Secondary Ion Mass Spectral Imaging of Metals and Alloys
Abstract
1. Introduction
2. Development of SIMS
2.1. History of SIMS
2.2. Recent Development of SIMS
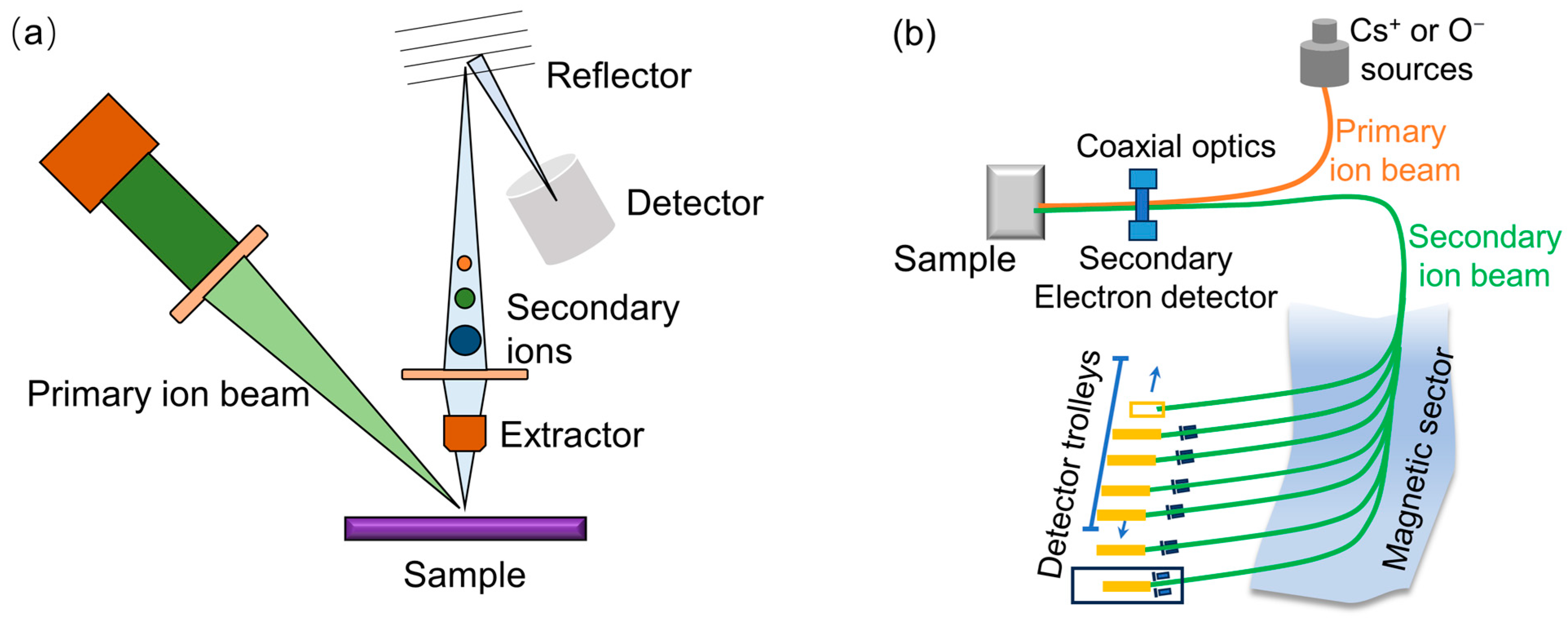
3. SIMS Principles
3.1. Mass Analyzers
| Types of SIMS | Energy of the Primary Ion Source | Primary Ion | Mode of the Primary Ion Source | Mass Analyzer | Spatial Resolution | Sensitive | References |
|---|---|---|---|---|---|---|---|
| ToF SIMS V (IONTOF) | tens of keV | Arn+, Bin+, Cs+, C60+, O2+ | Pulse | ToF | <300 nm | ppm to ppb | [43,44] |
| M6 Hybrid SIMS (IONTOF) | A few keV to tens of keV | O2+, Arn+, Xe+, MCs+, Bin+, Bi3++ | Pulse | ToF and OrbitrapTM | <50 nm | ppm to ppb | [45] |
| PHI nanoTOF3 | A few keV to tens of keV | Bi3++, LMIG with Bi and Au, Ga emitter, Arn+, O2+ | Pulse | ToF and ToF | <500 nm in high mass resolution mode <50 nm in high spatial resolution mode | ppm to ppb | [46,47] |
| J105 SIMS (IONOPTIKA) | A few keV to tens of keV | Arn+, (CO2)n+, (H2O)n+ C60+, C60++, C60+++, Au+, Au++, Au2+, Au3+, O2+, Cs+ | Continuous | ToF | Tens to hundreds of nanometers | ppm to ppb | [48] |
| NanoSIMS 50L (CAMECA) | A few keV to tens of keV | Cs+, O− | Continuous | Magnetic | <50 nm | ppm | [49] |
| SHRIMP-II | ~10 keV | O2− | Continuous | Magnetic | 5–30 μm | ppm | [50,51] |
3.2. In Situ and Operando SIMS
3.3. SIMS Measurement Modality
4. SIMS Applications in Metals and Alloys
4.1. ToF-SIMS
4.1.1. Corrosion Behavior
| Type | Field of Study | Instrument Make and Model | Metallic Ions Studied | References |
|---|---|---|---|---|
| ToF-SIMS | Corrosion Behavior | IONTOF V | Al, Cu, Li, Ga, Fe, Mn, Mg Zn, Pb, Sn, Y | [76,77,79,80,81,82] |
| Thin Film/Oxide Properties | IONTOF IV, V | Cr, Ni, Cu, Al, Li, V, Ag Ti | [78,83,84,85,86,87,88,89] | |
| Biomedical Implants | IONTOF V, Physical Electronics PHI 7200 | Fe, Cr, Ti, Nb, Ca | [90,91,92,93,94] | |
| Magnetic SIMS | Semiconductor Analysis | CAMECA IMS-6F, CAMECA IMS-4FE7 CAMECA IMS-4f | Hf, Cu, In, Ga | [95,96,97,98,99,100] |
| Additive Transport | CAMECA IMS-6F, CAMECA IMS 5F, DTDC SHRIMP | Al, Li, Cu, Y, Mn, Zn, Mg, Zr, Ga, Fe, Cr, Ni, U | [101,102,103,104,105,106] | |
| Geologic Analysis | CAMECA IMS-6F, DTDC SHRIMP | U, Pb, Hf, Lu | [106,107,108,109,110] | |
| Cosmochemical Analysis | CAMECA IMS 1280, CAMECA IMS 1270 DTDC SHRIMP | Pb, Fe, Ni, Mn, Cr | [111,112,113,114,115] | |
| Nuclear Safeguard Analysis | CAMECA IMS 1280, CAMECA IMS 1280-HR | U | [116,117,118,119,120] | |
| NanoSIMS | Segregation Analysis | CAMECA NanoSIMS 50L, CAMECA NanoSIMS 50 | Mo, Cr, Fe, Nb, Ti, Al, Mg, Ni | [98,121,122,123,124,125,126] |
| Hydrogen Isotopic Analysis | CAMECA NanoSIMS 50 | Mn, Cr, Mo, Ni, Fe | [127,128,129,130,131,132] |
4.1.2. Thin Films and Oxide Layers
4.1.3. Metals and Alloys for Biomedical Applications
4.2. Magnetic SIMS
4.2.1. Semiconductor Materials
4.2.2. Small Additive Transport and Incorporation in Materials
4.2.3. Geologic Formations and Minerals
4.3. Large Geometry SIMS
4.3.1. Extraterrestrial Materials
4.3.2. Particle Heterogeneity/Homogeneity
4.4. NanoSIMS
4.4.1. Study of Elemental Segregation in Metals and Alloys Using NanoSIMS
4.4.2. Hydrogen Isotopes in Metals and Alloys
4.5. Correlative Imaging Using SIMS
4.5.1. Irradiated Materials
4.5.2. Semiconductors Using
4.5.3. SIMS Complementary Techniques
4.6. Nanomaterials
5. SIMS Data Challenge
5.1. Multivariate Analysis (MVA)
5.2. ML to Address SIMS Data Challenge
6. Outlook and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Al | Aluminum |
| APT | Atom Probe Tomography |
| B | Boron |
| CIGS | Cu(In,Ga)Se2 |
| Cu | Copper |
| EDX | Energy-Dispersive X-ray |
| EF-TEM | Energy Filtered Transmission Electron Microscopy |
| FIB | Focused Ion Beam |
| FOV | Field of View |
| FTIR | Fourier Transform Infrared |
| HIM | Helium Ion Microscopy |
| Li | Lithium |
| ML | Machine Learning |
| MVA | Multivariate Analysis |
| MCR | Multivariate Curve Resolution |
| NMF | Non-Negative Matrix Factorization |
| NRA | Nuclear Reaction Analysis |
| PEELS | Parallel Electron Energy Loss Spectroscopy |
| PCA | Principal Component Analysis |
| P | Phosphorus |
| RBS | Rutherford Backscattering Spectrometry |
| SECM | Scanning Electrochemical microscopy |
| SEM | Scanning Electron Microscopy |
| STEM | Scanning Transmission Electron Microscopy |
| SIMS | Secondary Ion Mass Spectrometry |
| SHRIMP | Sensitive High-Resolution Ion Microprobe |
| ToF | Time-of-Flight |
| TEM | Transmission Electron Microscopy |
| V | Vanadium |
| XRD | X-ray Diffraction |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Gilmore, I.S.; Seah, M.P. Static SIMS: Towards unfragmented mass spectra—The G-SIMS procedure. Appl. Surf. Sci. 2000, 161, 465–480. [Google Scholar] [CrossRef]
- Green, F.M.; Gilmore, I.S.; Seah, M.P. TOF-SIMS: Accurate mass scale calibration. J. Am. Soc. Mass Spectrom. 2006, 17, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Schaepe, K.; Jungnickel, H.; Heinrich, T.; Tentschert, J.; Luch, A.; Unger, W.E.S. Secondary ion mass spectrometry. In Characterization of Nanoparticles: Measurement Processes for Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 481–509. [Google Scholar]
- Parker, G.D.; Hanley, L.; Yu, X.Y. Mass spectral imaging to map plant-microbe interactions. Microorganisms 2023, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Benninghoven, A. The development of SIMS and international SIMS conferences: A personal retrospective view. Surf. Interface Anal. 2011, 43, 2–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Ni, Z.; Zhao, Y.; Jia, F.; Luo, Q.; Mao, L.; Zhu, Z.; Wang, F. Real-time characterization of the fine structure and dynamics of an electrical double layer at electrode-electrolyte interfaces. J. Phys. Chem. Lett. 2021, 12, 5279–5285. [Google Scholar] [CrossRef]
- Cheng, C.; Zhou, Y.; Nelson, H.M.; Ahmadullah, T.; Piao, H.; Wang, Z.; Guo, W.; Wang, J.G.; Lai, G.; Zhu, Z. Molecular identification of wines using in situ liquid SIMS and PCA analysis. Front. Chem. 2023, 11, 1124229. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, J.; Grovenor, C.R.M.; Moore, K.L. NanoSIMS imaging and analysis in materials science. Annu. Rev. Anal. Chem. 2020, 13, 273–292. [Google Scholar] [CrossRef]
- Gardner, W.; Winkler, D.A.; Muir, B.W.; Pigram, P.J. Applications of multivariate analysis and unsupervised machine learning to ToF-SIMS images of organic, bioorganic, and biological systems. Biointerphases 2022, 17, 020802. [Google Scholar] [CrossRef]
- Fisher, G.L.; Bruinen, A.L.; Ogrinc Potocnik, N.; Hammond, J.S.; Bryan, S.R.; Larson, P.E.; Heeren, R.M. A new method and mass spectrometer design for TOF-SIMS parallel imaging MS/MS. Anal. Chem. 2016, 88, 6433–6440. [Google Scholar] [CrossRef]
- Passarelli, M.K.; Pirkl, A.; Moellers, R.; Grinfeld, D.; Kollmer, F.; Havelund, R.; Newman, C.F.; Marshall, P.S.; Arlinghaus, H.; Alexander, M.R.; et al. The 3D OrbiSIMS—Label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power. Nat. Methods 2017, 14, 1175–1183. [Google Scholar] [CrossRef]
- Kollmer, F.; Pirkl, A.; Zakel, J.; Arlinghaus, H.; Niehuis, E. Hybrid SIMS: Secondary Ion Mass Spectrometry Imaging with High Mass Resolving Power. Microsc. Microanal. 2023, 29 (Suppl. S1), 748. [Google Scholar] [CrossRef] [PubMed]
- Ogrinc Potocnik, N.; Fisher, G.L.; Prop, A.; Heeren, R.M.A. Sequencing and identification of endogenous neuropeptides with matrix-enhanced Secondary Ion Mass Spectrometry Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 8223–8227. [Google Scholar] [CrossRef]
- Finšgar, M. Surface analysis by gas cluster ion beam XPS and ToF-SIMS tandem MS of 2-mercaptobenzoxazole corrosion inhibitor for brass. Corros. Sci. 2021, 182, 109269. [Google Scholar] [CrossRef]
- Van Nuffel, S.; Quatredeniers, M.; Pirkl, A.; Zakel, J.; Le Caer, J.P.; Elie, N.; Vanbellingen, Q.P.; Dumas, S.J.; Nakhleh, M.K.; Ghigna, M.R.; et al. Multimodal imaging mass spectrometry to identify markers of pulmonary arterial hypertension in human lung tissue using MALDI-ToF, ToF-SIMS, and Hybrid SIMS. Anal. Chem. 2020, 92, 12079–12087. [Google Scholar] [CrossRef] [PubMed]
- Van Nuffel, S.; Brunelle, A. TOF-SIMS imaging of biological tissue sections and structural determination using tandem MS. Methods Mol. Biol. 2022, 2437, 77–86. [Google Scholar] [PubMed]
- Fisher, G. Applications of 2D/3D TOF-SIMS with fast MS/MS imaging and keV-CID identification for research and industrial problem solving: Low-abundance molecules, stereoisomers, monolayers & devices. Microsc. Microanal. 2020, 26 (Suppl. S2), 80–81. [Google Scholar]
- Iida, S.I.; Murakami, T.; Kurosawa, Y.; Suzuri, Y.; Fisher, G.L.; Miyayama, T. Time-of-flight secondary ion tandem mass spectrometry depth profiling of organic light-emitting diode devices for elucidating the degradation process. Rapid Commun. Mass Spectrom. 2020, 34, e8640. [Google Scholar] [CrossRef]
- Spampinato, V.; Dialameh, M.; Franquet, A.; Fleischmann, C.; Conard, T.; van der Heide, P.; Vandervorst, W. A correlative ToF-SIMS/SPM methodology for probing 3D devices. Anal. Chem. 2020, 92, 11413–11419. [Google Scholar] [CrossRef]
- Casula, G.; Busby, Y.; Franquet, A.; Spampinato, V.; Houssiau, L.; Bonfiglio, A.; Cosseddu, P. A flexible organic memory device with a clearly disclosed resistive switching mechanism. Org. Electron. 2019, 64, 209–215. [Google Scholar] [CrossRef]
- Koyun, A.N.; Zakel, J.; Kayser, S.; Stadler, H.; Keutsch, F.N.; Grothe, H. High resolution nanoscale chemical analysis of bitumen surface microstructures. Sci. Rep. 2021, 11, 13554. [Google Scholar] [CrossRef] [PubMed]
- Melkonyan, D.; Fleischmann, C.; Veloso, A.; Franquet, A.; Bogdanowicz, J.; Morris, R.J.H.; Vandervorst, W. Wet-chemical etching of atom probe tips for artefact free analyses of nanoscaled semiconductor structures. Ultramicroscopy 2018, 186, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal of polyethylene glycols from wastewater: A comparison of different approaches. Chemosphere 2021, 273, 129725. [Google Scholar] [CrossRef] [PubMed]
- Priebe, A.; Michler, J. Review of recent advances in gas-assisted focused ion beam time-of-flight secondary Mass spectrometric studies of solid surfaces ion mass spectrometry (FIB-TOF-SIMS). Materials 2023, 16, 2090. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, T.; Philipp, P.; Audinot, J.N.; Dowsett, D.; Eswara, S. High-resolution high-sensitivity elemental imaging by secondary ion mass spectrometry: From traditional 2D and 3D imaging to correlative microscopy. Nanotechnology 2015, 26, 434001. [Google Scholar] [CrossRef] [PubMed]
- Audinot, J.-N.; Ost, A.D.; Stoffels, C.; Philipp, P.; De Castro, O.; Biesemeier, A.; Hoang, Q.H.; Wirtz, T. SIMS performed on focused ion beam instruments: In-situ correlative structural and chemical imaging. Micros. Microanal. 2022, 28 (Suppl. S1), 30–31. [Google Scholar] [CrossRef]
- Honig, R.E. Advances in Mass Spectrometry; Pergamon: Oxford, UK, 1963; pp. 25–37. [Google Scholar]
- Liebl, H.J.; Herzog, R.F.K. Sputtering Ion Source for Solids. Jpn. J. Appl. Phys. 1963, 34, 2893–2896. [Google Scholar] [CrossRef]
- Liebl, H. Ion microprobe mass analyzer. Jpn. J. Appl. Phys. 1967, 38, 5277–5283. [Google Scholar] [CrossRef]
- Evans, C.A. Ion probe mass-spectrometry—Overview. Thin Solid Film. 1973, 19, 11–19. [Google Scholar] [CrossRef]
- Pillatsch, L.; Östlund, F.; Michler, J. FIBSIMS: A review of secondary ion mass spectrometry for analytical dual beam focussed ion beam instruments. Prog. Cryst. Growth Charact. Mater. 2019, 65, 1–19. [Google Scholar] [CrossRef]
- Fisher, G.L.; Hammond, J.S.; Larson, P.E.; Bryan, S.R.; Heeren, R.M.A. Parallel imaging MS/MS TOF-SIMS instrument. J. Vac. Sci. Technol. B 2016, 34, 03H126. [Google Scholar] [CrossRef]
- Davies, N.; Weibel, D.E.; Blenkinsopp, P.; Lockyer, N.; Hill, R.; Vickerman, J.C. Development and experimental application of a gold liquid metal ion source. Appl. Surf. Sci. 2003, 203–204, 223–227. [Google Scholar] [CrossRef]
- Walker, A.V.; Winograd, N. Prospects for imaging with TOF-SIMS using gold liquid metal ion sources. Appl. Surf. Sci. 2003, 203–204, 198–200. [Google Scholar] [CrossRef]
- Wong, S.C.C.; Hill, R.; Blenkinsopp, P.; Lockyer, N.P.; Weibel, D.E.; Vickerman, J.C. Development of a C60+ ion gun for static SIMS and chemical imaging. Appl. Surf. Sci. 2003, 203–204, 219–222. [Google Scholar] [CrossRef]
- Jia, F.; Zhao, X.; Zhao, Y. Advancements in ToF-SIMS imaging for life sciences. Front. Chem. 2023, 11, 1237408. [Google Scholar] [CrossRef]
- Tian, H.; Maciazek, D.; Postawa, Z.; Garrison, B.J.; Winograd, N. CO2 cluster ion beam, an alternative projectile for secondary ion mass spectrometry. J. Am. Soc. Mass Spectrom. 2016, 27, 1476–1482. [Google Scholar] [CrossRef]
- Wucher, A.; Tian, H.; Winograd, N. A mixed cluster ion beam to enhance the ionization efficiency in molecular secondary ion mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 396–400. [Google Scholar] [CrossRef]
- Angerer, T.B.; Blenkinsopp, P.; Fletcher, J.S. High energy gas cluster ions for organic and biological analysis by time-of-flight secondary ion mass spectrometry. Int. J. Mass Spectrom. 2015, 377, 591–598. [Google Scholar] [CrossRef]
- Nayak, D.; Pourrezaei, K.; Francois, M.; Bahasadri, A. Measurement of charge-to-mass ratio (Q/m) distribution of an ionized cluster beam by a special type of quadrupole mass analyzer. Rev. Sci. Instrum. 1987, 58, 2249–2255. [Google Scholar] [CrossRef]
- Schwartz, J.C.; Senko, M.W.; Syka, J.E. A two-dimensional quadrupole ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 2002, 13, 659–669. [Google Scholar] [CrossRef] [PubMed]
- CAMECA. Available online: https://www.cameca.com/products (accessed on 17 December 2023).
- Time-of-Flight SIMS—ION-TOF SIMS 5. Available online: https://www.aif.ncsu.edu/tof-sims/ (accessed on 17 December 2023).
- ToF.SIMS 5 Mass Spectrometer. Available online: https://data.pnnl.gov/group/nodes/data-source/13428 (accessed on 17 December 2023).
- IONTOF M6 Brochure. Available online: https://www.iontof.com/download/IONTOF_M6_Brochure.pdf (accessed on 17 December 2023).
- PHI nanoTOF3. Available online: https://www.ulvac-phi.com/en/products/tof-sims/nanotof3%2B/ (accessed on 17 December 2023).
- PHInanoTOF3. Available online: https://www.ulvac-phi.com/files/1416/9708/6890/PHI_nanoTOF3_en_231012.pdf (accessed on 17 December 2023).
- J105SIMS. Available online: https://ionoptika.com/products/j105-sims/ (accessed on 17 December 2023).
- NanoSIMS 50L. Available online: https://www.cameca.com/products/sims/nanosims (accessed on 17 December 2023).
- Sensitive High-Resolution Ion Microprobe. Available online: https://en.wikipedia.org/wiki/Sensitive_high-resolution_ion_microprobe (accessed on 17 December 2023).
- Marsden, R.C.; Kirkland, C.L.; Danišík, M.; Daggitt, M.L.; Ahn, U.-S.; Friedrichs, B.; Evans, N.J. A new approach to SHRIMP II zircon U-Th disequilibrium dating. Comput. Geosci. 2022, 158, 104947. [Google Scholar] [CrossRef]
- Chen, S.; Ma, L.; Huang, Z.; Liang, G.; Zhi, C. In situ/operando analysis of surface reconstruction of transition metal-based oxygen evolution electrocatalysts. Cell Rep. Phys. Sci. 2022, 3, 100729. [Google Scholar] [CrossRef]
- Shen, Y.; Fu, Y.; Yao, J.; Lao, D.; Nune, S.; Zhu, Z.; Heldebrant, D.; Yao, X.; Yu, X.Y. Revealing the structural evolution of green rust synthesized in ionic liquids by in situ molecular imaging. Adv. Mater. Interfaces 2020, 7, 2000452. [Google Scholar] [CrossRef]
- Yang, L.; Yu, X.Y.; Zhu, Z.; Iedema, M.J.; Cowin, J.P. Probing liquid surfaces under vacuum using SEM and ToF-SIMS. Lab Chip 2011, 11, 2481–2484. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, X.-Y.; Zhu, Z.; Thevuthasan, T.; Cowin, J.P. Making a hybrid microfluidic platform compatible for in situ imaging by vacuum-based techniques. J. Vac. Sci. Technol. A 2011, 29, 061101. [Google Scholar] [CrossRef]
- Yu, X.-Y. In situ, in vivo, and in operando imaging and spectroscopy of liquids using microfluidics in vacuum. J. Vac. Sci. Technol. A 2020, 38, 040804. [Google Scholar] [CrossRef]
- Fearn, S. Characterisation of biological material with ToF-SIMS: A review. Mater. Sci. Technol. 2014, 31, 148–161. [Google Scholar] [CrossRef]
- Trzyna-Sowa, M.; Berchenko, N.; Dziawa, P.; Cebulski, J. Molecular speciation analysis of oxidized metal surfaces by TOF SIMS. Appl. Surf. Sci. 2022, 577, 151855. [Google Scholar] [CrossRef]
- Mei, H.; Laws, T.S.; Mahalik, J.P.; Li, J.; Mah, A.H.; Terlier, T.; Bonnesen, P.; Uhrig, D.; Kumar, R.; Stein, G.E.; et al. Entropy and enthalpy mediated segregation of bottlebrush copolymers to interfaces. Macromolecules 2019, 52, 8910–8922. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Kawashima, T.; Ohkawa, M.; Iwai, H.; Aoyagi, S. Extraction of hidden information of ToF-SIMS data using different multivariate analyses. Surf. Interface Anal. 2015, 47, 439–446. [Google Scholar] [CrossRef]
- Gajos, K.; Awsiuk, K.; Budkowski, A. Controlling orientation, conformation, and biorecognition of proteins on silane monolayers, conjugate polymers, and thermo-responsive polymer brushes: Investigations using TOF-SIMS and principal component analysis. Colloid Polym. Sci. 2020, 299, 385–405. [Google Scholar] [CrossRef]
- Liu, S.; Weng, L.T.; Chan, C.M.; Li, L.; Ho, N.K.; Jiang, M. Quantitative surface characterization of poly(styrene)/poly(4-vinyl phenol) random and block copolymers by ToF-SIMS and XPS. Surf. Interface Anal. 2001, 31, 745–753. [Google Scholar] [CrossRef]
- Yin, Y.-S.; Chen, B.-J.; Ling, Y.-C. ToF-SIMS study of official seals from Han Dynasty. Appl. Surf. Sci. 2008, 255, 1534–1537. [Google Scholar] [CrossRef]
- Seah, M.P.; Shard, A.G. The matrix effect in secondary ion mass spectrometry. Appl. Surf. Sci. 2018, 439, 605–611. [Google Scholar] [CrossRef]
- Dowsett, M.; Adriaens, A. The role of SIMS in cultural heritage studies. Nucl. Instrum. Methods Phys. Res. B 2004, 226, 38–52. [Google Scholar] [CrossRef]
- Smith, D.H.; Christie, W.H. A comparison of a theoretical model and sensitivity factor calculations for quantification of sims data. Int. J. Mass Spectrom. 1978, 26, 61–76. [Google Scholar] [CrossRef]
- Seah, M.P.; Gilmore, I.S. Simplified equations for correction parameters for elastic scattering effects in AES and XPS for Q, β and attenuation lengths. Surf. Interface Anal. 2001, 31, 835–846. [Google Scholar] [CrossRef]
- Kim, K.J.; Moon, D.W.; Park, C.J.; Simons, D.; Gillen, G.; Jin, H.; Kang, H.J. Quantitative surface analysis of Fe-Ni alloy films by XPS, AES and SIMS. Surf. Interface Anal. 2007, 39, 665–673. [Google Scholar] [CrossRef]
- Gu, C.J.; Stevie, F.A.; Hitzman, C.J.; Saripalli, Y.N.; Johnson, M.; Griffis, D.P. SIMS quantification of matrix and impurity species in AlxGa1−xN. Appl. Surf. Sci. 2006, 252, 7228–7231. [Google Scholar] [CrossRef]
- Py, M.; Barnes, J.P.; Hartmann, J.M. Quantification of germanium in Si1−xGex alloys by negative mode ToF-SIMS: The interest of the full spectrum method. Surf. Interface Anal. 2011, 43, 539–542. [Google Scholar] [CrossRef]
- Zhu, Z.; Ronsheim, P.; Turansky, A.; Hatzistergos, M.; Madan, A.; Pinto, T.; Holt, J.; Reznicek, A. SIMS quantification of SiGe composition with low-energy ion beams. Surf. Interface Anal. 2011, 43, 657–660. [Google Scholar] [CrossRef]
- Stevie, F.A.; Griffis, D.P. Quantification in dynamic SIMS: Current status and future needs. Appl. Surf. Sci. 2008, 255, 1364–1367. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Yang, C.; Gao, J.; Xiong, Z.; Zhong, L.; Zhang, Y.; Son, J. Molecular detection of per-and polyfluoroalkyl substances in water using time-of-flight secondary ion mass spectrometry. Front. Chem. 2023, 11, 1253685. [Google Scholar] [CrossRef]
- Belu, A.M.; Graham, D.J.; Castner, D.G. Time-of-flight secondary ion mass spectrometry: Techniques and applications for the characterization of biomaterial surfaces. Biomaterials 2003, 24, 3635–3653. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, I.S.; Seah, M.P. Static SIMS inter-laboratory study. Surf. Interface Anal. 2000, 29, 624–637. [Google Scholar] [CrossRef]
- Li, M.; Seyeux, A.; Wiame, F.; Marcus, P.; Światowska, J. Localized corrosion induced surface modifications of Al-Cu-Li alloy studied by ToF-SIMS 3D imaging. npj Mater. Degrad. 2021, 5, 23. [Google Scholar] [CrossRef]
- Esmaily, M.; Malmberg, P.; Shahabi-Navid, M.; Svensson, J.E.; Johansson, L.G. A ToF-SIMS investigation of the corrosion behavior of Mg alloy AM50 in atmospheric environments. Appl. Surf. Sci. 2016, 360, 98–106. [Google Scholar] [CrossRef]
- Seyeux, A.; Liu, M.; Schmutz, P.; Song, G.; Atrens, A.; Marcus, P. ToF-SIMS depth profile of the surface film on pure magnesium formed by immersion in pure water and the identification of magnesium hydride. Corros. Sci. 2009, 51, 1883–1886. [Google Scholar] [CrossRef]
- Gulbrandsen, E.; Taftø, J.; Olsen, A. The passive behaviour of Mg in alkaline fluoride solutions. Electrochemical and electron microscopical investigations. Corros. Sci. 1993, 34, 1423–1440. [Google Scholar] [CrossRef]
- Seyeux, A.; Frankel, G.S.; Missert, N.; Unocic, K.A.; Klein, L.H.; Galtayries, A.; Marcus, P. ToF-SIMS imaging study of the early stages of corrosion in Al-Cu thin films. J. Electrochem. Soc. 2011, 158, C165. [Google Scholar] [CrossRef]
- Sun, R.; Yao, M.; Zhang, J.; Wang, H.; Lin, X.; Zhang, W.; Qiu, Y.; Yang, J.; Cheng, G.; Dong, J. Effect of Y on the corrosion behavior of Fe22Cr5Al3Mo alloy in 500 °C super-heated steam. Corros. Sci. 2022, 196, 110022. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, L.; Wang, J.; Zeng, Y.; Li, J. Characterization of nitride-based LED materials and devices using TOF-SIMS. Surf. Interface Anal. 2014, 46 (Suppl. S1), 299–302. [Google Scholar] [CrossRef]
- Bamford, S.E.; Jones, R.T.; Gardner, W.; Muir, B.W.; Winkler, D.A.; Pigram, P.J. Profiling a low emissivity glass coating with ToF-SIMS and machine learning. Adv. Mater. Interfaces 2023, 2300645. [Google Scholar] [CrossRef]
- Byrne, C.; Brennan, B.; Lundy, R.; Bogan, J.; Brady, A.; Gomeniuk, Y.Y.; Monaghan, S.; Hurley, P.K.; Hughes, G. Physical, chemical and electrical characterisation of the diffusion of copper in silicon dioxide and prevention via a CuAl alloy barrier layer system. Mater. Sci. Semicond. Process. 2017, 63, 227–236. [Google Scholar] [CrossRef]
- Marseilhan, D.; Barnes, J.P.; Fillot, F.; Hartmann, J.M.; Holliger, P. Quantification of SiGe layer composition using MCs+ and MCs2+ secondary ions in ToF-SIMS and magnetic SIMS. Appl. Surf. Sci. 2008, 255, 1412–1414. [Google Scholar] [CrossRef]
- Mihara, I.; Nakagawa, K.; Kudo, M.; Aoyagi, S. Evaluation of layered titanate nanosheets using TOF-SIMS and G-SIMS analysis. Surf Interface Anal. 2012, 45, 453–456. [Google Scholar] [CrossRef]
- Światowska-Mrowiecka, J.; Martin, F.; Maurice, V.; Zanna, S.; Klein, L.; Castle, J.; Marcus, P. The distribution of lithium intercalated in V2O5 thin films studied by XPS and ToF-SIMS. Electrochim. Acta 2008, 53, 4257–4266. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Yao, J.; Matthews, B.; Spurgeon, S.R.; Riechers, S.; Sevigny, G.; Zhu, Z.; Jiang, W.; Luscher, W. Evidence of lithium mobility under neutron irradiation. J. Mater. Res. Technol. 2021, 14, 475–483. [Google Scholar] [CrossRef]
- Jiang, S.P.; Zhen, Y. Mechanism of Cr deposition and its application in the development of Cr-tolerant cathodes of solid oxide fuel cells. Solid State Ion. 2008, 179, 1459–1464. [Google Scholar] [CrossRef]
- Eriksson, C.; Börner, K.; Nygren, H.; Ohlson, K.; Bexell, U.; Billerdahl, N.; Johansson, M. Studies by imaging TOF-SIMS of bone mineralization on porous titanium implants after 1 week in bone. Appl. Surf. Sci. 2006, 252, 6757–6760. [Google Scholar] [CrossRef]
- Göttlicher, M.; Rohnke, M.; Helth, A.; Leichtweiss, T.; Gemming, T.; Gebert, A.; Eckert, J.; Janek, J. Controlled surface modification of Ti-40Nb implant alloy by electrochemically assisted inductively coupled RF plasma oxidation. Acta Biomater. 2013, 9, 9201–9210. [Google Scholar] [CrossRef]
- Lu, X.; Leng, Y.; Weng, L.-T. TOF-SIMS study of bone mineralization on alkali-treated Ti alloy. J. Mater. Sci. 2004, 39, 6809–6811. [Google Scholar] [CrossRef]
- Malmberg, P.; Lopes, V.R.; Billstrom, G.H.; Gallinetti, S.; Illies, C.; Linder, L.K.B.; Birgersson, U. Targeted ToF-SIMS analysis of macrophage content from a human cranial triphasic calcium phosphate implant. ACS Appl. Bio Mater. 2021, 4, 6791–6798. [Google Scholar] [CrossRef]
- Malmberg, P.; Nygren, H. Methods for the analysis of the composition of bone tissue, with a focus on imaging mass spectrometry (TOF-SIMS). Proteomics 2008, 8, 3755–3762. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Stevie, F.A.; Bennett, J.; Garcia, R.; Griffis, D.P. Back side SIMS analysis of hafnium silicate. Appl. Surf. Sci. 2006, 252, 7179–7181. [Google Scholar] [CrossRef]
- Holliger, P.; Laugier, F.; Dupuy, J.C. SIMS depth profiling of ultrashallow P, Ge and As implants in Si using MCs2+ ions. Surf. Interface Anal. 2002, 34, 472–476. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Kim, H.; Lee, K.-B.; Lee, Y. Effect of the impurity incorporation on the performance of Cu(In,Ga)Se2 semiconductor solar cells. J. Nanosci. Nanotechnol. 2016, 16, 10748–10752. [Google Scholar] [CrossRef]
- Seol, J.B.; Lee, B.H.; Choi, P.; Lee, S.G.; Park, C.G. Combined nano-SIMS/AFM/EBSD analysis and atom probe tomography, of carbon distribution in austenite/epsilon-martensite high-Mn steels. Ultramicroscopy 2013, 132, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Anderson, S. Using direct solid sampling ICP-MS to complement SEM-EDX and SIMS in characterizing semiconductor materials. AIP Conf. Proc. 2003, 683, 715–719. [Google Scholar]
- Napolitani, E.; Carnera, A.; Privitera, V.; Priolo, F. Ultrashallow profiling of semiconductors by secondary ion mass spectrometry. Mater. Sci. Semicond. Process. 2001, 4, 55–60. [Google Scholar] [CrossRef]
- Andersen, D.; Chen, H.; Pal, S.; Cressa, L.; De Castro, O.; Wirtz, T.; Schmitz, G.; Eswara, S. Correlative high-resolution imaging of hydrogen in Mg2Ni hydrogen storage thin films. Int. J. Hydrog. Energy 2023, 48, 13943–13954. [Google Scholar] [CrossRef]
- De Castro, O.; Audinot, J.N.; Hoang, H.Q.; Coulbary, C.; Bouton, O.; Barrahma, R.; Ost, A.; Stoffels, C.; Jiao, C.; Dutka, M.; et al. Magnetic sector secondary ion mass spectrometry on FIB-SEM instruments for nanoscale chemical imaging. Anal. Chem. 2022, 94, 10754–10763. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, K.L.; Bennison, S.J.; Mikeska, K.R.; Levi-Setti, R. Grain boundary chemistry of alumina by high-resolution imaging SIMS. Acta Mater. 1999, 47, 4031–4039. [Google Scholar] [CrossRef]
- Gu, C.J. SIMS Quantification of Matrix and Impurity Species in III-Nitride Alloys; North Carolina State University: Raleigh, NC, USA, 2005. [Google Scholar]
- Matsuda, M. Lattice and grain boundary diffusion of Ca in polycrystalline yttria-stabilized ZrO2 determined by employing SIMS technique. Solid State Ion. 1998, 111, 301–306. [Google Scholar] [CrossRef]
- Mazdab, F.K. Characterization of flux-grown trace-element-doped titanite using the high-mass-resolution ion microprobe (Shrimp-Rg). Can. Mineral. 2009, 47, 813–831. [Google Scholar] [CrossRef][Green Version]
- Lan, Z.; Li, X.-H.; Zhang, Q.; Li, Q.-L. Global synchronous initiation of the 2nd episode of Sturtian glaciation: SIMS zircon U–Pb and O isotope evidence from the Jiangkou Group, South China. Precambrian Res. 2015, 267, 28–38. [Google Scholar] [CrossRef]
- Lobach-Zhuchenko, S.B.; Kaulina, T.V.; Marin, Y.B.; Yurchenko, A.V.; Skublov, S.G.; Egorova, Y.S.; Galankina, O.L.; Sergeev, S.A. Paleoarchean U–Pb (SIMS SHRIMP-II) age of mafic granulites from the bug complex, Ukrainian Shield. Dokl. Earth Sci. 2019, 484, 101–104. [Google Scholar] [CrossRef]
- Tung, K.; Yang, H.-J.; Yang, H.-Y.; Liu, D.; Zhang, J.; Wan, Y.; Tseng, C.-Y. SHRIMP U-Pb geochronology of the zircons from the Precambrian basement of the Qilian Block and its geological significances. Chin. Sci. Bull. 2007, 52, 2687–2701. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Zhang, D.; Miao, L.; Li, T.; Xie, H.; Meng, Q.; Liu, D. Zircon SHRIMP U-Pb dating of meta-diorite from the basement of the Songliao Basin and its geological significance. Chin. Sci. Bull. 2006, 51, 1877–1883. [Google Scholar] [CrossRef]
- McKibbin, S.J.; Ireland, T.R.; Amelin, Y.; Holden, P.; Sugiura, N. A re-evaluation of the Mn–Cr systematics of olivine from the angrite meteorite D’Orbigny using secondary ion mass spectrometry. Geochim. Cosmochim. Acta. 2013, 123, 181–194. [Google Scholar] [CrossRef]
- McKibbin, S.J.; Ireland, T.R.; Amelin, Y.; O’Neill, H.S.C.; Holden, P. Mn–Cr relative sensitivity factors for secondary ion mass spectrometry analysis of Mg–Fe–Ca olivine and implications for the Mn–Cr chronology of meteorites. Geochim. Cosmochim. Acta 2013, 110, 216–228. [Google Scholar] [CrossRef]
- Merle, R.E.; Nemchin, A.A.; Whitehouse, M.J.; Snape, J.F.; Kenny, G.G.; Bellucci, J.J.; Connelly, J.N.; Bizzarro, M. Pb-Pb ages and initial Pb isotopic composition of lunar meteorites: NWA 773 clan, NWA 4734, and Dhofar 287. Meteorit. Planet. Sci. 2020, 55, 1808–1832. [Google Scholar] [CrossRef] [PubMed]
- Pack, A.; Vogel, I.; Rollion-Bard, C.; Luais, B.; Palme, H. Silicon in iron meteorite metal. Meteorit. Planet. Sci. 2011, 46, 1470–1483. [Google Scholar] [CrossRef]
- Soens, B.; Suttle, M.D.; Maeda, R.; Vanhaecke, F.; Yamaguchi, A.; Van Ginneken, M.; Debaille, V.; Claeys, P.; Goderis, S. Evidence for the presence of chondrule- and CAI-derived material in an isotopically anomalous Antarctic micrometeorite. Meteorit. Planet. Sci. 2020, 55, 2703–2726. [Google Scholar] [CrossRef]
- Asplanato, P.; Zannouh, W.; Fauré, A.L.; Imbert, P.H.; Lautru, J.; Cornaton, M.; Dacheux, N.; Pointurier, F.; Clavier, N. Hydrothermal synthesis of homogenous and size-controlled uranium-thorium oxide micro-particles for nuclear safeguards. J. Nucl. Mater. 2023, 573, 154142. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Weber, P.K.; Pidduck, A.J.; Gaffney, A.M.; Girard, P.; Pointurier, F.; Hedberg, M.; Simons, A.J.; Stebelkov, V.; Kell, T.; et al. Uncovering uranium isotopic heterogeneity of fuel pellets from the fifth collaborative materials exercise of The Nuclear Forensics International Technical Working Group. J. Radioanal. Nucl. Chem. 2020, 326, 1853–1866. [Google Scholar] [CrossRef]
- Kips, R.; Weber, P.K.; Kristo, M.J.; Jacobsen, B.; Ramon, E.C. Microscale isotopic variation in uranium fuel pellets with implications for nuclear forensics. Anal. Chem. 2019, 91, 11598–11605. [Google Scholar] [CrossRef]
- Stebelkov, V.; Elantyev, I.; Hedberg, M.; Wallenius, M.; Fauré, A.L. Determination of isotopic composition of uranium in the CMX-4 samples by SIMS. J. Radioanal. Nucl. Chem. 2018, 315, 417–423. [Google Scholar] [CrossRef]
- Varga, Z.; Wallenius, M.; Nicholl, A.; Mayer, K. Laser ablation inductively coupled plasma mass spectrometry analysis of isotopically heterogeneous uranium materials. J. Radioanal. Nucl. Chem. 2022, 331, 4377–4385. [Google Scholar] [CrossRef]
- Alam, T.; Felfer, P.J.; Chaturvedi, M.; Stephenson, L.T.; Kilburn, M.R.; Cairney, J.M. Segregation of B, P, and C in the Ni-based superalloy, inconel 718. Phys. Metall. Mater. Sci. A 2012, 43, 2183–2191. [Google Scholar] [CrossRef]
- Da Rosa, G.; Maugis, P.; Portavoce, A.; Drillet, J.; Valle, N.; Lentzen, E.; Hoummada, K. Grain-boundary segregation of boron in high-strength steel studied by nano-SIMS and atom probe tomography. Acta Mater. 2020, 182, 226–234. [Google Scholar] [CrossRef]
- Seol, J.B.; Lim, N.S.; Lee, B.H.; Renaud, L.; Park, C.G. Atom probe tomography and nano secondary ion mass spectroscopy investigation of the segregation of boron at austenite grain boundaries in 0.5 wt.% carbon steels. Met. Mater. Int. 2011, 17, 413–416. [Google Scholar] [CrossRef]
- Usiobo, O.J.; Kanda, H.; Gratia, P.; Zimmermann, I.; Wirtz, T.; Nazeeruddin, M.K.; Audinot, J.-N. Nanoscale mass-spectrometry imaging of grain boundaries in perovskite semiconductors. J. Mater. Chem. C 2020, 124, 23230–23236. [Google Scholar] [CrossRef]
- Valle, N.; Drillet, J.; Bouaziz, O.; Migeon, H.N. Study of the carbon distribution in multi-phase steels using the NanoSIMS 50. Appl. Surf. Sci. 2006, 252, 7051–7053. [Google Scholar] [CrossRef]
- Xu, X.; Jiao, C.; Li, K.; Hao, M.; Moore, K.L.; Burnett, T.L.; Zhou, X. Application of high-spatial-resolution secondary ion mass spectrometry for nanoscale chemical mapping of lithium in an Al-Li alloy. Mater. Charact. 2021, 181, 111442. [Google Scholar] [CrossRef]
- Aboura, Y.; Martelo, D.F.; Morana, R.; Akid, R.; Moore, K.L. Characterising hydrogen induced cracking of alloy 625+ using correlative SEM—EDX and NanoSIMS. Corros. Sci. 2021, 181, 109228. [Google Scholar] [CrossRef]
- Li, K.; Aarholt, T.; Liu, J.; Hulme, H.; Garner, A.; Preuss, M.; Lozano-Perez, S.; Grovenor, C. 3D-characterization of deuterium distributions in zirconium oxide scale using high-resolution SIMS. Appl. Surf. Sci. 2019, 464, 311–320. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Sayers, J.; Aarholt, T.; He, G.; Hulme, H.; Garner, A.; Preuss, M.; Nordin, H.; Partezana, J.M.; et al. Characterisation of deuterium distributions in corroded zirconium alloys using high-resolution SIMS imaging. Acta Mater. 2020, 200, 581–596. [Google Scholar] [CrossRef]
- McMahon, G.; Miller, B.D.; Burke, M.G. High resolution NanoSIMS imaging of deuterium distributions in 316 stainless steel specimens after fatigue testing in high pressure deuterium environment. npj Mater. Degrad. 2018, 2, 2. [Google Scholar] [CrossRef]
- McMahon, G.; Miller, B.D.; Burke, M.G. Correlative NanoSIMS and electron microscopy methods for understanding deuterium distributions after fatigue testing of 304/304L stainless steel in deuterated water. Int. J. Hydrog. Energy 2020, 45, 20042–20052. [Google Scholar] [CrossRef]
- Tarzimoghadam, Z.; Rohwerder, M.; Merzlikin, S.V.; Bashir, A.; Yedra, L.; Eswara, S.; Ponge, D.; Raabe, D. Multi-scale and spatially resolved hydrogen mapping in a Ni–Nb model alloy reveals the role of the δ phase in hydrogen embrittlement of alloy 718. Acta Mater. 2016, 109, 69–81. [Google Scholar] [CrossRef]
- Mazenc, A.; Galtayries, A.; Seyeux, A.; Marcus, P.; Leclercq, S. ToF-SIMS study of the behavior of thermally oxidized films formed on nickel-based 690 alloy in high-temperature water. Surf. Interface Anal. 2012, 45, 583–586. [Google Scholar] [CrossRef]
- Felloni, L.; Fratesi, R.; Quadrini, E.; Roventi, G. Electrodeposition of zinc-nickel alloys from chloride solution. J. Appl. Electrochem. 1987, 17, 574–582. [Google Scholar] [CrossRef]
- de Jesus, R.A.; de Assis, G.C.; de Oliveira, R.J.; Bilal, M.; Bharagava, R.N.; Iqbal, H.M.; Ferreira, L.F.; Figueiredo, R.T. Metal oxide nanoparticles for environmental remediation. In Biodegradation and Biodeterioration at the Nanoscale; Iqbal, H.M.N., Bilal, M., Nguyen, T.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 529–560. [Google Scholar]
- Lee, Y.-H.; Kim, I.-H.; Kim, H.-K.; Kim, H.-G. Role of ZrO2 oxide layer on the fretting wear resistance of a nuclear fuel rod. Tribol. Int. 2020, 145, 106146. [Google Scholar] [CrossRef]
- Krings, L.H.M.; Tamminga, Y.; van Berkum, J.; Labohm, F.; van Veen, A.; Arnoldbik, W.M. Lithium depth profiling in thin electrochromic WO3 films. J. Vac. Sci. Technol. A 1999, 17, 198–205. [Google Scholar] [CrossRef]
- Weisener, C.; Gerson, A. An investigation of the Cu (II) adsorption mechanism on pyrite by ARXPS and SIMS. Miner. Eng. 2000, 13, 1329–1340. [Google Scholar] [CrossRef]
- Voras, Z.E.; deGhetaldi, K.; Wiggins, M.B.; Buckley, B.; Baade, B.; Mass, J.L.; Beebe, T.P., Jr. ToF-SIMS imaging of molecular-level alteration mechanisms in Le Bonheur de vivre by Henri Matisse. Appl. Phys. A 2015, 121, 1015–1030. [Google Scholar] [CrossRef]
- Bich, C.; Touboul, D.; Brunelle, A. Biomedical studies by TOF-SIMS imaging. Biointerphases 2014, 10, 018901. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.H.; Lee, K.-B.; Min, B.K.; Lee, Y. Improved quantitative analysis of Cu(In,Ga)Se2 thin films using MCs+-SIMS depth profiling. Appl. Phys. A 2014, 115, 1355–1364. [Google Scholar] [CrossRef]
- Priebe, A.; Barnes, J.P.; Edwards, T.E.J.; Huszar, E.; Petho, L.; Michler, J. Elemental characterization of Al nanoparticles buried under a Cu thin film: TOF-SIMS vs. STEM/EDX. Anal. Chem. 2020, 92, 12518–12527. [Google Scholar] [CrossRef]
- Schöppe, P.; Schönherr, S.; Wuerz, R.; Wisniewski, W.; Martínez-Criado, G.; Ritzer, M.; Ritter, K.; Ronning, C.; Schnohr, C.S. Rubidium segregation at random grain boundaries in Cu(In,Ga)Se2 absorbers. Nano Energy 2017, 42, 307–313. [Google Scholar] [CrossRef]
- Shatkov, G.A.; Berezhnaya, N.G.; Lepekhina, Y.N.; Rodionov, N.V.; Paderin, I.P.; Sergeyev, S.A. U-Pb (SIMS SHRIMP-II) age of volcanic rocks from the Tulukuev caldera (Streltsov Uranium-Ore Cluster, Eastern Transbaikalia). Dokl. Earth Sci. 2010, 432, 587–592. [Google Scholar] [CrossRef]
- Koga, K.; Hauri, E.; Hirschmann, M.; Bell, D. Hydrogen concentration analyses using SIMS and FTIR: Comparison and calibration for nominally anhydrous minerals. Geochem. Geophys. Geosyst. 2003, 4, 1019. [Google Scholar] [CrossRef]
- Hauri, E.; Wang, J.; Dixon, J.E.; King, P.L.; Mandeville, C.; Newman, S. SIMS analysis of volatiles in silicate glasses. Chem. Geol. 2002, 183, 99–114. [Google Scholar] [CrossRef]
- Devine, J.D.; Gardner, J.E.; Brack, H.P.; Layne, G.D.; Rutherford, M.J. Comparison of microanalytical methods for estimating H2O contents of silicic volcanic glasses. Am. Mineral. 1995, 80, 319–328. [Google Scholar] [CrossRef]
- Wasson, J.T.; Willis, J.; Wai, C.M.; Kracher, A. Origin of iron meteorite groups IAB and IIICD. Z. Naturforsch. A 1980, 35, 781–795. [Google Scholar] [CrossRef]
- Westbrook, J.H.; Wood, D.L. Embrittlement of grain boundaries by equilibrium segregation. Nature 1961, 192, 1280–1281. [Google Scholar] [CrossRef]
- Hauri, E.H.; Papineau, D.; Wang, J.; Hillion, F. High-precision analysis of multiple sulfur isotopes using NanoSIMS. Chem. Geol. 2016, 420, 148–161. [Google Scholar] [CrossRef]
- Dadfarnia, M.; Nagao, A.; Wang, S.; Martin, M.L.; Somerday, B.P.; Sofronis, P. Recent advances on hydrogen embrittlement of structural materials. Int. J. Fract. 2015, 196, 223–243. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Morita, H.; Nomura, Y.; Igaki, E. Visualizing lithiation of graphite composite anodes in all-solid-state batteries using operando time-of-flight secondary ion mass spectrometry. J. Phys. Chem. Lett. 2021, 12, 4623–4627. [Google Scholar] [CrossRef]
- Oudriss, A.; Le Guernic, S.; Wang, Z.; Osman Hoch, B.; Bouhattate, J.; Conforto, E.; Zhu, Z.; Li, D.S.; Feaugas, X. Meso-scale anisotropic hydrogen segregation near grain-boundaries in polycrystalline nickel characterized by EBSD/SIMS. Mater. Lett. 2016, 165, 217–222. [Google Scholar] [CrossRef]
- Perego, M.; Seguini, G.; Arduca, E.; Frascaroli, J.; De Salvador, D.; Mastromatteo, M.; Carnera, A.; Nicotra, G.; Scuderi, M.; Spinella, C.; et al. Thermodynamic stability of high phosphorus concentration in silicon nanostructures. Nanoscale 2015, 7, 14469–14475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Otto, S.-K.; Moryson, Y.; Krauskopf, T.; Peppler, K.; Sann, J.; Janek, J.; Henss, A. In-depth characterization of lithium-metal surfaces with XPS and ToF-SIMS: Toward better understanding of the passivation layer. Chem. Mater. 2021, 33, 859–867. [Google Scholar] [CrossRef]
- Amadelli, R.; Armelao, L.; Tondello, E.; Daolio, S.; Fabrizio, M.; Pagura, C.; Velichenko, A. A SIMS and XPS study about ions influence on electrodeposited PbO2 films. Appl. Surf. Sci. 1999, 142, 200–203. [Google Scholar] [CrossRef]
- Kellner, F.J.J.; Killian, M.S.; Yang, G.; Spiecker, E.; Virtanen, S. TEM and ToF-SIMS studies on the corrosion behavior of vanadium and chromium containing WC–Co hard metals in alkaline solutions. Int. J. Refract. Met. Hard Mater. 2011, 29, 376–383. [Google Scholar] [CrossRef]
- Grovenor, C.R.M.; Ni, N.; Hudson, D.; Yardley, S.S.; Moore, K.L.; Smith, G.D.W.; Lozano-Perez, S.; Sykes, J.M. Mechanisms of oxidation of fuel cladding alloys revealed by high resolution APT, TEM and SIMS analysis. MRS Proc. 2012, 1383, 101–112. [Google Scholar] [CrossRef]
- Tian, G.; Wang, J.; Zhang, C.; Wang, S.; Wang, B.; StJohn, D. Ultra-strong Mg alloy with nano-grain structures produced by a high-throughput magnetron co-sputtering method for the full chemistry spectra. J. Mater. Sci. 2022, 57, 21813–21827. [Google Scholar] [CrossRef]
- Choi, K.-J.; Shin, S.-H.; Kim, J.-J.; Jung, J.-A.; Kim, J.-H. Nano-structural and nano-chemical analysis of Ni-base alloy/low alloy steel dissimilar metal weld interfaces. Nucl. Eng. Technol. 2012, 44, 491–500. [Google Scholar] [CrossRef]
- Higgins, K.; Lorenz, M.; Ziatdinov, M.; Vasudevan, R.K.; Ievlev, A.V.; Lukosi, E.D.; Ovchinnikova, O.S.; Kalinin, S.V.; Ahmadi, M. Exploration of electrochemical reactions at organic–inorganic halide perovskite interfaces via machine learning in in situ time-of-flight secondary ion mass spectrometry. Adv. Funct. Mater. 2020, 30, 2001995. [Google Scholar] [CrossRef]
- Ievlev, A.V.; Belianinov, A.; Jesse, S.; Allison, D.P.; Doktycz, M.J.; Retterer, S.T.; Kalinin, S.V.; Ovchinnikova, O.S. Automated interpretation and extraction of topographic information from time of flight secondary ion mass spectrometry data. Sci. Rep. 2017, 7, 17099. [Google Scholar] [CrossRef]
- Pachuta, S.J. Enhancing and automating TOF-SIMS data interpretation using principal component analysis. App. Surf. Sci. 2004, 231–232, 217–223. [Google Scholar] [CrossRef]
- Pacholski, M.L. Principal component analysis of TOF-SIMS spectra, images and depth profiles: An industrial perspective. Appl. Surf. Sci. 2004, 231–232, 235–239. [Google Scholar] [CrossRef]
- Smentkowski, V.S.; Keenan, M.R.; Ohlhausen, J.A.; Kotula, P.G. Multivariate statistical analysis of concatenated time-of-flight secondary ion mass spectrometry spectral images. Complete description of the sample with one analysis. Anal. Chem. 2005, 77, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Vanden Eynde, X.; Bertrand, P. ToF-SIMS quantification of polystyrene spectra based on principal component analysis (PCA). Surf. Interface Anal. 1997, 25, 878–888. [Google Scholar] [CrossRef]
- Cossement, D.; Gouttebaron, R.; Cornet, V.; Viville, P.; Hecq, M.; Lazzaroni, R. PLA-PMMA blends: A study by XPS and ToF-SIMS. Appl. Surf. Sci. 2006, 252, 6636–6639. [Google Scholar] [CrossRef]
- Médard, N.; Poleunis, C.; Eynde, X.V.; Bertrand, P. Characterization of additives at polymer surfaces by ToF-SIMS. Surf. Interface Anal. 2002, 34, 565–569. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Paepegaey, P.Y.; McIntyre, N.S.; Harbottle, R.R.; Petersen, N.O. Principal component analysis of TOF-SIMS images of organic monolayers. Anal. Chem. 2002, 74, 5711–5716. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B. Interpretation of TOF-SIMS images: Multivariate and univariate approaches to image de-noising, image segmentation and compound identification. Appl. Surf. Sci. 2003, 203–204, 825–831. [Google Scholar] [CrossRef]
- Yang, L.; Lua, Y.Y.; Jiang, G.; Tyler, B.J.; Linford, M.R. Multivariate analysis of TOF-SIMS spectra of monolayers on scribed silicon. Anal. Chem. 2005, 77, 4654–4661. [Google Scholar] [CrossRef]
- Ferrari, S.; Ratner, B.D. ToF-SIMS quantification of albumin adsorbed on plasma-deposited fluoropolymers by partial least-squares regression. Surf. Interface Anal. 2000, 29, 837–844. [Google Scholar] [CrossRef]
- Sanni, O.D.; Wagner, M.S.; Briggs, D.; Castner, D.G.; Vickerman, J.C. Classification of adsorbed protein static ToF-SIMS spectra by principal component analysis and neural networks. Surf. Interface Anal. 2002, 33, 715–728. [Google Scholar] [CrossRef]
- Brüning, C.; Hellweg, S.; Dambach, S.; Lipinsky, D.; Arlinghaus, H.F. Improving the interpretation of ToF-SIMS measurements on adsorbed proteins using PCA. Surf. Interface Anal. 2006, 38, 191–193. [Google Scholar] [CrossRef]
- von Gradowski, M.; Wahl, M.; Förch, R.; Hilgers, H. Multivariate characterization of ultra-thin nanofunctional plasma polymer films using ToF-SIMS analysis. Surf. Interface Anal. 2004, 36, 1114–1118. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Henderson, A.; Jarvis, R.M.; Lockyer, N.P.; Vickerman, J.C.; Goodacre, R. Rapid discrimination of the causal agents of urinary tract infection using ToF-SIMS with chemometric cluster analysis. Appl. Surf. Sci. 2006, 252, 6869–6874. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.; Komorek, R.; Plymale, A.; Yu, R.; Wang, B.; Zhu, Z.; Liu, F.; Yu, X.Y. Characterization of syntrophic Geobacter communities using ToF-SIMS. Biointerphases 2017, 12, 05G601. [Google Scholar] [CrossRef] [PubMed]
- Sabale, S.; Barpaga, D.; Yao, J.; Kovarik, L.; Zhu, Z.; Chatterjee, S.; McGrail, B.P.; Motkuri, R.K.; Yu, X.Y. Understanding time dependence on Zinc metal-organic framework growth using in situ liquid secondary ion mass spectrometry. ACS Appl. Mater. Interfaces 2020, 12, 5090–5098. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wei, W.; Liu, F.; Yu, X.Y. Peak selection matters in principal component analysis: A case study of syntrophic microbes. Biointerphases 2019, 14, 051004. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhou, Y.; Zhang, F.; Zhang, Y.; Chen, J.; Zhu, Z.; Yu, X.Y. ToF-SIMS characterization of glyoxal surface oxidation products by hydrogen peroxide: A comparison between dry and liquid samples. Surf. Interface Anal. 2018, 50, 927–938. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, X.; Chen, J.; Zhu, Z.; Yu, X.-Y. Dark air–liquid interfacial chemistry of glyoxal and hydrogen peroxide. npj Clim. Atmos. Sci. 2019, 2, 28. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis; Springer: New York, NY, USA, 1986. [Google Scholar]
- Jaumot, J.; Tauler, R. Potential use of multivariate curve resolution for the analysis of mass spectrometry images. Analyst 2015, 140, 837–846. [Google Scholar] [CrossRef]
- Navarro-Reig, M.; Jaumot, J.; Baglai, A.; Vivo-Truyols, G.; Schoenmakers, P.J.; Tauler, R. Untargeted Comprehensive Two-Dimensional Liquid Chromatography Coupled with High-Resolution Mass Spectrometry Analysis of Rice Metabolome Using Multivariate Curve Resolution. Anal. Chem. 2017, 89, 7675–7683. [Google Scholar] [CrossRef]
- Lee, J.L.S.; Gilmore, I.S.; Seah, M.P. Quantification and methodology issues in multivariate analysis of ToF-SIMS data for mixed organic systems. Surf. Interface Anal. 2008, 40, 1–14. [Google Scholar] [CrossRef]
- Gallagher, N.B.; Shaver, J.M.; Martin, E.B.; Morris, J.; Wise, B.M.; Windig, W. Curve resolution for multivariate images with applications to TOF-SIMS and Raman. Chemometr. Intell. Lab. Syst. 2004, 73, 105–117. [Google Scholar] [CrossRef]
- de Juan, A.; Tauler, R. Chemometrics applied to unravel multicomponent processes and mixtures. Anal. Chim. Acta 2003, 500, 195–210. [Google Scholar] [CrossRef]
- Graham, D.J.; Castner, D.G. Multivariate analysis of ToF-SIMS data from multicomponent systems: The why, when, and how. Biointerphases 2012, 7, 49. [Google Scholar] [CrossRef]
- Karande, P.; Gallagher, B.; Han, T.Y.-J. A strategic approach to machine learning for material science: How to tackle real-world challenges and avoid pitfalls. Chem. Mater. 2022, 34, 7650–7665. [Google Scholar] [CrossRef]
- Schmidt, J.; Marques, M.R.G.; Botti, S.; Marques, M.A.L. Recent advances and applications of machine learning in solid-state materials science. npj Comput. Mater. 2019, 5, 83. [Google Scholar] [CrossRef]
- Trindade, G.F.; Abel, M.L.; Lowe, C.; Tshulu, R.; Watts, J.F. A time-of-flight secondary ion mass spectrometry/multivariate analysis (ToF-SIMS/MVA) approach to identify phase segregation in blends of incompatible but extremely similar resins. Anal. Chem. 2018, 90, 3936–3941. [Google Scholar] [CrossRef]
- Zhao, Y.; Otto, S.K.; Lombardo, T.; Henss, A.; Koeppe, A.; Selzer, M.; Janek, J.; Nestler, B. Identification of lithium compounds on surfaces of lithium metal anode with machine-learning-assisted analysis of ToF-SIMS spectra. ACS Appl. Mater. Interfaces 2023, 15, 50469–50478. [Google Scholar] [CrossRef]
- Heller, D.; Hagenhoff, B.; Engelhard, C. Time-of-flight secondary ion mass spectrometry as a screening method for the identification of degradation products in lithium-ion batteries—A multivariate data analysis approach. J. Vac. Sci. Technol. B 2016, 34, 03H138. [Google Scholar] [CrossRef]
- Griffin, L.A.; Gaponenko, I.; Zhang, S.; Bassiri-Gharb, N. Smart machine learning or discovering meaningful physical and chemical contributions through dimensional stacking. npj Comput. Mater. 2019, 5, 85. [Google Scholar] [CrossRef]
- Lombardo, T.; Kern, C.; Sann, J.; Rohnke, M.; Janek, J. Bridging the gap: Electrode microstructure and interphase characterization by combining ToF-SIMS and machine learning. Adv. Mater. Interfaces 2023, 10, 2300640. [Google Scholar] [CrossRef]
- Kornilov, A.; Safonov, I. An overview of watershed algorithm implementations in open source libraries. J. Imaging 2018, 4, 123. [Google Scholar] [CrossRef]
- Abbasi, K.; Smith, H.; Hoffman, M.; Farghadany, E.; Bruckman, L.S.; Sehirlioglu, A. Dimensional stacking for machine learning in ToF-SIMS analysis of heterostructures. Adv. Mater. Interfaces 2021, 8, 2001648. [Google Scholar] [CrossRef]








| Mass Analyzer | Resolution | Practical Mass Range | Transmission | Mass Detection | Relative Sensitivity |
|---|---|---|---|---|---|
| Quadrupole | 102–103 | <103 | 0.01–0.1 | Sequential | 1 |
| Magnetic sector | 104 | >104 | 0.1–0.5 | Sequential | 10 |
| Time-of-flight | 100,000 | 103–104 | >0.5 | Parallel | 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Howard, L.; Yu, X.-Y. Secondary Ion Mass Spectral Imaging of Metals and Alloys. Materials 2024, 17, 528. https://doi.org/10.3390/ma17020528
Shen Y, Howard L, Yu X-Y. Secondary Ion Mass Spectral Imaging of Metals and Alloys. Materials. 2024; 17(2):528. https://doi.org/10.3390/ma17020528
Chicago/Turabian StyleShen, Yanjie, Logan Howard, and Xiao-Ying Yu. 2024. "Secondary Ion Mass Spectral Imaging of Metals and Alloys" Materials 17, no. 2: 528. https://doi.org/10.3390/ma17020528
APA StyleShen, Y., Howard, L., & Yu, X.-Y. (2024). Secondary Ion Mass Spectral Imaging of Metals and Alloys. Materials, 17(2), 528. https://doi.org/10.3390/ma17020528







