Advancing Sustainability: Geraniol-Enhanced Waterborne Acrylic Pressure-Sensitive Adhesives without Chemical Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Emulsion Polymerization Procedure
2.2. Latex Characterization
2.2.1. Nuclear Magnetic Resonance
2.2.2. Fourier-Transform Infrared Spectroscopy
2.2.3. Differential Scanning Calorimetry Analysis (DSC)
2.2.4. Thermal Gravimetric Analysis (TGA)
2.2.5. Particle Size and Viscosity
2.2.6. Molar Mass Determination
2.2.7. Conversion and Solid Content
2.2.8. Peeling Test
3. Results and Discussion
3.1. Particle Size and Viscosity
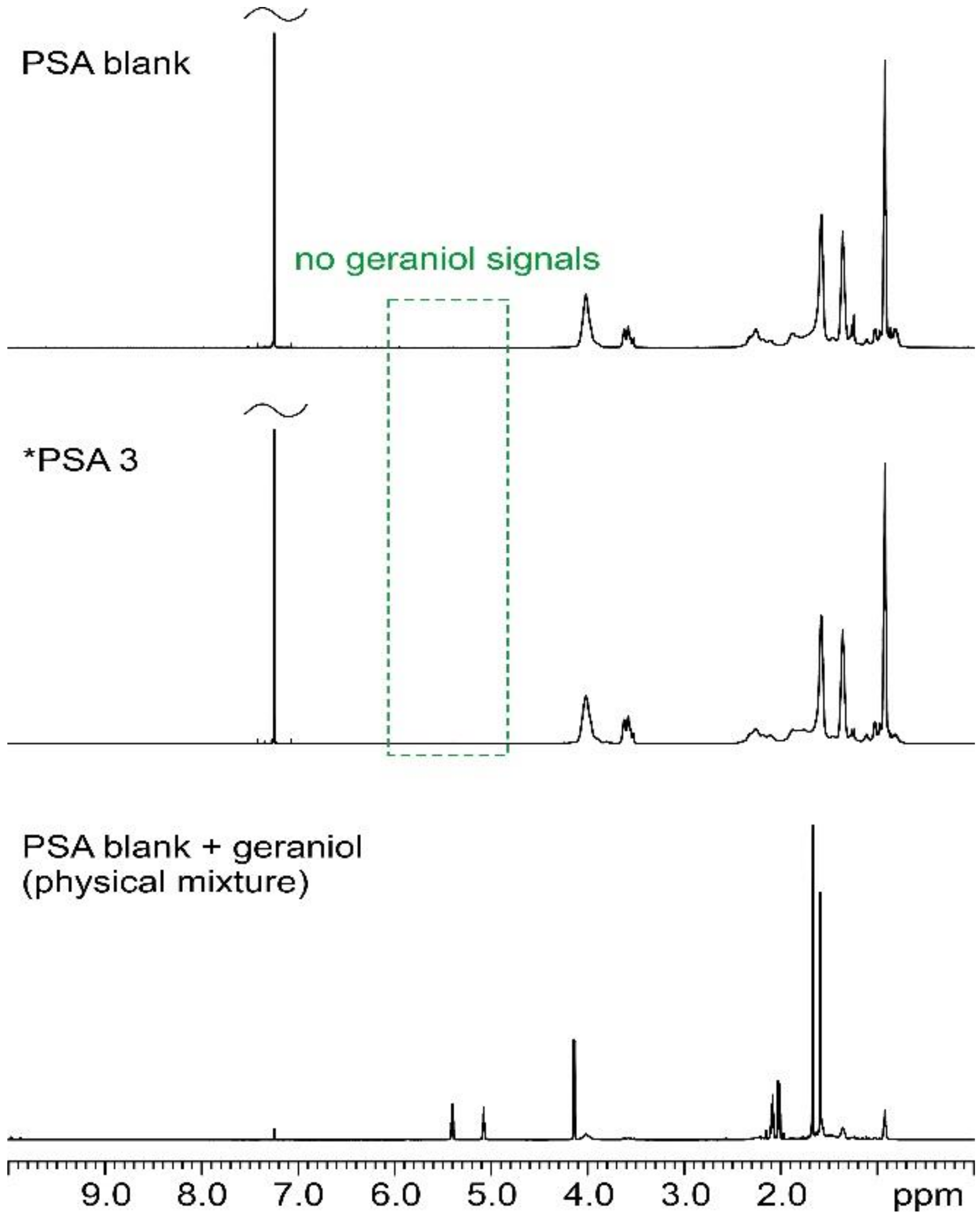
3.2. Nuclear Magnetic Resonance
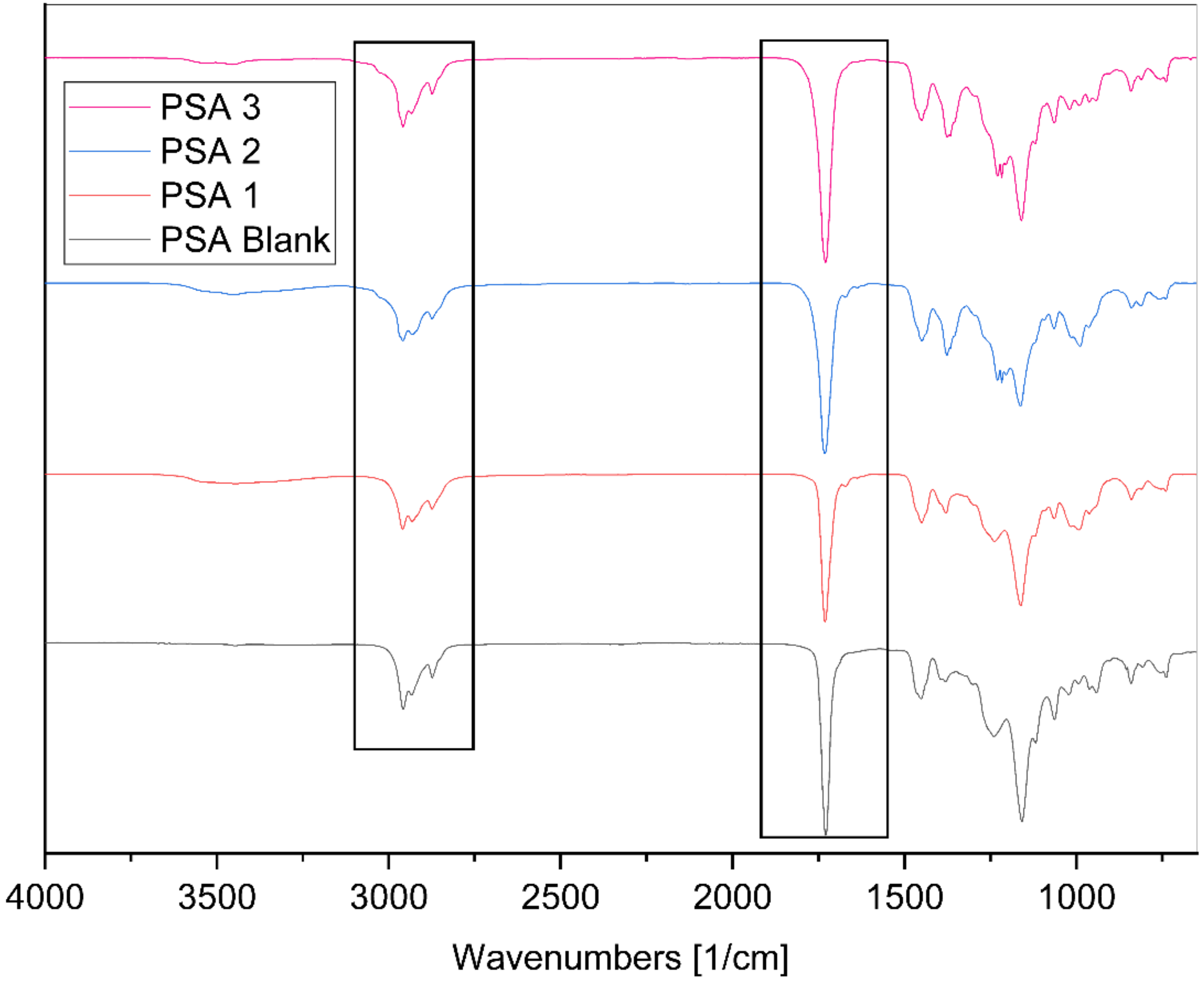
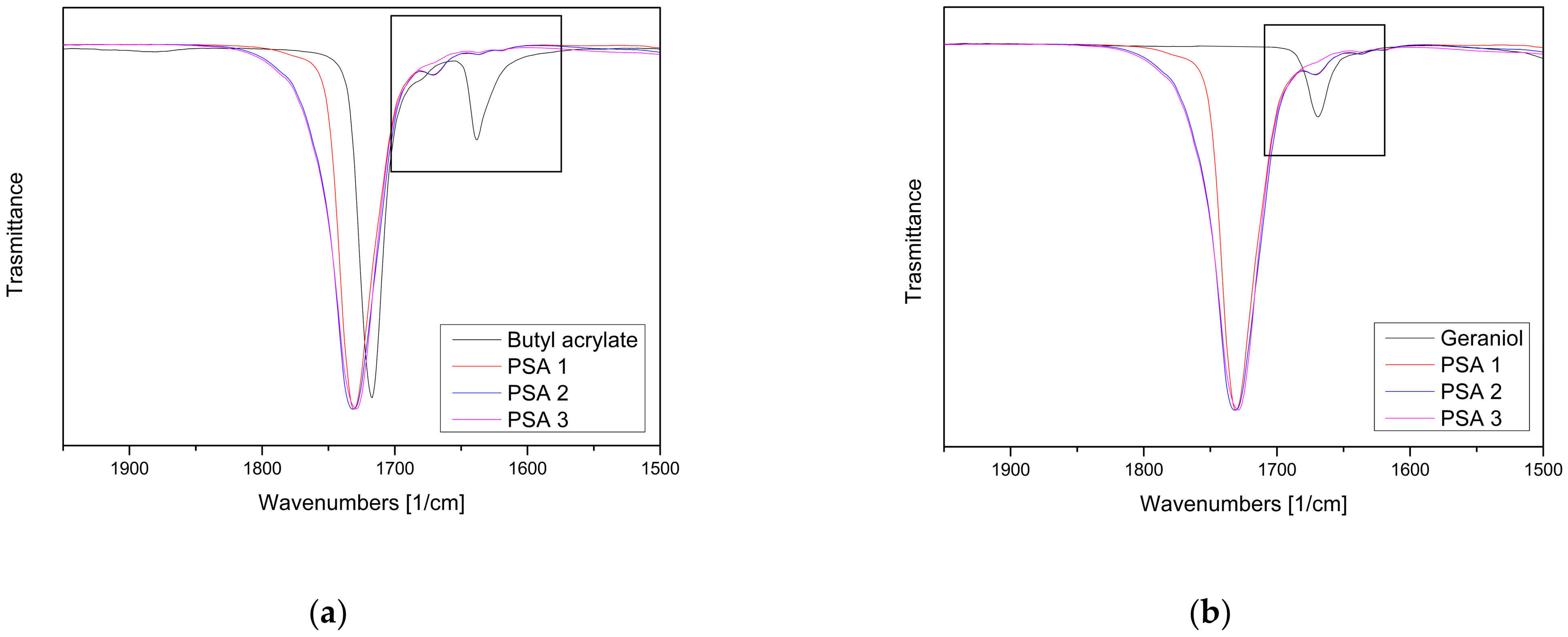
3.3. Fourier-Transform Infrared Spectroscopy (FTIR)
3.4. Differential Scanning Calorimetry (DSC)
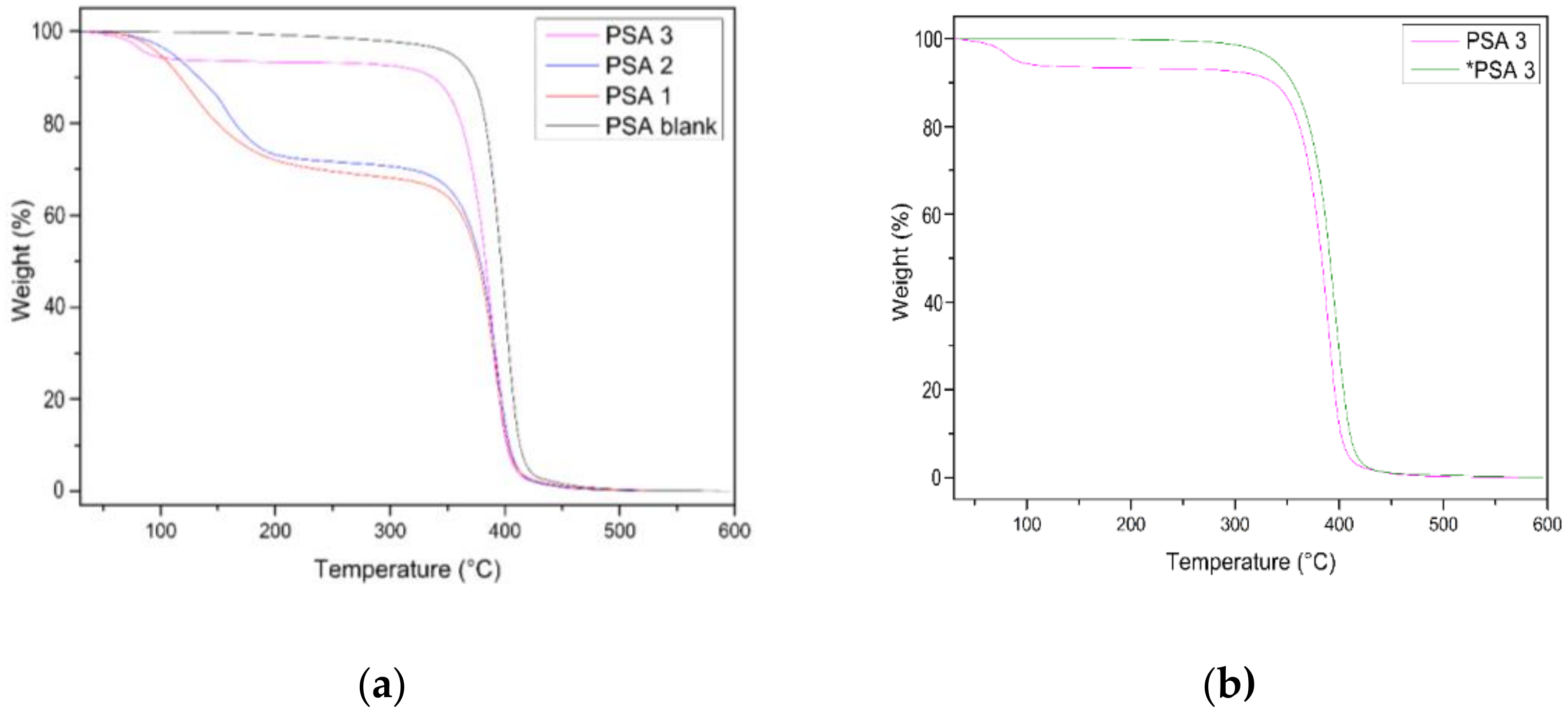
3.5. Thermogravimetric Analysis (TGA)
3.6. Molar Mass Determination
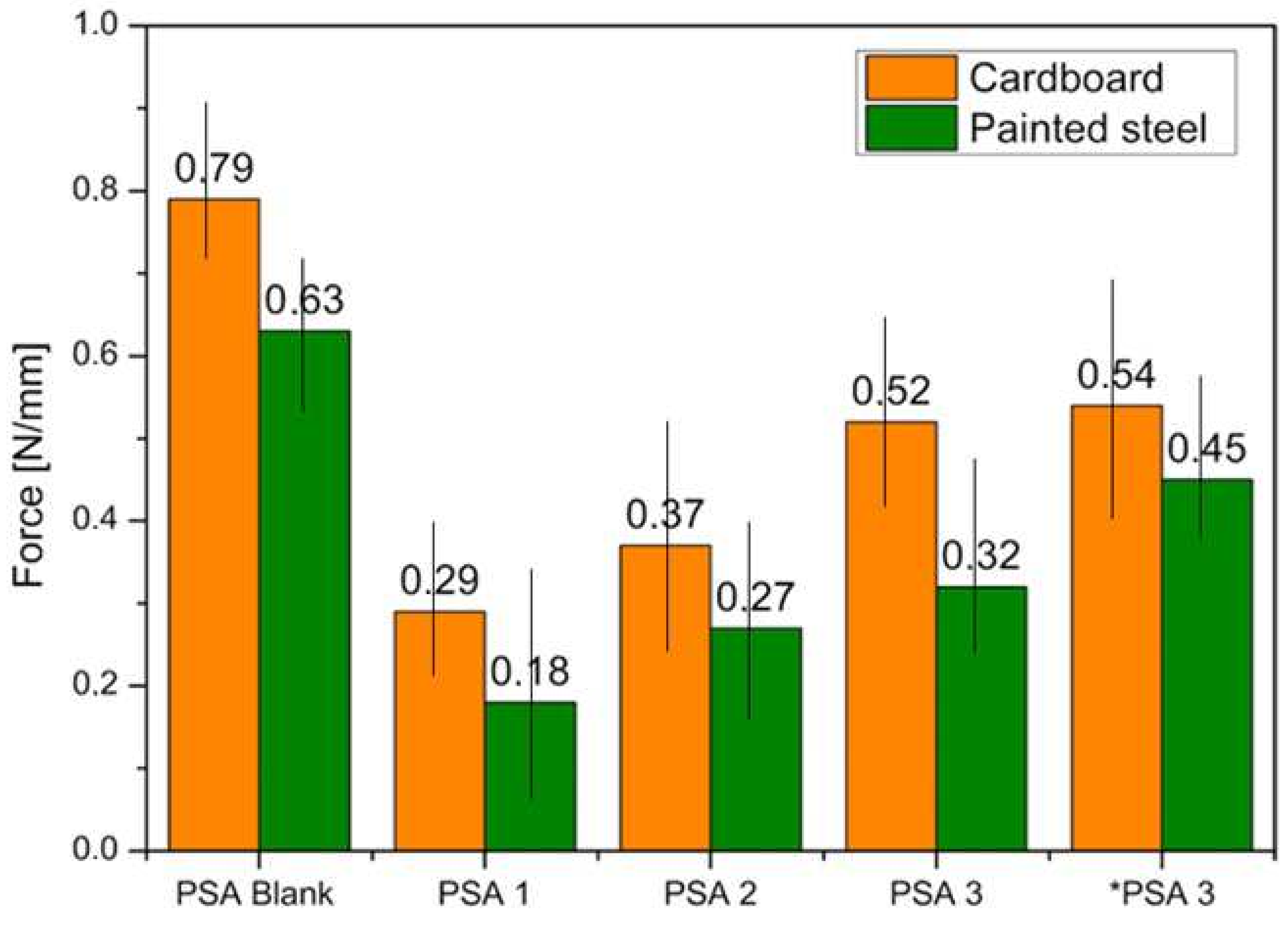
3.7. Peeling Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Creton, C. Pressure-Sensitive Adhesives: An Introductory Course. MRS Bull. 2003, 28, 434–439. [Google Scholar] [CrossRef]
- Allied Market Research. Available online: https://www.alliedmarketresearch.com/pressure-sensitive-adhesives-market (accessed on 10 March 2024).
- Mapari, S.; Mestry, S.; Mhaske, S.T. Developments in Pressure-Sensitive Adhesives: A Review. Polym. Bull. 2021, 78, 4075–4108. [Google Scholar] [CrossRef]
- Droesbeke, M.A.; Aksakal, R.; Simula, A.; Asua, J.M.; Du Prez, F.E. Biobased Acrylic Pressure-Sensitive Adhesives. Prog. Polym. Sci. 2021, 117, 101396. [Google Scholar] [CrossRef]
- Pradeep, S.V.; Kandasubramanian, B.; Sidharth, S. A Review on Recent Trends in Bio-Based Pressure-Sensitive Adhesives. J. Adhes. 2023, 99, 2145–2166. [Google Scholar] [CrossRef]
- Satas, D. (Ed.) Handbook of Pressure Sensitive Adhesive Technology, 3rd ed.; Satas & Associates: Warwick, RI, USA, 1999. [Google Scholar]
- Chadwick, S.S. Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Jovanović, R.; Dubé, M.A. Emulsion-Based Pressure-Sensitive Adhesives: A Review. J. Macromol. Sci. Part C Polym. Rev. 2004, 44, 1–51. [Google Scholar] [CrossRef]
- Fonseca, G.E.; McKenna, T.F.; Dubé, M.A. Influence of Particle Nucleation in Pressure-Sensitive Adhesive Properties: Miniemulsion versus Emulsion Polymerization. Macromol. Symp. 2008, 271, 83–93. [Google Scholar] [CrossRef]
- Vendamme, J.; Eevers, W. Recent Synthetic Approaches and Emerging Bio-Inspired Strategies for the PSA Industry. J. Appl. Polym. Sci. 2014, 131, 40669. [Google Scholar]
- Dastjerdi, Z.; Cranston, E.D.; Dubé, M.A. Pressure-Sensitive Adhesive Property Modification Using Cellulose Nanocrystals. Int. J. Adhes. Adhes. 2018, 81, 36–42. [Google Scholar] [CrossRef]
- Badía, A.; Movellan, J.; Barandiaran, M.J.; Leiza, J.R. High Biobased Content Latexes for Development of Sustainable Pressure-Sensitive Adhesives. Ind. Eng. Chem. Res. 2018, 57, 14509–14516. [Google Scholar] [CrossRef]
- Callies, X.; Buguin, A.; Cottin-Bizonne, C.; Brochard-Wyart, F. Combined Effect of Chain Extension and Supramolecular Interactions on Rheological and Adhesive Properties of Acrylic Pressure-Sensitive Adhesives. ACS Appl. Mater. Interfaces 2016, 8, 33307–33315. [Google Scholar] [CrossRef]
- Kircher, M. Bioeconomy: Markets, Implications, and Investment Opportunities. Economies 2019, 7, 73. [Google Scholar] [CrossRef]
- Gallagher, J.J.; Hillmyer, M.A.; Reineke, T.M. Acrylic Triblock Copolymers Incorporating Isosorbide for Pressure-Sensitive Adhesives. ACS Sustain. Chem. Eng. 2016, 4, 3379–3387. [Google Scholar] [CrossRef]
- Imam, S.H.; Bilbao-Sainz, C.; Chiou, B.S.; Glenn, G.M.; Orts, W.J. Biobased Adhesives, Gums, Emulsions, and Binders: Current Trends and Future Prospects. J. Adhes. Sci. Technol. 2013, 27, 1972–1997. [Google Scholar] [CrossRef]
- Maassen, W.; Meier, M.A.R.; Willenbacher, N. Unique Adhesive Properties of Pressure-Sensitive Adhesives from Plant Oils. Int. J. Adhes. Adhes. 2016, 64, 65–71. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent Advances in Vegetable Oil-Based Polymers and Their Composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Sahoo, S.; Mohanty, S.; Nayak, S.K. Biobased Polyurethane Adhesive over Petroleum-Based Adhesive: Use of Renewable Resource. J. Macromol. Sci. Part A 2018, 55, 36–48. [Google Scholar] [CrossRef]
- Vendamme, R.; Eevers, W. Sweet Solution for Sticky Problems: Chemorheological Design of Self-Adhesive Gel Materials Derived from Lipid Biofeedstocks and Adhesion Tailoring via Incorporation of Isosorbide. Macromolecules 2013, 46, 3395–3405. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Kim, Y.-W.; Shin, J. Preparation and Characterization of a Renewable Pressure-Sensitive Adhesive System Derived from ε-Decalactone, L-Lactide, Epoxidized Soybean Oil, and Rosin Ester. ACS Sustain. Chem. Eng. 2015, 3, 2309–2320. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef]
- Winnacker, M.; Rieger, B. Recent Progress in Sustainable Polymers Obtained from Cyclic Terpenes: Synthesis, Properties, and Application Potential. ChemSusChem 2015, 8, 2455–2471. [Google Scholar] [CrossRef]
- Thomsett, M.R.; Storr, T.E.; Monaghan, O.R.; Stockman, R.A.; Howdle, S.M. Progress in the Synthesis of Sustainable Polymers from Terpenes and Terpenoids. Green Mater. 2016, 4, 115–134. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. From Monomers to Polymers from Renewable Resources: Recent Advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Sainz, M.F.; Souto, J.A.; Regentova, D.; Johansson MK, G.; Timhagen, S.T.; Irvine, D.J.; Buijsen, P.; Koning, C.E.; Stockman, R.A.; Howdle, S. A Facile and Green Route to Terpene-Derived Acrylate and Methacrylate Monomers and Simple Free Radical Polymerization to Yield New Renewable Polymers and Coatings. Polym. Chem. 2016, 7, 2882–2887. [Google Scholar] [CrossRef]
- Takahashi, T.; Hirayama, K.; Teramoto, N.; Shibata, M. Biocomposites Composed of Epoxidized Soybean Oil Cured with Terpene-Based Acid Anhydride and Cellulose Fibers. J. Appl. Polym. Sci. 2008, 108, 1596–1602. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.; Tolman, W.B.; Hillmyer, M.A. Thermoplastic Elastomers Derived from Menthide and Tulipalin A. Biomacromolecules 2012, 13, 3833–3840. [Google Scholar] [CrossRef]
- Ahn, B.K.; Kraft, S.; Wang, D.; Sun, X.S. Thermally Stable, Transparent, Pressure-Sensitive Adhesives from Epoxidized and Dihydroxyl Soybean Oil. Biomacromolecules 2011, 12, 1839–1843. [Google Scholar] [CrossRef]
- Badía, A.; Santos, J.I.; Agirre, A.; Barandiaran, M.J.; Leiza, J.R. UV-Tunable Biobased Pressure-Sensitive Adhesives Containing Piperonyl Methacrylate. ACS Sustain. Chem. Eng. 2019, 7, 19122–19130. [Google Scholar] [CrossRef]
- Dicks, J.A.; Woolard, C. Biodegradable Polymeric Foams Based on Modified Castor Oil, Styrene, and Isobornyl Methacrylate. Polymers 2021, 13, 1872. [Google Scholar] [CrossRef]
- Demchuk, Z.; Mora, A.-S.; Choudhary, S.; Caillol, S.; Voronov, A. Biobased Latexes from Natural Oil Derivatives. Ind. Crops Prod. 2021, 162, 113237. [Google Scholar] [CrossRef]
- Fang, C.; Zhu, X.; Cao, Y.; Xu, X.; Wang, S.; Dong, X. Toward Replacement of Methyl Methacrylate by Sustainable Bio-Based Isobornyl Methacrylate in Latex Pressure-Sensitive Adhesive. Int. J. Adhes. Adhes. 2020, 100, 102623. [Google Scholar] [CrossRef]
- Atanase, L.-I.; Larraya, C.; Tranchant, J.-F.; Save, M. Rational Design of Tetrahydrogeraniol-Based Hydrophobically Modified Poly(Acrylic Acid) as Emulsifier of Terpene-in-Water Transparent Nanoemulsions. Eur. Polym. J. 2017, 94, 248–258. [Google Scholar] [CrossRef]
- Watanabe, T.; Karita, K.; Manabe, M.; Ono, T. Preparation of Monodisperse Poly(Methyl Methacrylate)/Polystyrene Composite Particles by Seeded Emulsion Polymerization Using a Sequential Flow Process. Front. Chem. Eng. 2021, 3, 742447. [Google Scholar] [CrossRef]
- Márquez, I.; Paredes, N.; Alarcia, F.; Velasco, J.I. Adhesive Performance of Acrylic Pressure-Sensitive Adhesives from Different Preparation Processes. Polymers 2021, 13, 2627. [Google Scholar] [CrossRef]
- Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=cnmr&sdbsno=2200 (accessed on 21 March 2024).
- Zhang, X.; Liu, H.; Yue, L.; Bai, Y.; He, J. Fabrication of Acrylic Pressure-Sensitive Adhesives Containing Maleimide for Heat-Resistant Adhesive Applications. Polym. Bull. 2019, 76, 3093–3112. [Google Scholar] [CrossRef]
- Worzakowska, M. UV Polymerization of Methacrylates—Preparation and Properties of Novel Copolymers. Polymers 2021, 13, 1659. [Google Scholar] [CrossRef]
- Schiavi, C.; Barbaro, M.; Giorgini, L. Bio-Based Polymeric Materials for Additive Manufacturing: Recent Developments and Emerging Applications. J. Polym. Environ. 2023, 31, 3755–3771. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shim, G.-S.; Kim, H.-J.; Kim, Y. Adhesion Performance and Recovery of Acrylic PSA with Acrylic Elastomer (AE) Blends via Thermal Crosslinking for Application in Flexible Displays. Polymers 2019, 11, 1959. [Google Scholar] [CrossRef]
- Simões, B.D.; Da Silva, R.M.P.; Pinto, R.J.B.; Martins, M.A.; Pires, R.A.; Vilela, C.; Silvestre, A.J.D.; Sousa, H.C.D. Rheological and Mechanical Properties of an Acrylic PSA. Polymers 2023, 15, 3843. [Google Scholar] [CrossRef]

| Synthesized PSA Latexes | Formulation (wt.% Monomers) | Reaction Time (h) |
|---|---|---|
| PSA blank | BA:MMA:AA:HEMA (87:10:1:2) | 2.5 |
| PSA 1 | BA:GER:MMA:AA:HEMA (67:20:10:1:2) | 4 |
| PSA 2 | BA:GER:MMA:AA:HEMA (67:20:10:1:2) | 5 |
| PSA 3 | BA:GER:MMA:AA:HEMA (67:20:10:1:2) | 6 |
| Role/Function | Constituent | Acronyms | Weight (g) |
|---|---|---|---|
| 1 Soft monomer | Butyl acrylate | BA | 67–87 |
| 2 Soft monomer | Geraniol | GER | 0–20 |
| Hard monomer | Methyl methacrylate | MMA | 10 |
| Functional monomer | Acrylic acid | AA | 1 |
| Hydroxy ethyl methacrylate | HEMA | 2 | |
| Emulsifier | Sodium dodecyl sulfate | SDS | 1.5 |
| Initiator | Potassium persulfate | KPS | 0.7 |
| Buffer | Sodium bicarbonate | NaHCO3 | 0.15 |
| Continuous phase | Water | H2O | 95 |
| PSA ID | Density (g/mL) | Solid Content | Conversion (%) | Viscosity (cP) | Particle Size (nm) | Đ |
|---|---|---|---|---|---|---|
| PSA blank | 1.06 | 55% | 97% | 5000 | 145.7 | 0.043 |
| PSA 1 | 0.96 | 52% | 73% | 4000 | 108.9 | 0.044 |
| PSA 2 | 0.93 | 52% | 81% | 4300 | 115.6 | 0.045 |
| PSA 3 | 1.12 | 52% | 94% | 4800 | 121.9 | 0.043 |
| PSA ID | Tg (°C) |
|---|---|
| PSA blank | −31 |
| PSA 1_4 h | −53 |
| PSA 2_5 h | −51 |
| PSA 3_6 h | −49 |
| *PSA 3_6 h | −48 |
| PSA ID | Weight Change a | Tonset (1%) (°C) | Tmax (°C) b | Residual Mass (%) @ 600 °C |
|---|---|---|---|---|
| PSA blank | - | 379 °C | 400 | 3.9 |
| PSA 1_4 h | 24% | 90 °C | 388 | 7.2 |
| PSA 2_5 h | 18% | 116 °C | 393 | 3.2 |
| PSA 3_6 h | 6% | 118 °C | 399 | 6.8 |
| *PSA 3_6 h | - | 358 °C | 399 | 6.8 |
| Reaction Time | GER Amount (wt.%) | Mw (g/mol) | Mn (g/mol) | Đ | |
|---|---|---|---|---|---|
| PSA blank | 2.5 h | 20 | 98,806.00 | 52,241 | 1.77 |
| PSA 1 | 4 h | 20 | 44,318.00 | 22,052 | 2 |
| PSA 2 | 5 h | 20 | 68,699.00 | 39,207 | 1.75 |
| PSA 3 | 6 h | 2 | 83,415.00 | 42,829 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorenzo, L.; Bordignon, S.; Chierotti, M.R.; Alfeo, I.A.; Antosik, A.K.; Brunella, V. Advancing Sustainability: Geraniol-Enhanced Waterborne Acrylic Pressure-Sensitive Adhesives without Chemical Modification. Materials 2024, 17, 4957. https://doi.org/10.3390/ma17204957
Di Lorenzo L, Bordignon S, Chierotti MR, Alfeo IA, Antosik AK, Brunella V. Advancing Sustainability: Geraniol-Enhanced Waterborne Acrylic Pressure-Sensitive Adhesives without Chemical Modification. Materials. 2024; 17(20):4957. https://doi.org/10.3390/ma17204957
Chicago/Turabian StyleDi Lorenzo, Ludovica, Simone Bordignon, Michele R. Chierotti, Ignazio Andrea Alfeo, Adrian Krzysztof Antosik, and Valentina Brunella. 2024. "Advancing Sustainability: Geraniol-Enhanced Waterborne Acrylic Pressure-Sensitive Adhesives without Chemical Modification" Materials 17, no. 20: 4957. https://doi.org/10.3390/ma17204957







