Preparation of Sol–Gel-Derived CaO-B2O3-SiO2 Glass/Al2O3 Composites with High Flexural Strength and Low Dielectric Constant for LTCC Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CaO–B2O3–SiO2 Glass
2.2. Preparation of CBS glass/Al2O3 Composites
2.3. Characterizations of the Glass and Composites
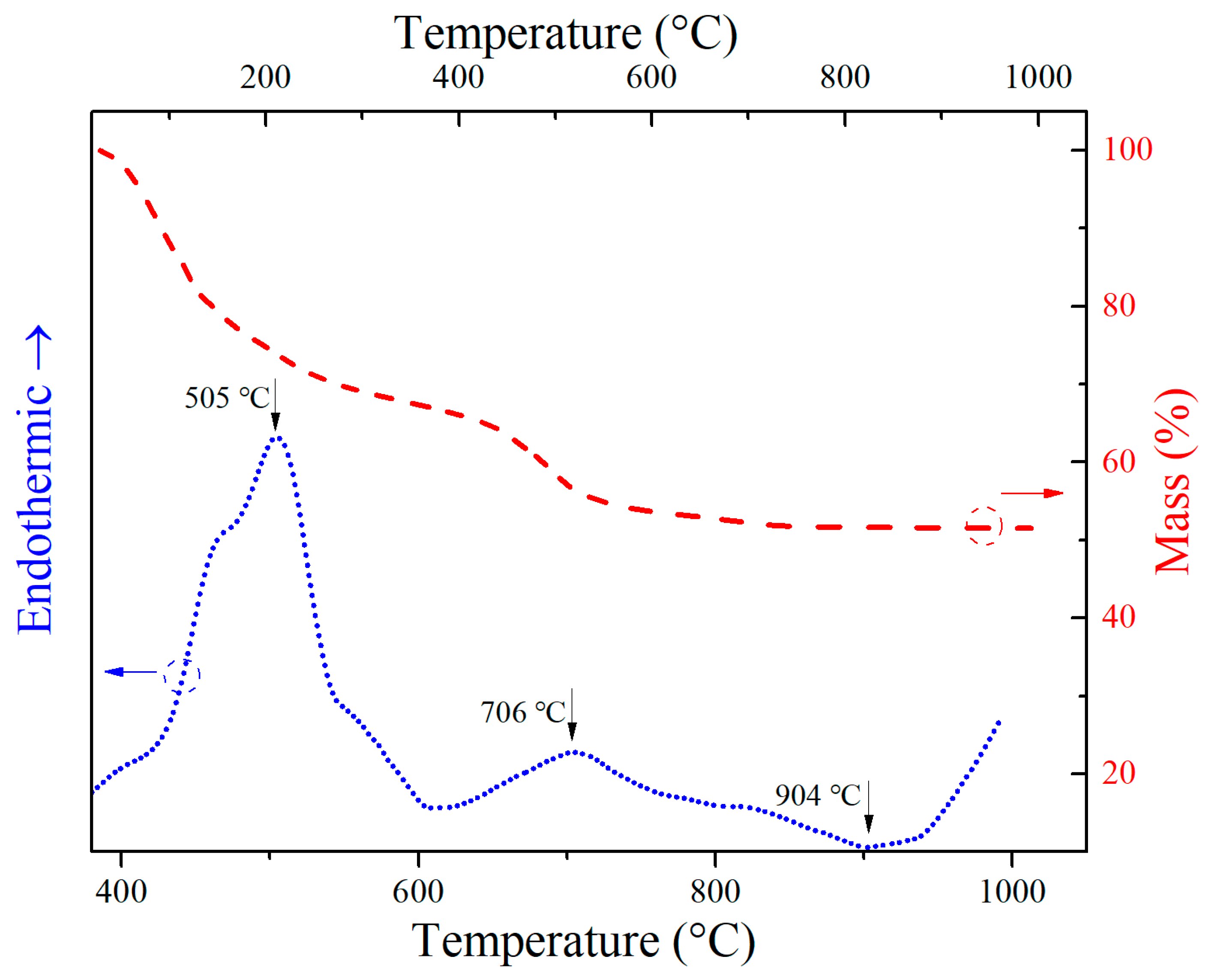
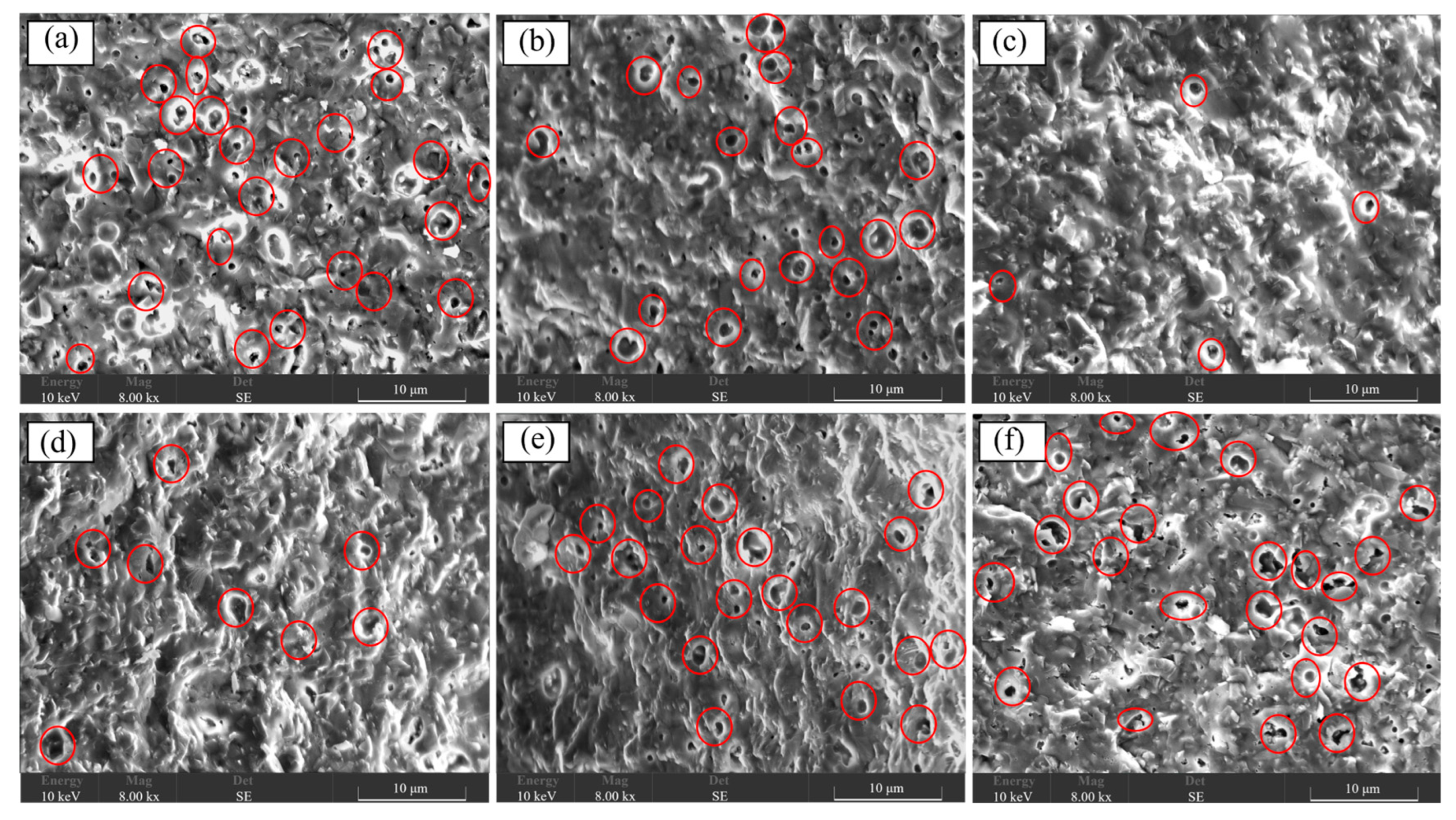
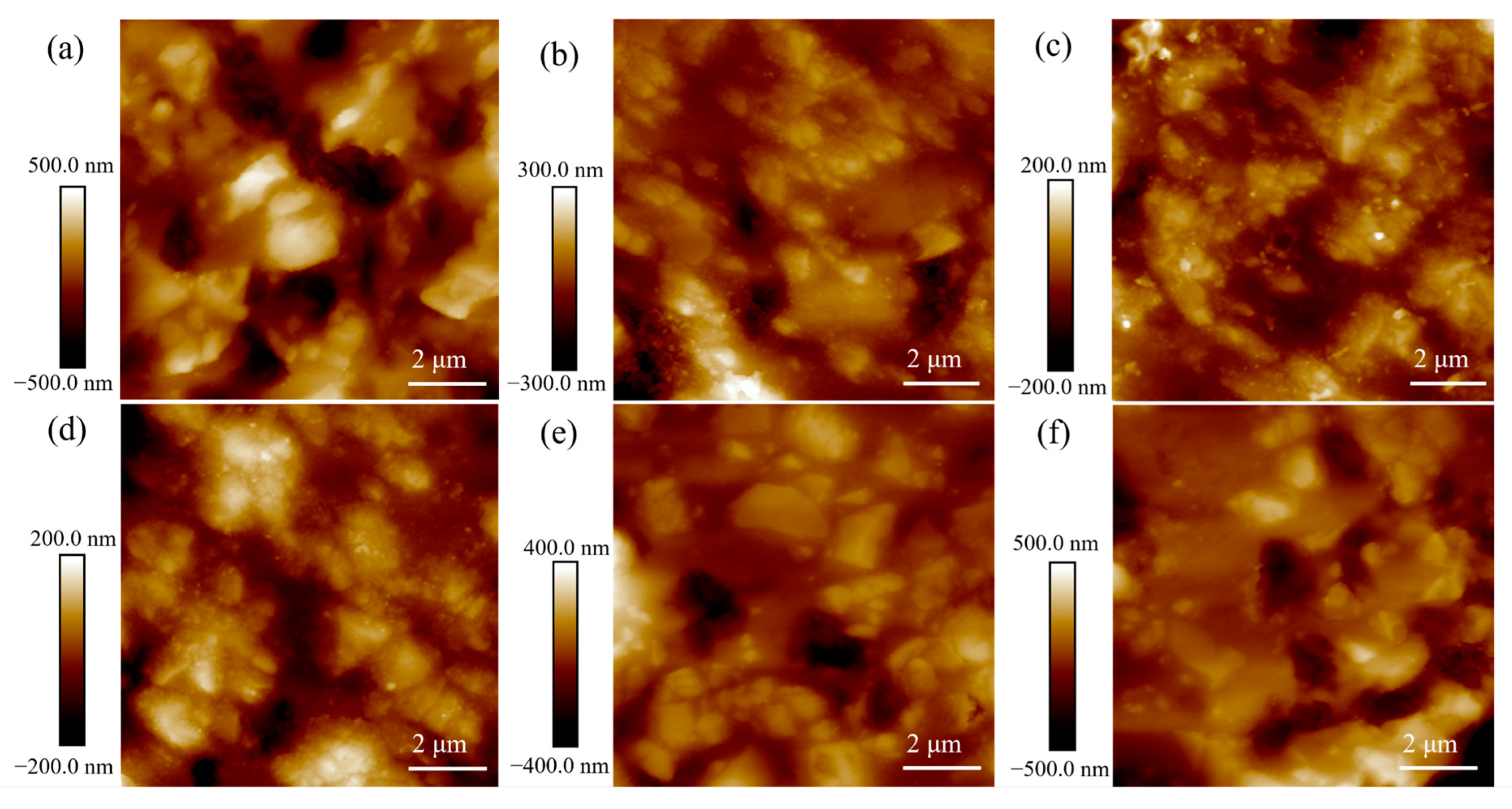
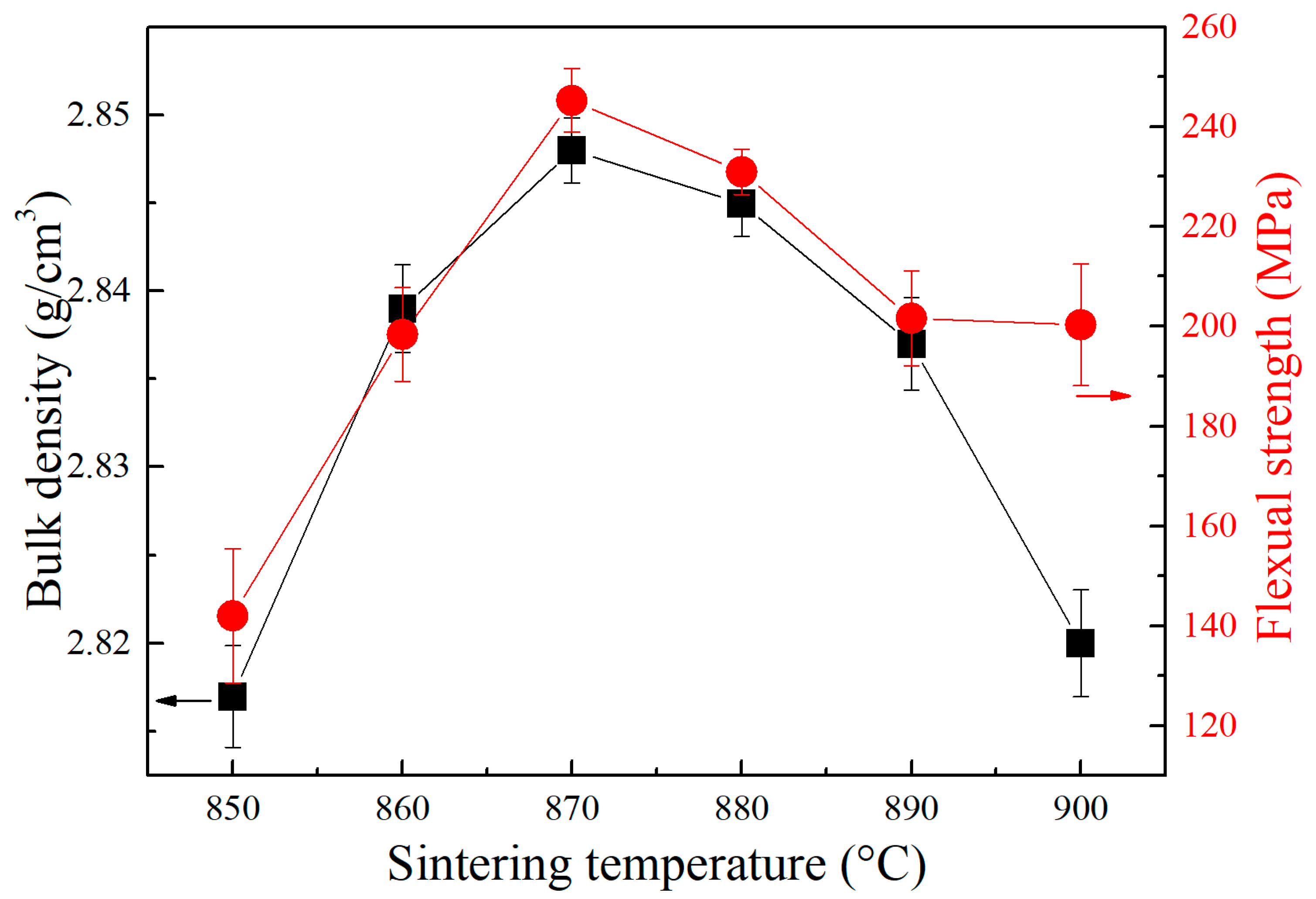
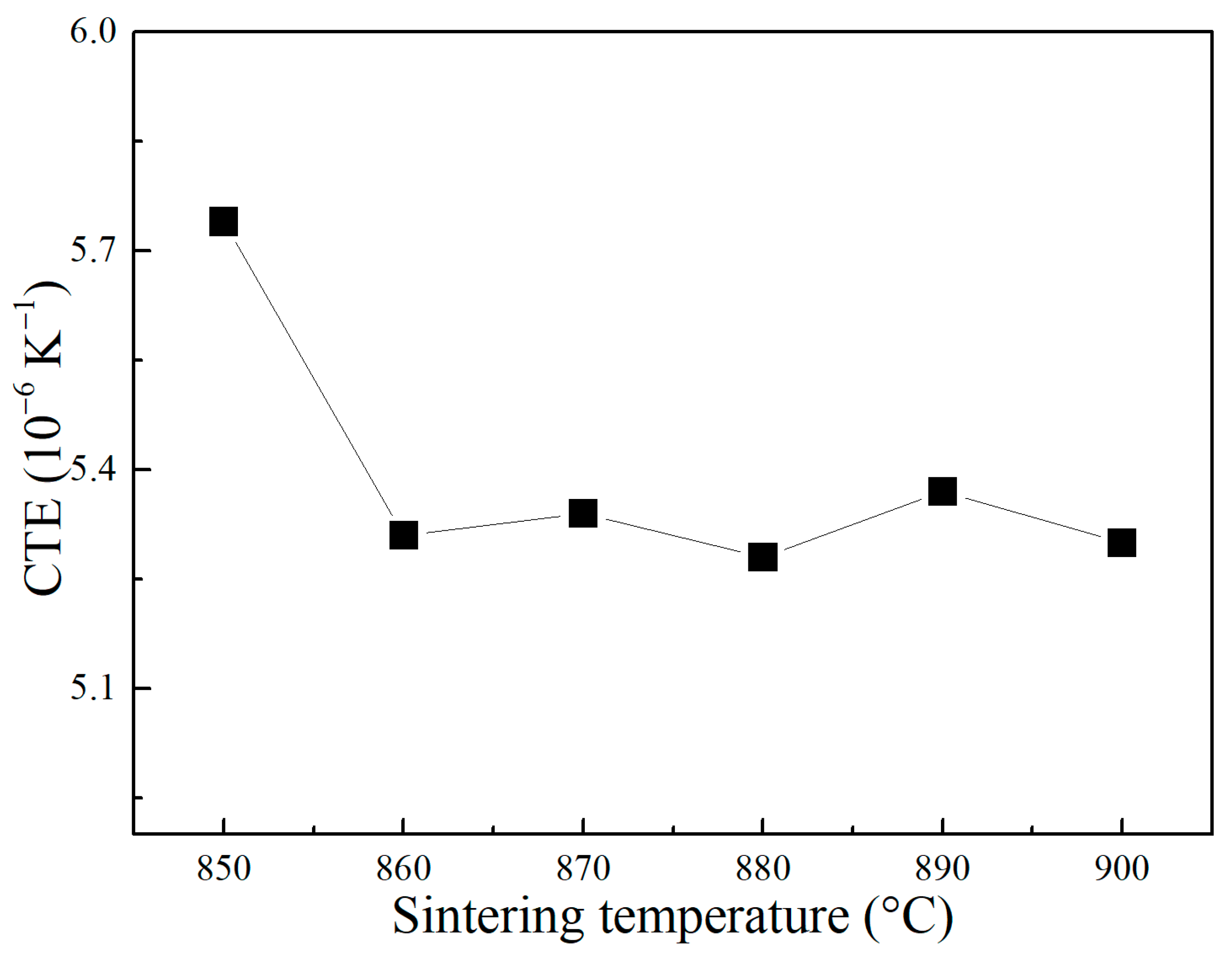
3. Results
| Dielectric Constants | Dielectric Loss | Flexural Strength (MPa) | CTE (10−6 K−1) | References |
|---|---|---|---|---|
| 7.82 (7 GHz) | 0.13% | 205 | 5.64 | [15] |
| 8.08 (7 GHz) | 0.09% | 206 | 5.35 | [14] |
| 6.95 (10 GHz) | 0.456% | 223 | - | [2] |
| 6.71 (10 GHz) | 0.459% | 205 | - | [2] |
| 8.06 (7 GHz) | 0.12% | 204 | 5.47 | [5] |
| 7.92 (7 GHz) | 0.16% | - | 5.6 | [39] |
| 6.87 (13 GHz) | 0.22% | 241 | 6.13 | [3] |
| 6.93 (13 GHz) | 0.26% | 262 | 6.63 | [3] |
| 6.3 (10 GHz) | 0.2% | 245 | 5.3 | This work |
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sebastian, M.T.; Wang, H.; Jantunen, H. Low temperature co-fired ceramics with ultra-low sintering temperature: A review. Curr. Opin. Solid State Mater. Sci. 2016, 20, 151–170. [Google Scholar] [CrossRef]
- Zhu, X.L.; Mao, H.J.; Wang, F.L.; Liang, R.R.; Chen, X.Y.; Liu, Z.F.; Li, W.; Zhang, W.J. Preparation of a CaO-Al2O3-B2O3-SiO2 glass/Al2O3 LTCC substrate material with high flexural strength for microwave application. J. Mater. Sci.-Mater. Electron. 2023, 34, 1125. [Google Scholar] [CrossRef]
- Wang, F.; Lou, Y.H.; Li, Z.J.; Lei, W.; Lu, Y.; Dong, Z.W.; Lu, W.Z. Improved flexural strength and dielectric loss in Al2O3-based LTCC with La2O3-CaO-B2O3-SiO2 glass. Ceram. Int. 2021, 47, 9955–9960. [Google Scholar] [CrossRef]
- Anjana, P.S.; Sebastian, M.T. Microwave Dielectric Properties and Low-Temperature Sintering of Cerium Oxide for LTCC Applications. J. Am. Ceram. Soc. 2009, 92, 96–104. [Google Scholar] [CrossRef]
- Luo, X.; Ren, L.; Xia, Y.; Hu, Y.; Gong, W.; Cai, M.; Zhou, H. Microstructure, sinterability and properties of CaO-B2O3-SiO2 glass/Al2O3 composites for LTCC application. Ceram. Int. 2017, 43, 6791–6795. [Google Scholar] [CrossRef]
- Baker, A.; Lanagan, M.; Randall, C.; Semouchkina, E.; Semouchkin, G.; Rajab, K.Z.; Eitel, R.; Mittra, R.; Rhee, S.; Geggier, P.; et al. Integration concepts for the fabrication of LTCC structures. Int. J. Appl. Ceram. Technol. 2005, 2, 514–520. [Google Scholar] [CrossRef]
- Dernovsek, O.; Naeini, A.; Preu, G.; Wersing, W.; Eberstein, M.; Schiller, W.A. LTCC glass-ceramic composites for microwave application. J. Eur. Ceram. Soc. 2001, 21, 1693–1697. [Google Scholar] [CrossRef]
- Todd, M.G.; Shi, F.G. Molecular basis of the interphase dielectric properties of microelectronic and optoelectronic packaging materials. IEEE Trans. Compon. Packag. Technol. 2003, 26, 667–672. [Google Scholar] [CrossRef]
- Sebastian, M.T.; Jantunen, H. Low loss dielectric materials for LTCC applications: A review. Int. Mater. Rev. 2008, 53, 57–90. [Google Scholar] [CrossRef]
- Ichinose, N.; Yamamoto, H. Effect of additives on microwave dielectric properties in low-temperature firing (Mg, Ca)TiO3 based ceramics. Ferroelectrics 1997, 201, 255–262. [Google Scholar] [CrossRef]
- Sakamaki, R.; Hoshina, T.; Kakemoto, H.; Yasuda, K.; Takeda, H.; Akedo, J.; Tsurumi, T. Heat-cycle endurance and in-plane thermal expansion of Al2O3/Al substrates formed by aerosol deposition method. J. Ceram. Soc. Jpn. 2008, 116, 1299–1303. [Google Scholar] [CrossRef]
- Seo, Y.J.; Shin, D.J.; Cho, Y.S. Phase evolution and microwave dielectric properties of lanthanum borate-based low-temperature co-fired ceramics materials. J. Am. Ceram. Soc. 2006, 89, 2352–2355. [Google Scholar] [CrossRef]
- Terao, K. Effects of impurities on dielectric properties and plasma resistance of Al2O3 ceramics for application of microwave window. Key Eng. Mater. 1999, 2, 513–516. [Google Scholar]
- Luo, X.F.; Tao, H.J.; Li, P.Z.; Fu, Y.; Zhou, H.Q. Properties of borosilicate glass/Al2O3 composites with different Al2O3 concentrations for LTCC applications. J. Mater. Sci.-Mater. Electron. 2020, 31, 14069–14077. [Google Scholar] [CrossRef]
- Liu, M.; Xu, X.Y.; Zhou, H.Q.; Yue, Z.X.; An, Z.Q. Sintering characteristics, microstructures and dielectric properties of borosilicate-based glass/alpha-Al2O3 composites for LTCC application with different MgO and Na2O contents. J. Mater. Sci.-Mater. Electron. 2020, 31, 11195–11203. [Google Scholar] [CrossRef]
- Santha, N.; Nideep, T.K.; Rejisha, S.R. Synthesis and characterization of barium borosilicate glass-Al2O3 composites. J. Mater. Sci.-Mater. Electron. 2012, 23, 1435–1441. [Google Scholar] [CrossRef]
- Santha, N.; Shamsudeen, S.; Karunakaran, N.T.; Naseemabeevi, J.I. Spectroscopic, Dielectric and Optical Properties of 60ZnO-30B2O3-10SiO2 Glass-Al2O3 Composites. Int. J. Appl. Ceram. Technol. 2011, 8, 1042–1049. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, Y.J.; Park, J.H.; Park, J.G. Low-fire dielectric compositions with permittivity 20–60 for LTCC applications. Mater. Chem. Phys. 2004, 88, 308–312. [Google Scholar] [CrossRef]
- Induja, I.J.; Abhilash, P.; Arun, S.; Surendran, K.P.; Sebastian, M.T. LTCC tapes based on Al2O3-BBSZ glass with improved thermal conductivity. Ceram. Int. 2015, 41, 13572–13581. [Google Scholar] [CrossRef]
- Cho, Y.S.; Jo, Y.H.; Choi, H.R.; Shin, D.W.; Chung, K.W. Influences of alkali oxides on crystallization and dielectric properties of anorthite-based low temperature dielectrics. J. Ceram. Soc. Jpn. 2008, 116, 825–828. [Google Scholar] [CrossRef]
- Wang, M.; Zuo, R.Z.; Jia, J.A.; Su, S.; Zhai, J.W. Investigation of the structure evolution process in sol-gel derived CaO-B2O3-SiO2 glass ceramics. J. Non-Cryst. Solids 2011, 375, 1160–1163. [Google Scholar] [CrossRef]
- Stolyarova, V.L.; Ivanov, G.G.; Stolyar, S.V. Vaporization and thermodynamic properties of melts in the Na2O-B2O3-SiO2 system. Glass Phys. Chem. 2002, 28, 112–116. [Google Scholar] [CrossRef]
- Zhou, X.H.; Li, B.; Zhang, S.R.; Ning, H.Y. Effect of Ca/Si ratio on the microstructures and properties of CaO-B2O3-SiO2 glass-ceramics. J. Mater. Sci.-Mater. Electron. 2009, 20, 262–266. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Miola, M.; Verné, E. Bioactive sol-gel glasses: Processing, properties, and applications. Int. J. Appl. Ceram. Technol. 2018, 15, 841–860. [Google Scholar] [CrossRef]
- McFarland, B.; Opila, E. Investigations into the thermal stability of sol-gel-derived glasses as models for thermally grown oxides. J. Am. Ceram. Soc. 2020, 103, 7041–7055. [Google Scholar] [CrossRef]
- Carbajal, N.; Mujika, F. Determination of compressive strength of unidirectional composites by three-point bending tests. Polym. Test. 2009, 28, 150–156. [Google Scholar] [CrossRef]
- Tellez, C.A.; Felcman, J.; Silva, A.D. Fourier transform infrared and Raman spectra of N-di-isopropylphosphorylguanidine (DPG). Spectrochim. Acta A 2000, 56, 1563–1574. [Google Scholar] [CrossRef]
- Gomez, L.M.; Osorio, C.; Amman, E.; Hernandez, S.P.; Castro, M.E. The spectroscopic fingerprint of TNT between 395 and 495 nm determined from transmission near field optical microscopy measurements. Chem. Phys. Lett. 2006, 422, 313–316. [Google Scholar] [CrossRef]
- Muralidharan, P.; Venkateswarlu, M.; Satyanarayana, N. AC conductivity studies of lithium borosilicate glasses: Synthesized by sol-gel process with various concentrations of nitric acid as a catalyst. Mater. Chen. Phys. 2004, 88, 138–144. [Google Scholar] [CrossRef]
- Aravindan, V.; Karthikeyan, K.; Ravi, S.; Amaresh, S.; Kim, W.S.; Lee, Y.S. Adipic acid assisted sol-gel synthesis of Li2MnSiO4 nanoparticles with improved lithium storage properties. J. Mater. Chem. 2010, 20, 7340–7343. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Goryczka, T.; Pisarski, W.A. Spectroscopic Properties of Eu3+ Ions in Sol-Gel Materials Containing Calcium Fluoride Nanocrystals. Phys. Status Solidi B 2020, 257, 1900478. [Google Scholar] [CrossRef]
- Huda, S.; Saha, A.; Karmakar, A. Ultra wideband (UWB) dielectric resonator antenna using fractal-inspired feeding mechanism. Int. J. Commun. Syst. 2023, 36, e5519. [Google Scholar] [CrossRef]
- Kumar, C.J.D.; Sunny, E.K.; Raghu, N.; Venkataramani, N.; Kulkarni, A.R. Synthesis and characterization of crystallizable anorthite-based glass for a low-temperature cofired ceramic application. J. Am. Ceram. Soc. 2008, 91, 652–655. [Google Scholar] [CrossRef]
- Choi, I.J.; Cho, Y.S. Effects of various oxide fillers on physical and dielectric properties of calcium aluminoborosilicate-based dielectrics. J. Electroceram. 2009, 23, 185–190. [Google Scholar] [CrossRef]
- Xia, G.B.; He, L.T.; Yang, D.A. Preparation and characterization of CaO-Al2O3-SiO2 glass/fused silica composites for LTCC application. J. Alloys Compd. 2012, 531, 70–76. [Google Scholar] [CrossRef]
- Chu, G.; Zhai, X.J.; Fu, Y.; Lü, Z.J.; Bi, S.W. Lattice thermal expansion coefficients of combustion synthesized α-Al2O3 nanoparticles. J. Inorg. Mater. 2005, 20, 755–758. [Google Scholar]
- Chen, G.H.; Tang, L.J.; Cheng, J.; Jiang, M.H. Synthesis and characterization of CBS glass/ceramic composites for LTCC application. J. Alloys Compd. 2009, 478, 858–862. [Google Scholar] [CrossRef]
- Chiang, C.C.; Wang, S.F.; Wang, Y.R.; Hsu, Y.F. Characterizations of CaO-B2O3-SiO2 glass-ceramics: Thermal and electrical properties. J. Alloys Compd. 2008, 461, 612–616. [Google Scholar] [CrossRef]
- Ren, L.C.; Luo, X.F.; Xia, Y.S.; Hu, Y.K.; Zhou, H.Q. Fabrication of a high-performance film based borosilicate glass/Al2O3 ceramics for LTCC application. J. Eur. Ceram. Soc. 2017, 37, 2415–2421. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Y.; Li, S.; Hou, B.; Zhuo, W.; Wen, W. Preparation of Sol–Gel-Derived CaO-B2O3-SiO2 Glass/Al2O3 Composites with High Flexural Strength and Low Dielectric Constant for LTCC Application. Materials 2024, 17, 511. https://doi.org/10.3390/ma17020511
Ni Y, Li S, Hou B, Zhuo W, Wen W. Preparation of Sol–Gel-Derived CaO-B2O3-SiO2 Glass/Al2O3 Composites with High Flexural Strength and Low Dielectric Constant for LTCC Application. Materials. 2024; 17(2):511. https://doi.org/10.3390/ma17020511
Chicago/Turabian StyleNi, Yiqun, Shanshan Li, Bo Hou, Weizhuang Zhuo, and Weijia Wen. 2024. "Preparation of Sol–Gel-Derived CaO-B2O3-SiO2 Glass/Al2O3 Composites with High Flexural Strength and Low Dielectric Constant for LTCC Application" Materials 17, no. 2: 511. https://doi.org/10.3390/ma17020511




