Experimental Study and Mechanism Analysis of Paraffin/Sisal Composite Phase Change Energy Storage Fiber Prepared by Vacuum Adsorption Method
Abstract
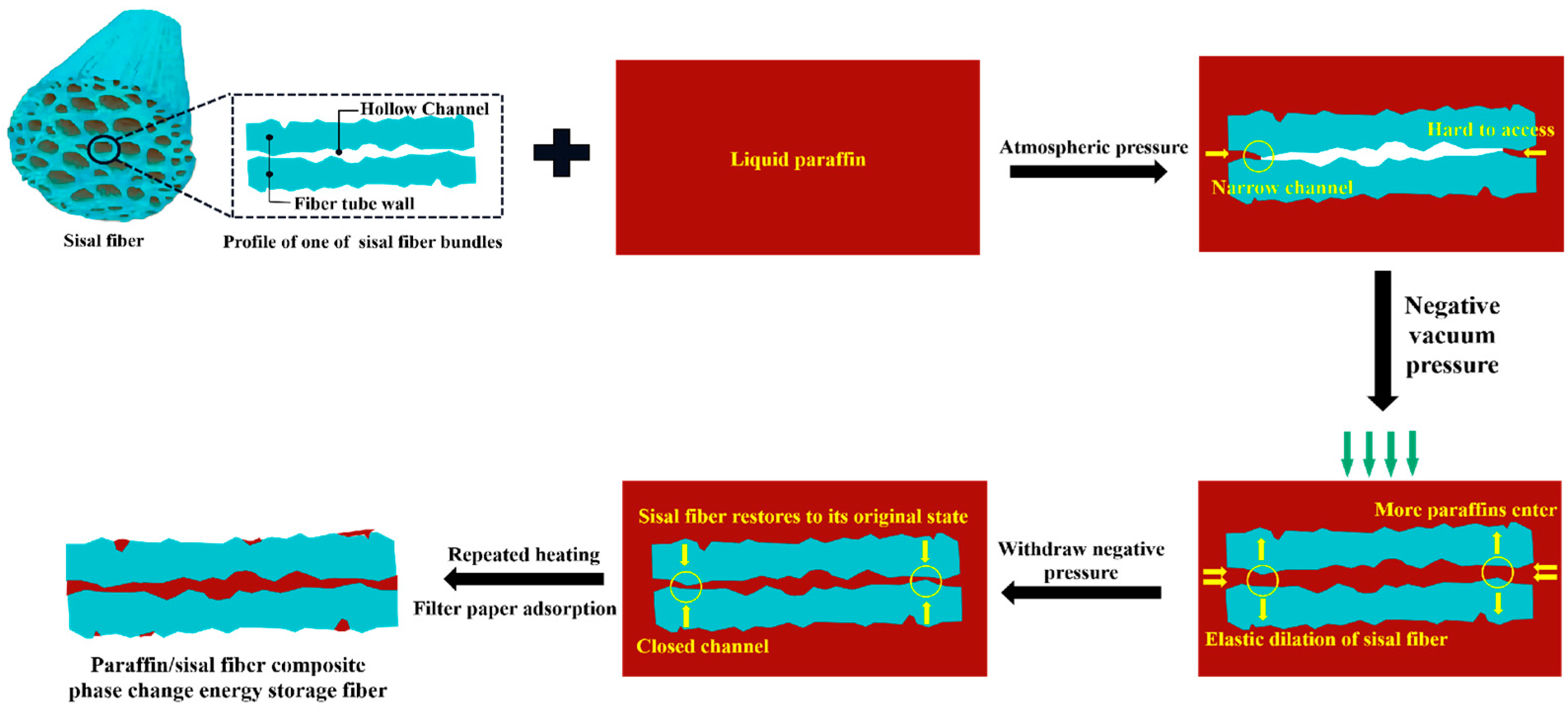
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Sisal Fiber
| Density (g/cm3) | Fiber Diameter (μm) | Tensile Strength (MPa) | Elastic Modulus (GPa) | Rupture Elongation (%) | Stiffness (KN/mm) | Hygroscopicity (%) |
|---|---|---|---|---|---|---|
| 1.47 | 150–300 | 470–720 | 17–22 | 2–5 | 30–38 | 11 |
| Cellulose (wt%) | Lignin (wt%) | Hemicellulose (wt%) | Pectin (wt%) | Wax (wt%) |
|---|---|---|---|---|
| 67–78 | 8–11 | 10–15 | 10 | 2 |

2.1.2. Paraffin
2.1.3. Nanosilicon Dioxide
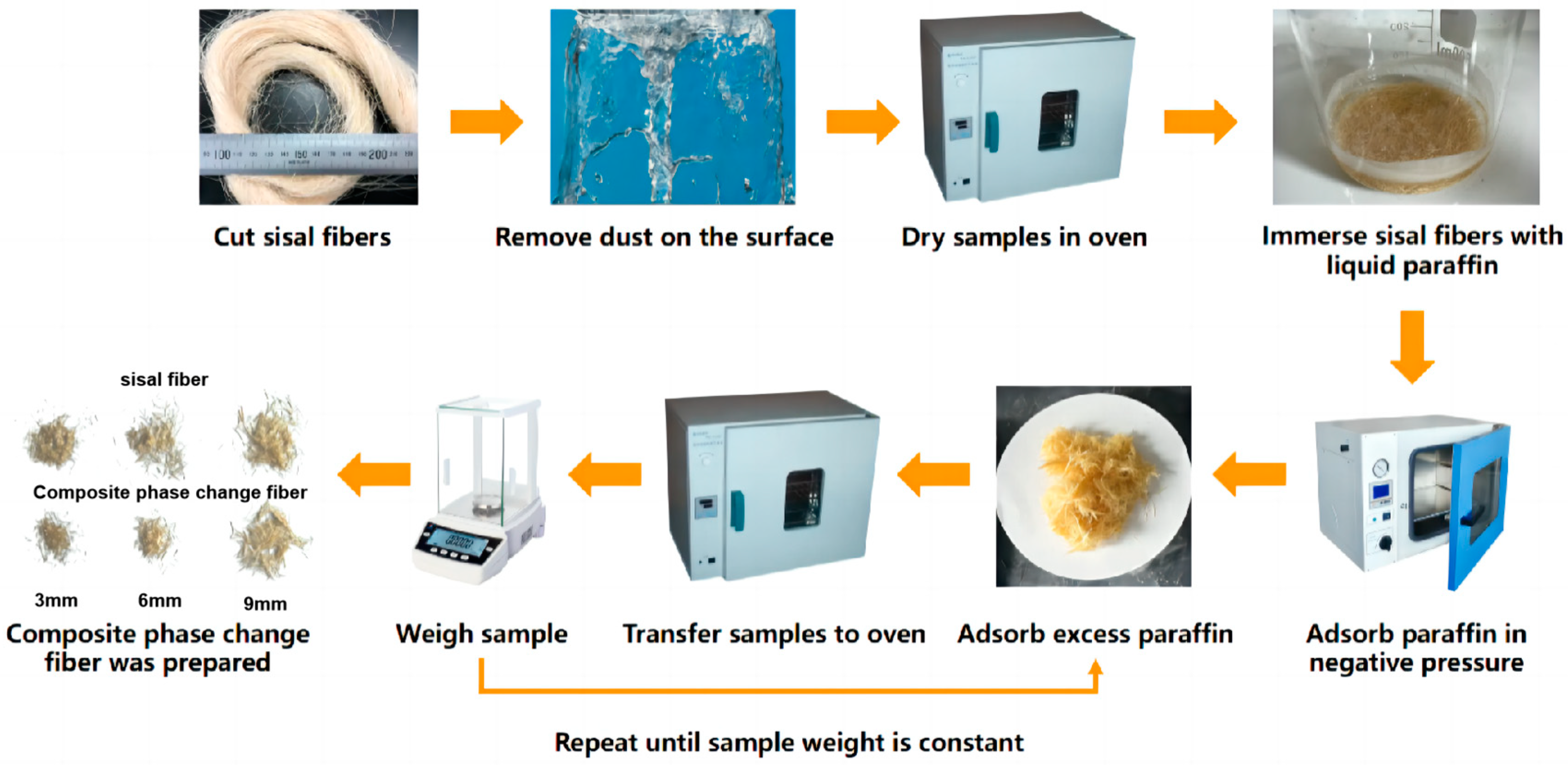
2.2. Specimen Preparation
2.3. Experimental Procedure
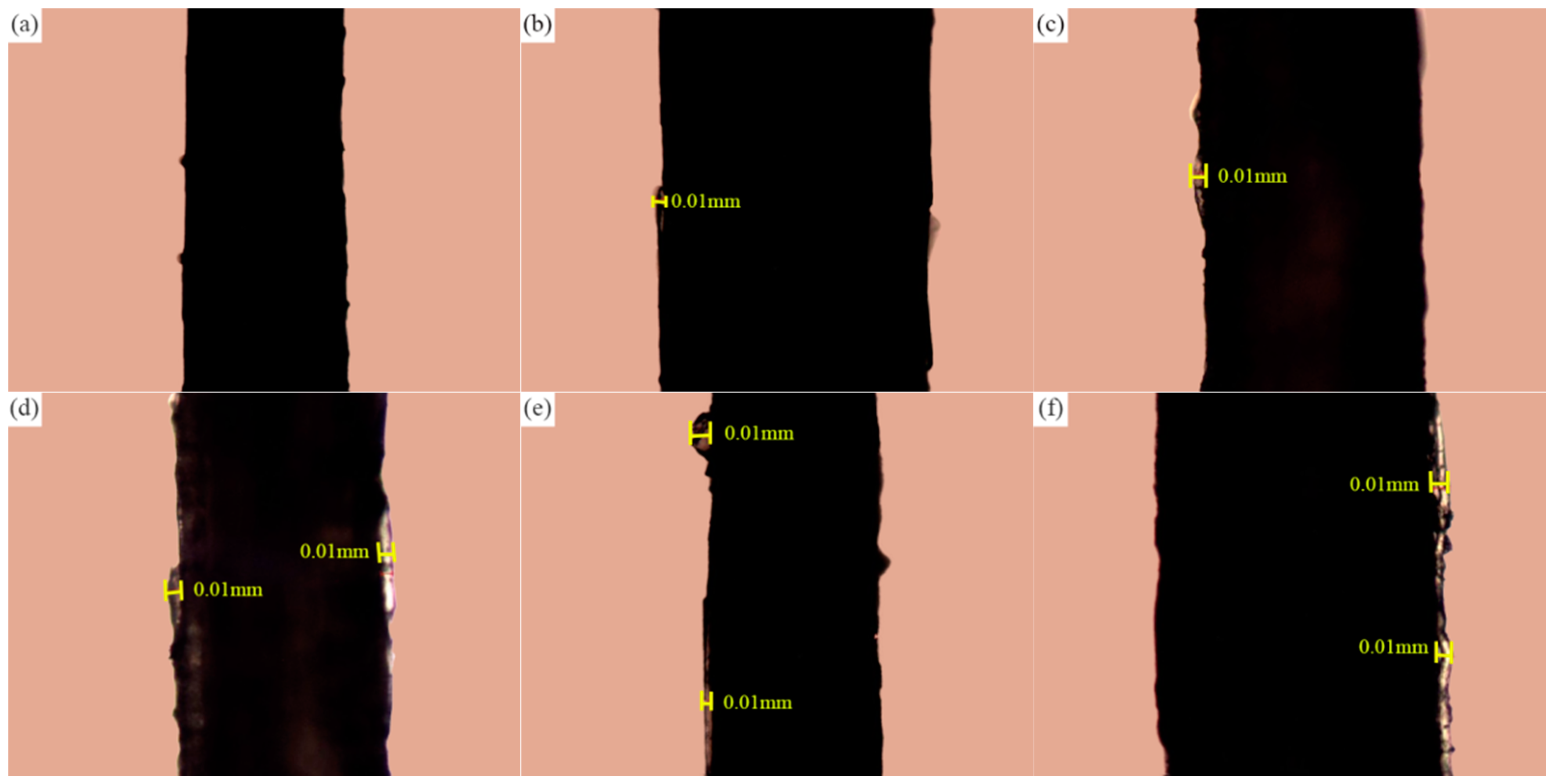
2.3.1. Petrographic Microscope Observation
2.3.2. Three-Dimensional X-ray Microscopy
3. Results and Discussion
3.1. Influencing Factors and Changing Rules of Paraffin Loading Rate
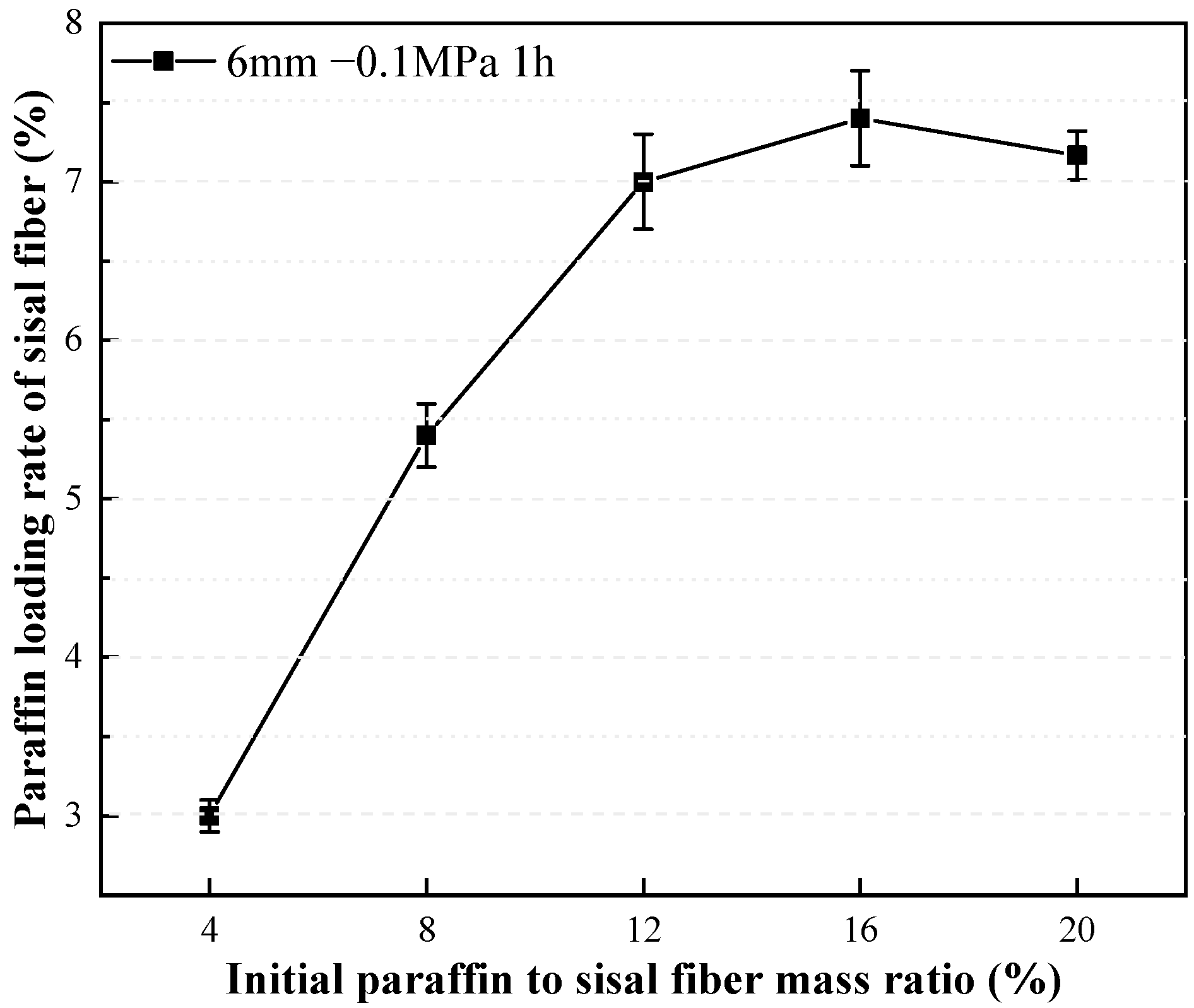
3.1.1. Effect of Mass Ratio between Paraffin and Sisal Fiber on Paraffin Loading Rate
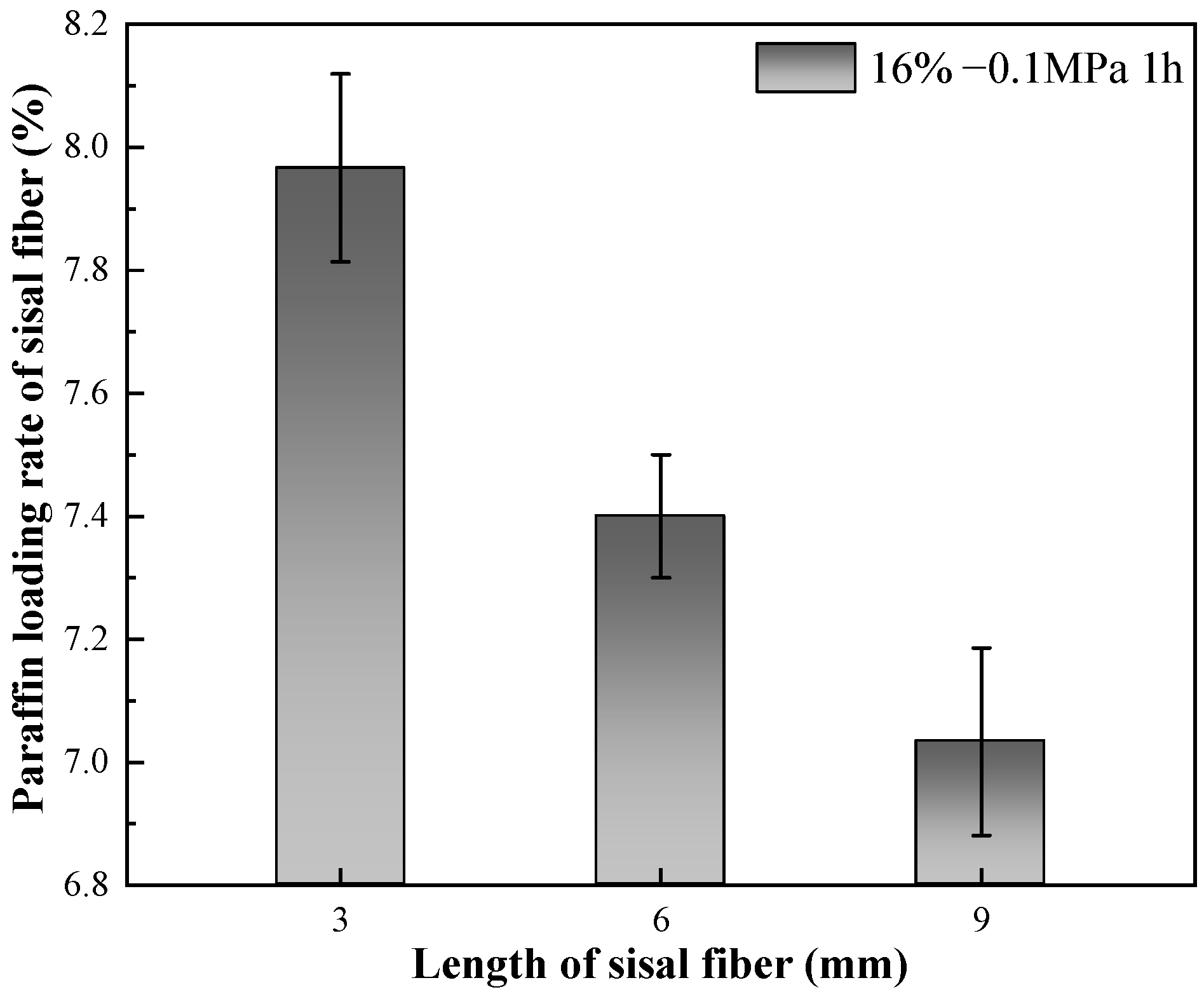
3.1.2. Effect of Sisal Fiber Length on Paraffin Loading Rate
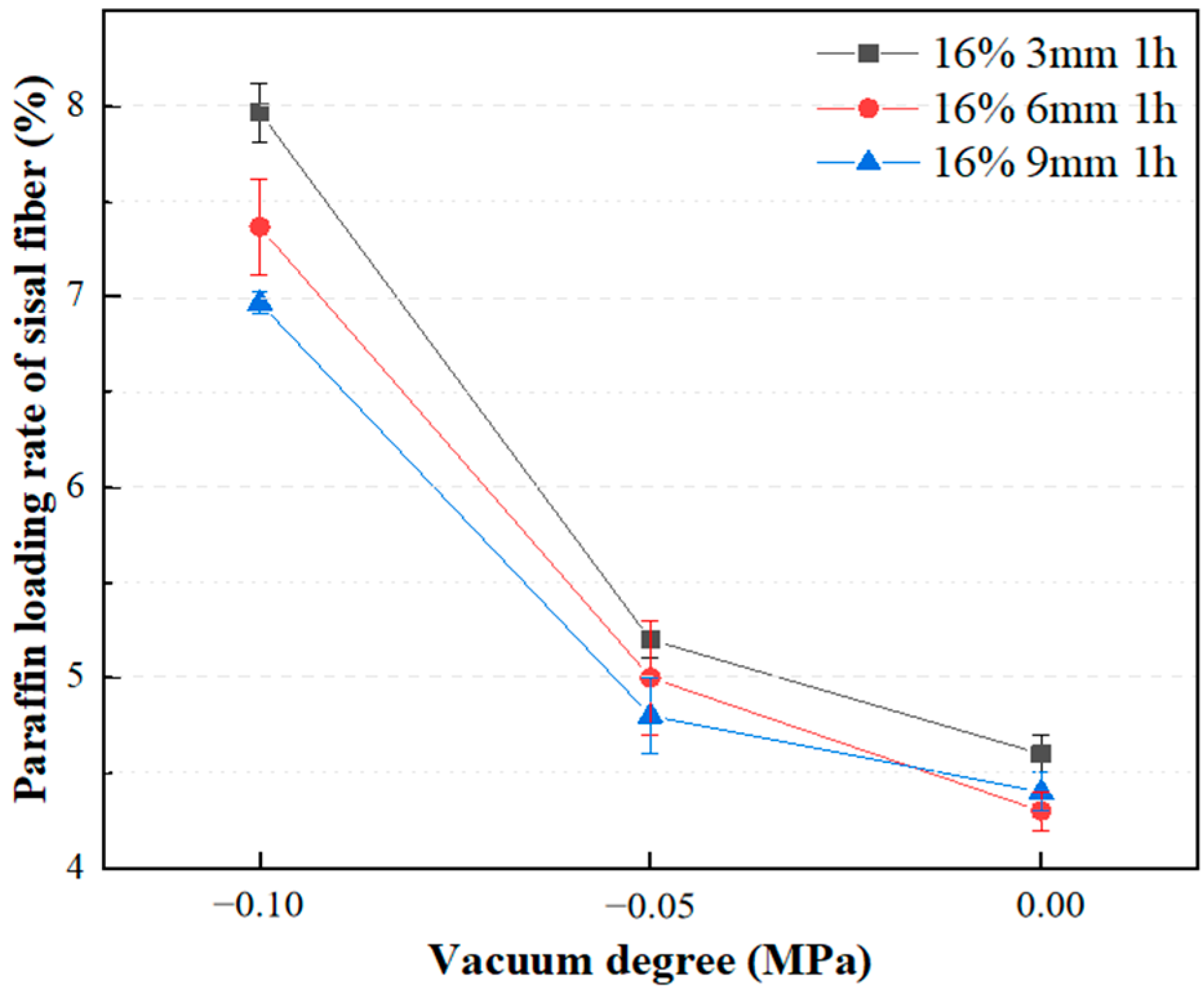
3.1.3. Effect of Vacuum Degree on Adsorption Rate of Paraffin
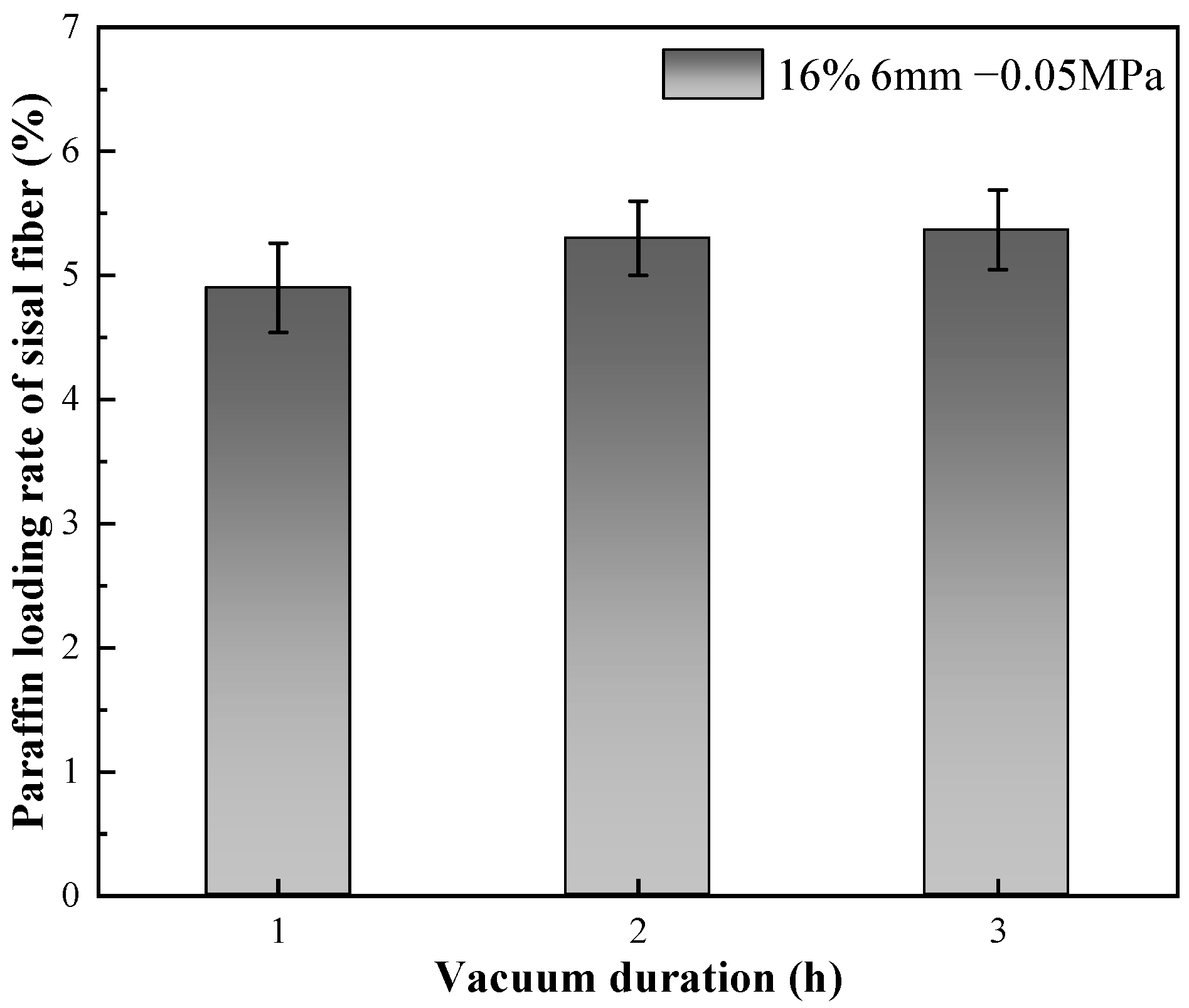
3.1.4. Effect of Negative Pressure Time on Paraffin Loading Rate
3.2. Storage Mechanism and Vacuum Adsorption Mechanism of Paraffin in Sisal Fiber
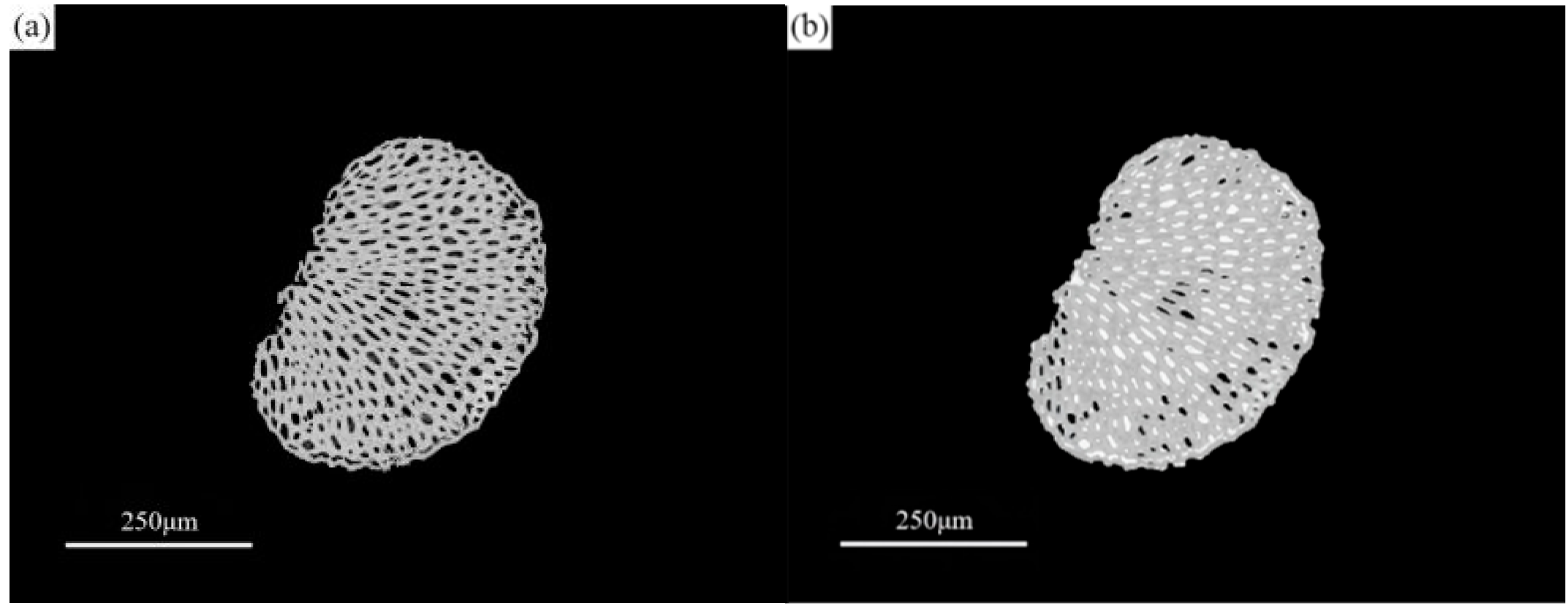
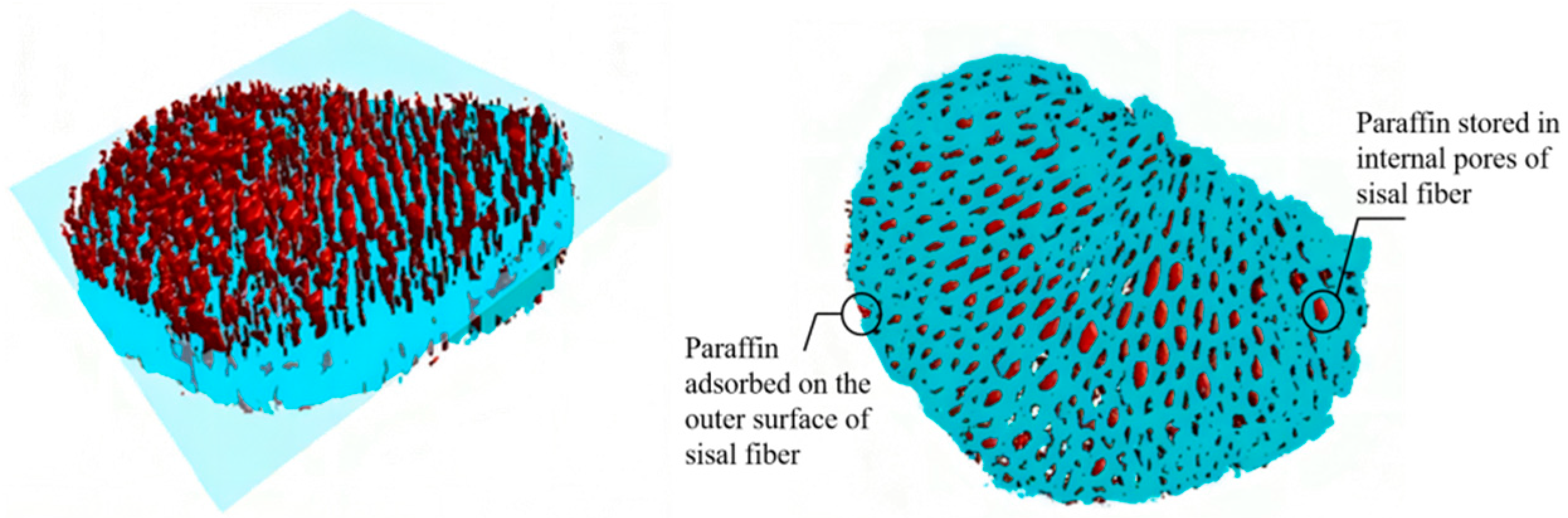
3.2.1. Experimental Study on the Storage Location of Paraffin in Sisal Fiber
3.2.2. Vacuum Adsorption Mechanism
4. Conclusions
- Increasing the mass ratio of paraffin to sisal fibers leads to a decrease in the rate of paraffin loading by sisal fibers, indicating a limited capacity for paraffin storage within them. Vacuum adsorption allows paraffin to impregnate the internal areas of sisal fibers, which are typically inaccessible under normal pressure, thereby increasing the storage space and enhancing the loading rate. The efficiency of vacuum adsorption depends on the vacuum level and the length of the sisal fibers. Higher vacuum levels are advantageous for increasing the paraffin loading rate. However, longer sisal fibers exhibit a lower increase in loading rate compared to shorter fibers at the same vacuum level. The influence of vacuum degree on the paraffin loading rate surpasses the impact of fiber length on the paraffin loading rate. Importantly, extending the duration of vacuum adsorption does not effectively improve the paraffin loading rate. Their relatively low loading capacity may be offset by using more fibers. These composite phase change fibers also exhibit excellent strength enhancement effects, and further work will enable a more thorough quantitative assessment of their thermal performance in applications such as building insulation, in particular their cost-effectiveness and long-term stability.
- By utilizing nano-CT and image processing techniques, the composite phase change fibers were reconstructed in three dimensions. These observations were corroborated with results from petrographic microscopy. The study confirms the potential of the fibrous porous structure and rough surface of sisal fibers to serve as storage spaces for PCMs, with a primary focus on the internal pores of sisal fibers. The mechanism of internal pore storage, combined with the ideal mechanical strength of these fibers and the presence of reserved spaces, enables the microscopic solid–liquid phase change of paraffin while maintaining the macroscopic integrity of the composite phase change fibers in a fully solid state. This mechanism ensures the structural stability of the material, even under compression and stress, and reduces the risk of paraffin leakage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, L.; Xiao, J.; Sun, J.; Xia, B.; Cao, W. Thermal conductivity of 3D printed concrete with recycled fine aggregate composite phase change materials. J. Clean. Prod. 2022, 364, 132598. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Metselaar, H.S.C.; Silakhori, M. Shape-stabilized phase change materials with high thermal conductivity based on paraffin/graphene oxide composite. Energy Convers. Manag. 2013, 67, 275–282. [Google Scholar] [CrossRef]
- Lin, S.C.; Al-Kayiem, H.H. Evaluation of copper nanoparticles—Paraffin wax compositions for solar thermal energy storage. Sol. Energy 2016, 132, 267–278. [Google Scholar] [CrossRef]
- Selimefendigil, F.; Şirin, C. Energy and exergy analysis of a hybrid photovoltaic/thermal-air collector modified with nano-enhanced latent heat thermal energy storage unit. J. Energy Storage 2021, 45, 103467. [Google Scholar] [CrossRef]
- Kalbasi, R.; Afrand, M. Which one is more effective to add to building envelope: Phase change material, thermal insulation, or their combination to meet zero-carbon-ready buildings? J. Clean. Prod. 2022, 367, 133032. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Passive thermal control in residential buildings using phase change materials. Renew. Sustain. Energy Rev. 2016, 55, 371–398. [Google Scholar] [CrossRef]
- Malik, F.K.; Khan, M.M.; Ahmed, H.F.; Irfan, M.; Ahad, I.U. Performance characteristics of PCM based thermal energy storage system for fluctuating waste heat sources. Case Stud. Therm. Eng. 2022, 34, 102012. [Google Scholar] [CrossRef]
- Gasia, J.; Miró, L.; De Gracia, A.; Barreneche, C.; Cabeza, L.F. Experimental Evaluation of a Paraffin as Phase Change Material for Thermal Energy Storage in Laboratory Equipment and in a Shell-and-Tube Heat Exchanger. Appl. Sci. 2016, 6, 112. [Google Scholar] [CrossRef]
- Sarbu, I.; Dorca, A. Review on heat transfer analysis in thermal energy storage using latent heat storage systems and phase change materials. Int. J. Energy Res. 2018, 43, 29–64. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar]
- Wang, S.; Liu, H.; Wu, D.; Wang, X. Temperature and pH dual-stimuli-responsive phase-change microcapsules for multi-purpose applications in smart drug delivery. J. Colloid. Interf. Sci. 2021, 583, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Chandel, S.S.; Agarwal, T. Review of current state of research on energy storage, toxicity, health hazards and commer-cialization of phase changing materials. Renew. Sustain. Energy Rev. 2017, 67, 581–596. [Google Scholar] [CrossRef]
- Fang, G.H.; Sun, P.B.; Yu, M.H.; Zhang, W.T.; Tan, X. Research progress in improving thermal properties of paraffin phase change materials. Appl. Chem. Ind. 2022, 51, 2433–2436. [Google Scholar]
- Ramakrishnan, S.; Sanjayan, J.; Wang, X.; Alam, M.; Wilson, J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl. Energy 2015, 157, 85–94. [Google Scholar] [CrossRef]
- Yataganbaba, A.; Ozkahraman, B.; Kurtbas, I. Worldwide trends on encapsulation of phase change materials: A bibliometric analysis (1990–2015). Appl. Energy 2017, 185, 720–731. [Google Scholar] [CrossRef]
- Yuan, K.; Shi, J.; Aftab, W.; Qin, M.; Usman, A.; Zhou, F.; Lv, Y.; Gao, S.; Zou, R. Engineering the Thermal Conductivity of Functional Phase-Change Materials for Heat Energy Conversion, Storage, and Utilization. Adv. Funct. Mater. 2019, 30, 1904228. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Min, X.; Fang, M.; Huang, Z.; Liu, Y.; Huang, Y.; Wen, R.; Qian, T.; Wu, X. Enhanced thermal properties of novel shape-stabilized PEG composite phase change materials with radial mesoporous silica sphere for thermal energy storage. Sci. Rep. 2015, 5, 12964. [Google Scholar] [CrossRef]
- Tumirah, K.; Hussein, M.Z.; Zulkarnain, Z.; Rafeadah, R. Nano-encapsulated organic phase change material based on co-polymer nanocomposites for thermal energy storage. Energy 2014, 66, 881–890. [Google Scholar] [CrossRef]
- Trivedi, G.; Parameshwaran, R. Microencapsulated phase change material suspensions for cool thermal energy storage. Mater. Chem. Phys. 2019, 242, 122519. [Google Scholar] [CrossRef]
- Drissi, S.; Mo, K.H.; Falchetto, A.C.; Ling, T. Understanding the compressive strength degradation mechanism of ce-ment-paste incorporating phase change material. Cem. Concr. Compos. 2021, 124, 104249. [Google Scholar] [CrossRef]
- Lak, S.N.; Ahmed, S.; Shamberger, P.J.; Pentzer, E.B. Encapsulation of hygroscopic liquids via polymer precipitation in non-aqueous emulsions. J. Colloid Interface Sci. 2022, 628, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, C.; Chen, Z.; Zhu, R.; Sheng, N.; Rao, Z. Anisotropically thermal transfer improvement and shape stabilization of paraffin supported by SiC-coated biomass carbon fiber scaffolds for thermal energy storage. J. Energy Storage 2022, 46, 103866. [Google Scholar] [CrossRef]
- Balaji, A.; Saravanan, R.; Purushothaman, R.; Vijayaraj, S.; Balasubramanian, P. Investigation of Thermal Energy Storage (TES) with lotus stem biocomposite block using PCM. Clean. Eng. Technol. 2021, 4, 100146. [Google Scholar] [CrossRef]
- Song, S.; Zhao, T.; Zhu, W.; Qiu, F.; Wang, Y.; Dong, L. Natural Microtubule-Encapsulated Phase-Change Material with Simultaneously High Latent Heat Capacity and Enhanced Thermal Conductivity. ACS Appl. Mater. Interfaces 2019, 11, 20828–20837. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Ramírez, C.; Jaramillo, F.; Gómez, M. Systematic review of encapsulation and shape-stabilization of phase change materials. J. Energy Storage 2020, 30, 101495. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sarı, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Chung, O.; Jeong, S.-G.; Kim, S. Preparation of energy efficient paraffinic PCMs/expanded vermiculite and perlite composites for energy saving in buildings. Sol. Energy Mater. Sol. Cells 2015, 137, 107–112. [Google Scholar] [CrossRef]
- Vandana, L.P.; Ramadoss, R. Extraction of natural myristic acid from nutmeg with expanded vermiculite for thermal energy storage. J. Energy Storage 2022, 52, 104770. [Google Scholar] [CrossRef]
- Govindasamy, D.; Kumar, A. Experimental analysis of solar panel efficiency improvement with composite phase change materials. Renew. Energy 2023, 212, 175–184. [Google Scholar] [CrossRef]
- Wu, Y.; Ji, Z.J.; Wang, Y.C. Preparation and performance analysis of sodium sulfate decahydrate/expanded vermiculite film phase change composite. Energy Storage Sci. Technol. 2022, 11, 3142–3150. [Google Scholar]
- Voronin, D.V.; Ivanov, E.; Gushchin, P.; Fakhrullin, R.; Vinokurov, V. Clay Composites for Thermal Energy Storage: A Review. Molecules 2020, 25, 1504. [Google Scholar] [CrossRef] [PubMed]
- Jafaripour, M.; Sadrameli, S.; Pahlavanzadeh, H.; Mousavi, S.S. Fabrication and optimization of kaolin/stearic acid composite as a form-stable phase change material for application in the thermal energy storage systems. J. Energy Storage 2020, 33, 102155. [Google Scholar] [CrossRef]
- Islam, A.; Pandey, A.; Saidur, R.; Aljafari, B.; Tyagi, V. Advancements in foam-based phase change materials: Unveiling leakage control, enhanced thermal conductivity, and promising applications. J. Energy Storage 2023, 74. [Google Scholar] [CrossRef]
- Mert, M.S.; Mert, H.H.; Arıcı, M. Development and properties of n-octadecane / kaolinite composites as form-stabilized phase change materials for energy storage. J. Clean. Prod. 2023, 410, 137304. [Google Scholar] [CrossRef]
- Yan, T.; Li, Z.; Pan, W. 3D network structural shape-stabilized composite PCMs for integrated enhancement of thermal conductivity and photothermal properties. Sol. Energy Mater. Sol. Cells 2022, 240, 111645. [Google Scholar] [CrossRef]
- Chinnasamy, V.; Heo, J.; Jung, S.; Lee, H.; Cho, H. Shape stabilized phase change materials based on different support structures for thermal energy storage applications—A review. Energy 2023, 262, 125463. [Google Scholar] [CrossRef]
- Nomura, T.; Okinaka, N.; Akiyama, T. Impregnation of porous material with phase change material for thermal energy storage. Mater. Chem. Phys. 2009, 115, 846–850. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Chen, X.; Wang, G.; Huang, X.; Li, A.; Dong, W. Nanoconfinement effects on thermal properties of na-noporous shape-stabilized composite PCMs: A review. Nano Energy 2018, 53, 769–797. [Google Scholar] [CrossRef]
- Feng, Y.; Wei, R.; Huang, Z.; Zhang, X.; Wang, G. Thermal properties of lauric acid filled in carbon nanotubes as shape-stabilized phase change materials. Phys. Chem. Chem. Phys. 2018, 20, 7772–7780. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Dong, W.; Berardi, U.; Kim, S. Phase change materials stabilized by porous metal supramolecular gels: Gelation effect on loading capacity and thermal performance. Chem. Eng. J. 2020, 394, 124806. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Zhang, C.; Mo, J.; Fu, Q.; Liu, Y.; Wang, S.; Nie, S. Wood-cellulose-fiber-based functional materials for triboelectric nanogen-erators. Nano Energy 2021, 81, 105637. [Google Scholar] [CrossRef]
- De Amorim, J.D.P.; De Souza, K.C.; Duarte, C.R.; Da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; De Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Sundarakannan, R.; John, K.; Johnson, R.D.J.; Prasath, K.A.; Ajith, S.; Arumugaprabu, V.; Uthayakumar, M. Recent advancement in the natural fiber polymer composites: A comprehensive review. J. Clean. Prod. 2020, 277, 124109. [Google Scholar] [CrossRef]
- Huang, Q.L.; Tan, F.; Ma, F.R.; Li, M.Y. Experimental study on the composites performance of sisal fiber-cement concrete. J. Guangxi Univ. 2008, 33, 27–30. [Google Scholar]
- He, J.; Li, C. Fabrication of metal-nanoparticle/carbon-fiber composites having a microtube-array morphology. J. Nanoparticle Res. 2006, 9, 931–937. [Google Scholar] [CrossRef]
- Vargas, V.H.; Paveglio, R.R.; Pauletto, P.d.S.; Salau, N.P.G.; Dotto, L.G. Sisal fiber as an alternative and cost-effective adsorbent for the removal of methylene blue and reactive black 5 dyes from aqueous solutions. Chem. Eng. Commun. 2019, 207, 523–536. [Google Scholar] [CrossRef]
- Narayana, V.L.; Rao, L.B. A brief review on the effect of alkali treatment on mechanical properties of various natural fiber reinforced polymer composites. Mater. Today Proc. 2021, 44, 1988–1994. [Google Scholar]
- Kabir, M.M.; Wang, H.; Cardona, K.T.L.A. Chemical treatments on plant-based natural fibre reinforced polymer com-posites: An overview. Compos. Part B Eng. 2012, 43, 2883–2892. [Google Scholar]
- Ren, G.; Chen, T.; Gao, X.; Su, A. Insights into thermal stability and interface bond performance of sisal fiber in ultra-high performance concrete under different curing conditions. Cem. Concr. Compos. 2023, 137, 104910. [Google Scholar] [CrossRef]
- Kampschulte, M.; Langheinirch, A.C.; Sender, J.; Litzlbauer, H.D.; Althöhn, U.; Schwab, J.D.; Alejandre-Lafont, E.; Martels, G.; Krombach, G.A. Nano-Computed Tomography: Technique and Applications. RöFo Fortschritte Auf Dem Geb. Röntgenstrahlen Und Bildgeb. Verfahr. 2016, 188, 146. [Google Scholar]
- Peng, Y.; Zhao, G.; Qi, Y.; Zeng, Q. In-situ assessment of the water-penetration resistance of polymer modified cement mortars by μ-XCT, SEM and EDS. Cem. Concr. Compos. 2020, 114, 103821. [Google Scholar] [CrossRef]
- Crica, L.E.; Wengenroth, J.; Tiainen, H.; Ionita, M.; Haugen, H.J. Enhanced X-ray absorption for micro-CT analysis of low density polymers. J. Biomater. Sci. Polym. Ed. 2016, 27, 805–823. [Google Scholar] [CrossRef]
- Tuziuti, T.; Yasui, K.; Lee, J.; Kozuka, T.; Towata, A.; Iida, Y. Mechanism of Enhancement of Sonochemical-Reaction Efficiency by Pulsed Ultrasound. J. Phys. Chem. A 2008, 112, 4875–4878. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.H.; Yang, H.T.; Xu, Y.Z.; Liu, J.T. Preparation and Influencing Factors of Diatomite Shape Phase Change Materi-al. Non-Met. Mines 2021, 44, 19–22. [Google Scholar]
- Song, S.; Dong, L.; Zhang, Y.; Chen, S.; Li, Q.; Guo, Y.; Deng, S.; Si, S.; Xiong, C. Lauric acid/intercalated kaolinite as form-stable phase change material for thermal energy storage. Energy 2014, 76, 385–389. [Google Scholar] [CrossRef]
- Shi, T.; Sun, W.; Wang, Q.N. Characterization of microstructures of expanded graphite and preparation of its phase change composites for energy storage. J. Southeast Univ. 2009, 39, 598–601. [Google Scholar]
- He, Q.; Fei, H.; Du, W.Q. Research progress on pore structure and thermal conductivity of EG composite phase change mate-rial. New Chem. Mater. 2021, 49, 11–15. [Google Scholar]


| Phase Transition Temperature (°C) | Solid Density (kg/L) | Liquid Density (kg/L) | Enthalpy (kJ/kg) | Specific Heat Capacity (J/(g·K)) | Thermal Conductivity (W/(m·K)) | Flash Point (°C) |
|---|---|---|---|---|---|---|
| 25 ± 2 | 0.76 | 0.86 | ≥140 | 2.14 | 0.21 | ≥110 |
| Groups | Sisal Fiber (g) | Sisal Fiber Length (mm) | Paraffin (g) | Paraffin to Sisal Fiber Mass Ratio (%) | Vacuum Degree (MPa) | Vacuum Duration (h) |
|---|---|---|---|---|---|---|
| 1# | 5 | 6 | 0 | 0 | −0.1 | 1 |
| 3# | 5 | 6 | 0.4 | 8 | −0.1 | 1 |
| 4# | 5 | 6 | 0.6 | 12 | −0.1 | 1 |
| 5# | 5 | 6 | 0.8 | 16 | −0.1 | 1 |
| 6# | 5 | 6 | 1.0 | 20 | −0.1 | 1 |
| 7# | 5 | 3 | 0.8 | 16 | −0.1 | 1 |
| 8# | 5 | 9 | 0.8 | 16 | −0.1 | 1 |
| 9# | 5 | 3 | 0.8 | 16 | 0 | 1 |
| 10# | 5 | 6 | 0.8 | 16 | 0 | 1 |
| 11# | 5 | 9 | 0.8 | 16 | 0 | 1 |
| 12# | 5 | 3 | 0.8 | 16 | −0.05 | 1 |
| 13# | 5 | 9 | 0.8 | 16 | −0.05 | 1 |
| 14# | 5 | 6 | 0.8 | 16 | −0.05 | 1 |
| 15# | 5 | 6 | 0.8 | 16 | −0.05 | 2 |
| 16# | 5 | 6 | 0.8 | 16 | −0.05 | 3 |
| Groups | Paraffin Loading Rate (wt%) | Groups | Paraffin Loading Rate (wt%) |
|---|---|---|---|
| 1# | 0 | 9# | 4.4 |
| 2# | 3 | 10# | 4.4 |
| 3# | 5.4 | 11# | 4.6 |
| 4# | 7 | 12# | 5 |
| 5# | 7.4 | 13# | 5.2 |
| 6# | 7.2 | 14# | 4.8 |
| 7# | 8 | 15# | 5.6 |
| 8# | 7 | 16# | 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Fu, Q.; Cao, R.; Chen, Z.; Zhang, Z.; Xia, K.; You, N.; Jiang, Y.; Zhang, Y. Experimental Study and Mechanism Analysis of Paraffin/Sisal Composite Phase Change Energy Storage Fiber Prepared by Vacuum Adsorption Method. Materials 2024, 17, 467. https://doi.org/10.3390/ma17020467
Chen C, Fu Q, Cao R, Chen Z, Zhang Z, Xia K, You N, Jiang Y, Zhang Y. Experimental Study and Mechanism Analysis of Paraffin/Sisal Composite Phase Change Energy Storage Fiber Prepared by Vacuum Adsorption Method. Materials. 2024; 17(2):467. https://doi.org/10.3390/ma17020467
Chicago/Turabian StyleChen, Chun, Qi Fu, Ruilin Cao, Zhenzhong Chen, Zedi Zhang, Kailun Xia, Nanqiao You, Yifan Jiang, and Yamei Zhang. 2024. "Experimental Study and Mechanism Analysis of Paraffin/Sisal Composite Phase Change Energy Storage Fiber Prepared by Vacuum Adsorption Method" Materials 17, no. 2: 467. https://doi.org/10.3390/ma17020467





