Spectroscopic Properties of TmF3-Doped CaF2 Crystals
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Optical Absorption Spectra
3.2. Judd–Ofelt Analysis
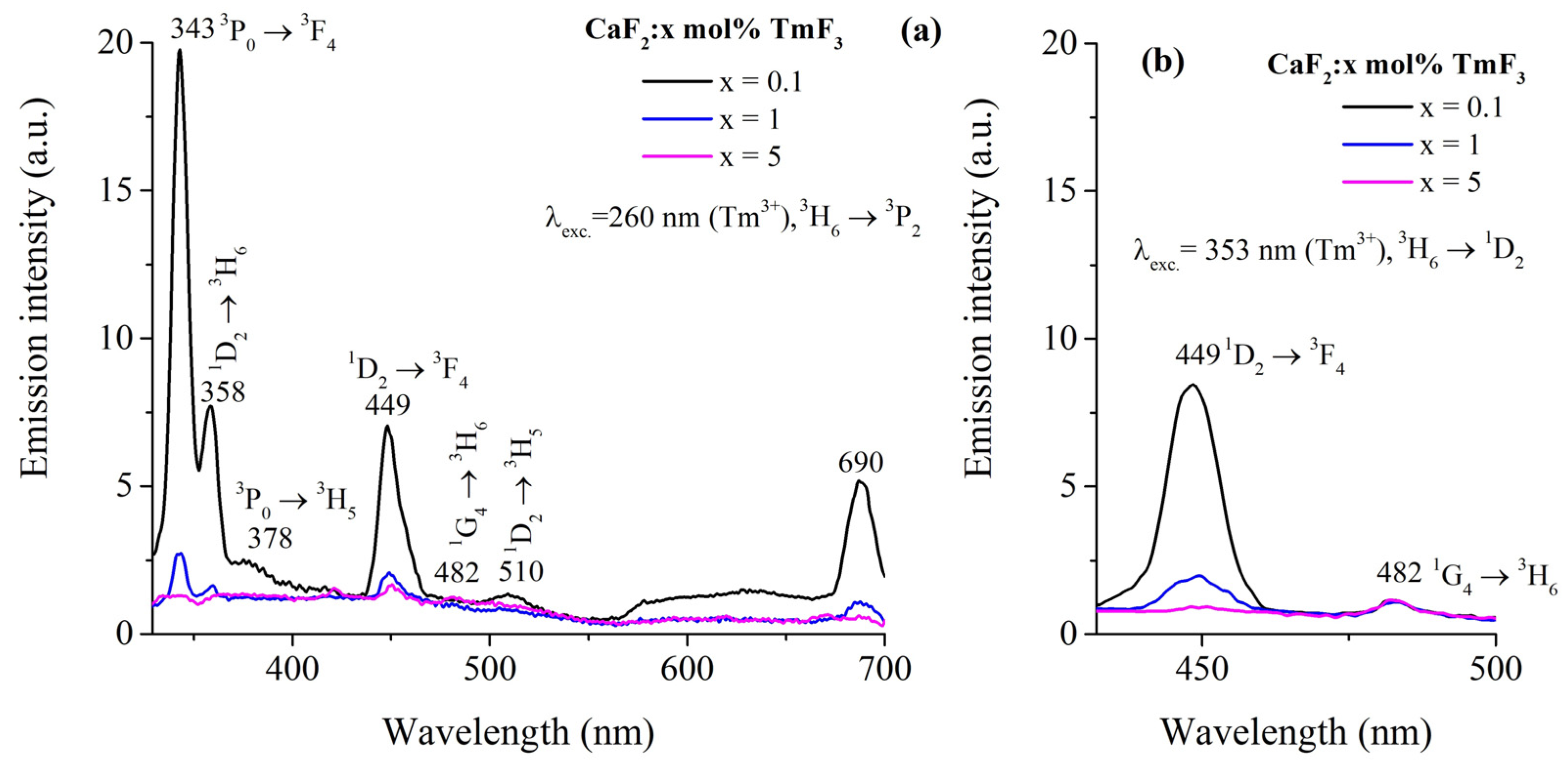
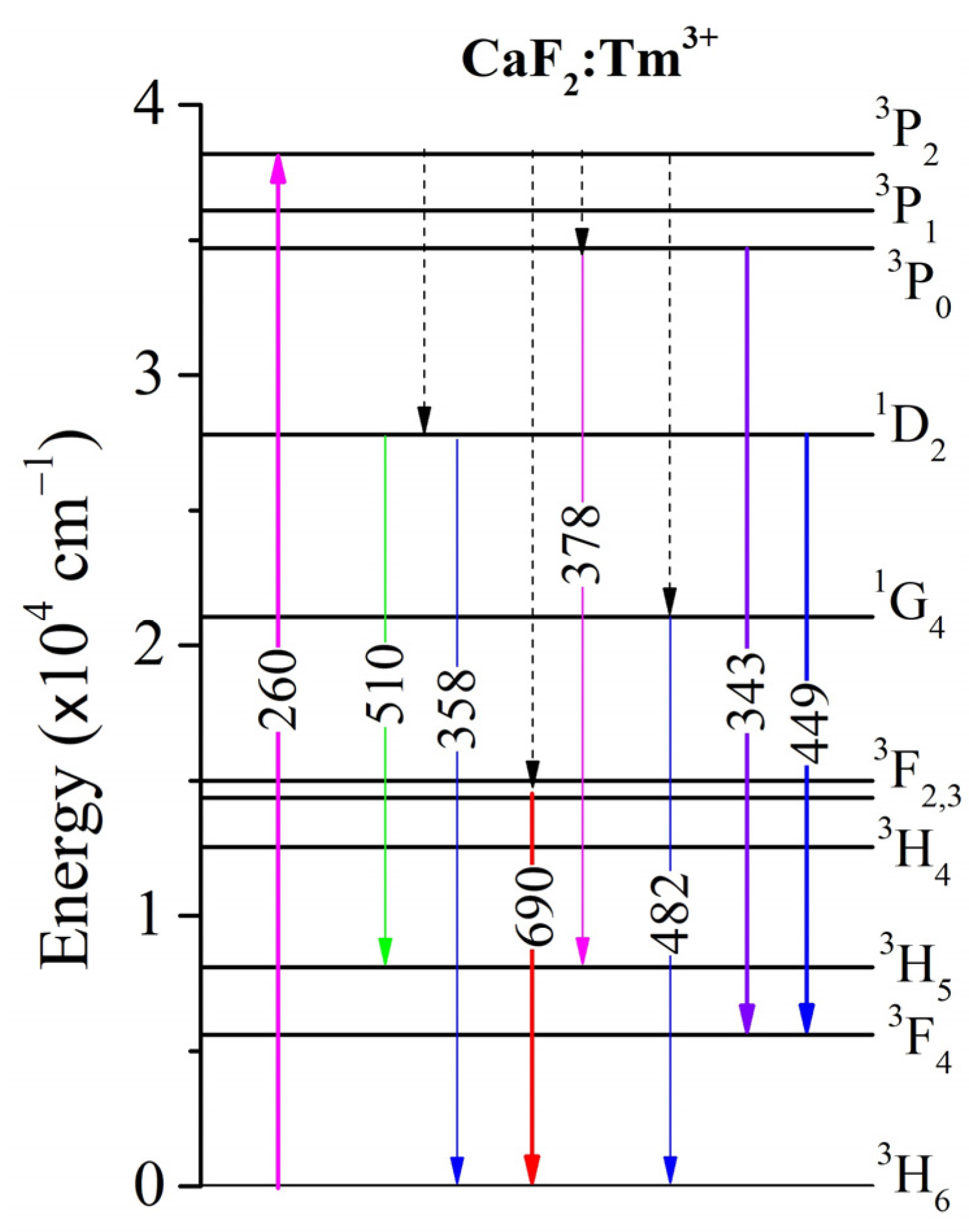
3.3. Emission Spectra of Tm3+ Ions
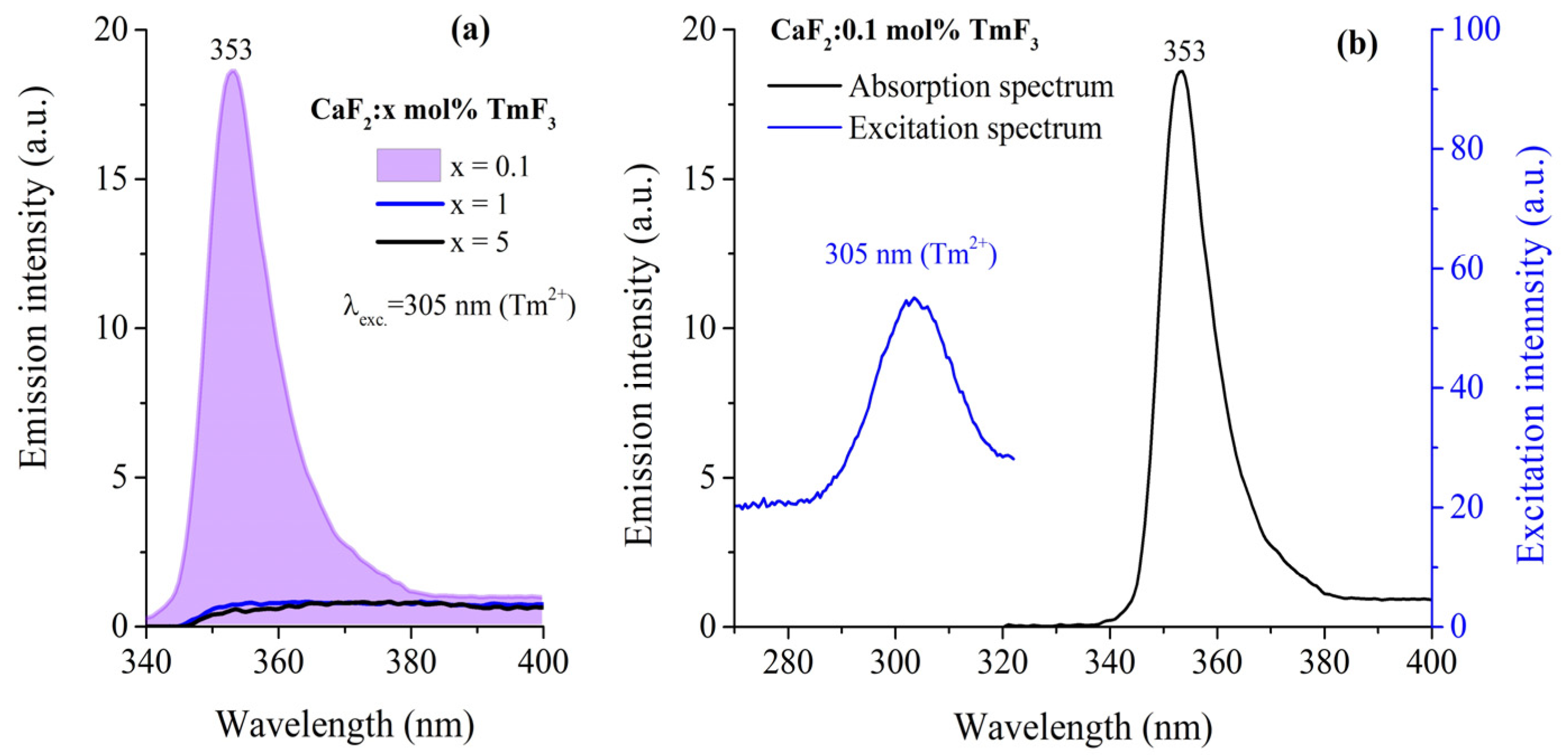
3.4. Emission Spectra of Tm2+ Ions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taikar, D.R.; Taikar, R.N.; Nagde, K.R.; Sarve, S.R. Synthesis and luminescence study of n-UV excitable Tm3+-activated blue phosphor. Macromol. Symp. 2021, 400, 2100019. [Google Scholar] [CrossRef]
- Reiter, J.; Körner, J.; Pejchal, J.; Yoshikawa, A.; Hein, J.; Kaluza, M.C. Temperature dependent absorption and emission spectra of Tm: CaF2. Opt. Mater. Express 2020, 10, 2142–2158. [Google Scholar] [CrossRef]
- Loiko, P.; Doualan, J.L.; Guillemot, L.; Moncorgé, R.; Starecki, F.; Benayad, A.; Dunina, E.; Kornienko, A.; Fomicheva, L.; Braud, A.; et al. Emission properties of Tm3+-doped CaF2, KY3F10, LiYF4, LiLuF4 and BaY2F8 crystals at 1.5 μm and 2.3 μm. J. Lumin. 2020, 225, 117279. [Google Scholar] [CrossRef]
- Zu, Y.; Zong, M.; Wang, Y.; Zhang, Z.; Wu, A.; Liu, J.; Su, L. Self-Q-switched and broad wavelength-tunable lasing in Tm3+-doped CaF2 single-crystal fiber. Appl. Phys. Express 2020, 13, 102003. [Google Scholar] [CrossRef]
- Sizova, T.; Radzhabov, E.; Shendrik, R.; Egranov, A.; Myasnikova, A. Optical absorption spectra of X-ray irradiated alkaline earth fluoride crystals doped with divalent rare-earth ions studied by thermal bleaching. Radiat. Meas. 2019, 125, 25–28. [Google Scholar] [CrossRef]
- Shendrik, R.; Yu, Y.; Myasnikova, A.S.; Egranov, A.V.; Radzhabov, E.A. Divalent Cerium and Praseodymium ions in crystals of alkaline earth fluorides. Opt. Spectrosc. 2014, 116, 777–782. [Google Scholar] [CrossRef]
- McClure, D.S.; Kiss, Z. Survey of the spectra of the divalent rare-earth ions in cubic crystals. J. Chem. Phys. 1963, 39, 3251–3257. [Google Scholar] [CrossRef]
- Radzhabov, E.A. F3− molecular ions in fluoride crystals. Opt. Spectrosc. 2016, 120, 294–299. [Google Scholar] [CrossRef]
- Nicoara, I.; Stef, M. Study of Na+ ions influence on the charge compensating defects in CaF2: YbF3 crystals using dielectric relaxation. Eur. Phys. J. B 2012, 85, 180. [Google Scholar] [CrossRef]
- Nicoara, I.; Stef, M. Charge compensating defects study of YbF3-doped BaF2 crystals using dielectric loss. Phys. Status Solidi B 2016, 253, 397–403. [Google Scholar] [CrossRef]
- Stef, M.; Nicoara, I.; Vizman, D. Distribution of Yb3+ and Yb2+ ions along YbF3-Doped BaF2 crystals. Cryst. Res. Technol. 2018, 53, 1800186. [Google Scholar] [CrossRef]
- Nicoara, I.; Stef, M.; Pruna, A. Growth of YbF3-doped CaF2 crystals and characterization of Yb3+/Yb2+ conversion. J Cryst Growth 2008, 310, 1470–1475. [Google Scholar] [CrossRef]
- Stef, M.; Pruna, A.; Pecingina-Garjoaba, N.; Nicoara, I. Infuence of various impurities on the optical properties of YbF3-doped CaF2 crystals. Acta Phys. Pol. A 2007, 112, 1007–1012. [Google Scholar] [CrossRef]
- Buse, G.; Preda, E.; Stef, M.; Nicoara, I. Influence of Yb3+ ions on the optical properties of double-doped Er, Yb: CaF2 crystals. Phys. Scr. 2011, 83, 025604. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic energy levels in the trivalent lanthanide aquo ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J. Chem. Phys. 1968, 49, 4424–4442. [Google Scholar] [CrossRef]
- Doroshenko, M.E.; Alimov, O.K.; Papashvili, A.G.; Martynova, K.A.; Konyushkin, V.A.; Nakladov, A.N.; Osiko, V.V. Spectroscopic and laser properties of Tm3+ optical centers in CaF2 crystal under 795 nm diode laser excitation. Laser Phys. Lett. 2015, 12, 125701. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Zu, Y.; Fan, X.; Liu, J.; Guo, X.; Qian, X.; Su, L. Efficient continuous-wave, broadly tunable and passive Q-switching lasers based on a Tm3+:CaF2 crystal. Laser Phys. Lett. 2018, 15, 045803. [Google Scholar] [CrossRef]
- Camy, P.; Doualan, J.L.; Renard, S.; Braud, A.; Ménard, V.; Moncorgé, R. Tm3+:CaF2 for 1.9 lm laser operation. Opt. Commun. 2004, 236, 395–402. [Google Scholar] [CrossRef]
- Renard, S.; Camy, P.; Braud, A.; Doualan, J.L.; Moncorgé, R. CaF2 doped with Tm3+: A cluster model. J. Alloys Compd. 2008, 451, 71–73. [Google Scholar] [CrossRef]
- Radzhabov, E.; Shendrik, R.; Pankratov, V. Emission of Tm2+ in alkaline-earth fluoride crystals. J. Lumin. 2022, 252, 119271. [Google Scholar] [CrossRef]
- Duncan, R.C., Jr.; Kiss, Z.J. Continuously operating CaF2:Tm2+ optical maser. Appl. Phys. Lett. 1963, 3, 23–24. [Google Scholar] [CrossRef]
- Strickland, N.M.; Jones, G.D. Site-selective spectroscopy of Tm3+ centers in CaF2:Tm3+. Phys. Rev. B 1997, 56, 10916–10929. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, X.; Wang, J.; Zhang, C.; Liu, C.; Su, L. High-efficiency 2 μm continuous-wave laser in laser diode-pumped Tm3+, La3+: CaF2 single crystal. Opt. Lett. 2018, 43, 4300–4303. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Dong, J.; Li, J.; Wu, M.; Song, Q.; Li, Q.; Wang, Q.; Xue, Y.; Xu, X.; Lin, H.; et al. Enhance and modulated near-infrared luminescence of Tm3+:CaF2 crystal by co-doping Y3+ ions. J. Lumin. 2023, 254, 119514. [Google Scholar] [CrossRef]
- Karel Veselský, K.; Sulc, J.; Jelínkov, H.; Doroshenko, M.E.; Konyushkin, V.A.; Pierpoint, K.A.; Prajzler, V. Spectroscopy and lasing of cryogenically cooled Tm3+, Lu3+: CaF2 crystal. J. Lumin. 2023, 255, 119563. [Google Scholar] [CrossRef]
- Vasconcelos, D.A.A.; Barros, V.S.M.; Khoury, H.J.; Azevedo, W.M.; Asfora, V.K.; Guzzo, P.L. Synthesis and thermoluminescent response of CaF2 doped with Tm3+. Radiat. Meas. 2014, 71, 51–54. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Wang, J.; Zhang, Z.; Zhang, Z.; Rongrong, L.R.; Zu, Y.; Liu, J.; Su, L. High-efficiency ~2 μm CW laser operation of LD-pumped Tm3+:CaF2 single-crystal fibers. Opt. Express 2020, 28, 6684–6695. [Google Scholar] [CrossRef]
- Peijzel, P.S.; Vergeer, P.; Meijerink, A. 4f n−15d-4f n emission of Ce3+, Pr3+, Nd3+, Er3+, and Tm3+ in LiYF4 and YPO4. Phys. Rev. B 2005, 71, 045116. [Google Scholar] [CrossRef]
- Walsh, B.M.; Barnes, N.P.; Di Bartolo, B. Branching ratios, cross sections, and radiative lifetimes of rare earth ions in solids: Application to Tm3+ and Ho3+ ions in LiYF4. J. Appl. Phys. 1998, 83, 2772–2787. [Google Scholar] [CrossRef]
- Özen, G.; Demirata, B.; Övecoglu, M.L.; Genc, A. Thermal and optical properties of Tm3+ doped tellurite glasses. Spectrochim. Acta P A 2001, 57, 273–280. [Google Scholar] [CrossRef]
- Mattarelli, M.; Tikhomirov, V.K.; Seddon, A.B.; Montagna, M.; Moser, E.; Chiasera, A.; Chaussedent, S.; Nunzi Conti, G.; Pelli, S.; Righini, G.C.; et al. Tm3+-activated transparent oxy-fluoride glass–ceramics: Structural and spectroscopic properties. J. Non-cryst. Solids 2024, 345, 354–358. [Google Scholar] [CrossRef]
- Wang, S.; Ruan, Y.; Tsuboi, T.; Zhang, S.; Wang, Y.; Wu, Z.; Tong, H. Studies on the growth and optical characterization of Tm3+-doped BaY2F8 single crystals. Cryst. Res. Technol. 2012, 47, 928–938. [Google Scholar] [CrossRef]
- Schlesinger, M.; Szczurek, T. Spectroscopic studies of excited Tm3+ ions in alkaline-earth fluorides. Phys. Rev. B 1987, 35, 8341–8347. [Google Scholar] [CrossRef]
- Khenata, R.; Daoudi, B.; Sahnoun, M.; Baltache, H.; Rerat, M.; Reshak, A.H.; Bouhafs, B.; Abid, H.; Driz, M. Structural, electronic and optical properties of fluorite-type compounds. Eur. Phys. J. B 2005, 47, 63–70. [Google Scholar] [CrossRef]
- Kiss, Z.J. Energy Levels of Divalent Thulium in CaF2. Phis. Rev. 1962, 127, 718. [Google Scholar] [CrossRef]
- Anlsimov, F.; Vala, A.; Dagys, R. The Shielding of Crystal Field at Tm2+ in CaF2. Phys. Status Solidi (A) 1971, 6, K15–K17. [Google Scholar] [CrossRef]
- Hayes, W.; Smith, P.H.S. Paramagnetic resonance in ground and optically excited states of Tm2+ in alkaline earth fluorides. J. Phys. C Solid St. Phys. 1971, 4, 63–70. [Google Scholar] [CrossRef]
- Choh, S.H.; Yi, K.S. Point-charge approximation of trigonal Tm2+ and Yb3+ centres in crystals of CaF2 structure. J. Phys. C Solid State Phys. 1978, 11, 840–850. [Google Scholar] [CrossRef]
- Nicoara, D.; Nicoara, I. An improved Bridgman-Stockbarger crystal-growth system. Mat. Sci. Eng. A 1978, 102, 725–733. [Google Scholar] [CrossRef]
- Sarkisova, S.E.; Yusima, V.A.; Pisarevskya, Y.V. Study of the formation of radiation-stimulated impurity defects in CaF2 crystals activated by trivalent rare-earth ions. Crystallogr. Rep. 2023, 68, 79–87. [Google Scholar] [CrossRef]
- Nicoara, I.; Stef, M.; Vizman, D. Influence of growth conditions on the optical spectra of gamma irradiated BaF2 and CaF2 crystals. J. Cryst. Growth 2019, 525, 125188. [Google Scholar] [CrossRef]
- Kaminskii, A. Crystalline Lasers Physical Processes and Operating Schemes; CRC Press: Boca Raton, FL, USA, 1996; pp. 284–297. [Google Scholar]
- Judd, B.R. Optical absorption intensities of rare-earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Li, H.H. Refractive index of alkaline earth halides and its wavelenght and temperature derivatives. J. Phys. Chem. 1980, 9, 161–289. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Z.; Hong, F.; Dou, J.; Dong, T.; Zhang, Y.; Li, D.; Liu, G.; Dong, X.; Wang, J. Enhanced UV–Vis–NIR composite photocatalysis of NaBiF4:Yb3+, Tm3+ upconversion nanoparticles loaded on Bi2WO6 microspheres. J. Solid State Chem. 2021, 300, 122248. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Zhang, X.; Li, X.; Zhang, J.; Xu, S.; Wang, X.; Zhang, Y.; Wang, L.; Li, D.; et al. Full color white light, temperature self-monitor, and thermochromatic effect of Cu+ and Tm3+ codoped germanate glasses. J. Am. Ceram. Soc. 2021, 104, 350–360. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Guan, X.; Luo, P.; Chen, F.; Yu, M.; Jiang, L. Dual-emitting Tm3+/Mn2+ co-doped glass ceramic for wide-range optical thermometer. J. Alloys Compd. 2020, 836, 155507. [Google Scholar] [CrossRef]
- Zhou, J.; Song, B.; Qin, H.; Lu, W.; Zhao, G.; Huang, Z.M.; Han, G. Color-tunable and white emission of Tm3+ doped transparent zinc silicate glass-ceramics embedding ZnO nanocrystals. J. Am. Ceram. Soc. 2020, 103, 1010–1017. [Google Scholar] [CrossRef]
- Alvesa, R.T.; Rego-Filhoa, F.G.; Santosa, F.P.S.; Silvaa, A.C.A.; Dantasa, N.O.; Vermelhoa, M.V.D.; Gouveia-Netoa, A.S. Luminescence and Tm3+ to Dy3+ energy transfer in TeO2:ZnO glass under NIR/UV excitation. J. Lumin. 2019, 215, 116706. [Google Scholar] [CrossRef]
- Thomas, J.K.R. Group on Computational Imaging and Electromagnetics (GoCIE—CIE Plot Utility). Available online: https://faculty.iitr.ac.in/~krjt8fcy/gocie.html#fea (accessed on 20 July 2024).
- Racu, A.; Stef, M.; Buse, G.; Nicoara, I.; Vizman, D. Luminescence properties and Judd-Ofelt analysis of various ErF3 concentration-doped BaF2 crystals. Materials 2021, 14, 4221. [Google Scholar] [CrossRef]



| Sample | CaF2:1 mol% TmF3 | CaF2:5 mol% TmF3 | |||||
|---|---|---|---|---|---|---|---|
| Transition, 3H6→ | λm [nm] | Σ [×10−20 cm2·nm] | Smeas [×10−20 cm2] | Scalc [×10−20 cm2] | Σ [×10−20 cm2·nm] | Smeas [×10−20 cm2] | Scalc [×10−20 cm2] |
| 3F4 | 1787 | 4.027 | 1.437 | 1.211 | 15.605 | 0.933 | 0.783 |
| 3H5 | 1234 | 14.1428 | 1.248 | 1.093 | 8.125 | 0.702 | 0.885 |
| 3H4 | 785 | 6.960 | 0.944 | 1.098 | 6.806 | 0.923 | 0.978 |
| 2F2 + 2F3 | 677 | 9.089 | 1.429 | 1.267 | 9.146 | 1.436 | 1.148 |
| 1G4 | 460 | 1.148 | 0.264 | 0.087 | 1.402 | 0.323 | 0.089 |
| 1D2 | 353 | 1.722 | 0.513 | 0.181 | 1.669 | 0.498 | 0.368 |
| ΔSrms = 0.298 × 10−20 cm2 | ΔSrms = 0.267 × 10−20 cm2 | ||||||
| Ωt (10−20 cm2) | CaF2:1 mol% TmF3 (This Paper) | CaF2:5 mol% TmF3 (This Paper) | CaF2: 0.5 at.% TmF3 [24] | CaF2:1.5 at.% TmF3, 4 at.% YF3 [3] | CaF2:3 at.% TmF3, 2 at.% LaF3 [23] | CaF2:3 at.% TmF3, 2 at.% LaF3 [27] |
|---|---|---|---|---|---|---|
| Ω2 | 1.25043 | 0.20294 | 3.28 | 0.891 | 1.429 | 1.283 |
| Ω4 | 0.15075 | 0.88504 | 0.99 | 1.251 | 1.485 | 1.323 |
| Ω6 | 1.44999 | 1.03299 | 1.52 | 0.959 | 1.163 | 2.159 |
| χ | 0.10397 | 0.85677 | 0.65 | 1.304 | 1.277 | 0.590 |
| Transition | λem. (nm) | Ref. | CaF2:1 mol% TmF3 | CaF2:5 mol% TmF3 | ||||
|---|---|---|---|---|---|---|---|---|
| τcalc. (ms) | AJJ′ (s−1) | βJJ′ | τcalc. (ms) | AJJ′ (s−1) | βJJ′ | |||
| 3F3→3H6 | 690 | [3,32] | 0.87 | 1010 | 0.87 ± 0.173 | 0.95 | 915.4 | 0.87 ± 0.129 |
| 1D2→3H5 | 510 | [1,32] | 0.20 | 78.7 | 0.02 ± 0.003 | 0.20 | 58.5 | 0.01 ± 0.002 |
| 1G4→3H6 | 482 | [1,3,32,46,47,48,49,50] | 0.83 | 175.7 | 0.15 ± 0.030 | 1.23 | 157.5 | 0.22 ± 0.033 |
| 1D2→3F4 | 449 | [1,3,32,46,47,48,49,50,51] | 0.20 | 2048.8 | 0.41 ± 0.081 | 0.20 | 1137.6 | 0.23 ± 0.034 |
| 3P0→3H5 | 378 | [32] | 0.78 | 0.10 | - | 0.95 | 0.10 | - |
| 1D2→3H6 | 358 | [3,46] | 0.20 | 1508.8 | 0.30 ± 0.060 | 0.20 | 3074.3 | 0.63 ± 0.093 |
| 3P0→3F4 | 343 | [32] | 0.78 | 11.4 | 0.01 ± 0.002 | 0.95 | 66.8 | 0.06 ± 0.009 |
| CaF2:x mol% TmF3 | λexc. (nm) | X-Coordinate | Y-Coordinate | λexc. (nm) | X-Coordinate | Y-Coordinate |
|---|---|---|---|---|---|---|
| x = 0.1 | 260 | 0.28 | 0.20 | 356 | 0.18 | 0.10 |
| x = 1 | 0.25 | 0.22 | 0.18 | 0.16 | ||
| x = 5 | 0.25 | 0.24 | 0.17 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schornig, C.; Stef, M.; Buse, G.; Poienar, M.; Veber, P.; Vizman, D. Spectroscopic Properties of TmF3-Doped CaF2 Crystals. Materials 2024, 17, 4965. https://doi.org/10.3390/ma17204965
Schornig C, Stef M, Buse G, Poienar M, Veber P, Vizman D. Spectroscopic Properties of TmF3-Doped CaF2 Crystals. Materials. 2024; 17(20):4965. https://doi.org/10.3390/ma17204965
Chicago/Turabian StyleSchornig, Carla, Marius Stef, Gabriel Buse, Maria Poienar, Philippe Veber, and Daniel Vizman. 2024. "Spectroscopic Properties of TmF3-Doped CaF2 Crystals" Materials 17, no. 20: 4965. https://doi.org/10.3390/ma17204965
APA StyleSchornig, C., Stef, M., Buse, G., Poienar, M., Veber, P., & Vizman, D. (2024). Spectroscopic Properties of TmF3-Doped CaF2 Crystals. Materials, 17(20), 4965. https://doi.org/10.3390/ma17204965








