Part A 2014, 49, 1588–1601. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Soldado, E.; Borsoi, G.; Mendes, M.P.; Flores-Colen, I. Nanomaterials Applied in the Construction Sector: Environmental, Human Health, and Economic Indicators. Appl. Sci. 2023, 13, 12896. [Google Scholar] [CrossRef]
- Inshakova, E.; Inshakova, A.; Goncharov, A. Engineered Nanomaterials for Energy Sector: Market Trends, Modern Applications and Future Prospects. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 971. [Google Scholar]
- Fu, C.; Xie, C.; Liu, J.; Wei, X.; Wu, D. A Comparative Study on the Effects of Three Nano-Materials on the Properties of Cement-Based Composites. Materials 2020, 13, 857. [Google Scholar] [CrossRef]
- Sabour, M.R.; Yekkalar, M.; Nikravan, M. Investigation of Effect of Nano-SiO2Consumption in Concrete on Its Environmental and Economic Functions. Ferdowsi Civ. Eng. 2014, 25, 12. [Google Scholar] [CrossRef]
- Reddy, A.N.; Reddy, P.N.; Kavyateja, B.V.; Reddy, G.G.K. Influence of Nanomaterial on High-Volume Fly Ash Concrete: A Statistical Approach. Innov. Infrastruct. Solut. 2020, 5, 88. [Google Scholar] [CrossRef]
- Imani, M.; Donn, M.; Balador, Z. Bio-Inspired Materials: Contribution of Biology to Energy Efficiency of Buildings. In Handbook of Ecomaterials; Springer: Berlin/Heidelberg, Germany, 2019; Volume 3. [Google Scholar]
- Reap, J.; Baumeister, D.; Bras, B. Holism, Biomimicry and Sustainable Engineering. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Orlando, FL, USA, 5–11 November 2005; Volume 2005. [Google Scholar]
- McLeod, K.J. Cats’ Paws and Catapults: Mechanical Worlds of Nature and People. Steven Vogel. Q Rev. Biol. 1999, 74, 212–213. [Google Scholar] [CrossRef]
- Fayemi, P.E.; Maranzana, N.; Aoussat, A.; Bersano, G. Bio-Inspired Design Characterisation and Its Links with Problem Solving Tools. In Proceedings of the 2014 13th International Design Conference, DESIGN, Dubrovnik, Croatia, 19–22 May 2014. [Google Scholar]
- Verbrugghe, N.; Rubinacci, E.; Khan, A.Z. Biomimicry in Architecture: A Review of Definitions, Case Studies, and Design Methods. Biomimetics 2023, 8, 107. [Google Scholar] [CrossRef]
- Badarnah, L. Towards the Living Envelope Biomimetics for Building Envelope Adaptation. Ph.D. Thesis, Israel Institute of Technology Geboren te Haifa, Haifa, Israel, 2019. [Google Scholar]
- El Albani, A.; Bengtson, S.; Canfield, D.E.; Bekker, A.; MacChiarelli, R.; Mazurier, A.; Hammarlund, E.U.; Boulvais, P.; Dupuy, J.J.; Fontaine, C.; et al. Large Colonial Organisms with Coordinated Growth in Oxygenated Environments 2.1 Gyr Ago. Nature 2010, 466, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Oguntona, O.A.; Aigbavboa, C.O. Biomimetic Reinvention of the Construction Industry: Energy Management and Sustainability. Energy Procedia 2017, 142, 2721–2727. [Google Scholar] [CrossRef]
- Horn, R.; Albrecht, S.; Haase, W.; Langer, M.; Schmeer, D.; Sobek, W.; Speck, O.; Leistner, P. Bio-Inspiration as a Concept for Sustainable Constructions Illustrated on Graded Concrete. J. Bionic. Eng. 2019, 16, 742–753. [Google Scholar] [CrossRef]
- Lu, M.; Lai, J. Review on Carbon Emissions of Commercial Buildings. Renew. Sustain. Energy Rev. 2020, 119, 109545. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; Davis, S.J.; Giron, C.; Ciais, P. Monitoring Global Carbon Emissions in 2021. Nat. Rev. Earth Environ. 2022, 3, 217–219. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; Davis, S.; Ciais, P. Monitoring Global Carbon Emissions in 2022. Nat. Rev. Earth Environ. 2023, 4, 205–206. [Google Scholar] [CrossRef]
- Shashwat, S.; Zingre, K.T.; Thurairajah, N.; Kumar, D.K.; Panicker, K.; Anand, P.; Wan, M.P. A Review on Bioinspired Strategies for an Energy-Efficient Built Environment. Energy Build. 2023, 296, 113382. [Google Scholar] [CrossRef]
- Chen, D.A.; Ross, B.E.; Klotz, L.E. Lessons from a Coral Reef: Biomimicry for Structural Engineers. J. Struct. Eng. 2015, 141, 02514002. [Google Scholar] [CrossRef]
- Rodríguez-Zaragoza, F.A.; Arias-González, J.E. Coral Biodiversity and Bio-Construction in the Northern Sector of the Mesoamerican Reef System. Front. Mar. Sci. 2015, 2, 13. [Google Scholar] [CrossRef]
- Ahamed, M.K.; Wang, H.; Hazell, P.J. From Biology to Biomimicry: Using Nature to Build Better Structures—A Review. Constr. Build. Mater. 2022, 320, 126195. [Google Scholar] [CrossRef]
- Shahsavari, R.; Hwang, S.H. Bioinspired Cementitious Materials: Main Strategies, Progress, and Applications. Front. Mater. 2020, 7, 62. [Google Scholar] [CrossRef]
- Toader, N.; Sobek, W.; Nickel, K.G. Energy Absorption in Functionally Graded Concrete Bioinspired by Sea Urchin Spines. J. Bionic. Eng. 2017, 14, 369–378. [Google Scholar] [CrossRef]
- Tee, Y.L.; Leary, M.; Tran, P. Porcupine Quill: Buckling Resistance Analysis and Design for 3d Printing. In Proceedings of the 16th East Asian-Pacific Conference on Structural Engineering and Construction, Lecture Notes in Civil Engineering, Brisbane, Australia, 3–6 December 2021; Volume 101. [Google Scholar]
- Sun, Y.; Yu, Z.; Wang, Z.; Liu, X. Novel Protective Covering to Enhance Concrete Resistance against Projectile Impact. Constr. Build. Mater. 2015, 96, 484–490. [Google Scholar] [CrossRef]
- Mayer, G. Mechanical Energy Dissipation in Natural Ceramic Composites. J. Mech. Behav. Biomed. Mater. 2017, 76, 21–29. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Wu, H.; Zhang, C. Low Velocity Impact Resistance of Bio-Inspired Building Ceramic Composites with Nacre-like Structure. Constr. Build. Mater. 2018, 169, 851–858. [Google Scholar] [CrossRef]
- Chen, I.H.; Kiang, J.H.; Correa, V.; Lopez, M.I.; Chen, P.Y.; McKittrick, J.; Meyers, M.A. Armadillo Armor: Mechanical Testing and Micro-Structural Evaluation. J. Mech. Behav. Biomed. Mater. 2011, 4, 713–722. [Google Scholar] [CrossRef]
- Tran, P.; Peng, C. Triply Periodic Minimal Surfaces Sandwich Structures Subjected to Shock Impact. J. Sandw. Struct. Mater. 2021, 23, 2146–2175. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Abu Al-Rub, R.K. Multifunctional Mechanical Metamaterials Based on Triply Periodic Minimal Surface Lattices. Adv. Eng. Mater. 2019, 21, 1900524. [Google Scholar] [CrossRef]
- Soltan, D.G.; Li, V.C. Nacre-Inspired Composite Design Approaches for Large-Scale Cementitious Members and Structures. Cem. Concr. Compos. 2018, 88, 172–186. [Google Scholar] [CrossRef]
- Soltan, D.G.; Ranade, R.; Li, V.C. A Bio-Inspired Cementitious Composite for High Energy Absorption in Infrastructural Applications. Blucher Mater. Sci. Proc. 2014, 1, 1–14. [Google Scholar]
- Gu, G.X.; Takaffoli, M.; Buehler, M.J. Hierarchically Enhanced Impact Resistance of Bioinspired Composites. Adv. Mater. 2017, 29, 1700060. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, H.; Ortiz, C.; Xu, J.; Dao, M. Bio-Inspired Interfacial Strengthening Strategy through Geometrically Interlocking Designs. J. Mech. Behav. Biomed. Mater. 2012, 15, 70–77. [Google Scholar] [CrossRef]
- Wani, K.M.N.S.; Mir, B.A. An Experimental Study on the Bio-Cementation and Bio-Clogging Effect of Bacteria in Improving Weak Dredged Soils. Geotech. Geol. Eng. 2021, 39, 317–334. [Google Scholar] [CrossRef]
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of Calcite (Calcium Carbonate) Crystals by Soil Bacteria Is a General Phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Schlangen, E. Development of a Bacteria-Based Self Healing Concrete. In Proceedings of the International FIB Symposium 2008—Tailor Made Concrete Structures: New Solutions for our Society, Amsterdam, The Netherlands, 19–22 May 2008. [Google Scholar]
- Sakai, K. Atomic Force Microscope (AFM). In Measurement Techniques and Practices of Colloid and Interface Phenomena; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Chen, C.J. Introduction to Scanning Tunneling Microscopy; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Bhushan, B.; Sayer, R.A. Gecko Feet: Natural Attachment Systems for Smart Adhesion; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Bhushan, B.; Sayer, R.A. Gecko Feet: Natural Attachment Systems for Smart Adhesion—Mechanism, Modeling, and Development of Bio-Inspired Materials. In Biosystems—Investigated by Scanning Probe Microscopy; Springer-Verlag: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Nosonovsky, M.; Bormashenko, E. Lotus Effect: Superhydrophobicity and Self-Cleaning. In Functional Properties of Bio-Inspired Surfaces: Characterization and Technological Applications; World Scientific: Singapore, 2009. [Google Scholar]
- Lee, H.J.; Michielsen, S. Lotus Effect: Superhydrophobicity. J. Text. Inst. 2006, 97, 455–462. [Google Scholar] [CrossRef]
- Garg, P.; Ghatmale, P.; Tarwadi, K.; Chavan, S. Influence of Nanotechnology and the Role of Nanostructures in Biomimetic Studies and Their Potential Applications. Biomimetics 2017, 2, 7. [Google Scholar] [CrossRef]
- Shapkin, N.P.; Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Simonenko, E.P.; Simonenko, N.P.; Zavjalov, A.P.; Belov, A.A.; Portnyagin, A.S.; Gerasimenko, A.V.; et al. Spark Plasma Sintering-Reactive Synthesis of SiC and SiC–HfB2 Ceramics Based on Natural Renewable Raw Materials. Russ. J. Inorg. Chem. 2021, 66, 629–637. [Google Scholar] [CrossRef]
- Weizmann, M.; Amir, O.; Grobman, Y.J. Topological Interlocking in Architecture: A New Design Method and Computational Tool for Designing Building Floors. Int. J. Archit. Comput. 2017, 15, 107–118. [Google Scholar] [CrossRef]
- Lazarus, B.S.; Velasco-Hogan, A.; Gómez-del Río, T.; Meyers, M.A.; Jasiuk, I. A Review of Impact Resistant Biological and Bioinspired Materials and Structures. J. Mater. Res. Technol. 2020, 9, 15705–15738. [Google Scholar] [CrossRef]
- Muhd Norhasri, M.S.; Hamidah, M.S.; Mohd Fadzil, A.; Megawati, O. Inclusion of Nano Metakaolin as Additive in Ultra High Performance Concrete (UHPC). Constr. Build. Mater. 2016, 127, 167–175. [Google Scholar] [CrossRef]
- Gu, C. Effect of Nano-TIO₂ on the Durability of Ultra-High Performance Concrete with and without a Flexural Load. Ceram. Silik. 2018, 62, 374–381. [Google Scholar] [CrossRef]
- Carmichael, M.J.; Arulraj, G.P. Impact Resistance of Concrete with Nano Materials. Mater. Today Proc. 2020, 37, 677–684. [Google Scholar] [CrossRef]
- Isfahani, F.T.; Redaelli, E.; Lollini, F.; Li, W.; Bertolini, L. Effects of Nanosilica on Compressive Strength and Durability Properties of Concrete with Different Water to Binder Ratios. Adv. Mater. Sci. Eng. 2016, 2016, 8453567. [Google Scholar] [CrossRef]
- Zhong, C.; Yu, Z.; Zhou, J.; Long, Y.; Tian, P.; Chen, J. Effect of Nano-TiO2 on Capillary Water Absorption of Recycled Aggregate Concrete. Coatings 2022, 12, 1833. [Google Scholar] [CrossRef]
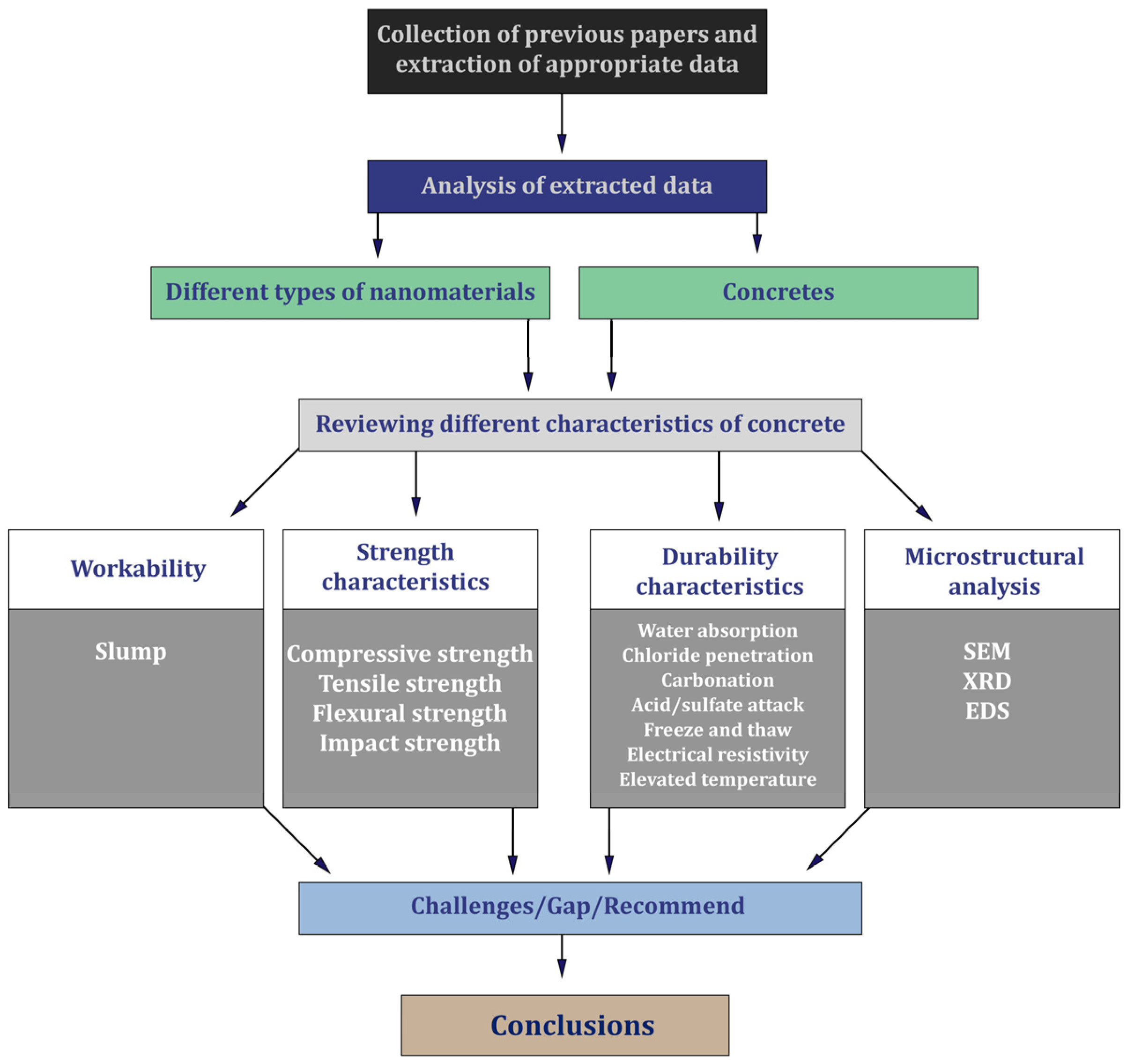


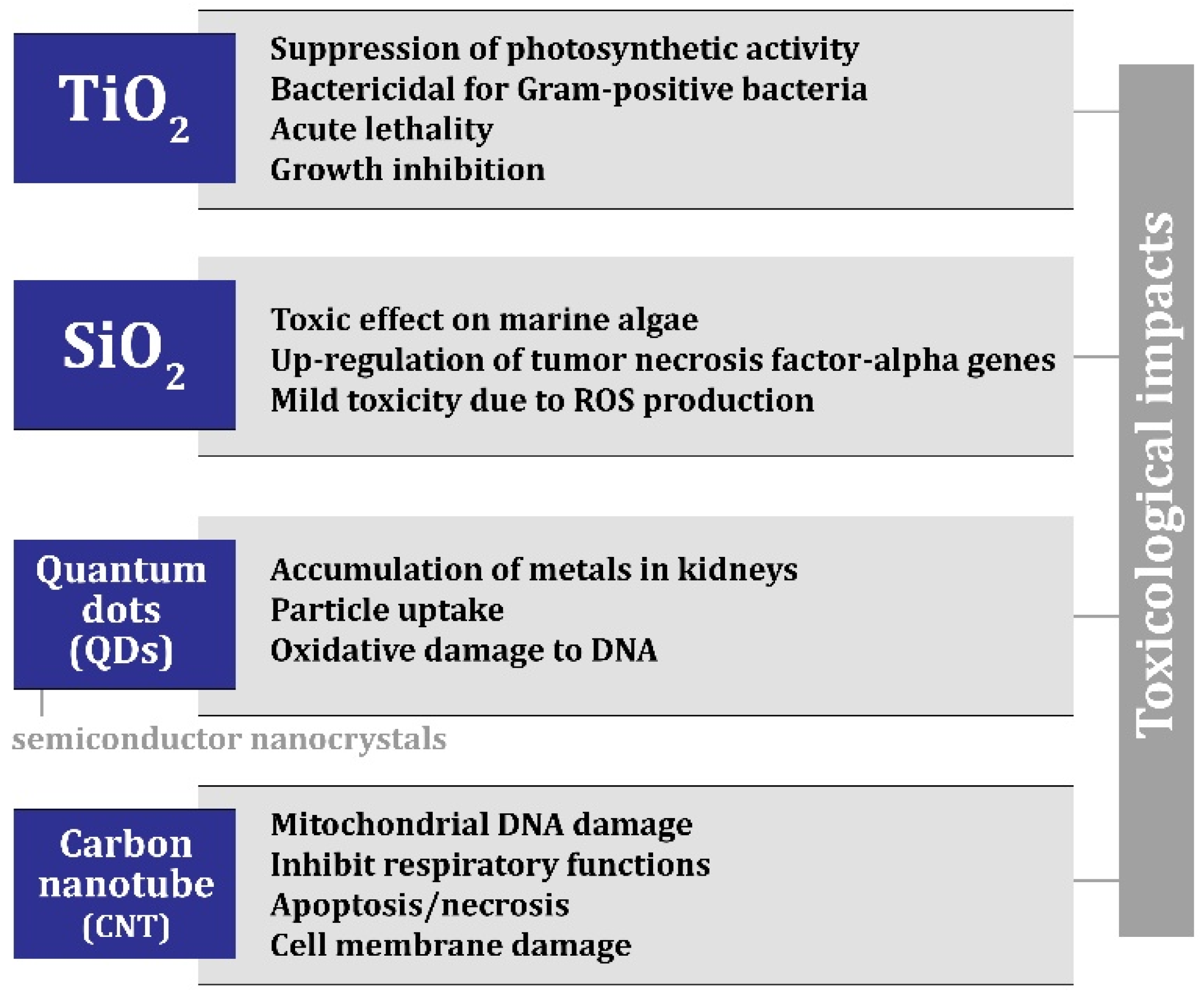
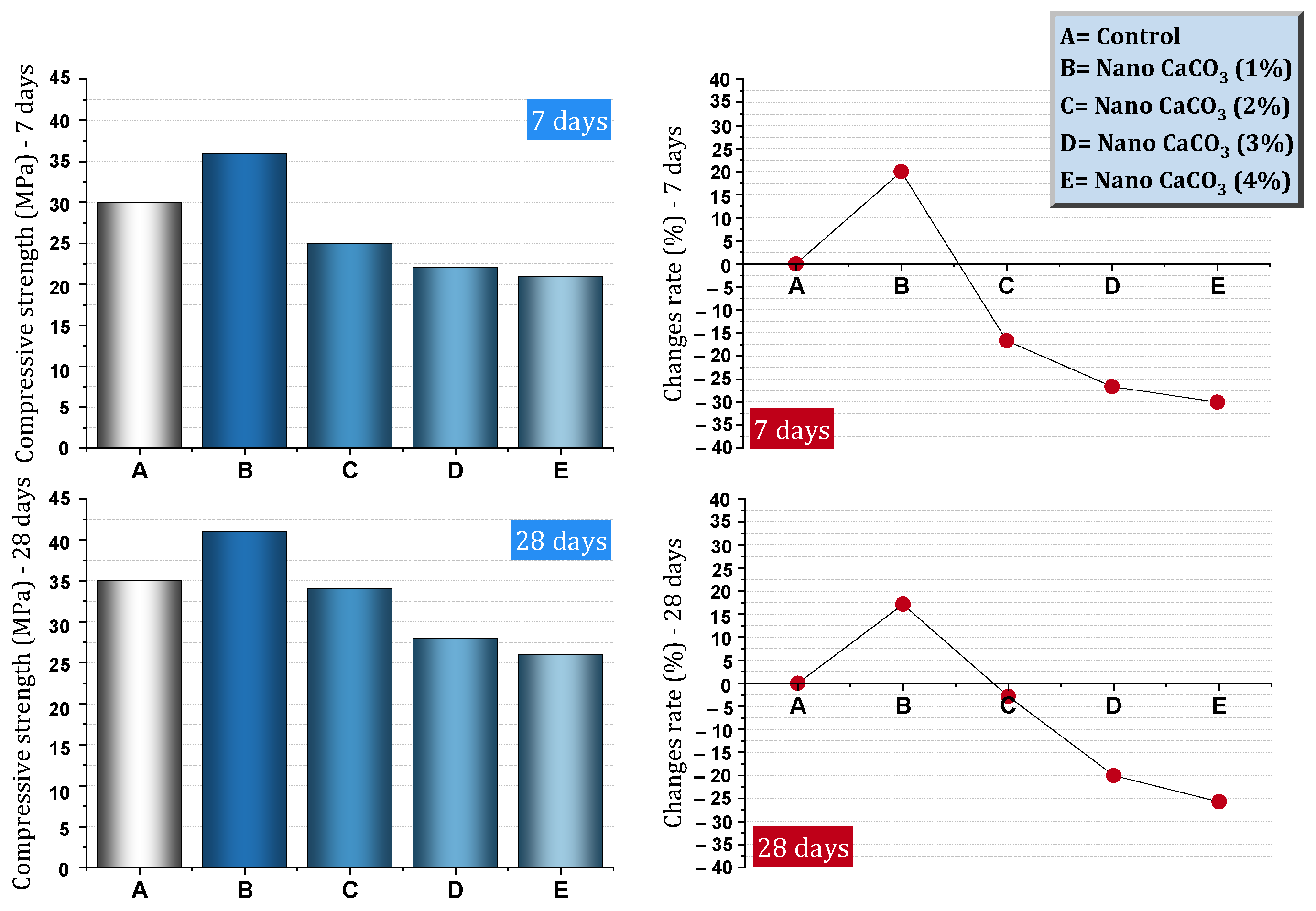


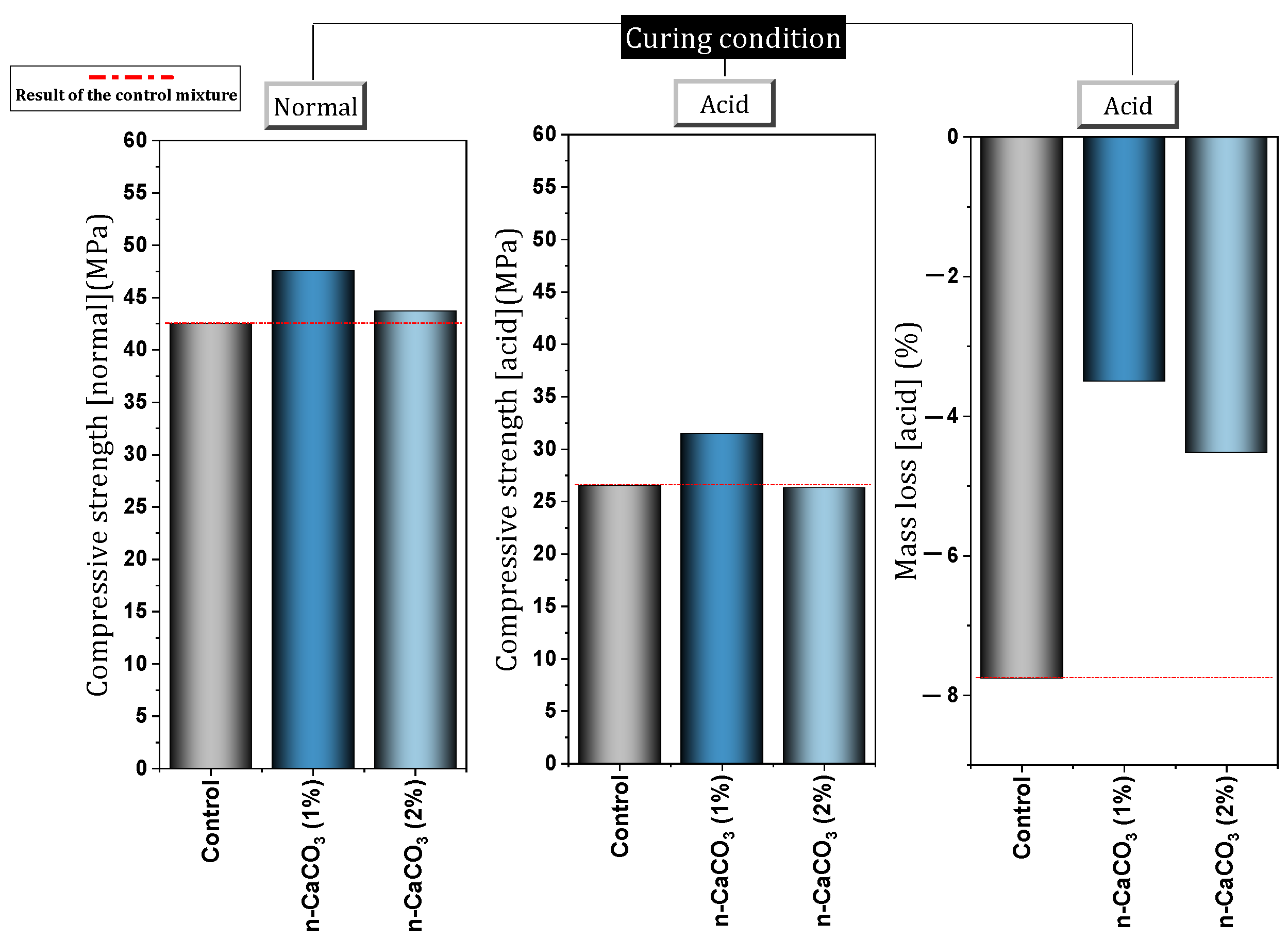
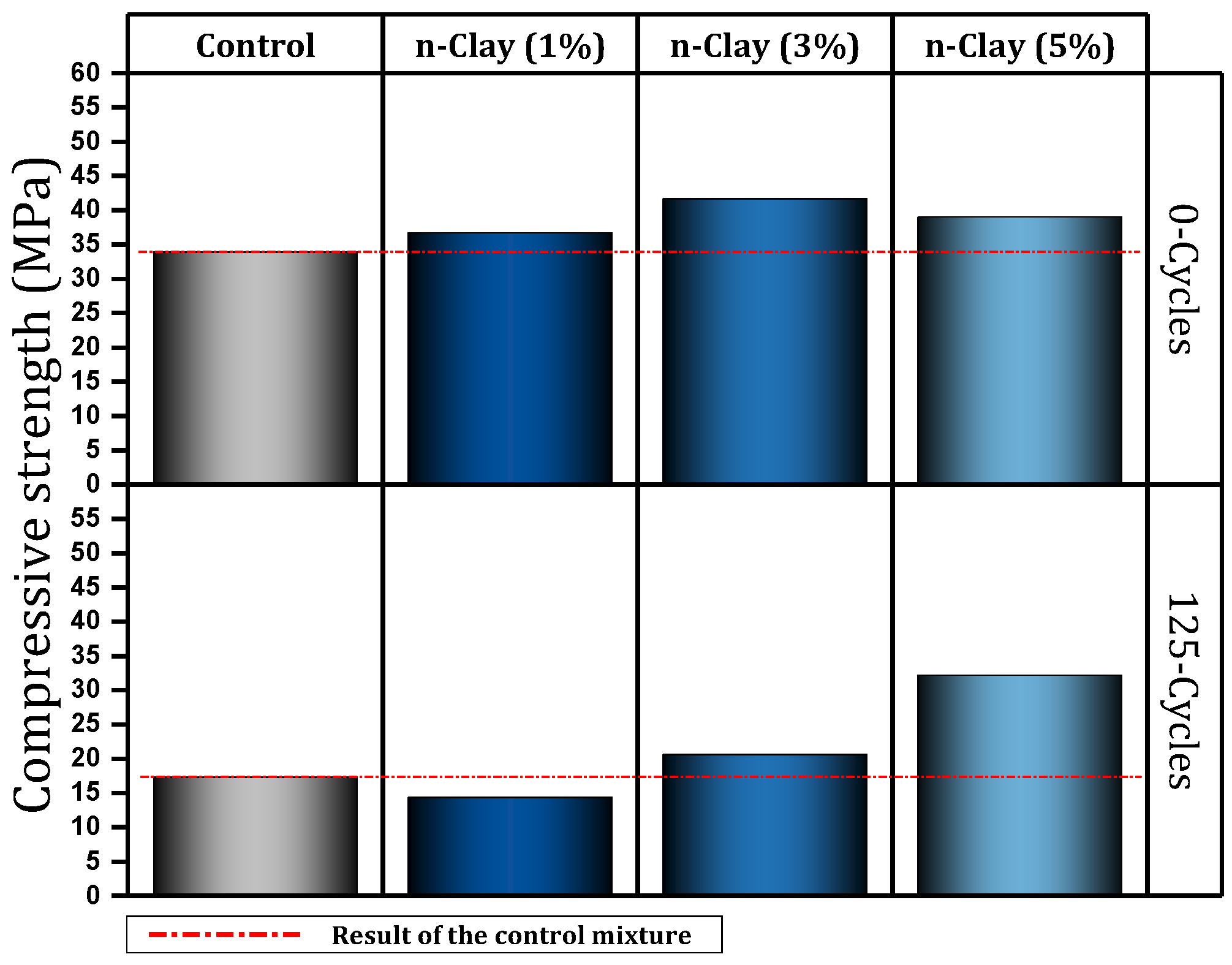
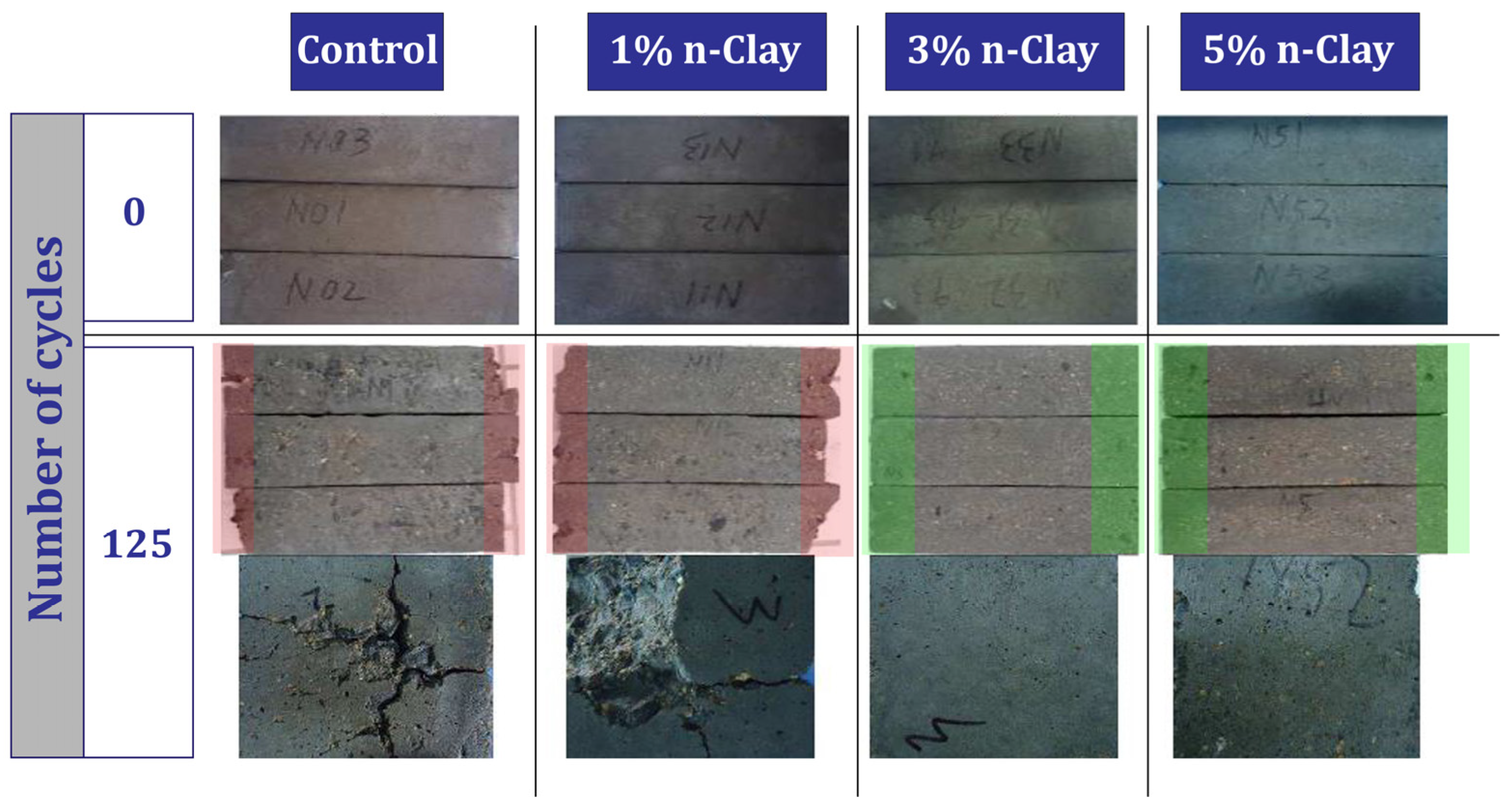
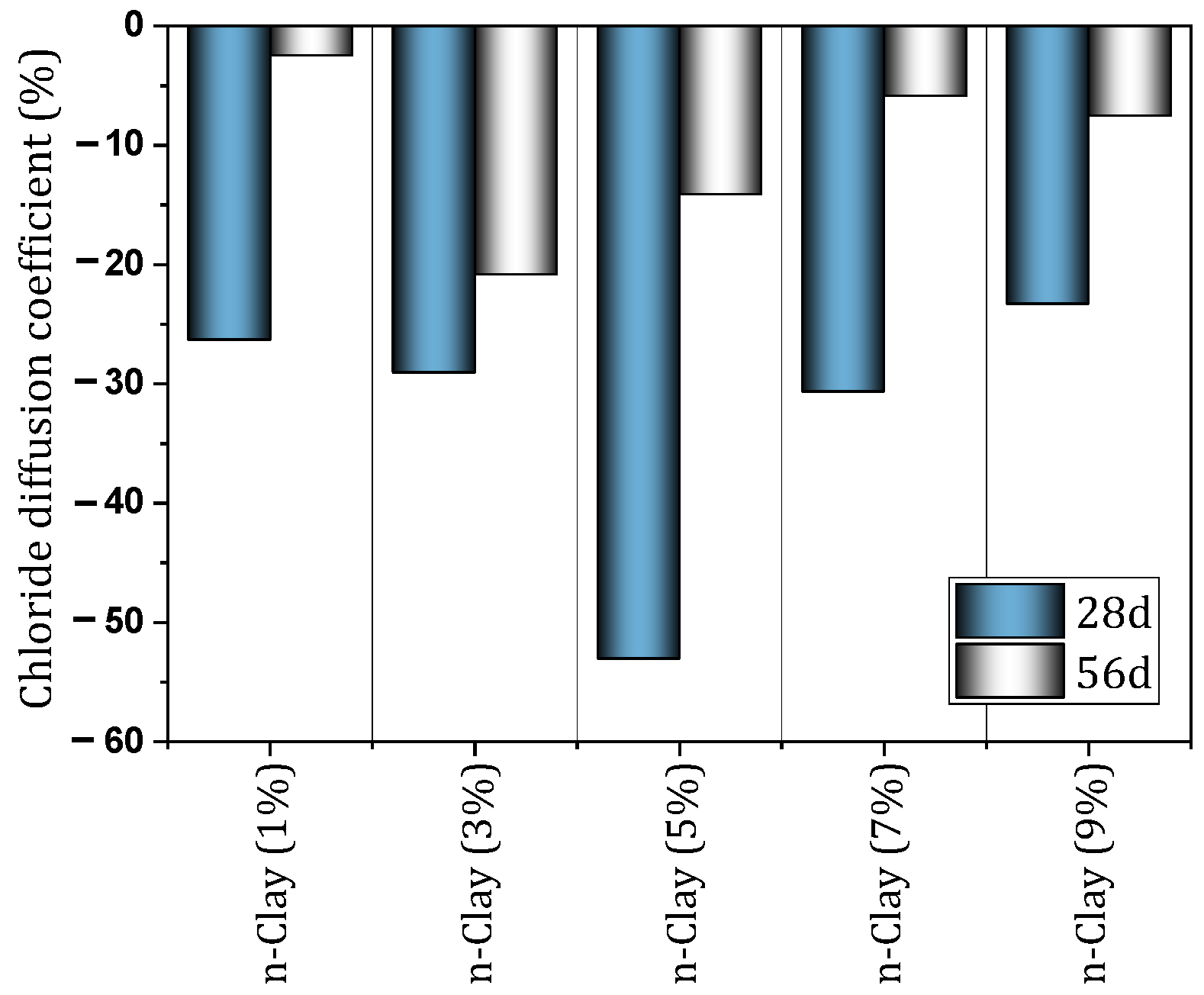
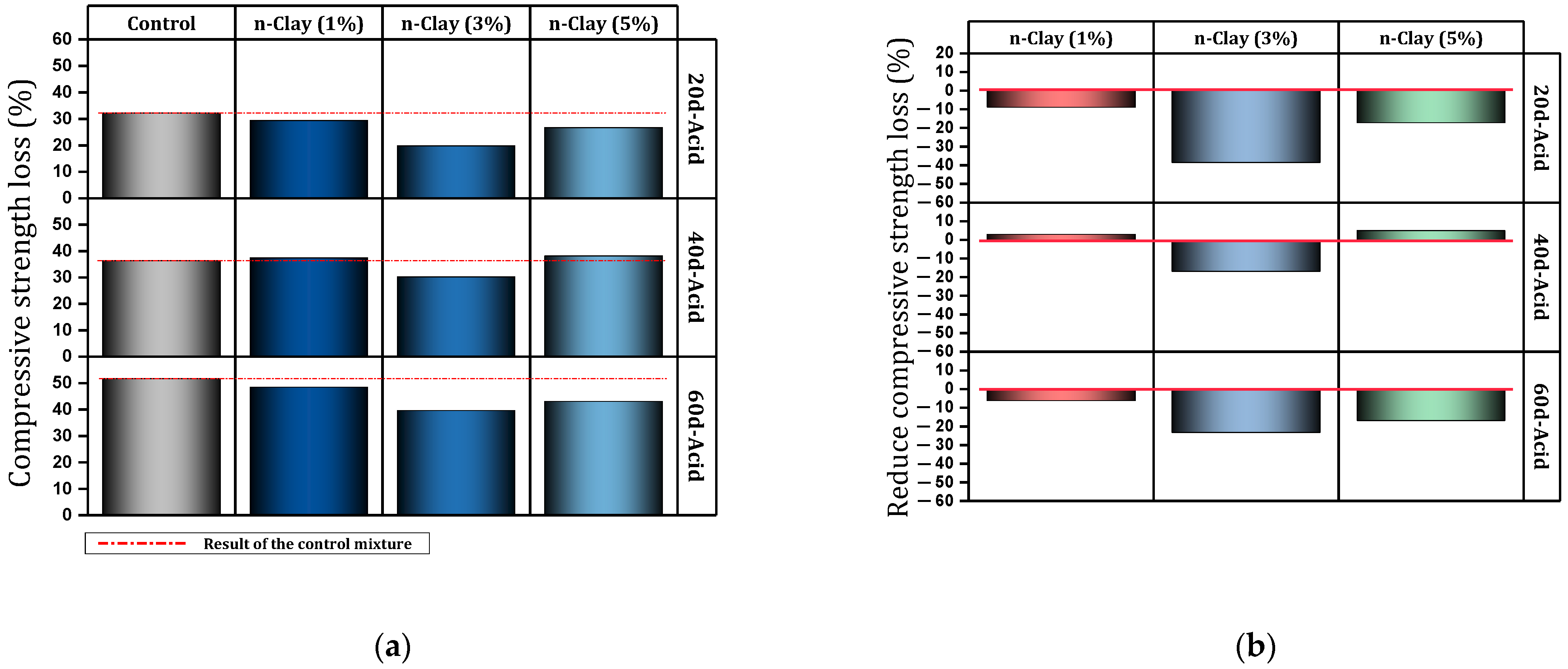
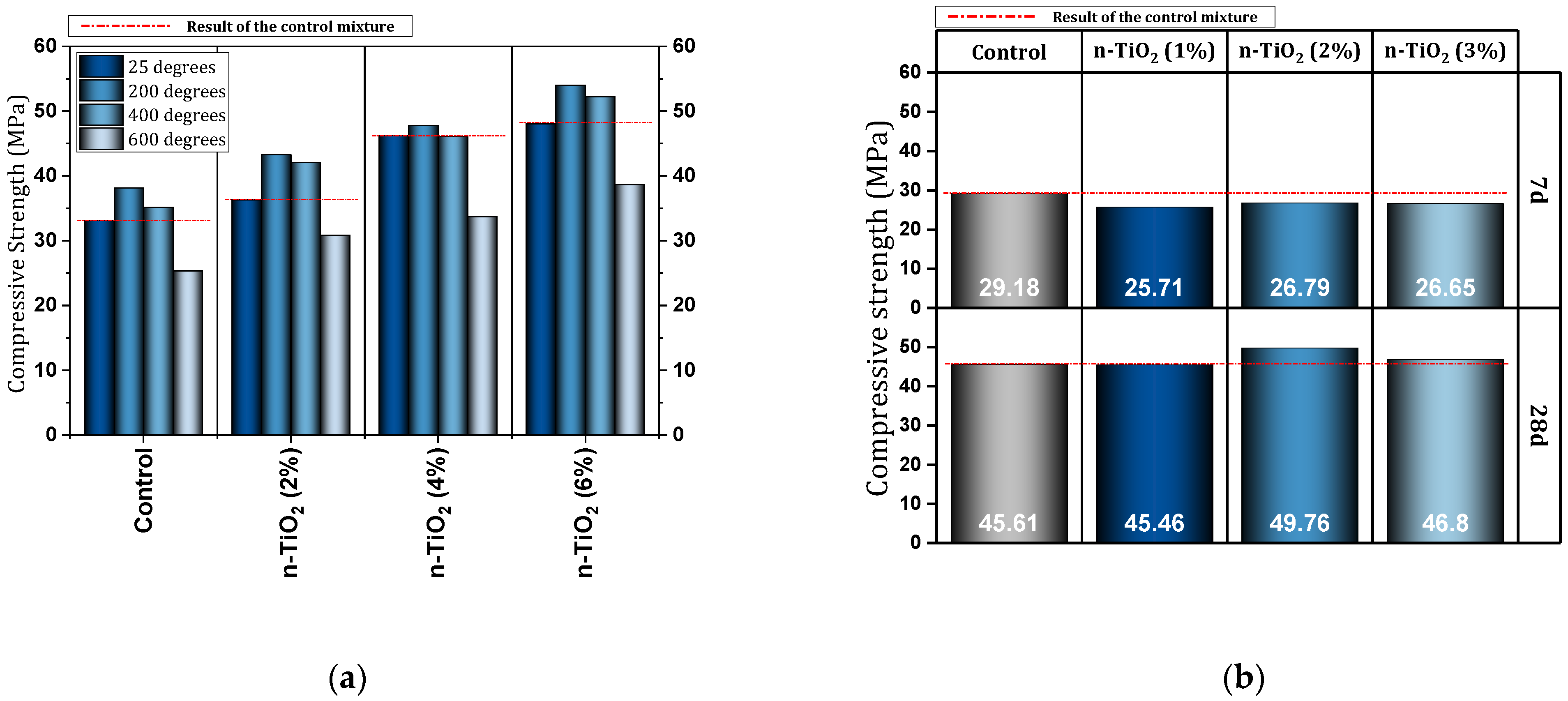
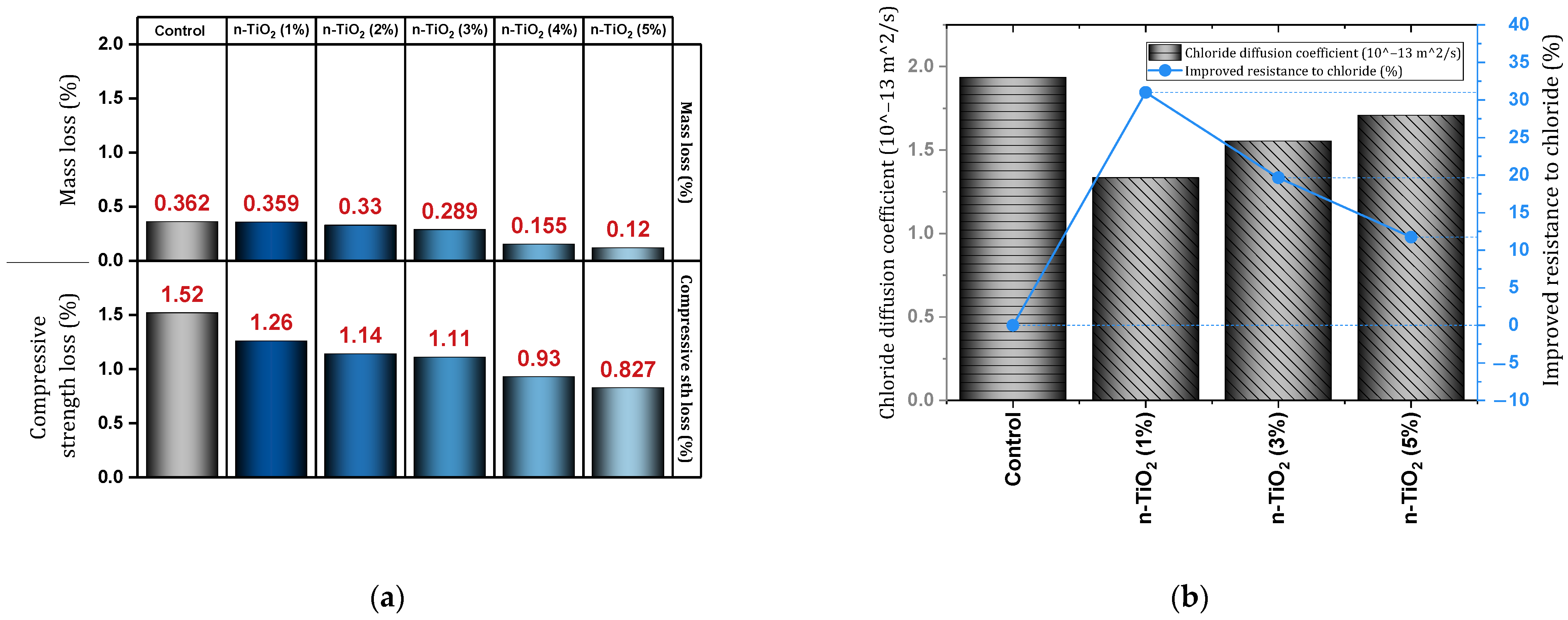



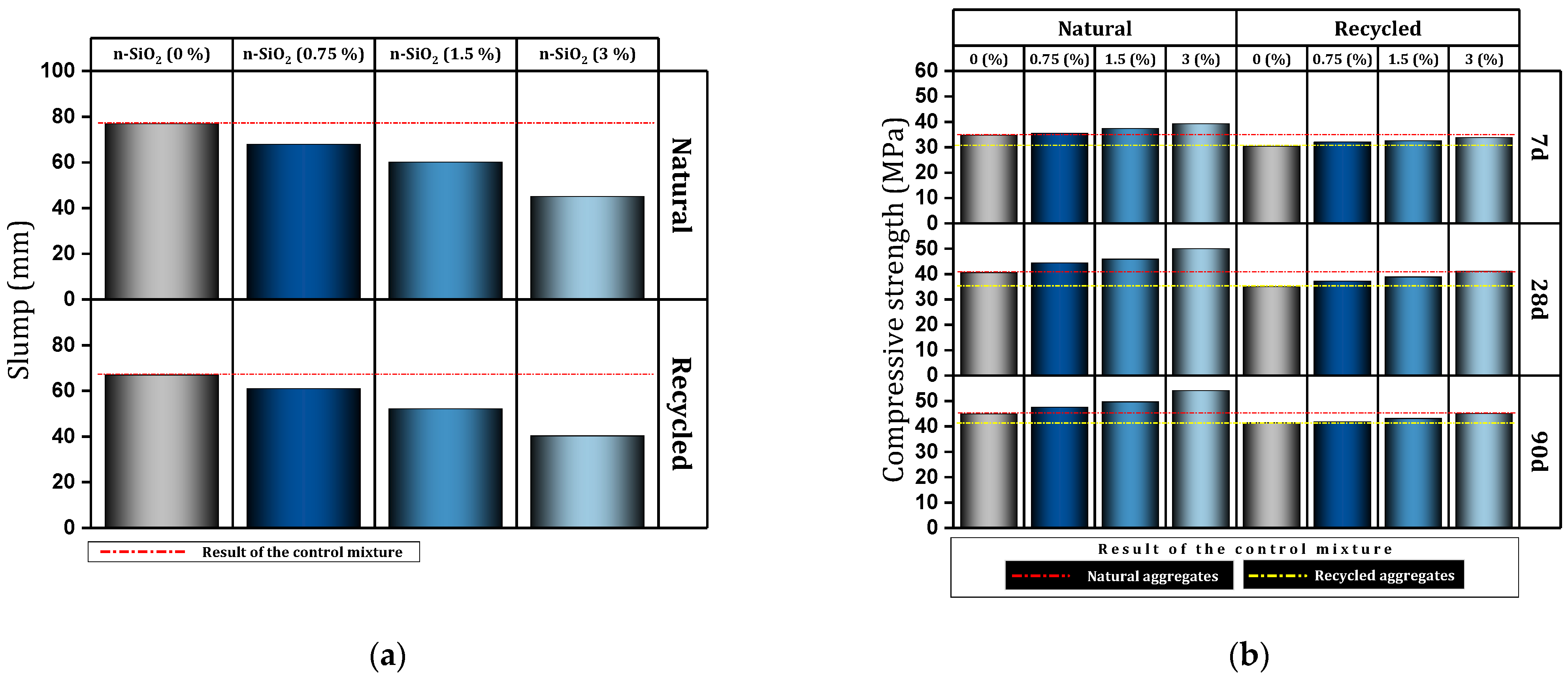

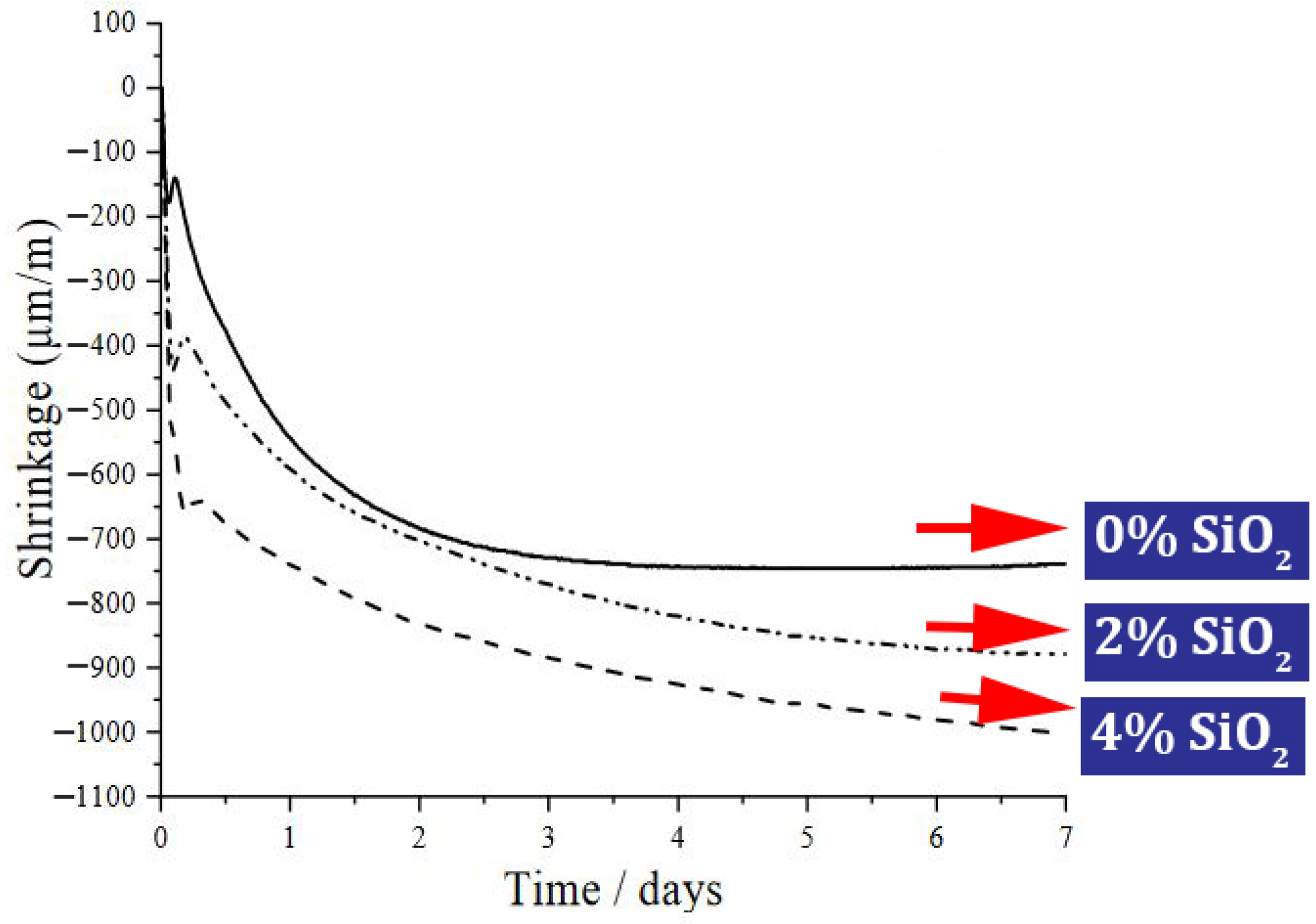
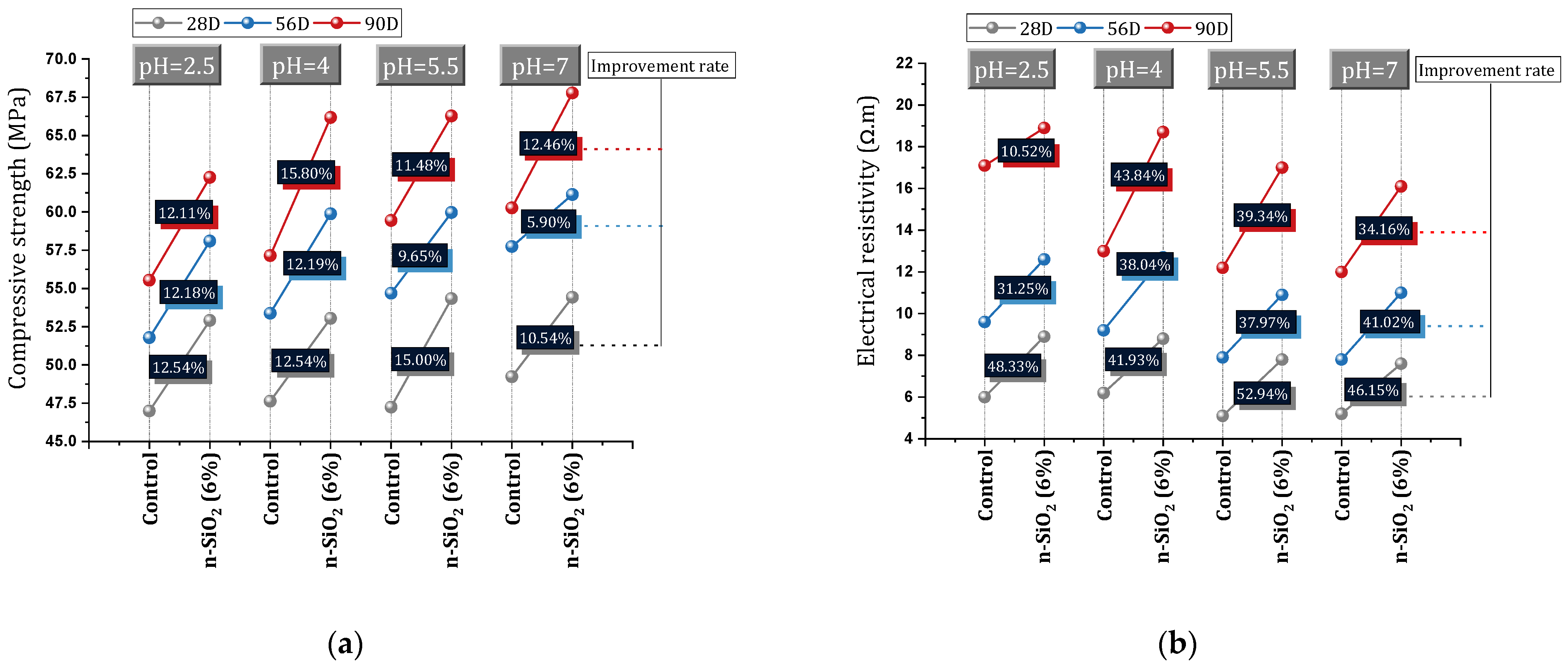

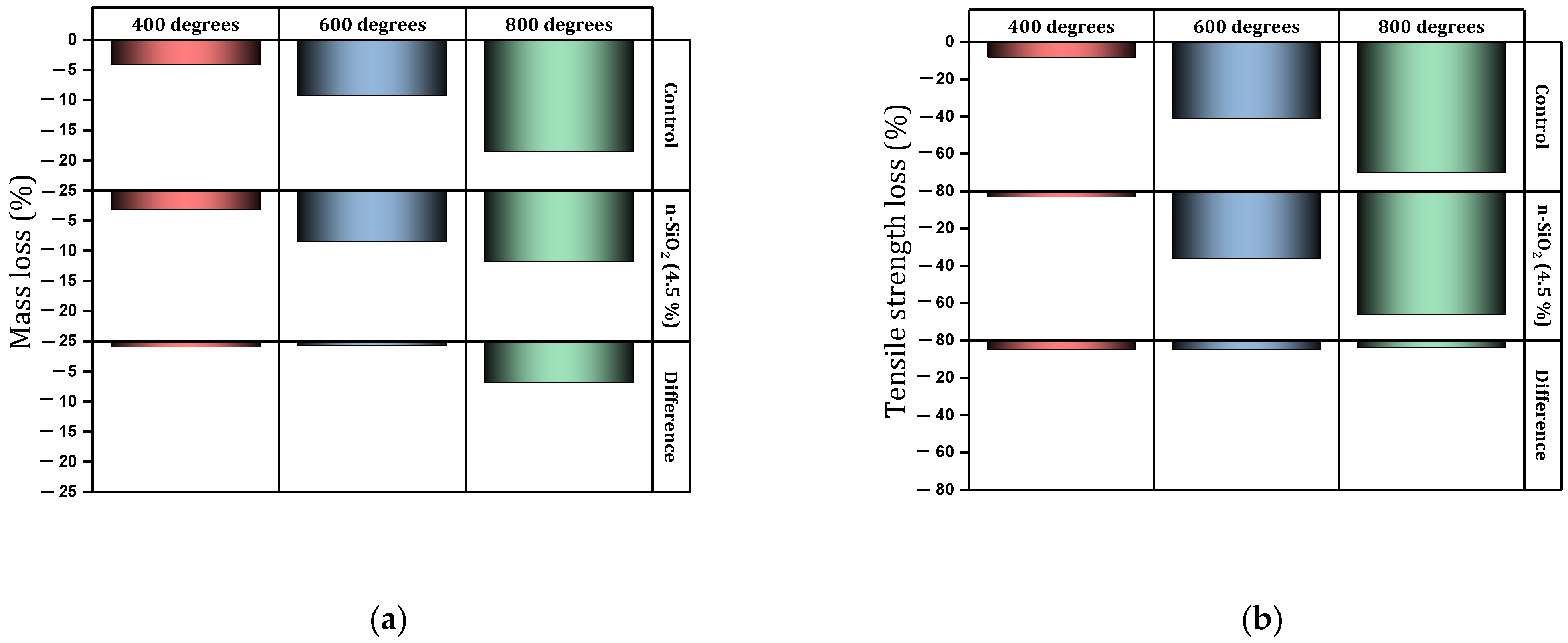
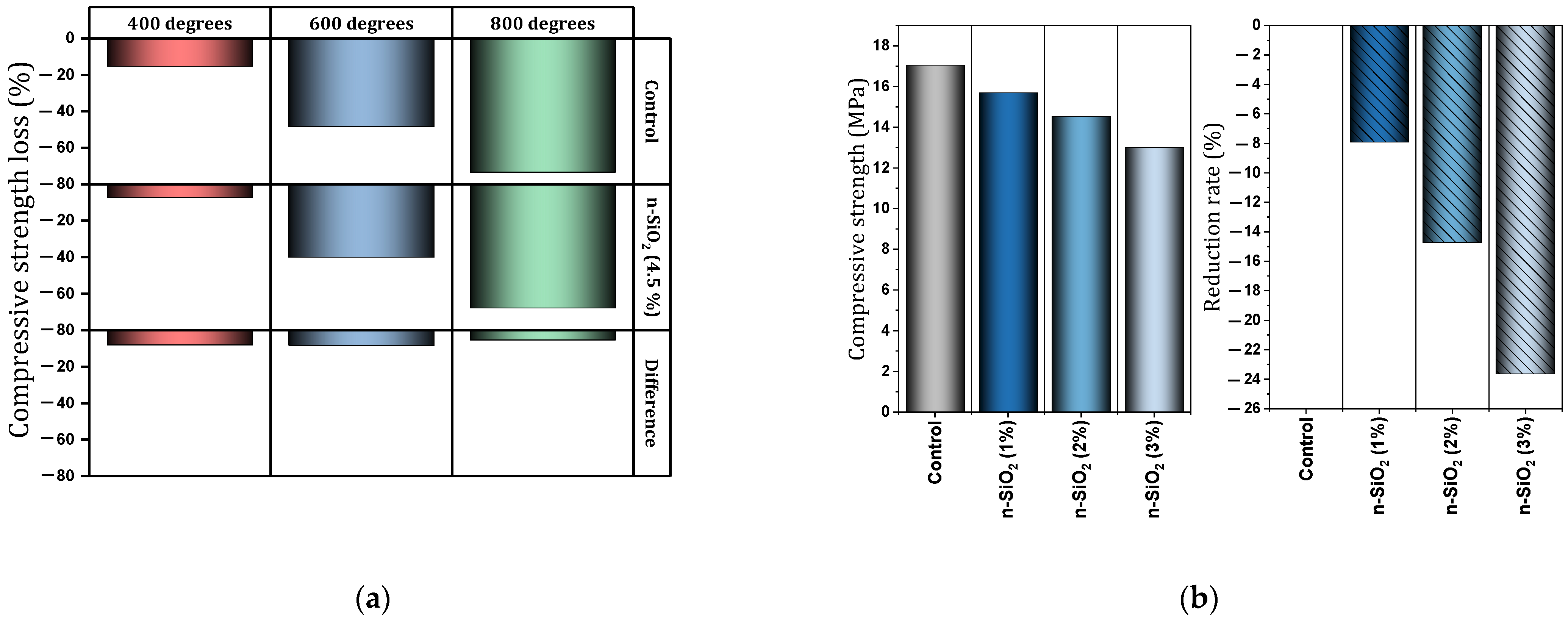
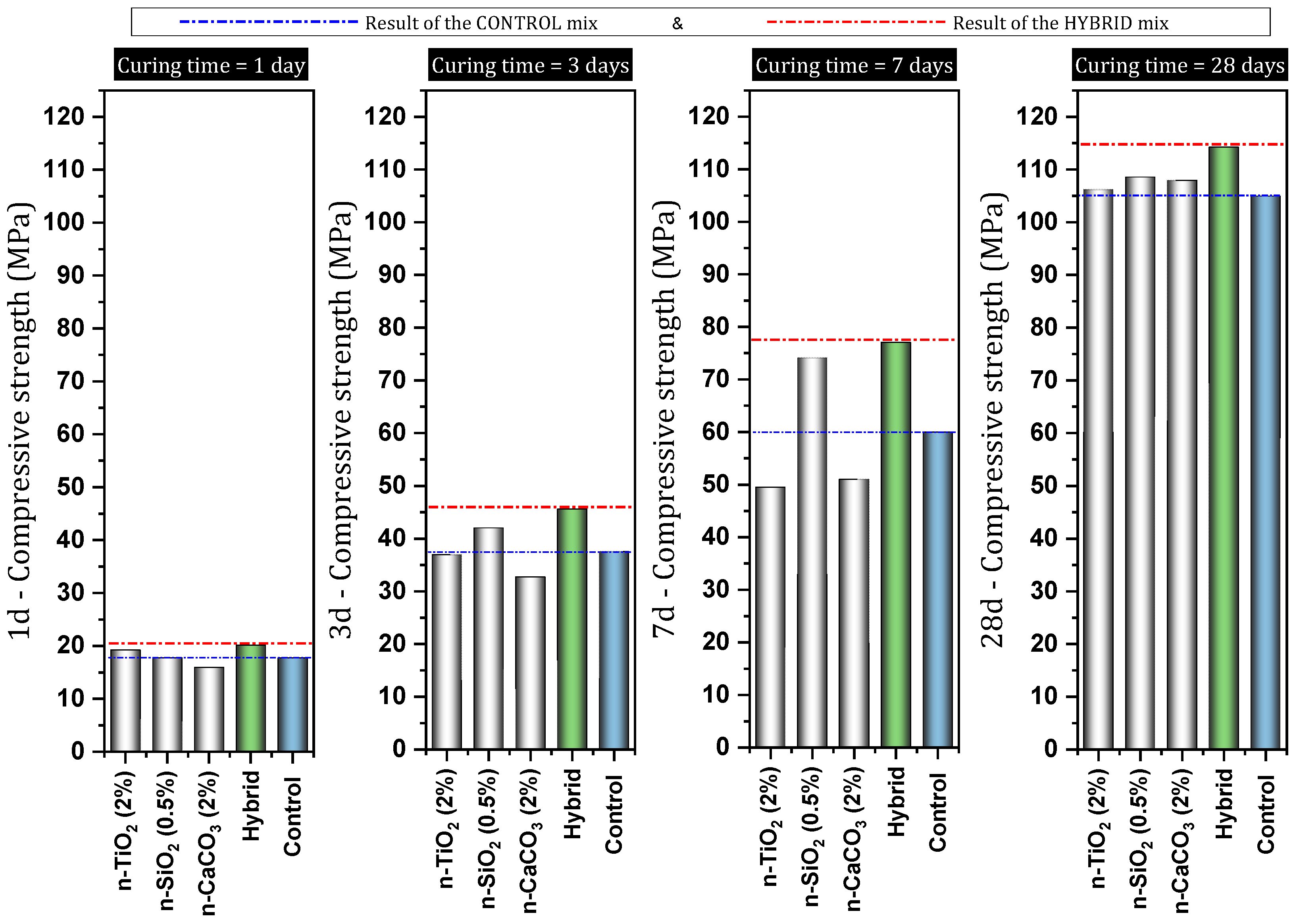

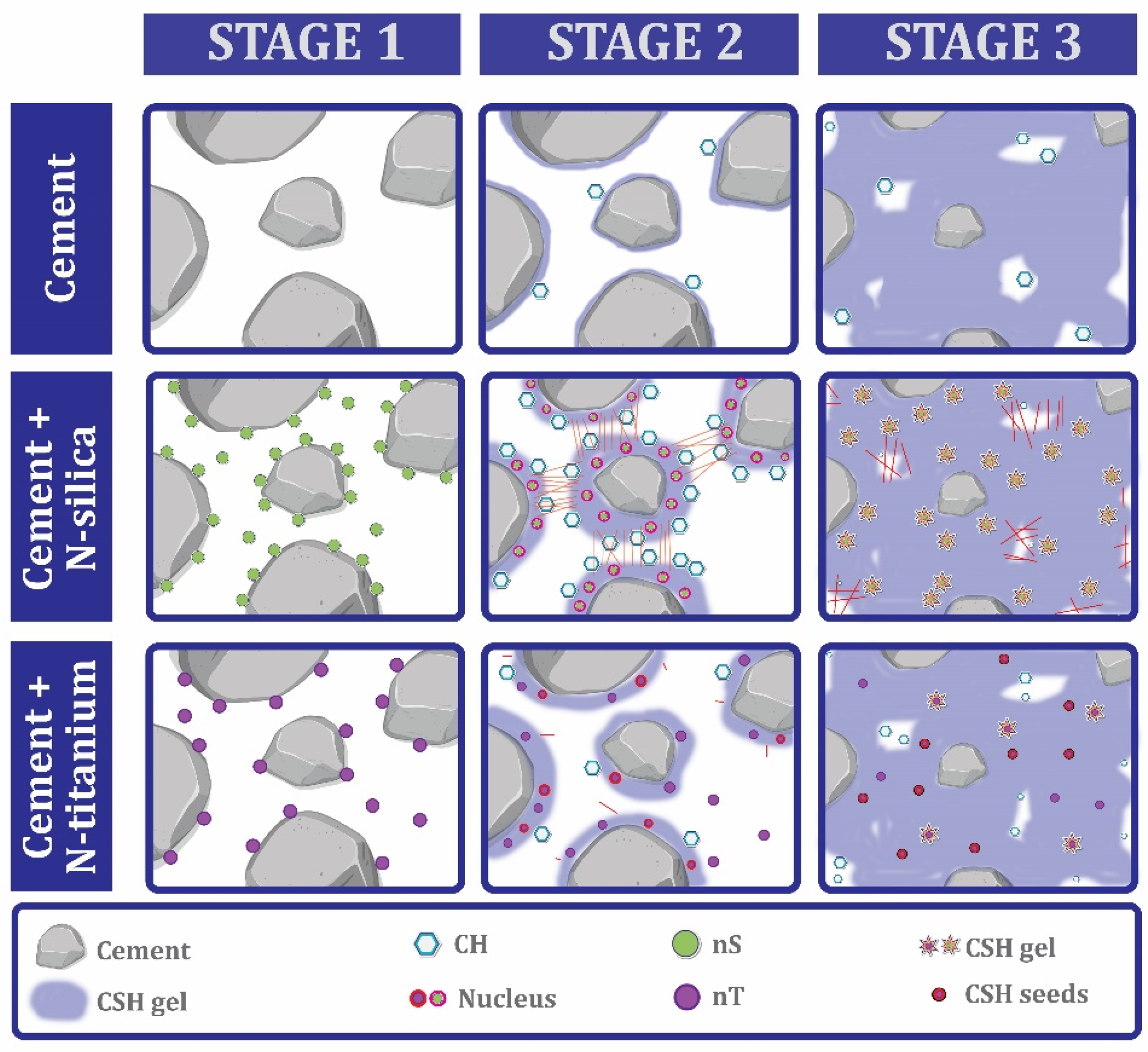
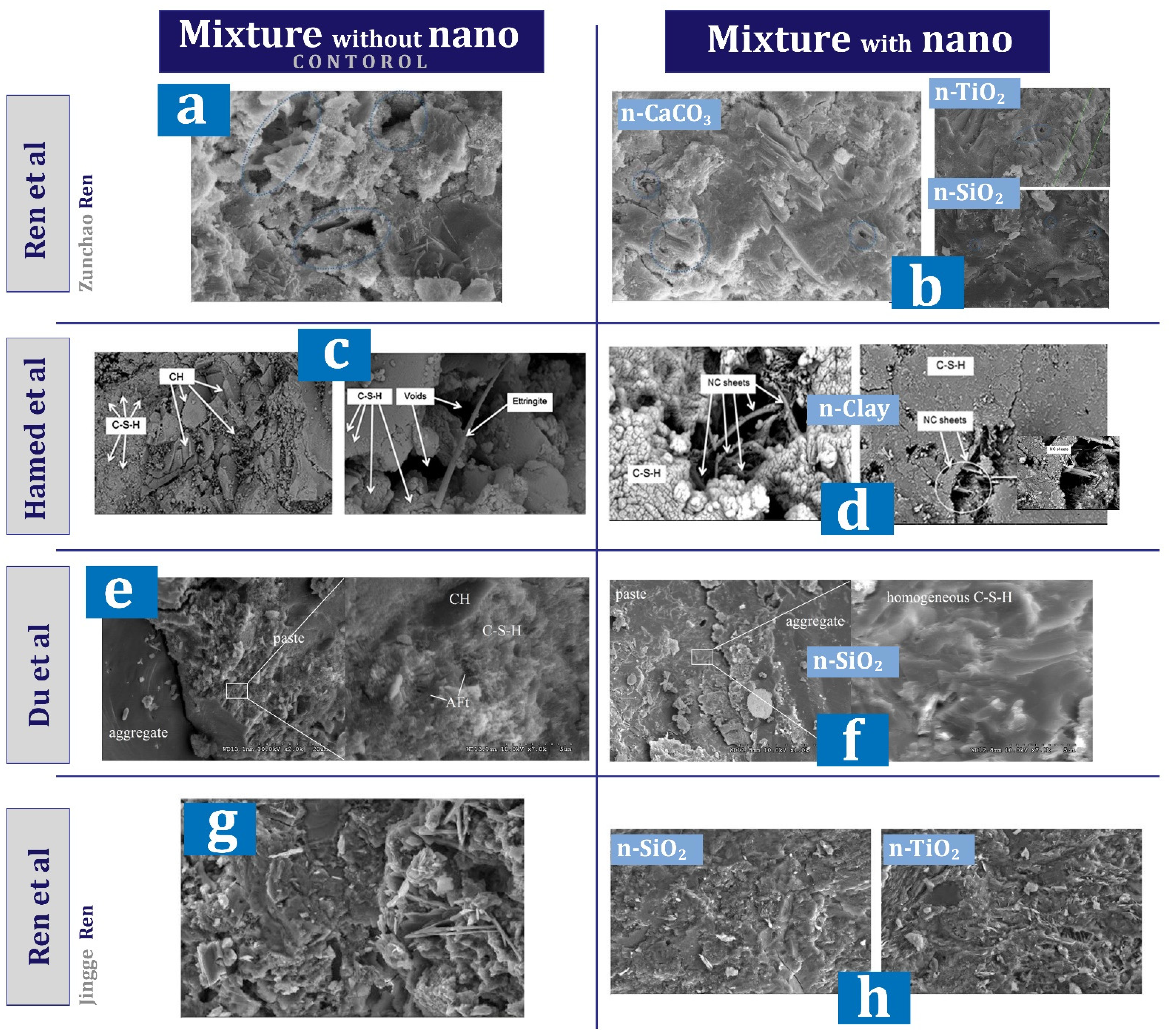
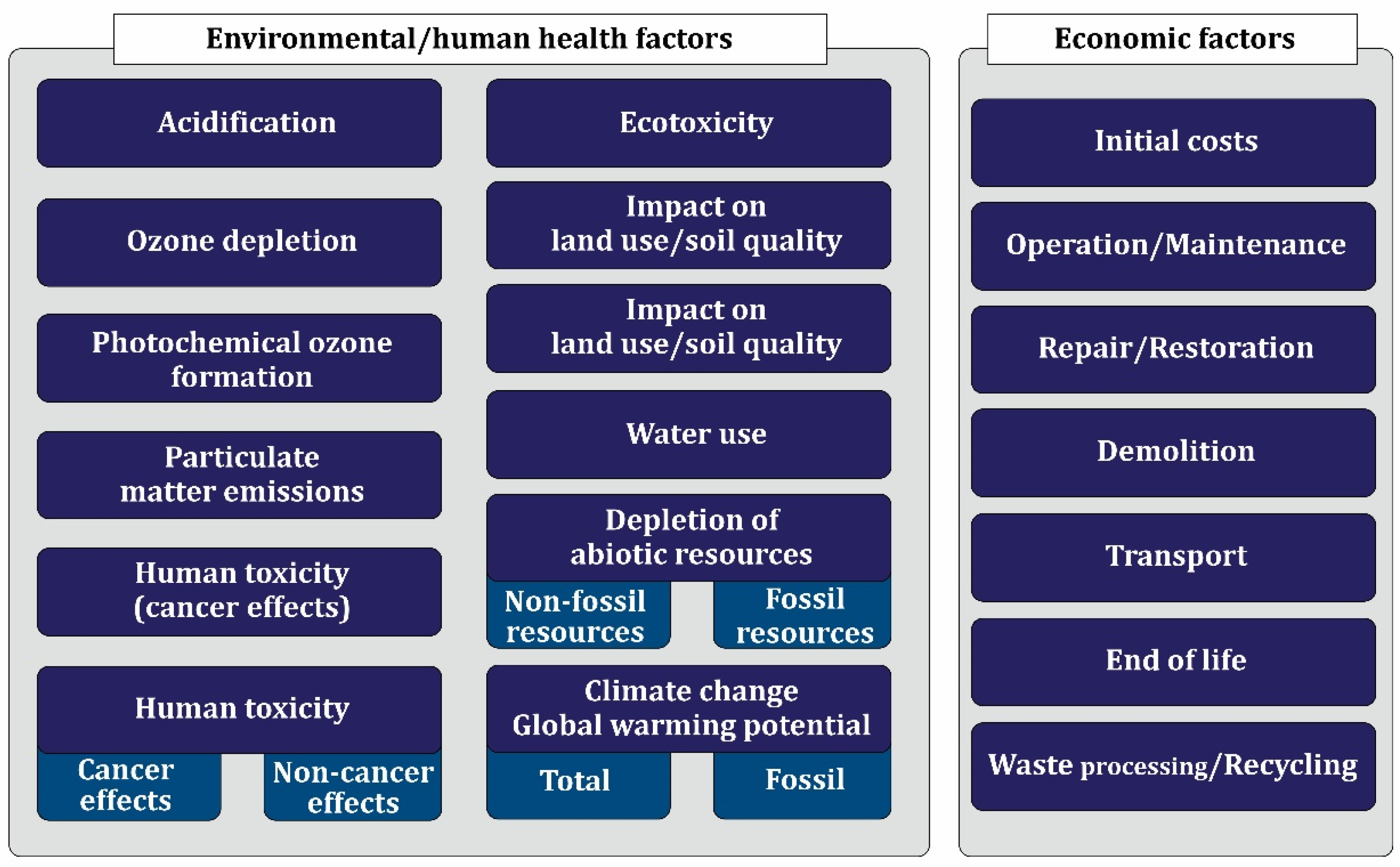

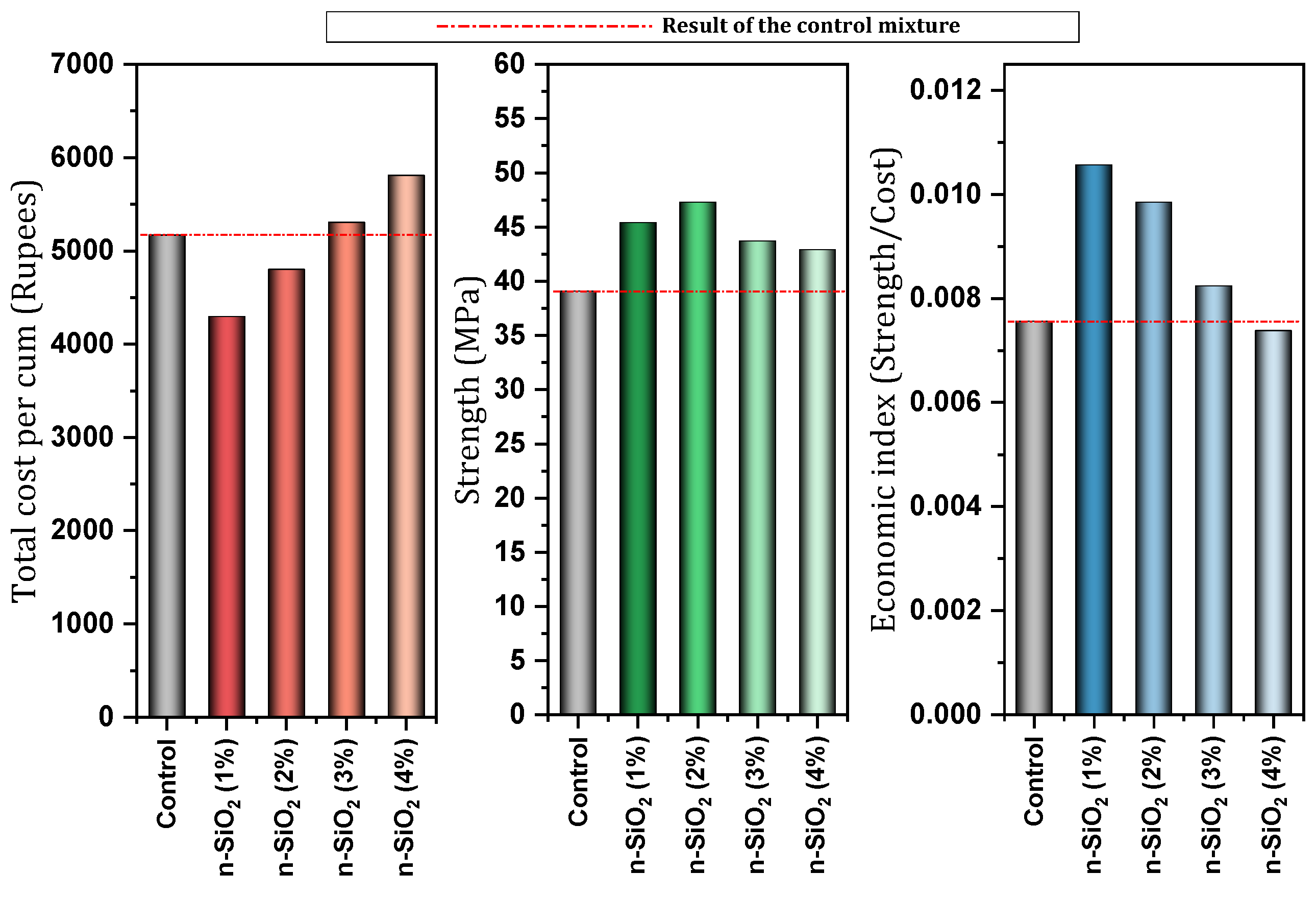

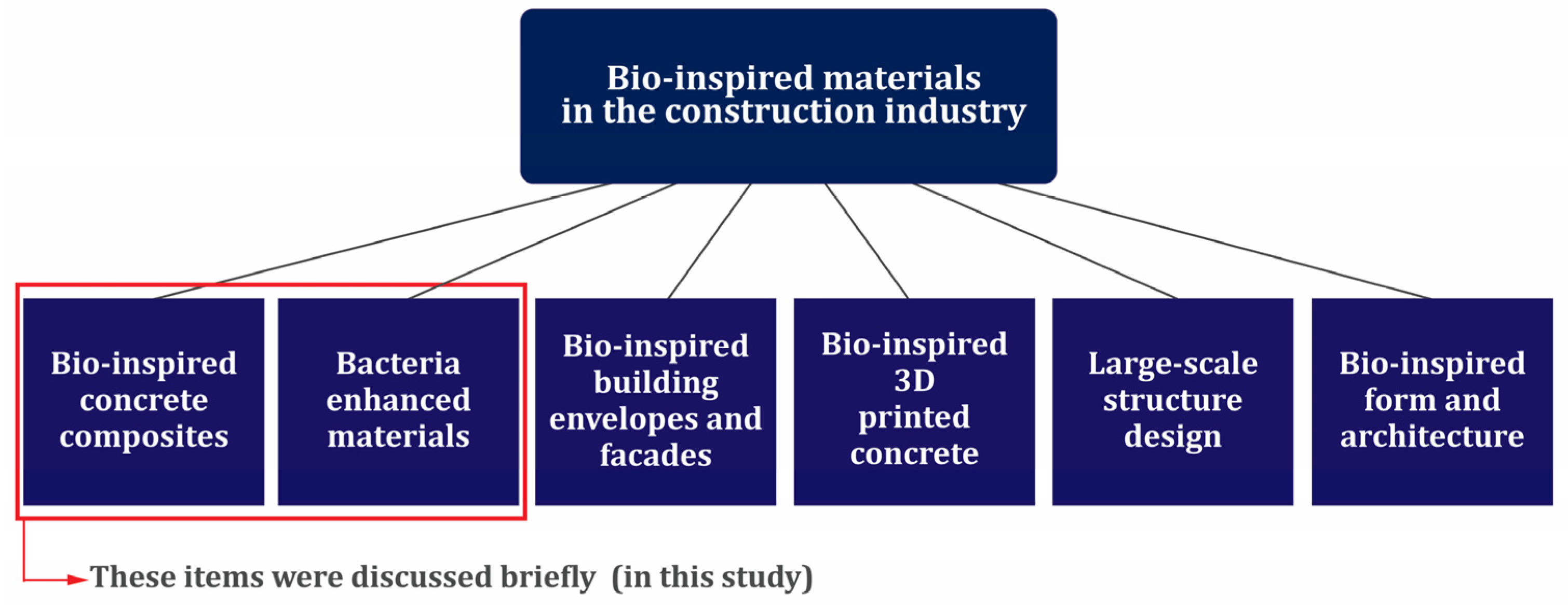

| N.O. | Characteristic | Recommended Dosages and Effects | Refs. |
|---|---|---|---|
| 1 | Workability | [1% and 2%] = Slump reduction by 3.5% and 14.28% | [82] |
| 2 | Compressive strength | [1%] (early age) = 146–148% ↑ [1%] (90d) = 40% ↑ | [82] |
| [1%] (300d) ↑ | [90] | ||
| [1%, 2%, 3% and 4%] (28d) = 3.75%, 10.73%, 14.47%, and 18.85% ↑ | [84] | ||
| [3%] (28d) = 54.9% ↑ | [86] | ||
| [3%] (90d) =59.8% ↑ | [77] | ||
| 3 | Flexural strength | [1%, 2%, 3% and 4%] (28d) = 17.55%, 24.6%, 26.86%, and 34.63% ↑ | [84] |
| 4 | Tensile strength | [1%, 2%, 3% and 4%] (28d) = 19.5%, 27.63%, 37.25%, and 35.75% ↑ | [84] |
| [3%] (28d) = 23.14% ↑ | [86] | ||
| [1%] (1–3–7–28d) ↑ | [77] | ||
| 5 | Impact strength | [2%] ↑ | [85] |
| 6 | Water absorption | [1%] (28d) = 17% ↓ [1%] (90d) = 30% ↓ | [82] |
| 7 | Chloride penetration | [1%] (28d) = 20% ↓ [1%] (90d) = 50% ↓ | [82] |
| [3%] (300d) = 70.08% improvement in resistance ↑ | [90] | ||
| 8 | Carbonation | [3%] (300d) = Normal concrete → 63% improvement in resistance Autoclave concrete → 66.8% improvement in resistance | [90] |
| 9 | Acid attack | Examining exclusively nano-CaCO3 in concrete = Research gap | --- |
| [1%] Nano-CaCO3 + [20%] Slag = prevented about 4.2% mass loss | [89] | ||
| 10 | Sulfate attack | Examining exclusively nano-CaCO3 in concrete = Research gap | --- |
| [1%] Nano-CaCO3 + [45 kg/m3] Fly ash = Improve resistance | [88] | ||
| 11 | Freeze and thaw | [5%] (300 cycles) = Improve resistance Reduction of about 83.72% compressive strength loss Reduction of about 26.69% decrease of length Reduction of about 17.17% mass loss Reduction of about 85.99% water absorption | [86] |
| 12 | Electrical resistivity | [3%] (28d) = 48.14% ↑ [3%] (90d) = 38.78% ↑ | [86] |
| 13 | Elevated temperature | Examining exclusively nano-CaCO3 in concrete = Research gap | --- |
| [1%] (800 °C): 76% prevention of mass loss | [87] | ||
| 14 | Shrinkage | [1%, 2%, and 3%] (28d) = 33.95%, 19.45%, and 10.5% ↓ | [77] |
| 15 | Microstructure | Effectively reduces capillary porosities and refines pores | [82,90] |
| N.O. | Characteristic | Recommended Dosages and Effects | Refs. |
|---|---|---|---|
| 1 | Workability | [1%, 2%, 3%, 4% and 5%] = Flow ability reduction by 10%, 13.10%, 16.15%, 19.1%, and 23.52% | [103] |
| [1%, 3%, 5%, 7%, 9%, and 10%] = Slump reduction by 6.75%, 8.98%, 10.67%, 12.92%, 14.60%, and 15.73% | [249] | ||
| 2 | Compressive strength | [0.3% and 0.5%] (7–90d) ↑ | [91] |
| [7.5%] (28d) = 24.52%, 55.47% ↑ | [95] | ||
| [1%] (28d) = 35% ↑ | [109] | ||
| 3 | Flexural strength | [7.5%] (28d) = 14.97%, 34.97% ↑ | [95] |
| [1%] (28d) = 31% ↑ | [109] | ||
| 4 | Tensile strength | [7.5%] (28d) = 9.76%, 28.05% ↑ | [95] |
| [1%] (14d) = 34% ↑ | [109] | ||
| 5 | Impact strength | Examining exclusively nano-clay in concrete = Research gap | --- |
| 6 | Water absorption | [1%] (28d) = 54% ↓ | [109] |
| 7 | Chloride penetration | Examining exclusively nano-clay in concrete = Research gap | --- |
| Mortar/[1%, 3%, 5%, 7% and 9%] (28d) = Improve resistance: 27%, 29%, 53%, 31%, and 23% ↑ | [103] | ||
| 8 | Carbonation | Examining exclusively nano-clay in concrete = Research gap | --- |
| 9 | Acid attack | Mortar/[3%] = reduction of about 17% compressive strength loss (after 60d) | [104] |
| [3%] = Improvement against nitric and sulfuric acid attack (pH = 1) Nitric acid attack (180, 270, and 360d): Reduction of about 0.6%, 1%, and 1.6% mass loss Sulfuric acid attack (60, 120, and 1500d): Reduction of about 2.1%, 2.6%, and 4.1% mass loss | [108] | ||
| 10 | Sulfate attack | [9%] = reduction of about 41.4% compressive strength loss (after 360d) | [108] |
| 11 | Freeze and thaw | [3% and 5%] (125 cycles) = good condition, 34% more resistance than control mix | [101,102] |
| 12 | Electrical resistivity | [1%] (7d, 28d and 56d) = 31%, 29% and 38.5% ↑ | [107] |
| 13 | Elevated temperature | [0.3% and 0.5%] (up to 300 °C): Improve resistance and thermal conductivity coefficient | [91] |
| 14 | Shrinkage | Examining exclusively nano-clay in concrete = Research gap | --- |
| Mortar/[1.5% and 3%]: 43% and 40% ↓ Significant reduction (1) number of cracks, (2) crack width, (3) crack length, (4) average cracking area, (5) unit cracking area of each crack | [106,107] | ||
| 15 | Microstructure | Pozzolanic reactivity/filling effect/nucleation effect/needle effect | [95,107] |
| Mortar/ increasing C-S-H and decreasing CH | [104] |
| N.O. | Characteristic | Recommended Dosages and Effects | Refs. |
|---|---|---|---|
| 1 | Workability | [0.5%, 1%, 1.5%, 2%, 2.5% and 3%] = Slump reduction by 16.34%, 21.40%, 31.5%, 42.7%, 50.6%, and 66.6% | [130] |
| 2 | Compressive strength | [3%] (28d) = 9% ↑ | [122] |
| [10%] (28d) = 17.3% ↑ | [123] | ||
| [1%] (28d) = 18.04% ↑ | [128] | ||
| [2%] (7, 28, 120d) = 12%, 22.71% and 27% ↑ | [129] | ||
| [1.5%] (7, 28, 56, 90d) = 18.67%, 6.45%, 10.5% and 7.88% ↑ | [130] | ||
| 3 | Flexural strength | [1%] (28d) = 10.28% ↑ | [128] |
| [1.5%] (7, 28, 56, 90d) = 7.86%, 10.47%, 7.55% and 5.22% ↑ | [130] | ||
| 4 | Tensile strength | [1.5%] (7, 28, 56, 90d) = 19.65%, 16.46%, 13.91% and 15.25% ↑ | [130] |
| 5 | Impact strength | [6%] = 35% ↑ | [119] |
| 6 | Water absorption | [2%] (28d) = 22% ↓ | [129] |
| [1.5%, 2%, 2.5%, and 3%] (28d) = 6.45%, 7.45%, 10.3%, and 12.09% ↓ | [130] | ||
| 7 | Chloride penetration | [0.9%] = 33% improvement resistance | [124] |
| [5%] nano-TiO2 + Fly ash = Reduction of 0.639% compressive strength loss Reduction of 0.242% mass loss | [127] | ||
| [1%] (28d) = Improved resistance by 31% ↑ | [128] | ||
| 8 | Carbonation | Examining exclusively nano-TiO2 in concrete = Research gap | --- |
| Paste/ [1%, 3% and 5%] nano-TiO2 + Fly ash = (28d) reduction of 6.40%, 16.22% and 32.49% carbonation depth ↓ (90d) reduction of 21.26%, 28.18%, and 45.74% carbonation depth ↓ (180d) reduction of 22.92%, 38.39%, and 57.88% carbonation depth ↓ | [132] | ||
| 9 | Acid attack | Examining exclusively nano-TiO2 in concrete = Research gap | --- |
| Mortar/ [3% and 5%] = 23.77% and 25.80% improvement in compressive strength (after 360d) | [133] | ||
| 10 | Sulfate attack | [3%] = Reduction of 3.87% compressive strength loss Reduction of 2.381% mass loss | [131] |
| [5%] nano-TiO2 + Fly ash = reduction of 1.36% compressive strength loss Reduction of 3.293% mass loss | [127] | ||
| 11 | Freeze and thaw | [6%] (25, 50, 75 cycles) = Improve resistance Minimum mass loss (1.52%, 2.66% and 3.99%) | [125] |
| [2%] (300 cycles) = Improve resistance Reduction of about 88.5% compressive strength loss Reduction of about 26.4% decrease of Length Reduction of about 78.65% mass loss Reduction of about 7.62% water absorption | [129] | ||
| [1%] nano-TiO2 + Fly ash + Steel fiber (0–190 d) = reduction of about 50% mass loss | [250] | ||
| 12 | Electrical resistivity | [3%] (180d) = 32.86 ↑ | [139] |
| Mortar/ [2%] (3d, 7d and 28d) = 33.70%, 28.50% and 42.50% ↑ [4%] (3d, 7d and 28d) = 93.87%, 77.19%, and 105.61% ↑ [6%] (3d, 7d and 28d) = 183%, 188%, and 189.2% ↑ [8%] (3d, 7d and 28d) = 211.6%, 225.6%, and 313.6% ↑ [10%] (3d, 7d and 28d) = 453.5%, 370%, and 350% ↑ | [135] | ||
| 13 | Elevated temperature | Preventing the reduction of residual compressive strength [0.5%, 1%, and 1.5%] (300 °C): 1.74%, 2.65% and 3.4% [0.5%, 1%, and 1.5%] (500 °C): 3.73%, 4.4% and 4.72% [0.5%, 1%, and 1.5%] (800 °C): 0.92%, 5.14% and 7.28% | [137] |
| Improve compressive strength [2%, 4%, and 6%] (200 °C): 13.5%, 25.4% and 41.7% [2%, 4%, and 6%] (400 °C): 19.8%, 30.99% and 48.65% [2%, 4%, and 6%] (600 °C): 21.45%, 32.80% and 52.48% | [120] | ||
| Mortar/[1%, 2%, and 3%] nano-TiO2 + Silica fume = (28–1000 °C): Improve compressive strength (28–300 °C): Reduce mass loss | [136] | ||
| 14 | Shrinkage | [3% and 5%] (90d) = 15.23% and 43.72% ↓ | [139] |
| [1%] nano-TiO2 + Fly ash + Steel fiber (0–190d) = 9.6% ↓ | [250] | ||
| Paste/ [1%, 3% and 5%] + Fly ash (0–28d) = Reduction of 11.03%, 36.03%, and 48.70% ↓ | [132] | ||
| 15 | Microstructure | Preventing pore and crack expansion | [126,127] |
| C-S-H gel formation, reducing pores, and improving microstructure | [130] |
| N.O. | Characteristic | Recommended Dosages and Effects | Refs. |
|---|---|---|---|
| 1 | Workability | [0.75%, 1.5% and 3%] = Slump reduction by 11.55%, 12.13%, and 41.35% | [150] |
| 2 | Compressive strength | [3%] (7, 28, 90 and 365d) ↑ | [149] |
| [3%] (7, 28, 90d) = 12–22% ↑ | [150] | ||
| [3%] (7 and 28d) = 26% and 31% ↑ | [151] | ||
| [10%] (3, 7 and 28d) = 11.84%, 17.24% and 24.59% ↑ | [166] | ||
| [3%] (7, 28, 120d) = 8%, 16.67% and 28% ↑ [5%] (7, 28, 120d) = 14.98%, 30.13% and 44.98% ↑ | [160] | ||
| 3 | Flexural strength | [3%] (28d) = 14.80% ↑ | [150] |
| [3%] (7 and 28d) = 18% and 25% ↑ | [151] | ||
| 4 | Tensile strength | [3%] (28d) ↑ | [149] |
| [3%] (28d) = 24% ↑ | [150] | ||
| 5 | Impact strength | [10%, 20%, 30%, and 40%] = Improve resistance | [251] |
| 6 | Water absorption | [3%] (28d) = 3.21% ↓ | [149] |
| [3%] = 64.5% ↓ | [151] | ||
| 7 | Chloride penetration | [0.9%] (28d) = 29% ↓ | [153] |
| 8 | Carbonation | [1%] = 23.03% ↓ | [252] |
| [2%] nano-SiO2 + [5%] marble dust = 20% ↓ | [154] | ||
| 9 | Acid attack | [2%] nano-SiO2 + [5%] Marble dust = minimal change in weight (6%) and compressive strength (22%) | [154] |
| Compressive: [6%] (90d) = 12.11% (pH = 2.5), 15.80% (pH = 4), 11.48% (pH = 5.5), and 12.46% (pH = 7) ↑ [6%] (56d) = 12.18% (pH = 2.5), 12.19% (pH = 4), 9.65% (pH = 5.5), and 5.90% (pH = 7) ↑ | [159] | ||
| Mass loss: [6%] (90d) = 18.32% (pH = 2.5), 9.40% (pH = 4), 28.97% (pH = 5.5), and 27.55% (pH = 7) ↓ [6%] (56d) = 21.05% (pH = 2.5), 23.76% (pH = 4), 55.1% (pH = 5.5), and 49.27% (pH = 7) ↓ | |||
| 10 | Sulfate attack | [8%] = reduction of about 2.51% mass loss (after 180d) | [155] |
| 11 | Freeze and thaw | [5%] (300 cycles) = Improve resistance Reduction of about 83.72% compressive strength loss Reduction of about 26.69% decrease of length Reduction of about 17.17% mass loss Reduction of about 85.99% water absorption | [160] |
| 12 | Electrical resistivity | [6%] (90d) = 10.52% (pH = 2), 43.84% (pH = 4), 39.34% (pH = 5.5), and 34.16% (pH = 7) ↑ [6%] (56d) = 31.25% (pH = 2), 30.04% (pH = 4), 37.97% (pH = 5.5), and 41.02% (pH = 7) ↑ | [159] |
| 13 | Elevated temperature | [1.5%] (0–400 °C): reduction of 14% compressive strength loss [3%] (0–400 °C): reduction of 5% compressive strength loss [1.5%] (400–600 °C): reduction of 22% compressive strength loss [3%] (400–600 °C): reduction of 12% compressive strength loss | [163] |
| [4.5%] nano-SiO2 + Silica fume = Reduction 8.13% (400 °C), 8.25% (600 °C), and 5.42% (800 °C) compressive strength loss Reduction of 5% (400 °C), 5.02% (600 °C), and 3.72% (800 °C) tensile strength loss Reduction of 0.93% (400 °C), 0.78% (600 °C), and 6.8% (800 °C) mass strength loss | [162] | ||
| 14 | Shrinkage | [2 and 4%] (7d) = 19.33% and 35.37% ↑ | [156] |
| [2%] (0–150d) = 5% ↓ | [157] | ||
| 15 | Microstructure | Improving the condition of weak and cracked attached mortar and porous ITZ | [78,154] |
| N.O. | Nanomaterials | Min (%) | Max (%) |
|---|---|---|---|
| 1 | Nano-CaCO3 | 0.5 | 2 |
| 2 | Nano-clay | 5 | 7.5 |
| 3 | Nano-TiO2 | 1 | 4 |
| 4 | Nano-SiO2 | 1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohtasham Moein, M.; Rahmati, K.; Saradar, A.; Moon, J.; Karakouzian, M. A Critical Review Examining the Characteristics of Modified Concretes with Different Nanomaterials. Materials 2024, 17, 409. https://doi.org/10.3390/ma17020409
Mohtasham Moein M, Rahmati K, Saradar A, Moon J, Karakouzian M. A Critical Review Examining the Characteristics of Modified Concretes with Different Nanomaterials. Materials. 2024; 17(2):409. https://doi.org/10.3390/ma17020409
Chicago/Turabian StyleMohtasham Moein, Mohammad, Komeil Rahmati, Ashkan Saradar, Jaeyun Moon, and Moses Karakouzian. 2024. "A Critical Review Examining the Characteristics of Modified Concretes with Different Nanomaterials" Materials 17, no. 2: 409. https://doi.org/10.3390/ma17020409








