A-C/Au Film with Low Humidity Sensitivity of Friction by Forming Au Transfer Film
Abstract
:1. Introduction
2. Materials and Methods
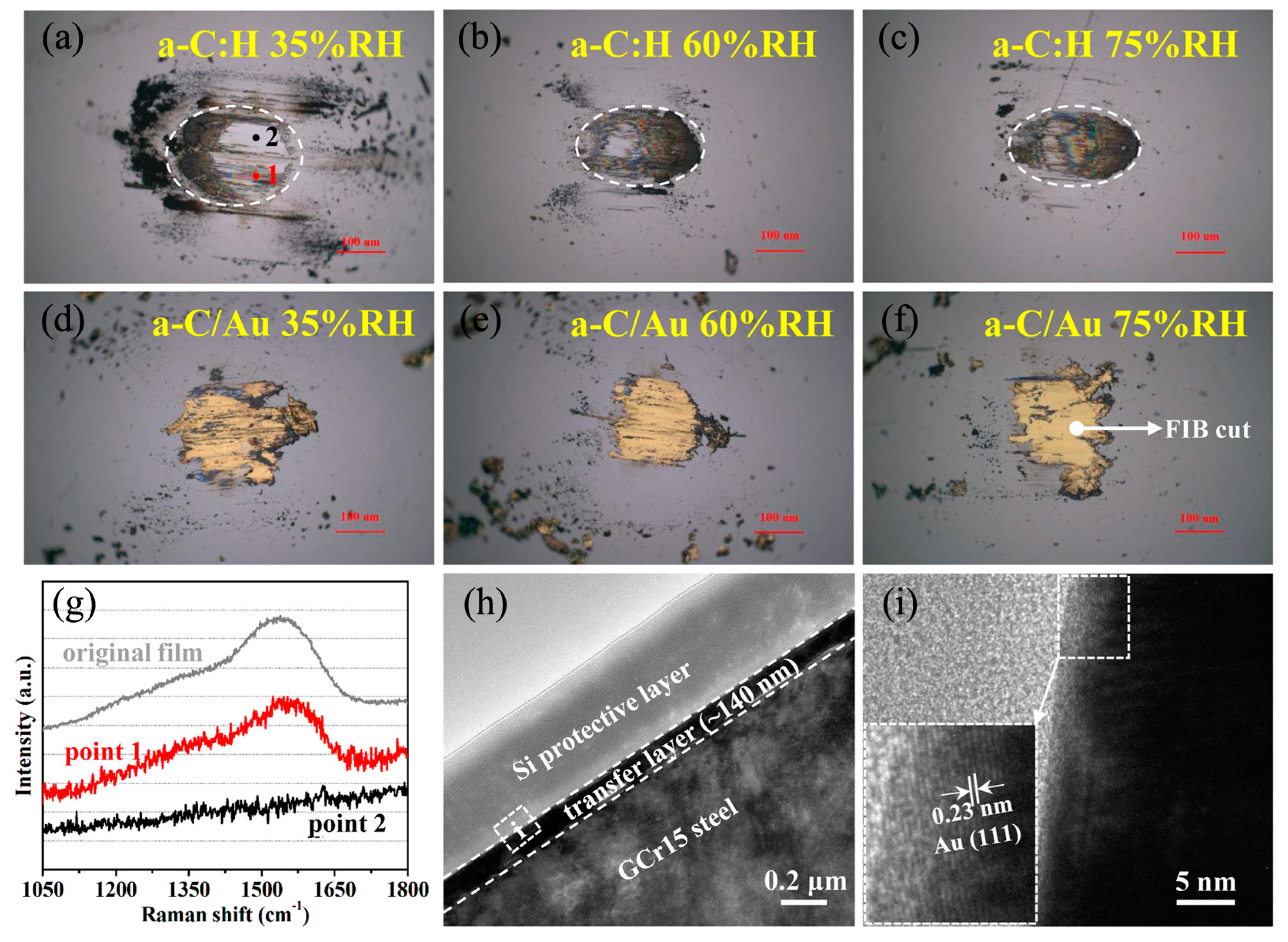
3. Results
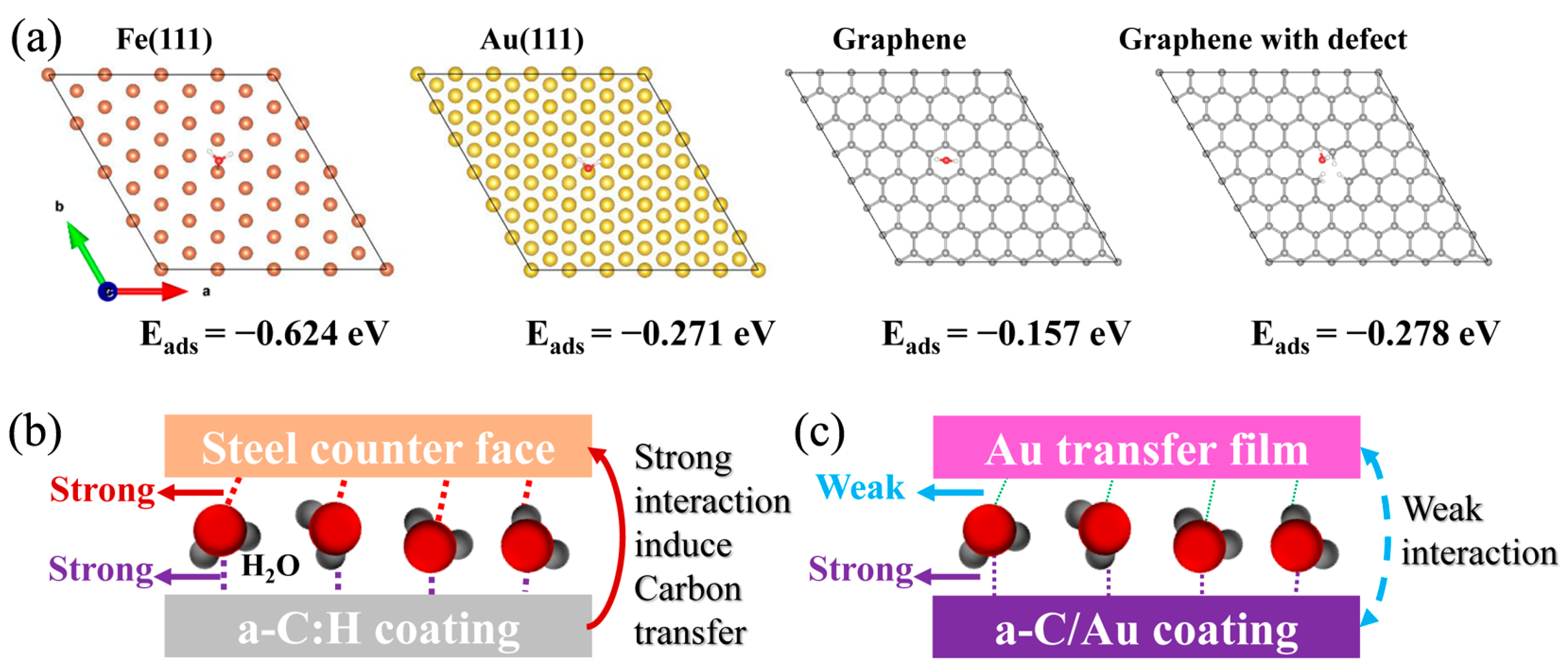
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajak, D.K.; Kumar, A.; Behera, A.; Menezes, P.L. Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Appl. Sci. 2021, 11, 4445. [Google Scholar] [CrossRef]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-Like Carbon Films. Materials 2021, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.X.; Li, H.X.; Ji, L.; Zhou, H.D.; Chen, J.M. Tribochemistry of superlubricating amorphous carbon films. Chem. Commun. 2021, 57, 11776–11786. [Google Scholar] [CrossRef] [PubMed]
- Ilberg, L.; Manis-Levy, H.; Raveh, A.; Lifshitz, Y.; Varenberg, M. Effect of structure of carbon films on their tribological properties. Diam. Relat. Mater. 2013, 38, 79–86. [Google Scholar] [CrossRef]
- Ronkainen, H.; Holmberg, K. Environmental and Thermal Effects on the Tribological Performance of DLC Coatings. In Tribology of Diamond-Like Carbon Films; Donnet, C., Erdemir, A., Eds.; Springer: Boston, MA, USA, 2008; pp. 155–200. [Google Scholar]
- Donnet, C.; Le Mogne, T.; Ponsonnet, L.; Belin, M.; Grill, A.; Patel, V.V.; Jahnes, C.V. The respective role of oxygen and water vapor on the tribology of hydrogenated diamond-like carbon coatings. Tribol. Lett. 1998, 4, 259–265. [Google Scholar] [CrossRef]
- Andersson, J.; Erck, R.A.; Erdemir, A. Frictional behavior of diamondlike carbon films in vacuum and under varying water vapor pressure. Surf. Coat. Tech. 2003, 163–164, 535–540. [Google Scholar] [CrossRef]
- Li, H.X.; Xu, T.; Wang, C.B.; Chen, J.M.; Zhou, H.D.; Liu, H.W. Friction behaviors of hydrogenated diamond-like carbon film in different environment sliding against steel ball. Appl. Surf. Sci. 2005, 249, 257–265. [Google Scholar] [CrossRef]
- Wang, J.J.; Cao, X.Q.; Zhang, G.G.; Lu, Z.B.; Xue, Q.J. The degradation of humidity sensitivity of friction for tetrahedral amorphous carbon film by spin-coating hexagonal boron nitride. Appl. Surf. Sci. 2020, 509, 145343. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xing, Z.Y.; Gao, K.X.; Yang, P.F.; Xu, C.L.; Wang, X.; Lin, Z. Tribology dependence on both structures of bulk a-C:H:Si films and transfer layer via adjustable Si content. Diam. Relat. Mater. 2023, 138, 110159. [Google Scholar] [CrossRef]
- Chen, X.C.; Yin, X.; Qi, W.; Zhang, C.H.; Choi, J.; Wu, S.D.; Wang, R.; Luo, J.B. Atomic-scale insights into the interfacial instability of superlubricity in hydrogenated amorphous carbon films. Sci. Adv. 2020, 6, 1272. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, L.; Xue, Q. Achieving Low Tribological Moisture Sensitivity by a-C:Si:Al Carbon-based Coating. Tribol. Lett. 2011, 43, 329–339. [Google Scholar] [CrossRef]
- Chen, T.; Wu, X.; Ge, Z.; Ruan, J.; Lv, B.; Zhang, J. Achieving low friction and wear under various humidity conditions by co-doping nitrogen and silicon into diamond-like carbon films. Thin Solid Films 2017, 638, 375–382. [Google Scholar] [CrossRef]
- Pei, L.L.; Chen, W.Q.; Ju, P.F.; Zhou, H.; Xu, Z.; Ji, L.; Ma, T.B.; Li, H.X.; Liu, X.H.; Zhou, H.D.; et al. Regulating Vacuum Tribological Behavior of a-C:H Film by Interfacial Activity. J. Phys. Chem. Lett. 2021, 12, 10333–10338. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.L.; Zhang, J.; Ji, L.; Ma, T.B.; Li, H.X.; Liu, X.H.; Zhou, H.D.; Chen, J.M. A novel strategy for improving tribological properties of a-C films in vacuum by Au doping and self-migration. Tribol. Int. 2024, 193, 109345. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. J. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Lee, W.Y.; Tokoroyama, T.; Umehara, N. Superlubricity with Graphitization in Ti-Doped DLC/Steel Tribopair: Response on Humidity and Temperature. ACS Appl. Mater. Interfaces 2023, 15, 19715–19729. [Google Scholar] [CrossRef] [PubMed]
- Scharf, T.W.; Prasad, S.V. Solid lubricants: A review. J. Mater. Sci. 2013, 48, 511–531. [Google Scholar] [CrossRef]
- Song, H.; Ji, L.; Li, H.X.; Liu, X.H.; Wang, W.Q.; Zhou, H.D.; Chen, J.M. External-Field-Induced Growth Effect of an a-C:H Film for Manipulating Its Medium-Range Nanostructures and Properties. ACS Appl. Mater. Interfaces 2016, 8, 6639–6645. [Google Scholar] [CrossRef]
- Gong, Z.B.; Shi, J.; Zhang, B.; Zhang, J.Y. Graphene nano scrolls responding to superlow friction of amorphous carbon. Carbon 2017, 116, 310–317. [Google Scholar] [CrossRef]


| Film Type | Deposition Pressure (Pa) | Source Gas | The Atomic Content of Au in the Film (at.%) | Film Thickness (μm) | Hardness (GPa) | Elasticity Modulus (GPa) | Surface Roughness (nm) |
|---|---|---|---|---|---|---|---|
| a-C:H | 0.64 | Ar:CH4 = 45:65 | 0 | 1.8 | 10.00 | 79.51 | 4.80 |
| a-C/Au | 0.5 | Only Ar | 16 | 1.18 | 5.55 | 96.84 | 6.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, L.; Ji, L.; Li, H.; Cai, H.; Xue, Y. A-C/Au Film with Low Humidity Sensitivity of Friction by Forming Au Transfer Film. Materials 2024, 17, 4941. https://doi.org/10.3390/ma17204941
Pei L, Ji L, Li H, Cai H, Xue Y. A-C/Au Film with Low Humidity Sensitivity of Friction by Forming Au Transfer Film. Materials. 2024; 17(20):4941. https://doi.org/10.3390/ma17204941
Chicago/Turabian StylePei, Lulu, Li Ji, Hongxuan Li, Haichao Cai, and Yujun Xue. 2024. "A-C/Au Film with Low Humidity Sensitivity of Friction by Forming Au Transfer Film" Materials 17, no. 20: 4941. https://doi.org/10.3390/ma17204941






