A Superparaelectric State in Relaxor Ferroelectric (Sr,Bi)TiO3-Bi(Mg,Ti)O3-Modified BaTiO3 Ceramics to Achieve High Energy Storage Performance
Abstract
1. Introduction
2. Materials and Methods
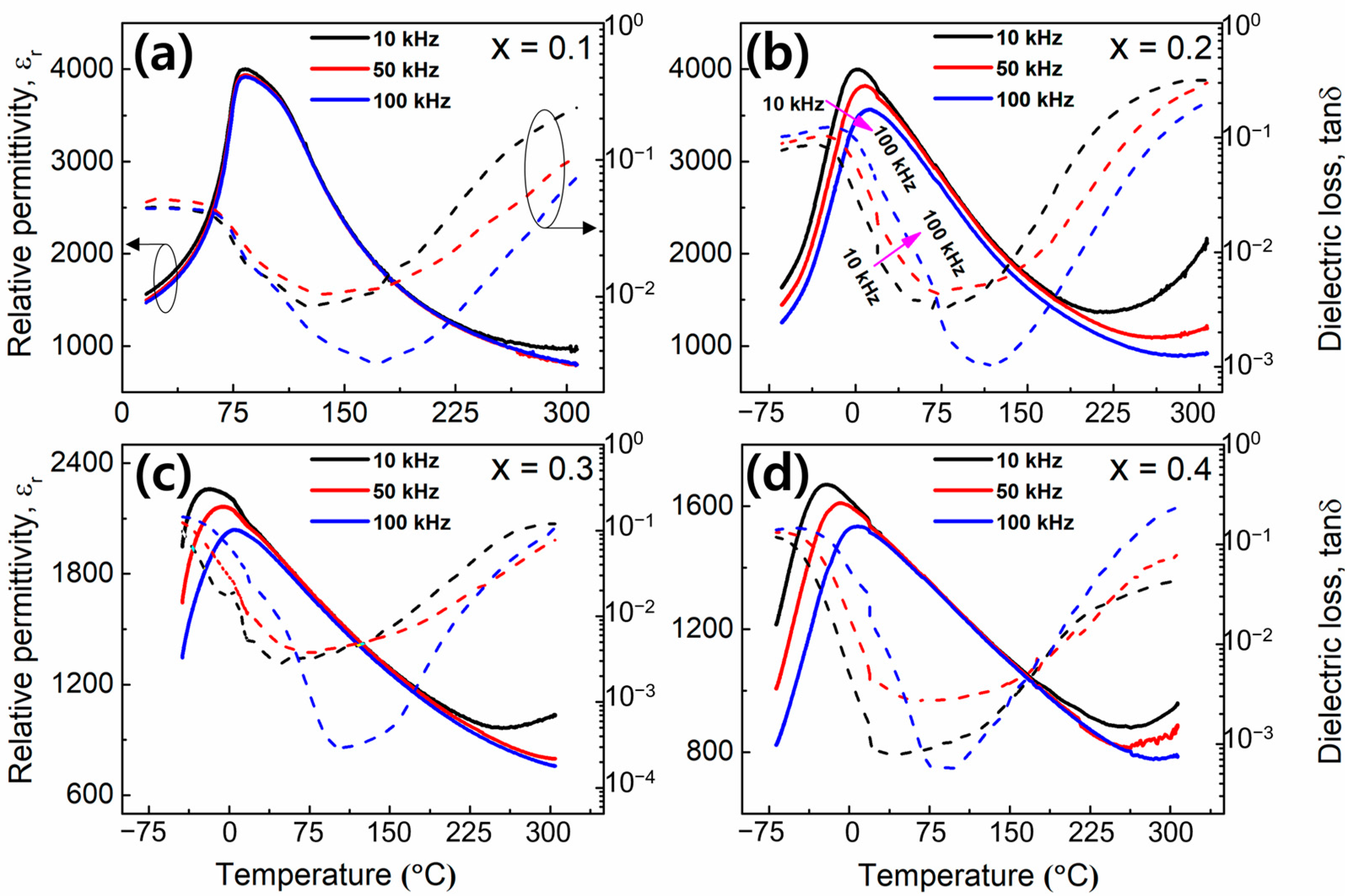
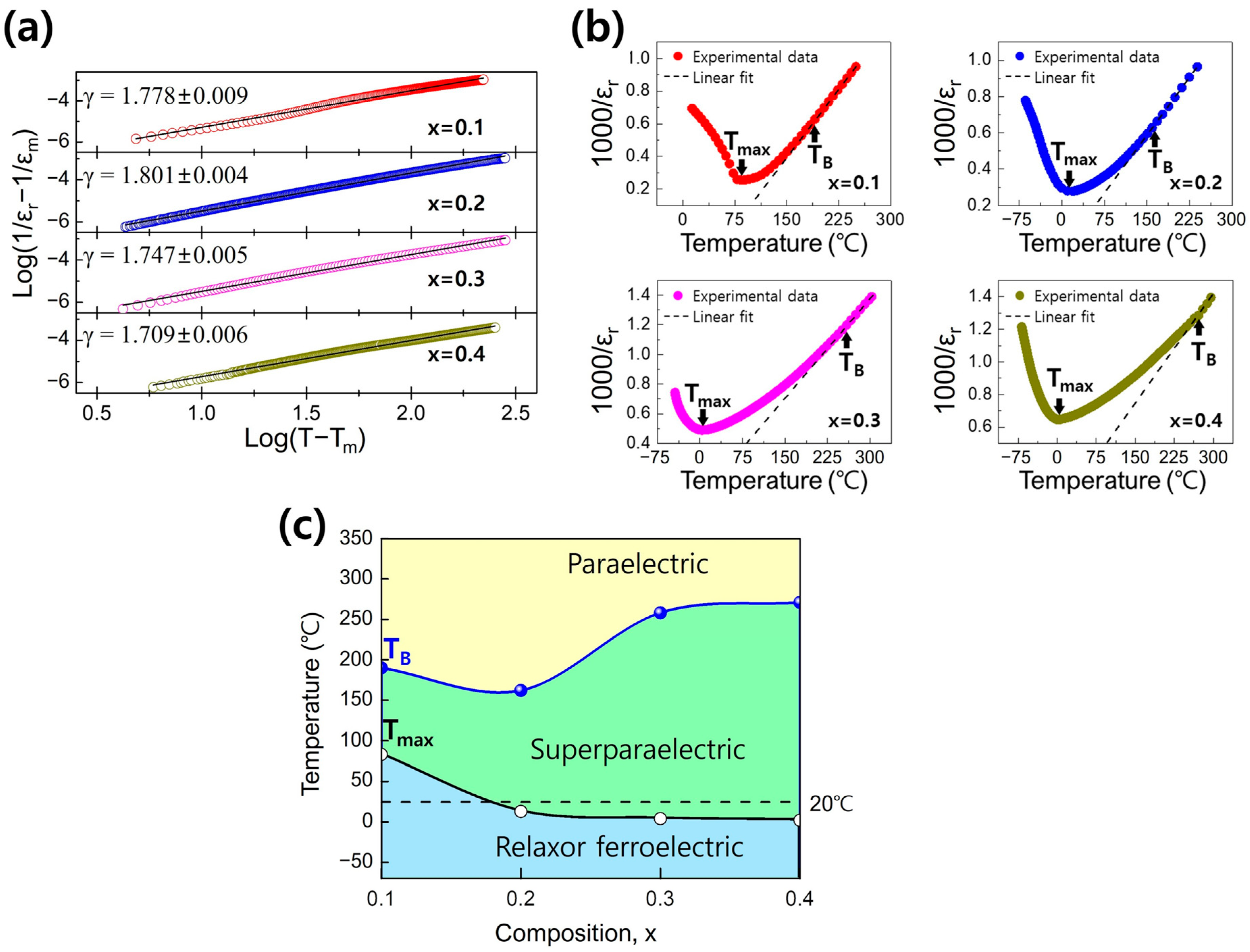
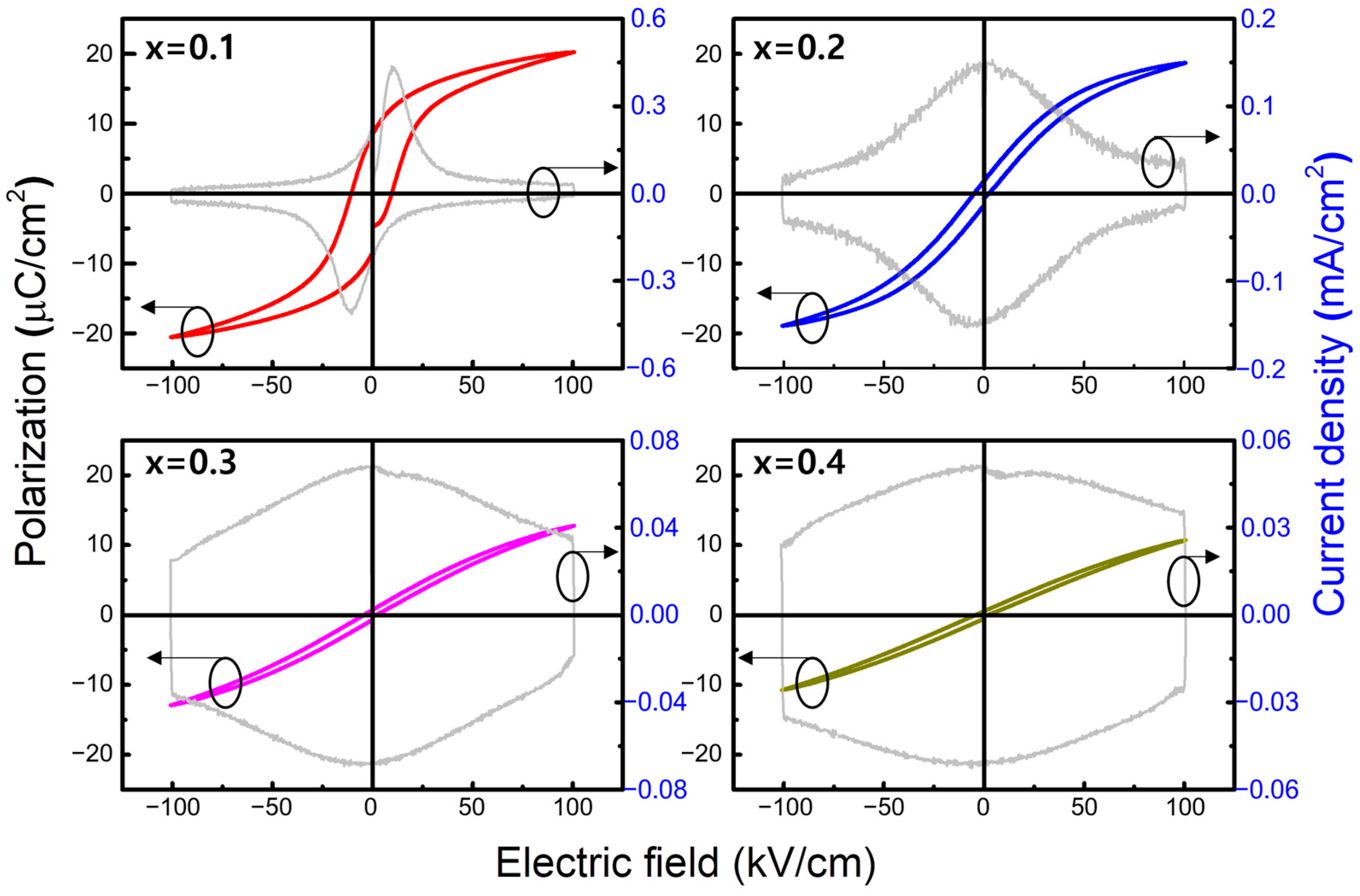
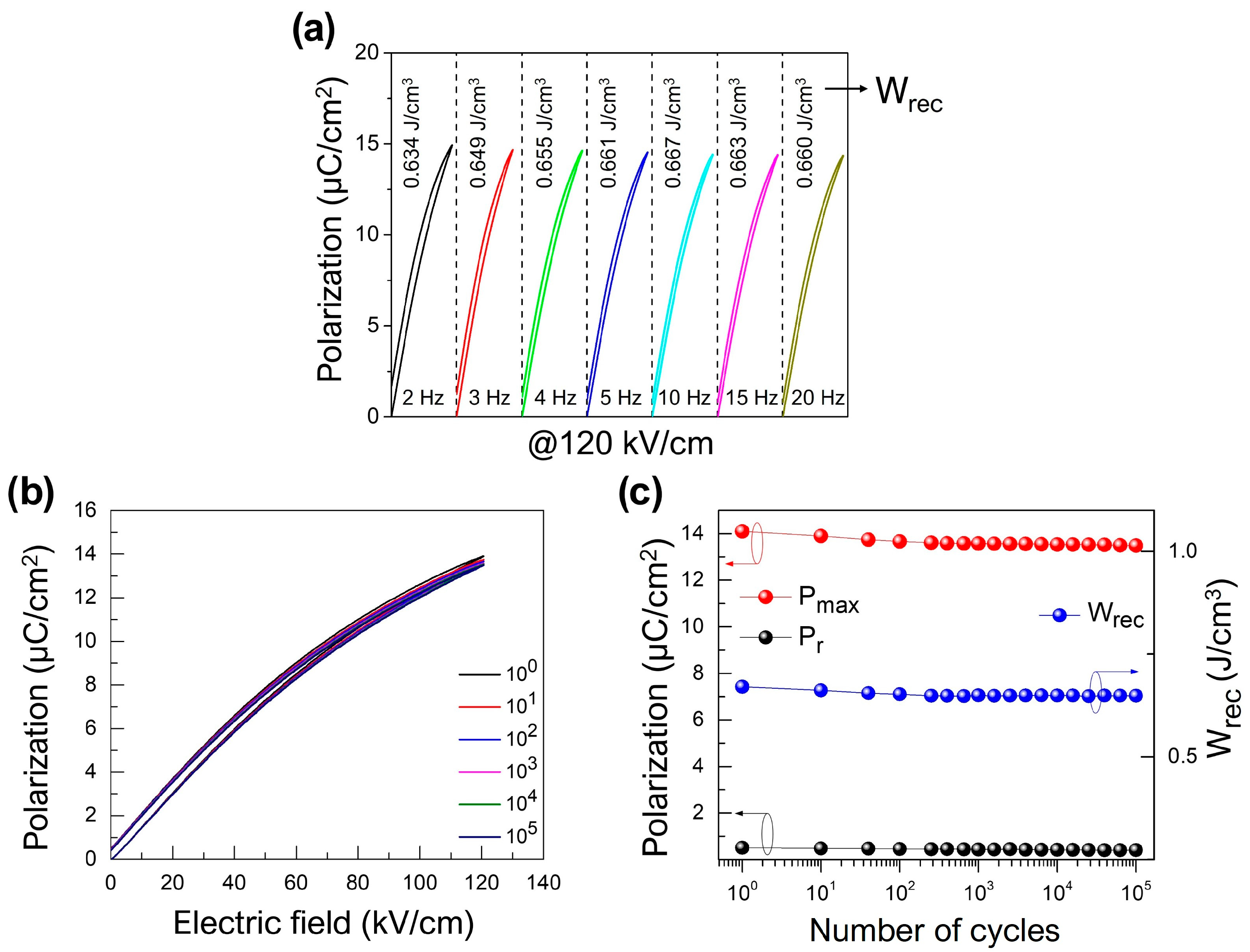
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, B.; Zhou, X.; Ren, K.; Neese, B.; Lin, M.; Wang, Q.; Bauer, F.; Zhang, Q.M. A Dielectric Polymer with High Electric Energy Density and Fast Discharge Speed. Science 2006, 313, 334. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, L.; Gadinski, M.R.; Zhang, S.; Zhang, G.; Li, H.U.; Iagodkine, E.; Haque, A.; Chen, L.-Q.; Jackson, T.N.; et al. Flexible high-temperature dielectric materials from polymer nanocomposites. Nature 2015, 523, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Palneedi, H.; Peddigari, M.; Hwang, G.-T.; Jeong, D.-Y.; Ryu, J. High-Performance Dielectric Ceramic Films for Energy Storage Capacitors: Progress and Outlook. Adv. Funct. Mater. 2018, 28, 1803665. [Google Scholar] [CrossRef]
- Pan, H.; Li, F.; Liu, Y.; Zhang, Q.; Wang, M.; Lan, S.; Zheng, Y.; Ma, J.; Gu, L.; Shen, Y.; et al. Ultrahigh–energy density lead-free dielectric films via polymorphic nanodomain design. Science 2019, 365, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Saremi, S.; Acharya, M.; Velarde, G.; Parsonnet, E.; Donahue, P.; Qualls, A.; Garcia, D.; Martin, L.W. Ultrahigh capacitive energy density in ion-bombarded relaxor ferroelectric films. Science 2020, 369, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Tian, J.; Tian, S.; Yu, F.; Ren, K. Composition design of BNBT-ST relaxor ferroelectric ceramics in superparaelectric state with ultrahigh energy density. Ceram. Int. 2023, 49 Pt A, 27750–27757. [Google Scholar] [CrossRef]
- Wang, K.; Ouyang, J.; Wuttig, M.; Zhao, Y.-Y.; Cheng, H.; Zhang, Y.; Su, R.; Yan, J.; Zhong, X.; Zeng, F. Superparaelectric (Ba0.95,Sr0.05)(Zr0.2,Ti0.8)O3 Ultracapacitors. Adv. Energy Mater. 2020, 10, 2001778. [Google Scholar] [CrossRef]
- Pan, H.; Lan, S.; Xu, S.; Zhang, Q.; Yao, H.; Liu, Y.; Meng, F.; Guo, E.-J.; Gu, L.; Yi, D.; et al. Ultrahigh energy storage in superparaelectric relaxor ferroelectrics. Science 2021, 374, 100–104. [Google Scholar] [CrossRef]
- Yang, L.; Kong, X.; Li, F.; Hao, H.; Cheng, Z.; Liu, H.; Li, J.-F.; Zhang, S. Perovskite lead-free dielectrics for energy storage applications. Prog. Mater Sci. 2019, 102, 72–108. [Google Scholar] [CrossRef]
- Cho, S.; Yun, C.; Kim, Y.S.; Wang, H.; Jian, J.; Zhang, W.; Huang, J.; Wang, X.; Wang, H.; MacManus-Driscoll, J.L. Strongly enhanced dielectric and energy storage properties in lead-free perovskite titanate thin films by alloying. Nano Energy 2018, 45, 398–406. [Google Scholar] [CrossRef]
- Ogihara, H.; Randall, C.A.; Trolier-McKinstry, S. High-Energy Density Capacitors Utilizing 0.7BaTiO3–0.3BiScO3 Ceramics. J. Am. Ceram. Soc. 2009, 92, 1719–1724. [Google Scholar] [CrossRef]
- Pan, H.; Ma, J.; Ma, J.; Zhang, Q.; Liu, X.; Guan, B.; Gu, L.; Zhang, X.; Zhang, Y.-J.; Li, L.; et al. Giant energy density and high efficiency achieved in bismuth ferrite-based film capacitors via domain engineering. Nat. Commun. 2018, 9, 1813. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhang, Q.; Li, X.; Sun, T.; Fan, H.; Ke, S.; Ye, M.; Wang, Y.; Lu, W.; Niu, H.; et al. Giant Electric Energy Density in Epitaxial Lead-Free Thin Films with Coexistence of Ferroelectrics and Antiferroelectrics. Adv. Electron. Mater. 2015, 1, 1500052. [Google Scholar] [CrossRef]
- Dong, X.; Li, X.; Chen, X.; Wu, J.; Zhou, H. Simultaneous enhancement of polarization and breakdown strength in lead-free BaTiO3-based ceramics. Chem. Eng. J. 2021, 409, 128231. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, G.; Yao, F.-Z.; Cheng, S.-D.; Wang, Y.; Ma, R.; Mi, S.-B.; Gu, M.; Wang, K.; Li, J.-F.; et al. Simultaneously achieved temperature-insensitive high energy density and efficiency in domain engineered BaTiO3-Bi(Mg0.5Zr0.5)O3 lead-free relaxor ferroelectrics. Nano Energy 2018, 52, 203–210. [Google Scholar] [CrossRef]
- Yang, Z.; Du, H.; Jin, L.; Hu, Q.; Wang, H.; Li, Y.; Wang, J.; Gao, F.; Qu, S. Realizing high comprehensive energy storage performance in lead-free bulk ceramics via designing an unmatched temperature range. J. Mater. Chem. A 2019, 7, 27256–27266. [Google Scholar] [CrossRef]
- Bell, A.J. Calculations of dielectric properties from the superparaelectric model of relaxors. J. Phys. Condens. Matter 1993, 5, 8773. [Google Scholar] [CrossRef]
- Li, F.; Zhang, S.; Damjanovic, D.; Chen, L.-Q.; Shrout, T.R. Ferroelectrics: Local Structural Heterogeneity and Electromechanical Responses of Ferroelectrics: Learning from Relaxor Ferroelectrics (Adv. Funct. Mater. 37/2018). Adv. Funct. Mater. 2018, 28, 1870262. [Google Scholar] [CrossRef]
- Bokov, A.A.; Ye, Z.-G. Dielectric Relaxation in Relaxor Ferroelectrics. J. Adv. Dielectr. 2012, 02, 1241010. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Cao, M.; Hao, H.; Liu, H. Energy Storage Characteristics in Sr(1-1.5x)BixTiO3 Ceramics. Ferroelectrics 2013, 447, 86–94. [Google Scholar] [CrossRef]
- Li, L.; Fan, P.; Wang, M.; Takesue, N.; Salamon, D.; Vtyurin, A.N.; Zhang, Y.; Tan, H.; Nan, B.; Lu, Y.; et al. Review of lead-free Bi-based dielectric ceramics for energy-storage applications. J. Phys. D Appl. Phys. 2021, 54, 293001. [Google Scholar] [CrossRef]
- Zuo, C.; Yang, S.; Cao, Z.; Yu, H.; Wei, X. Excellent energy storage and hardness performance of Sr0.7Bi0.2TiO3 ceramics fabricated by solution combustion-synthesized nanopowders. Chem. Eng. J. 2022, 442, 136330. [Google Scholar] [CrossRef]
- Ang, C.; Yu, Z.; Vilarinho, P.M.; Baptista, J.L. Bi:SrTiO3: A quantum ferroelectric and a relaxor. Phys. Rev. B 1998, 57, 7403–7406. [Google Scholar] [CrossRef]
- Okhay, O.; Wu, A.; Vilarinho, P.M.; Tkach, A. Dielectric relaxation of Sr1–1.5xBixTiO3 sol-gel thin films. J. Appl. Phys. 2011, 109, 064103. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, P.; Kang, R.; Li, S.; Wang, Z.; Qiao, W.; Zhang, X.; He, L.; Liu, Q.; Lou, X. Enhanced energy storage density of Sr0.7BixTiO3 lead-free relaxor ceramics via A-site defect and grain size tuning. Chem. Eng. J. 2021, 420, 129808. [Google Scholar] [CrossRef]
- Zhao, P.; Tang, B.; Si, F.; Yang, C.; Li, H.; Zhang, S. Novel Ca doped Sr0.7Bi0.2TiO3 lead-free relaxor ferroelectrics with high energy density and efficiency. J. Eur. Ceram. Soc. 2020, 40, 1938–1946. [Google Scholar] [CrossRef]
- Chao, M.; Liu, J.; Zeng, M.; Wang, D.; Yu, H.; Yuan, Y.; Zhang, S. High discharge efficiency of (Sr, Pb, Bi) TiO3 relaxor ceramics for energy-storage application. Appl. Phys. Lett. 2018, 112, 203903. [Google Scholar] [CrossRef]
- Kong, X.; Yang, L.; Cheng, Z.; Zhang, S. Bi-modified SrTiO3-based ceramics for high-temperature energy storage applications. J. Am. Ceram. Soc. 2020, 103, 1722–1731. [Google Scholar] [CrossRef]
- Yu, Z.; Ang, C.; Guo, R.; Bhalla, A.S. Dielectric properties and tunability of (Sr,Bi)TiO3 with MgO additive. Mater. Lett. 2003, 57, 2927–2931. [Google Scholar] [CrossRef]
- Xiong, B.; Hao, H.; Zhang, S.; Liu, H.; Cao, M. Structure, Dielectric Properties and Temperature Stability of BaTiO3–Bi(Mg1/2Ti1/2)O3 Perovskite Solid Solutions. J. Am. Ceram. Soc. 2011, 94, 3412–3417. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, H.; Han, W.; Wang, Z.; Li, F.; Li, J.; Xue, W. Facile preparation and dielectric properties of BaTiO3 with different particle sizes and morphologies. RSC Adv. 2023, 13, 11002–11009. [Google Scholar] [CrossRef]
- Yu, P.; Liu, W.; Gao, P.; Shao, T.; Zhao, S.; Han, Z.; Gu, X.; Zhang, J.; Wang, Y. Investigation on synthesis of tetragonal BaTiO3 nanopowders by a new wet chemical method. J. Mater. Sci. Mater. Electron. 2022, 33, 10828–10840. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Tkach, A.; Okhay, O. Comment on “Hole-pinned defect-dipoles induced colossal permittivity in Bi doped SrTiO3 ceramics with Sr deficiency”. J. Mater. Sci. Technol. 2021, 65, 151–153. [Google Scholar] [CrossRef]
- DiDomenico, M.; Wemple, S.H.; Porto, S.P.S.; Bauman, R.P. Raman Spectrum of Single-Domain BaTiO3. Phys. Rev. 1968, 174, 522–530. [Google Scholar] [CrossRef]
- Pasuk, I.; Neațu, F.; Neațu, Ș.; Florea, M.; Istrate, C.M.; Pintilie, I.; Pintilie, L. Structural Details of BaTiO3 Nano-Powders Deduced from the Anisotropic XRD Peak Broadening. Nanomaterials 2021, 11, 1121. [Google Scholar] [CrossRef]
- Yadav, A.K.; Fan, H.; Yan, B.; Wang, W.; Dong, W.; Wang, S. Structure evolutions with enhanced dielectric permittivity and ferroelectric properties of Ba(1−x)(La, Li)xTiO3 ceramics. J. Mater. Sci. Mater. Electron. 2021, 32, 23103–23115. [Google Scholar] [CrossRef]
- Shi, C.; Billinge, S.J.L.; Puma, E.; Bang, S.H.; Bean, N.J.H.; de Sugny, J.-C.; Gambee, R.G.; Haskell, R.C.; Hightower, A.; Monson, T.C. Barium titanate nanoparticles: Short-range lattice distortions with long-range cubic order. Phys. Rev. B 2018, 98, 085421. [Google Scholar] [CrossRef]
- Veerapandiyan, V.K.; Khosravi, H.S.; Canu, G.; Feteira, A.; Buscaglia, V.; Reichmann, K.; Deluca, M. B-site vacancy induced Raman scattering in BaTiO3-based ferroelectric ceramics. J. Eur. Ceram. Soc. 2020, 40, 4684–4688. [Google Scholar] [CrossRef]
- Pokorný, J.; Pasha, U.M.; Ben, L.; Thakur, O.P.; Sinclair, D.C.; Reaney, I.M. Use of Raman spectroscopy to determine the site occupancy of dopants in BaTiO3. J. Appl. Phys. 2011, 109, 114110. [Google Scholar] [CrossRef]
- Parsons, J.L.; Rimai, L. Raman spectrum of BaTiO3. Solid State Commun. 1967, 5, 423–427. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, Y.; Liu, N.; Peng, P.; Zhou, M.; Yan, S.; Cao, F.; Dong, X.; Wang, G. Enhanced energy storage properties in sodium bismuth titanate-based ceramics for dielectric capacitor applications. J. Mater. Chem. C 2019, 7, 6222–6230. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, H.; Yang, Y.; Zhou, E.; Zhang, S.; Wei, P.; Cao, M.; Yao, Z.; Liu, H. Structure and enhanced dielectric temperature stability of BaTiO3-based ceramics by Ca ion B site-doping. J. Mater. 2021, 7, 295–301. [Google Scholar] [CrossRef]
- Yao, G.; Wang, X.; Yang, Y.; Li, L. Effects of Bi2O3 and Yb2O3 on the Curie Temperature in BaTiO3-Based Ceramics. J. Am. Ceram. Soc. 2010, 93, 1697–1701. [Google Scholar] [CrossRef]
- Liu, J.; Ma, C.; Zhao, X.; Ren, K.; Zhang, R.; Shang, F.; Du, H.; Wang, Y. Structure, dielectric, and relaxor properties of BaTiO3-modified high-entropy (Bi0.2Na0.2K0.2Ba0.2Ca0.2)TiO3 ceramics for energy storage applications. J. Alloys Compd. 2023, 947, 169626. [Google Scholar] [CrossRef]
- Hu, Q.; Tian, Y.; Zhu, Q.; Bian, J.; Jin, L.; Du, H.; Alikin, D.O.; Shur, V.Y.; Feng, Y.; Xu, Z.; et al. Achieve ultrahigh energy storage performance in BaTiO3–Bi(Mg1/2Ti1/2)O3 relaxor ferroelectric ceramics via nano-scale polarization mismatch and reconstruction. Nano Energy 2020, 67, 104264. [Google Scholar] [CrossRef]
- Zuo, J.; Yang, H.; Chen, J.; Li, C. Highly-reliable dielectric capacitors with excellent comprehensive energy-storage properties using Bi0.5Na0.5TiO3-based relaxor ferroelectric ceramics. J. Eur. Ceram. Soc. 2023, 43, 2452–2459. [Google Scholar] [CrossRef]
- Hu, D.; Pan, Z.; Zhang, X.; Ye, H.; He, Z.; Wang, M.; Xing, S.; Zhai, J.; Fu, Q.; Liu, J. Greatly enhanced discharge energy density and efficiency of novel relaxation ferroelectric BNT–BKT-based ceramics. J. Mater. Chem. C 2020, 8, 591–601. [Google Scholar] [CrossRef]
- Huang, N.; Liu, H.; Hao, H.; Yao, Z.; Cao, M.; Xie, J. Energy storage properties of MgO-doped 0.5Bi0·5Na0·5TiO3-0.5SrTiO3 ceramics. Ceram. Int. 2019, 45, 14921–14927. [Google Scholar] [CrossRef]
- Brown, E.; Ma, C.; Acharya, J.; Ma, B.; Wu, J.; Li, J. Controlling Dielectric and Relaxor-Ferroelectric Properties for Energy Storage by Tuning Pb0.92La0.08Zr0.52Ti0.48O3 Film Thickness. ACS Appl. Mater. Interfaces 2014, 6, 22417–22422. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mahajan, A.; Riekehr, L.; Zhang, H.; Yang, B.; Meng, N.; Zhang, Z.; Yan, H. Perovskite Srx(Bi1−xNa0.97−xLi0.03)0.5TiO3 ceramics with polar nano regions for high power energy storage. Nano Energy 2018, 50, 723–732. [Google Scholar] [CrossRef]
- Petzelt, J. Dielectric Grain-Size Effect in High-Permittivity Ceramics. Ferroelectrics 2010, 400, 117–134. [Google Scholar] [CrossRef]
- Huamán, J.L.C.; Rivera, V.A.G.; Pinto, A.H.; Marega, E. 11-Multiferroic perovskite ceramics: Properties and applications. In Perovskite Ceramics; Huamán, J.L.C., Rivera, V.A.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 339–381. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, X.; Luo, B.; Li, L. BaTiO3–BiYbO3 perovskite materials for energy storage applications. J. Mater. Chem. A 2015, 3, 18146–18153. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, H.; Zhang, S.; Lv, J.; Cao, M.; Yao, Z.; Liu, H. Enhanced energy storage and fast discharge properties of BaTiO3 based ceramics modified by Bi(Mg1/2Zr1/2)O3. J. Eur. Ceram. Soc. 2019, 39, 1103–1109. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.; Li, L. Lead-free BaTiO3–Bi(Zn2/3Nb1/3)O3 weakly coupled relaxor ferroelectric materials for energy storage. RSC Adv. 2016, 6, 14273–14282. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Yan, F.; Qin, Y.; Lin, Y.; Wang, T. Enhanced energy storage properties of BaTiO3-Bi0.5Na0.5TiO3 lead-free ceramics modified by SrY0.5Nb0.5O3. J. Alloys Compd. 2019, 778, 97–104. [Google Scholar] [CrossRef]
- Zhao, H.; Duan, X.; Yu, T.; Xu, D.; Zhao, W. Enhanced energy storage efficiency of Ba0.8Sr0.2TiO3 ceramics modified by BiTaO3. Solid State Commun. 2023, 360, 115050. [Google Scholar] [CrossRef]
- Borkar, S.N.; Deshpande, V.K. Effect of Mg substitution on microstructural, dielectric, ferroelectric and energy storage properties of BaTiO3. Phys. B Condens. Matter. 2022, 644, 414244. [Google Scholar] [CrossRef]
- Dong, X.; Chen, H.; Wei, M.; Wu, K.; Zhang, J. Structure, dielectric and energy storage properties of BaTiO3 ceramics doped with YNbO4. J. Alloys Compd. 2018, 744, 721–727. [Google Scholar] [CrossRef]
- Yi, X.; Ji, C.; Chen, G.; Yang, H.; Yong, H.; Fu, C.; Cai, W.; Gao, R.; Fan, T.; Wang, Z.; et al. Effects of Sintering Method and BiAlO3 Dopant on Dielectric Relaxation and Energy Storage Properties of BaTiO3–BiYbO3 Ceramics. Phys. Status Solidi A 2020, 217, 1900721. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Bai, H.-R.; Li, P.; Li, Y.-C.; Han, W.-F.; Hao, J.-G.; Li, W.; Wang, C.-M.; Fu, P. Preeminent energy storage properties and superior stability of (Ba(1–x)Bix)(Ti(1–x)Mg2x/3Tax/3)O3 relaxor ferroelectric ceramics via elongated rod-shaped grains and domain structural regulation. J. Mater. Sci. Technol. 2024, 184, 207–220. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Z.; Liang, R.; Liu, F.; Dong, X. High-energy storage performance in lead-free (1-x)BaTiO3-xBi(Zn0.5Ti0.5)O3 relaxor ceramics for temperature stability applications. Ceram. Int. 2017, 43, 9060–9066. [Google Scholar] [CrossRef]



| Composition | Space Group | Lattice Parameters | Volume of Unit Cell |
|---|---|---|---|
| x = 0.1 | P4mm | a = b = 3.99555 ± 0.00003 Å c = 4.01797 ± 0.00004 Å c/a = 1.00561 ± 0.00001 | 64.145 ± 0.001 Å3 |
| x = 0.2 | a = b = c = 3.99556 ± 0.00001 Å c/a = 1 | 63.787 ± 0.001 Å3 | |
| x = 0.3 | a = b = c = 3.98836 ± 0.00001 Å c/a = 1 | 63.443 ± 0.001 Å3 | |
| x = 0.4 | a = b = c = 3.98101 ± 0.00001 Å c/a = 1 | 63.093 ± 0.001 Å3 |
| Composition | Energy Storage Density, Wrec (J/cm3) | Efficiency, η (%) | Applied Field (kV/cm) | Reference |
|---|---|---|---|---|
| 0.91BaTiO3-0.09BiYbO3 | 0.71 | 82.6 | 93 | [54] |
| 0.85BaTiO3-0.15Bi(Mg1/2Zr1/2)O3 | 1.25 | 95.4 | 185 | [55] |
| 0.85BaTiO3-0.15Bi(Zn2/3Nb1/3)O3 | 0.79 | 93.5 | 131 | [56] |
| 0.92(0.65BaTiO3-0.35Bi0.5Na0.5TiO3)-0.08SrY0.5Nb0.5O3 | 1.36 | 74.3 | 152 | [57] |
| 0.88Ba0.8Sr0.2TiO3-0.12BiTaO3 | 0.526 | 98 | 130 | [58] |
| BaTi0.95Mg0.05O3 | 1.04 | 89.65 | 350 | [59] |
| 0.93BaTiO3-0.07YNbO4 | 0.614 | 86.8 | 173 | [60] |
| 0.86BaTiO3-0.1BiYbO3-0.04BiAlO3 | 0.59 | 97.44 | 110 | [61] |
| (Ba0.9Bi0.1)(Ti0.9Mg0.2/3Ta0.1/3)O3 | 5.97 | 87.4 | 710 | [62] |
| 0.86BaTiO3-0.14Bi(Zn0.5Ti0.5)O3 | 0.81 | 94 | 120 | [63] |
| 0.7BaTiO3-0.3[0.75(Sr0.88Bi0.08)TiO3-0.25Bi(Mg0.5Ti0.5)O3] | 1.12 | 94 | 170 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, I.-R.; Choi, S.-H.; Park, J.-Y.; Kim, M.-S.; Yadav, A.K.; Cho, K.-H. A Superparaelectric State in Relaxor Ferroelectric (Sr,Bi)TiO3-Bi(Mg,Ti)O3-Modified BaTiO3 Ceramics to Achieve High Energy Storage Performance. Materials 2024, 17, 426. https://doi.org/10.3390/ma17020426
Yoo I-R, Choi S-H, Park J-Y, Kim M-S, Yadav AK, Cho K-H. A Superparaelectric State in Relaxor Ferroelectric (Sr,Bi)TiO3-Bi(Mg,Ti)O3-Modified BaTiO3 Ceramics to Achieve High Energy Storage Performance. Materials. 2024; 17(2):426. https://doi.org/10.3390/ma17020426
Chicago/Turabian StyleYoo, Il-Ryeol, Seong-Hui Choi, Je-Yeon Park, Min-Seok Kim, Arun Kumar Yadav, and Kyung-Hoon Cho. 2024. "A Superparaelectric State in Relaxor Ferroelectric (Sr,Bi)TiO3-Bi(Mg,Ti)O3-Modified BaTiO3 Ceramics to Achieve High Energy Storage Performance" Materials 17, no. 2: 426. https://doi.org/10.3390/ma17020426
APA StyleYoo, I.-R., Choi, S.-H., Park, J.-Y., Kim, M.-S., Yadav, A. K., & Cho, K.-H. (2024). A Superparaelectric State in Relaxor Ferroelectric (Sr,Bi)TiO3-Bi(Mg,Ti)O3-Modified BaTiO3 Ceramics to Achieve High Energy Storage Performance. Materials, 17(2), 426. https://doi.org/10.3390/ma17020426






