Regeneration and Single Stage Batch Adsorber Design for Efficient Basic Blue-41 Dye Removal by Porous Clay Heterostructures Prepared from Al13 Montmorillonite and Pillared Derivatives
Abstract
:1. Introduction
2. Materials and Characterization
2.1. Chemicals and Materials
2.2. Preparation of Al13 Intercalated Materials and Its Pillared Derivatives
2.3. Procedure to Prepare PCH Material
2.4. Exchange with C12amine Solution
2.5. Removal Process to Remove BB-41
2.6. Regeneration Studies
2.7. Characterization
3. Results and Discussion
3.1. XRF Analysis
3.2. Powder XRD Data
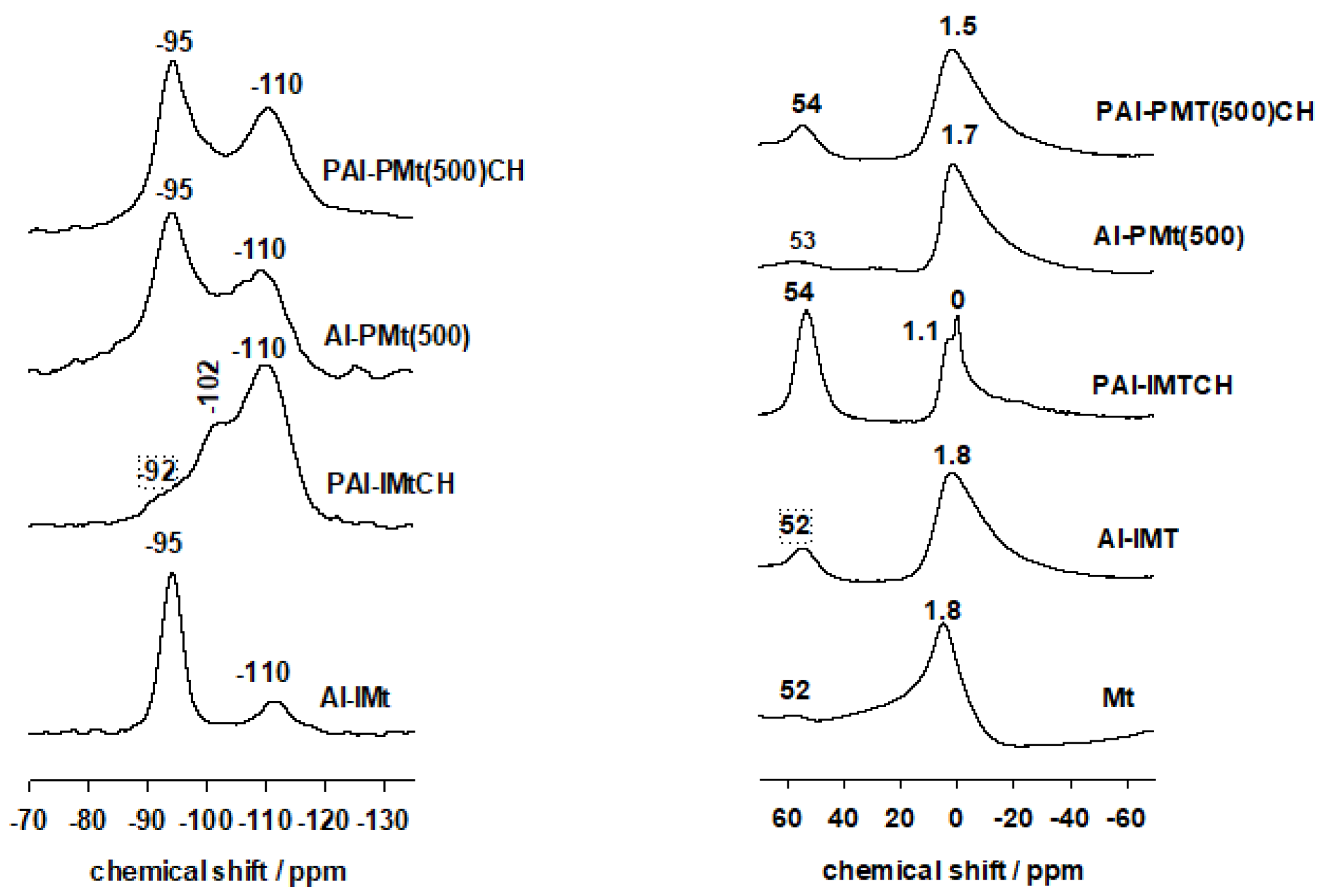
3.3. 29Si and 27Al MAS-NMR Data
3.4. Thermogravimetric Data
3.5. Textural Properties
3.6. Acidity Characterization
3.7. Influence of BB-41 Removal Parameters
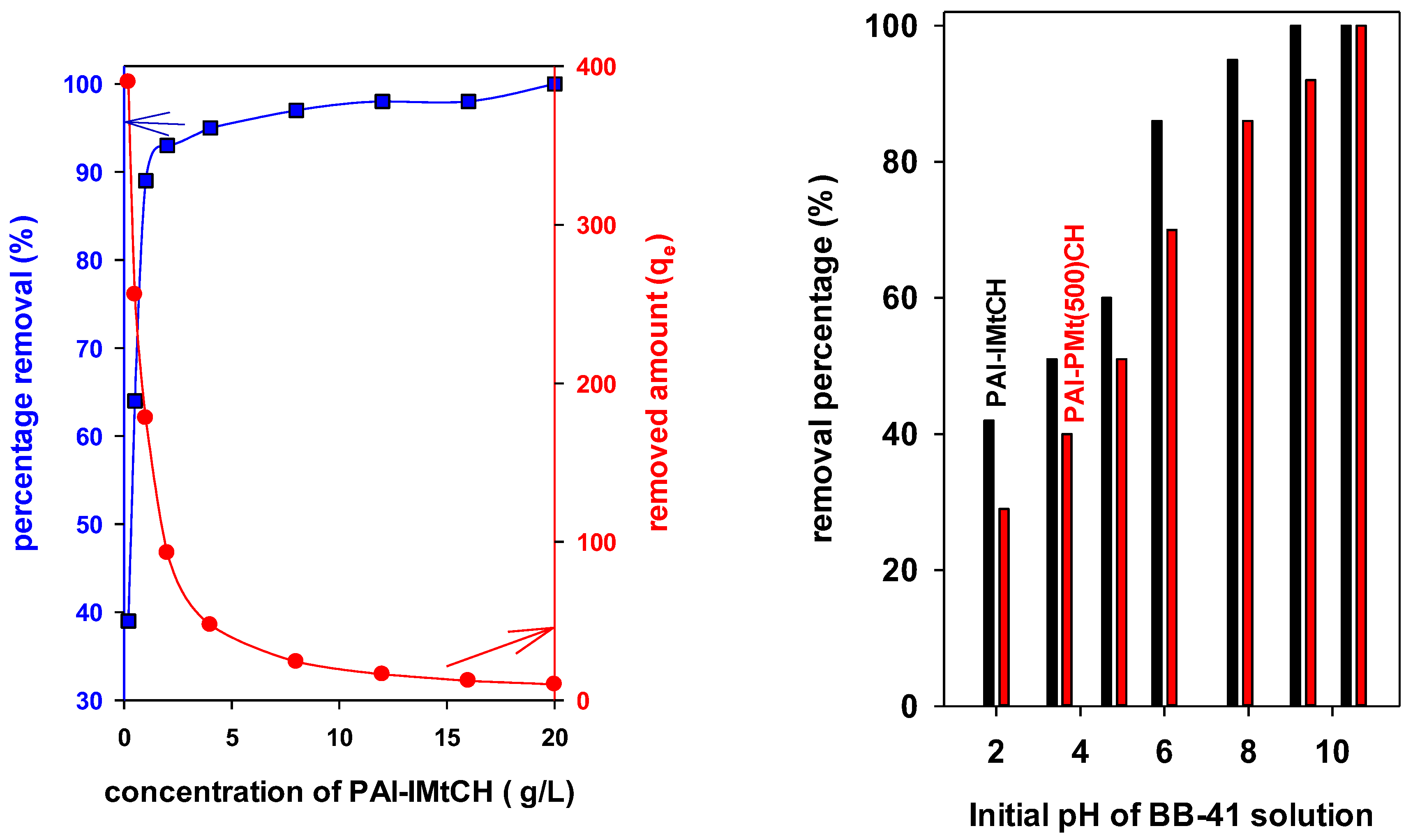
3.7.1. Effect of the Dose
3.7.2. Effect of Initial pH BB-41 Solution
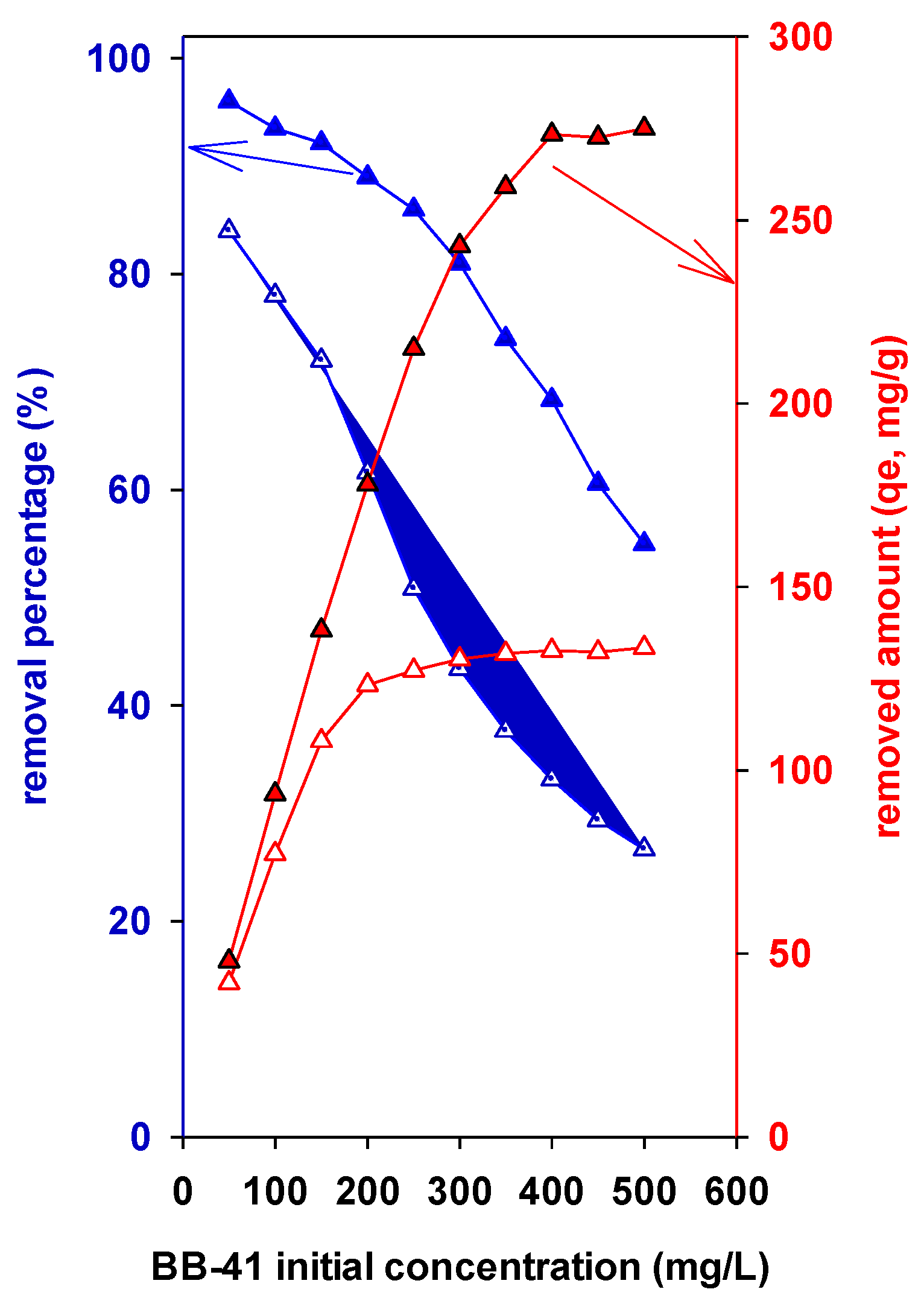
3.7.3. Effect of BB-41 Initial Concentration on Elimination Effectiveness and on the Removal Capacity
3.7.4. Effect of Calcination Temperature of Al-IMt
3.7.5. Modeling the Isotherm Data
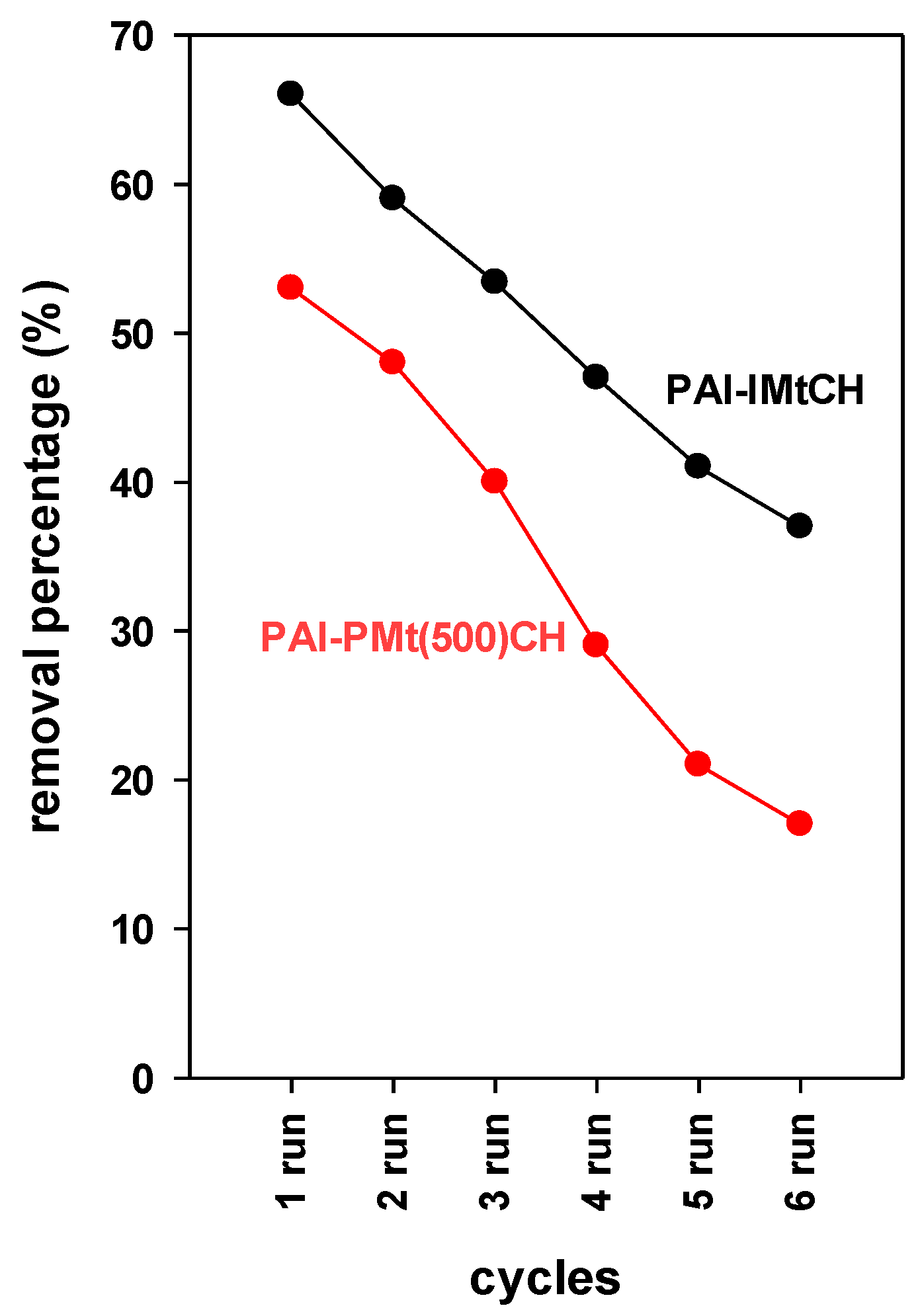
3.7.6. Regeneration Performance
3.7.7. Single-Stage Batch Design Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nisticò, R.A. Comprehensive Study on the Applications of Clays into Advanced Technologies, with a Particular Attention on Biomedicine and Environmental Remediation. Inorganics 2022, 10, 40. [Google Scholar] [CrossRef]
- Iva Ivanov, N.P.; Dran’kov, A.N.; Shichalin, O.O.; Lembikov, A.O.; Buravlev, I.Y.; Mayorov, V.Y.; Balanov, M.I.; Rogachev, K.A.; Kaspruk, G.D.; Pisarev, S.M.; et al. Composite magnetic sorbents based on magnetic Fe3O4 coated by Zn and Al layered double hydroxide for U(VI) removal from aqueous media. J. Radioanal. Nucl. Chem. 2024, 333, 1213–1230. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Yarusova, S.B.; Ivanov, N.P.; Papynov, E.K.; Belov, A.A.; Azon, S.A.; Yu Buravlev, I.; Myagchilov, A.V.; Fedorets, A.N.; Rastorguev, V.L.; et al. Calcium silicate solid-state matrices from boric acid production waste for 60Co removal and immobilization by spark plasma sintering. J. Water Process Eng. 2024, 59, 105042. [Google Scholar] [CrossRef]
- Qi, J.; Yu, J.; Shah, K.J.; Shah, D.D.; You, Z. Applicability of Clay/Organic Clay to Environmental Pollutants: Green Way-An Overview. Appl. Sci. 2023, 13, 9395. [Google Scholar] [CrossRef]
- Roy, M.; Saha, R. Dyes and their removal technologies from wastewater: A critical review. In Intelligent Data-Centric Systems, Intelligent Environmental Data Monitoring for Pollution Management; Bhattacharyya, S., Mondal, N.K., Platos, J., Snášel, V., Krömer, P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 127–160. [Google Scholar]
- Sivarajasekar, N.; Rajoo, B. Agriculture waste biomass valorisation for cationic dyes sequestration: A concise review. J. Chem. Pharm. Res. 2015, 79, 737–748. [Google Scholar]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.H.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Barakan, S.; Aghazadeh, V. The advantages of clay mineral modification methods for enhancing adsorption efficiency in wastewater treatment: A review. Environ. Sci. Pollut. Res. 2021, 28, 2572–2599. [Google Scholar] [CrossRef]
- Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: A review. RSC Adv. 2018, 8, 5197–5211. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Rengifo-Herrera, J.A.; Arredondo-López, S.M.; Marín-Flórez, A.; Sanabria-González, N.R. Research Trends on pillared interlayered clays (PILCs) used as catalysts in environmental and chemical processes: Bibliometric Analysis. Sci. World J. 2022, 2, 5728678. [Google Scholar] [CrossRef]
- Pandey, P.; Saini, V.K. Pillared interlayered clays: Sustainable materials for pollution abatement. Environ. Chem. Lett. 2019, 17, 721–727. [Google Scholar] [CrossRef]
- Najafi, H.; Farajfaed, S.; Zolgharnian, S.; Mosavi Mirak, S.H.; Asasian-Kolur, N.; Sharifian, S.A. comprehensive study on modified-pillared clays as an adsorbent in wastewater treatment processes. Process Saf. Environ. Prot. 2021, 147, 8–36. [Google Scholar] [CrossRef]
- Kloprogge, J.T. Synthesis of smectites and porous pillared clay catalysts: A review. J. Porous Mater. 1998, 5, 5–41. [Google Scholar] [CrossRef]
- Parodia, A.; Prasniski, J.A.; Bertella, F.; Pergher, S.B.C. Keggin-Al13 Polycations: Influence of Synthesis and Intercalation Parameters on the Structural Properties of Al-Pillared Clays. Minerals 2021, 11, 1211. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; Mccullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y. Brief history, preparation method, and biological application of mesoporous silica molecular sieves: A narrative review. Molecules 2023, 28, 2013. [Google Scholar] [CrossRef]
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis methods of mesoporous silica materials. Mater. Today-Proc. 2017, 4, 350–357. [Google Scholar] [CrossRef]
- Galarneau, A.; Barodawalla, A.; Pinnavaia, T. Porous clay heterostructures formed by gallery-templated synthesis. Nature 1995, 374, 529–531. [Google Scholar] [CrossRef]
- Garea, S.A.; Mihal, A.; Vasile, E.; Voicu, G. Synthesis and characterization of porous clay heterostructures. Rev. Chim. 2014, 65, 649–656. [Google Scholar]
- Cecilia, J.A.; García-Sancho, C.; Vilarrasa-García, E.; Jiménez-Jiménez, J.; Rodriguez-Castellón, E. Synthesis, characterization, uses and applications of porous clays heterostructures: A review. Chem. Rec. 2018, 18, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, L.; Kowalczyk, A.; Skoczek, M.; Rutkowska, M.; Gil, B.; Natkański, P.; Radko, M.; Motak, M.; Dębek, R.; Ryczkowski, J. Porous clay heterostructures intercalated with multicomponent pillars as catalysts for dehydration of alcohols. Appl. Clay Sci. 2018, 160, 116–125. [Google Scholar] [CrossRef]
- Yuan, M.; Deng, W.; Dong, S.; Li, Q.; Zhao, B.; Su, Y. Montmorillonite based porous clay heterostructures modified with Fe as catalysts for selective catalytic reduction of NO with propylene. Chem. Eng. J. 2018, 353, 839–848. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Soriano, M.D.; Natoli, A.; Rodríguez-Castellón, E.; López Nieto, J.M. Selective Oxidation of Hydrogen Sulfide to Sulfur Using Vanadium Oxide Supported on Porous Clay Heterostructures (PCHs) Formed by Pillars Silica, Silica-Zirconia or Silica-Titania. Materials 2018, 11, 1562. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Hbaieb, K.; Al-Faze, R. Preparation and catalytic activities of porous clay heterostructures from aluminium-intercalated clays: Effect of Al content. Clay Miner. 2017, 52, 521–535. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Hbaieb, K.; Al-Faze, R. Characterization and catalytic properties of porous clay heterostructures from zirconium intercalated clay and its pillared derivatives. Microp. Mesop. Mater. 2016, 226, 482–492. [Google Scholar] [CrossRef]
- Popoola, S.A.; Al Dmour, H.; Rakass, S.; Fatimah, I.; Liu, Y.; Mohmoud, A.; Kooli, F. Enhancement Properties of Zr Modified Porous Clay Heterostructures for Adsorption of Basic-Blue 41 Dye: Equilibrium, Regeneration, and Single Batch Design Adsorber. Materials 2022, 15, 5567. [Google Scholar] [CrossRef] [PubMed]
- Al Dmour, H.; Kooli, F.; Mohmoud, A.; Liu, Y.; Popoola, S.A. Al and Zr porous clay heterostructures as removal agents of Basic Blue 41 dye from an artificially polluted solution: Regeneration properties and batch design. Materials 2020, 14, 2528. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Jefri, O.A.; Gulfam Alam, M.; Al-Faze, R.; Kooli, F. Organo acid-activated clays for water treatment as removal agent of Eosin-Y: Properties, regeneration, and single batch design absorber. Heliyon 2024, 10, e30530. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Classification of Dye and Pigments, Dyes and Pigments; Springer: Berlin/Heidelberg, Germany, 2016; pp. 31–45. [Google Scholar]
- Kass, L. New stains for blood and bone marrow cells. Stain Technol. 1990, 65, 211–230. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Muñoz, H.J.; Blanco, C.; Gil, A.; Vicente, M.Á.; Galeano, L.A. Preparation of Al/Fe-Pillared Clays: Effect of the Starting Mineral. Materials 2017, 28, 1364. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Song, M.; Kim, D.; Jung, S.P.; Hwang, Y. Synthesis conditions of porous clay heterostructure (PCH) optimized for volatile organic compounds (VOC) adsorption. Korean J. Chem. Eng. 2019, 36, 1806–1813. [Google Scholar] [CrossRef]
- Byun, Y.; Seo, C.; Yun, T.; Joo, Y.; Jo, H.Y. Prediction of Na- and Ca-montmorillonite contents and swelling properties of clay mixtures using Vis-NIR spectroscopy. Geoderma 2023, 430, 116294. [Google Scholar] [CrossRef]
- Cardona, Y.; Korili, S.A.; Gil, A. Understanding the formation of Al13 and Al30 polycations to the development of microporous materials based on Al13-and Al30-PILC montmorillonites: A review. Appl. Clay Sci. 2021, 203, 10599. [Google Scholar] [CrossRef]
- Kooli, F. Pillared montmorillontes from unusual antiperspirant aqueous solutions: Characterization and catalytic tests. Microp. Mesop. Mater. 2013, 167, 228–236. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Wen, K.; Su, X.; Zhu, J.; He, H. A new insight into the compositional and structural control of porous clay heterostructures from the perspective of NMR and TEM. Microp. Mesop. Mater. 2016, 224, 285–293. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kustrowski, P.; Piwowarska, Z.; Dudek, B.; Gil, B.; Michalik, M. Montmorillonite, vermiculite and saponite based porous clay heterostructures modified with transition metals as catalysts for the DeNOx process. Appl. Catal. B Environ. 2009, 88, 331–340. [Google Scholar] [CrossRef]
- Rubiyanto, D.; Prakoso, N.I.; Sahroni, I.; Nurillahi, R.; Fatimah, I. ZnO-Porous Clay Heterostructure from Saponite as Green Catalyst for Citronellal Cyclization. Bull. Chem. React. Eng. Catal. 2020, 15, 137–145. [Google Scholar] [CrossRef]
- Rubiyanto, D.; Prakoso, N.I.; Citradewi, P.W.; Purwiandono, G.; Sagadevan, S.; Fatimah, I. Microwave-assisted synthesized porous clay heterostructure-Zn/Si from montmorillonite for citronellal conversion into isopulegol. Mater. Res. Express 2020, 7, 105006. [Google Scholar] [CrossRef]
- Son, Y.; Kim, T.H.; Kim, D.; Hwang, Y. Porous clay heterostructure with alginate encapsulation for toluene removal. Nanomaterials 2021, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F. Porous clay heterostructures (PCHs) from Al13-intercalated and Al13-pillared montmorillonites: Properties and heptane hydro-isomerization catalytic activity. Microp. Mesop. Mater. 2014, 184, 184–192. [Google Scholar] [CrossRef]
- Barakan, S.; Aghazadeh, V. Synthesis and characterization of hierarchical porous clay heterostructure from Al, Fe-pillared nano-bentonite using microwave and ultrasonic techniques. Microp. Mesop. Mater. 2019, 278, 138–148. [Google Scholar] [CrossRef]
- Pavón, E.; María, D. Alba, Swelling layered minerals applications: A solid state NMR overview. Prog. Nucl. Magn. Reson. Spectrosc. 2021, 124–125, 99–128. [Google Scholar] [CrossRef]
- Cuadros, J.; Wing-Dudekmas, T. NMR investigation of kaolinite-smectite structure using 6 Li and 29 Si with Mn exchange. Clay Miner. 2007, 42, 181–186. [Google Scholar] [CrossRef]
- Wang, F.; Nimmo, S.L.; Cao, B.; Mao, C. Oxide formation on biological nanostructures via a structure-directing agent: Towards an understanding of precise structural transcription. Chem. Sci. 2012, 3, 2639–2645. [Google Scholar] [CrossRef]
- Nunes, C.D.; Pires, J.; Carvalho, A.P.; Calhorda, M.J.; Ferreira, P. Synthesis and characterization of organo-silica hydrophobic clay heterostructures for volatile organic compounds removal. Microp. Mesop. Mater. 2008, 111, 612–619. [Google Scholar] [CrossRef]
- Plee, D.; Borg, F.; Gatineau, L.; Fripiat, J.J. High-resolution solid-state 27A1 and 29Si nuclear magnetic resonance study of pillared clays. J. Am. Chem. Soc. 1985, 107, 2362–2369. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Booy, E.; Jansen, J.B.H.; Geus, J.W. The Effect of Thermal Treatment on the Properties of Hydroxy-Al and Hydroxy-Ga Pillared Montmorillonite and Beidellite. Clay Miner. 1994, 29, 153–167. [Google Scholar] [CrossRef]
- Garea, S.A.; Mihai, A.I.; Vasile, E.; Nistor, C.; Sarbu, A.; Mitran, R. Synthesis of new porous clay heterostructures: The influence of co-surfactant type. Mater. Chem. Phys. 2016, 179, 17–26. [Google Scholar] [CrossRef]
- Kashif, M.; Chaeyeon Kang, C.; Siddhartha, T.R.; Che, C.A.; Su, Y.; Heynderickx, P.M. Investigating the effects of calcination temperature on porous clay heterostructure characteristics. Vacuum 2024, 227, 113145. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Hbaieb, K.; Al-Faze, R. Factors that affect the thermal stability and properties of Zr-porous clay heterostructures. J. Therm. Anal. Calorim. 2016, 126, 1143–1155. [Google Scholar] [CrossRef]
- Enotiadis, A.; Tsokaridou, M.; Chalmpes, N.; Sakavitsi, V.; Spyrou, K.; Gournis, D. Synthesis and characterization of porous clay-organic heterostructures. J. Sol-Gel Sci. Techn. 2019, 91, 295–301. [Google Scholar] [CrossRef]
- Garea, S.A.; Mihai, A.I.; Ghebaur, A.; Nistor, C.; Sarbu, A. Porous clay heterostructures: A new inorganic host for 5-fluorouracil 2 encapsulation. Int. J. Pharm. 2015, 491, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa-García, E.; Cecilia, J.A.; Azevedo, D.C.S.; Cavalcante, C.L.; Rodríguez-Castellón, E. Evaluation of porous clay heterostructures modified with amine species as adsorbent for the CO2 capture. Microp. Mesop. Mater. 2017, 249, 25–33. [Google Scholar] [CrossRef]
- Aguiar, J.E.; Cecilia, J.A.; Tavares, P.A.S.; Azevedo, D.C.S.; Castellón, E.R.; Lucena, S.M.P.; Silva, I.J. Adsorption study of reactive dyes onto porous clay heterostructures. Appl. Clay Sci. 2017, 135, 35–44. [Google Scholar] [CrossRef]
- Gil, A.; Trujillano, R.; Vicente, M.A.; Korili, S.A. Analysis of the structure of alumina-pillared clays by nitrogen and carbon dioxide adsorption. Adsorption Sci. Technol. 2007, 25, 217–226. [Google Scholar] [CrossRef]
- Mokaya, R.; Jones, W. Pillared Clays and Pillared Acid-Activated Clays: A Comparative-Study of Physical, Acidic, and Catalytic Properties. J. Catal. 1995, 153, 76–85. [Google Scholar] [CrossRef]
- Kooli, F.; Hian, P.C.; Weirong, Q.; Alshahateet, S.F.; Chen, F. Effect of the acid-activated clays on the properties of porous clay heterostructures. J. Porous Mater. 2006, 13, 319–324. [Google Scholar] [CrossRef]
- Perdigon, A.C.; Li, D.; Pesquera, C.; Gonzalez, F.; Ortiz, B.; Aguado, F.; Blanco, C. Synthesis of porous clay heterostructures from high charge mica-type aluminosilicates. J. Mater. Chem. A 2013, 1, 1213–1219. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Al Dmour, H.; Popoola, S.A.; Oudghiri Hassani, H.; Rakass, S.; Al-Faze, R.; Kooli, F. Parameters Synthesis of Na-Magadiite Materials for Water Treatment and Removal of Basic Blue-41: Properties and Single-Batch Design Adsorber. Inorganics 2023, 11, 423. [Google Scholar] [CrossRef]
- Al-Madanat, O.Y.; Popoola, S.A.; Al Dmour, H.; Al-Faze, R.; Kooli, F. Na-Kenyaite as Efficient Basic Blue-41 Dye Removal: Synthesis and Regeneration Studies. Water 2024, 16, 2056. [Google Scholar] [CrossRef]
- Mnasri Ghnimia, S.; Frini Strasra, N. A comparison of single and mixed pillared clays for -zinc and chromium cations removal. Appl. Clay Sci. 2018, 158, 150–157. [Google Scholar] [CrossRef]
- Aghazadeh, V.; Barakan, S.; Bidari, E. Determination of surface protonation-deprotonation behavior, surface charge, and total surface site concentration for natural, pillared and porous nano bentonite heterostructure. J. Mol. Struct. 2020, 1204, 127570. [Google Scholar] [CrossRef]
- Gil, A.; Assis, F.; Albeniz, S.; Korili, S. Removal of dyes from wastewaters by adsorption on pillared clays. Chem. Eng. Technol. 2011, 168, 1032–1040. [Google Scholar] [CrossRef]
- Daas, N.; Zaghouane-Boudiaf, H. Synthesis and characterization of porous bentonite adsorbent and its application to remove methylene blue dye from aqueous solutions. Desalin. Water Treat. 2022, 249, 271–280. [Google Scholar] [CrossRef]
- Guilhen, S.; Watanabe, T.; Silva, T.; Rovani, S.; Marumo, J.; Tenório, J.; Masek, O.; de Araujo, L. Role of point of zero charge in the adsorption of cationic textile dye on standard biochars from aqueous solutions: Selection criteria and performance assessment. Recent Progre. Mate. 2020, 4, 10. [Google Scholar] [CrossRef]
- Abbas, M.; Harrache, Z.; Trari, M. Mass-transfer processes in the adsorption of Crystal Violet (CV) by activated carbon derived from pomegranate peels: Kinetics and thermodynamic studies. J. Eng. Fibers Fabr. 2020, 15, 1–11. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A.; Dotto, G.L. Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Ávila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 19–51. [Google Scholar]
- Humelnicu, I.; Baiceanu, A.; Ignat, M.; Dulman, V. The removal of Basic Blue 41 textile dye from aqueous solution by adsorption onto natural zeolitic tuff: Kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 105, 274–287. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Al-Faze, R.; Al Suhaimi, A. Effect of acid activation of Saudi local clay mineral on removal properties of basic blue 41 from an aqueous solution. Appl. Clay Sci. 2015, 116–117, 23–30. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L.; Al-Faze, R.; Al-Sehimi, A. Removal enhancement of basic blue 41 by waste brick from an aqueous solution. Arabian J. Chem. 2015, 8, 333–342. [Google Scholar] [CrossRef]
- Gougazeh, M.; Kooli, F.; Buhl, J.-C. Removal efficiency of basicblue41 by three zeolites prepared from natural Jordanian kaolinite. Clays Clay Miner. 2019, 67, 143–153. [Google Scholar] [CrossRef]
- Kul, A.R.; Aldemir, A.; Koyuncu, H. An investigation of natural and modified diatomite performance for adsorption of Basic Blue 41: Isotherm, kinetic, and thermodynamic studies. Desalination Water Treat. 2021, 229, 384–394. [Google Scholar] [CrossRef]
- Lellou, S.; Kadi, S.; Ouadjenia, F.; Benhebal, H.; Schott, J.; Marouf, R. Reda Marouf. Synthesis and application of montmorillonite nanocomposites/phenolic resins for the elimination of Basic Blue 41. Desalination Water Treat. 2021, 218, 389–400. [Google Scholar] [CrossRef]
- Zarezadeh-Mehrizi, M.; Badiei, A.R. Highly efficient removal of Basic Blue 41 with nanoporous silica. Water Resour. Ind. 2014, 5, 49–57. [Google Scholar] [CrossRef]
- Rápó, E.; Tonk, S. Factors affecting synthetic dye adsorption; desorption studies: A review of results from the last five years (2017–2021). Molecules 2021, 26, 5419. [Google Scholar] [CrossRef]
- Santos, D.H.D.S.; Xiao, Y.; Chaukura, N.; Hill, J.M.; Selvasembian, R.; Zanta, C.L.P.S.; Meili, L. Regeneration of dye-saturated activated carbon through advanced oxidative processes: A review. Heliyon 2022, 8, e10205. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A comprehensive review on the chemical regeneration of biochar adsorbent for sustainable wastewater treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Vasanth Kumar, K.; Porkodi, K. A review on the adsorption isotherms and design calculations for the optimization of Batch adsorber design for different solution volume/adsorbent mass ratios using the experimental equilibrium data with fixed solution volume/adsorbent mass ratio of malachite green onto orange peel. Dyes Pigm. 2007, 74, e590–e594. [Google Scholar]
- Ledesma, B.; Sabio, E.; González-García, C.M.; Román, S.; Fernandez, M.E.; Bonelli, P.; Cukierman, A.L. Batch and Continuous Column Adsorption of p-Nitrophenol onto Activated Carbons with Different Particle Sizes. Processes 2023, 11, 2045. [Google Scholar] [CrossRef]
- Patel, H.; Batch Debnath, S.; Ballav, N.; Maity, A.; Pillay, K. Single stage batch adsorber design for efficient Eosin yellow removal by polyaniline coated ligno-cellulose. Int. J. Biol. Macromol. 2015, 72, 732–739. [Google Scholar]




| Samples | Mt | Al-IMt | Al-PMt(300) | Al-PMt(400) | Al-PMt(500) | Al-PMt(600) | Al-PMt(700) | Al-PMt(800) |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 56 | (79.9) * | 57.9 (63.3) | 56.4 (63.9) | 57.2 (62.2) | 57.7 (61.1) | 57.7 (61.1) | 58.8 (61.9) |
| Al2O3 | 22.4 | 22.2 (5.54) | 23.5 (17.9) | 22.8 (16.3) | 23.8 (18.7) | 23.3 (17.7) | 23.1 (18.6) | 24.1 (19.2) |
| MgO | 3.00 | 2.23 (0.66) | 2.27 (1.68) | 2.21 (1.59) | 2.28 (1.72) | 2.26 (1.68) | 2.28 (1.79) | 2.38 (1.87) |
| CaO | 1.77 | 0.05 (0.014) | 0.049 (0.042) | 0.055 (0.033) | 0.047 (0.026) | 0.047 (0.052) | 0.653 (0.523) | 0.692 (0.547) |
| Fe2O3 | 0.884 | 0.669 (0.139) | 0.0663 (0.435) | 0.628 (0.467) | 0.063 (0.528) | 0.643 (0.506) | 0.046 (0.044) | 0.040 (0.036) |
| TiO2 | 0.236 | 0.019 (0.03) | 0.213 (0.144) | 0.224 (0.164) | 0.204 (0.164) | 0.204 (0.164) | 0.206 (0.151) | 0.223 (0.169) |
| K2O | 0.108 | 0.062 (0.03) | 0.096 (0.054) | 0.084 (0.055) | 0.072 (0.062) | 0.088 (0.062) | 0.079 (0.069) | 0.066 (0.062) |
| Samples | SBET (m2/g) | Vµp (cm3/g) | Sext (m2/g) | T.P.V (cc/g) | A.P.D (nm) |
|---|---|---|---|---|---|
| Mt | 90 | 0.0 | 95 | 0.051 | 5.8 |
| Al-IMt | 880 (318) | 0.0 (0.098) | 880 (134) | 0.851 (0.257) | 3.82 (3.23) |
| Al-PMt(300) | 383 (269) | 0.024(0.078) | 335 (117) | 0.310 (0.216) | 3.22 (3.21) |
| Al-PMt(400) | 453 (285) | 0.024 (0.087) | 400 (121) | 0.389 (0.242) | 3.43 (3.34) |
| Al-PMt(500) | 360 (281) | 0.043 (0.086) | 275 (160) | 0.239 (0.237) | 2.65 (3.37) |
| Al-PMt(600) | 380 (227) | 0.020 (0.06) | 329 (113) | 0.245 (0.239) | 2.63 (3.58) |
| Al-PM(700) | 340 (165) | 0.01 (0.037) | 303 (94) | 0.170 (0.21) | 2.48 (4.1) |
| Al-PMt(800) | 244 (30) | 0.0 (0) | 244 (30) | 0.157 (0.075) | 2.57 (9.9) |
| Samples | Acidity * | Samples | Acidity * |
|---|---|---|---|
| Mt(500) | 0.51 | --- | --- |
| Al-IMt | 0.32 | PAl-IMtCH | 0.60 |
| Al-PMt(300) | 0.71 | PAl-PMt(300)CH | 0.73 |
| Al-PMt(400) | 0.58 | PAl-PMt(400)CH | 0.62 |
| Al-PMt(500) | 0.49 | PAl-PMt(500)CH | 0.58 |
| Al-PMt(600) | 0.47 | PAl-PMt(600)CH | 0.59 |
| Al-PMt(700) | 0.34 | PAl-PMt(700)CH | 0.59 |
| Al-PMt(800) | 0.29 | PAl-PMt(800)CH | 0.59 |
| Samples | qmax (mg/g) | KL (g/L) | R2 | PCC * | Γmax, mg/m2 |
|---|---|---|---|---|---|
| Mt | 57 | 0.0289 | 0.9854 | 0.9993 | 0.633 |
| Al-PMt(500) | 88 | 0.0564 | 0.9999 | 0.9999 | 0.313 |
| PAl-IMtCH | 274 | 0.2280 | 0.9996 | 0.9997 | 0.311 |
| PAl-PMt(300)CH | 142 | 0.1635 | 0.9998 | 0.9999 | 0.371 |
| PAl-PMt(400)CH | 160 | 0.1743 | 0.9998 | 0.9998 | 0.353 |
| PAl-PMt(500)CH | 120 | 0.0955 | 0.9996 | 0.9999 | 0.316 |
| PAl-PMt(600)CH | 140 | 0.1354 | 0.9998 | 0.9999 | 0.369 |
| PAl-PM(700)CH | 105 | 0.0787 | 0.9997 | 0.9999 | 0.309 |
| PAl-PM(800)CH | 76 | 0.054. | 0.9996 | 0.9997 | 0.311 |
| Samples | qmax (mg/g) | Reference |
|---|---|---|
| Montmorillonite (Mt) | 55 mg/g | [28] |
| Saudi Local clays | 50–70 | [75] |
| Brick wastes | 60–70 | [76] |
| Natural zeolite | 60–70 | [74] |
| Sodalite Zeolite | 39 | [77] |
| Mn modified diatomite | 77 | [78] |
| Bentonite * | 173 | [79] |
| Nanoporous silica | 345 | [80] |
| Zirconia pillared clay (500 °C)+ | 114 | [27] |
| Al-PCH | 274 | [28] |
| Zr-PCH | 224–346 | [27] |
| Alumina pillared clays (500 °C)+ | 88 | This study |
| PCH from pillared clays | 100–165 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popoola, S.A.; Al Dmour, H.; Al-Faze, R.; Alam, M.G.; Rakass, S.; Oudghiri Hassani, H.; Kooli, F. Regeneration and Single Stage Batch Adsorber Design for Efficient Basic Blue-41 Dye Removal by Porous Clay Heterostructures Prepared from Al13 Montmorillonite and Pillared Derivatives. Materials 2024, 17, 4948. https://doi.org/10.3390/ma17204948
Popoola SA, Al Dmour H, Al-Faze R, Alam MG, Rakass S, Oudghiri Hassani H, Kooli F. Regeneration and Single Stage Batch Adsorber Design for Efficient Basic Blue-41 Dye Removal by Porous Clay Heterostructures Prepared from Al13 Montmorillonite and Pillared Derivatives. Materials. 2024; 17(20):4948. https://doi.org/10.3390/ma17204948
Chicago/Turabian StylePopoola, Saheed A., Hmoud Al Dmour, Rawan Al-Faze, Mohd Gulfam Alam, Souad Rakass, Hicham Oudghiri Hassani, and Fethi Kooli. 2024. "Regeneration and Single Stage Batch Adsorber Design for Efficient Basic Blue-41 Dye Removal by Porous Clay Heterostructures Prepared from Al13 Montmorillonite and Pillared Derivatives" Materials 17, no. 20: 4948. https://doi.org/10.3390/ma17204948






