High-Temperature Creep Resistance of FeAlOY ODS Ferritic Alloy
Abstract
:1. Introduction
2. Materials and Methods
- M16RS: Hot rotary swaged Fe–9Al–13Cr–4Mo–4Y2O3
- M17: Hot rolled Fe–9Al–13Cr–4Mo–4Y2O3
- A18RS: Hot rotary swaged Fe–10Al–3Y2O3–1Ti
- Y9: Hot rolled Fe–10Al–4Cr–4Y2O3 (1% of metallic Y added)
3. Results
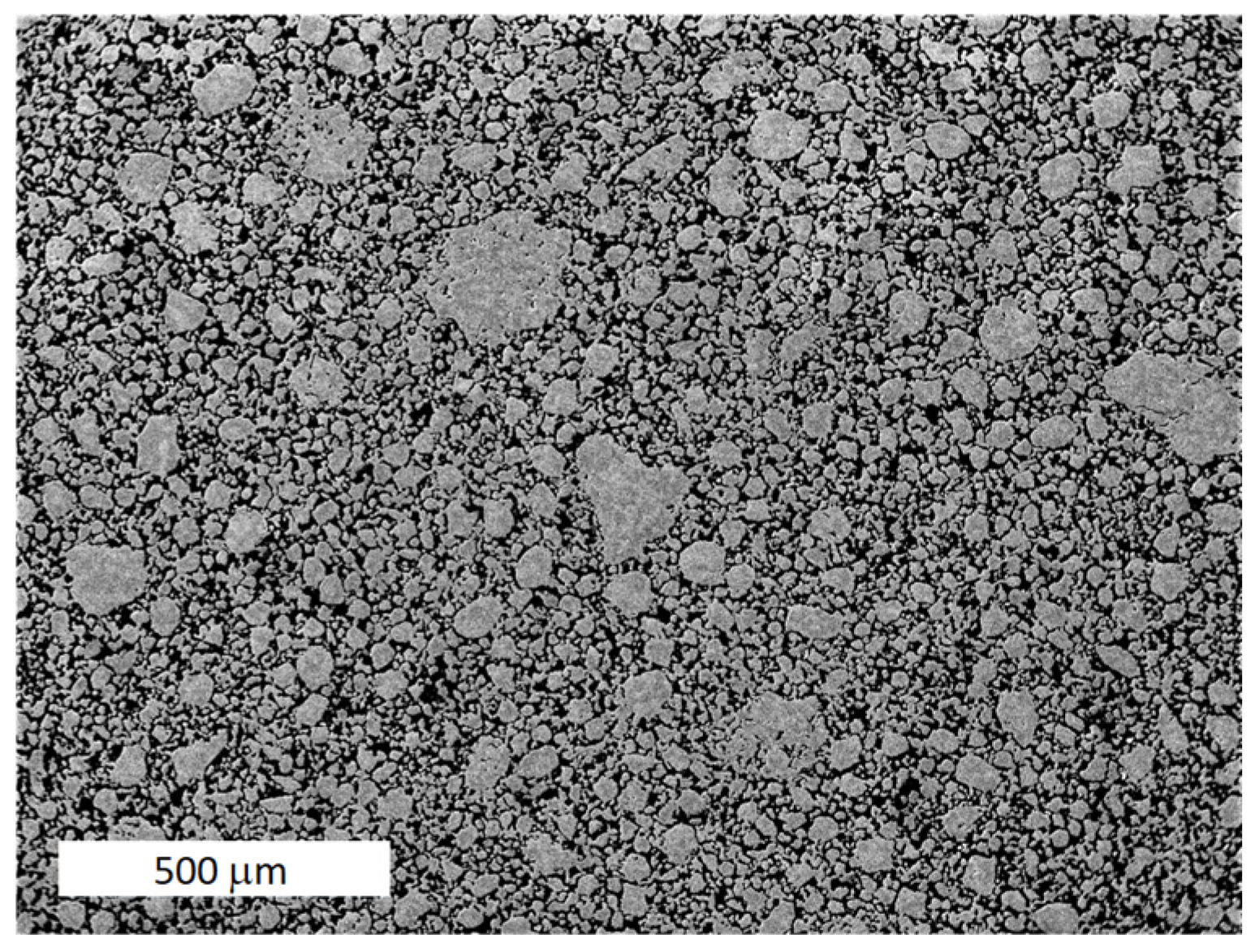
3.1. Powder Preparation and Processing of the FeAlOY
- (1)
- When the balls collide, small particles of powder are forged onto the balls and form larger and larger protrusions on their surface. When they grow sufficiently, they break off as large particles.
- (2)
- The large particles are gradually broken into small ones and are forged onto the balls to grow the protrusions again.
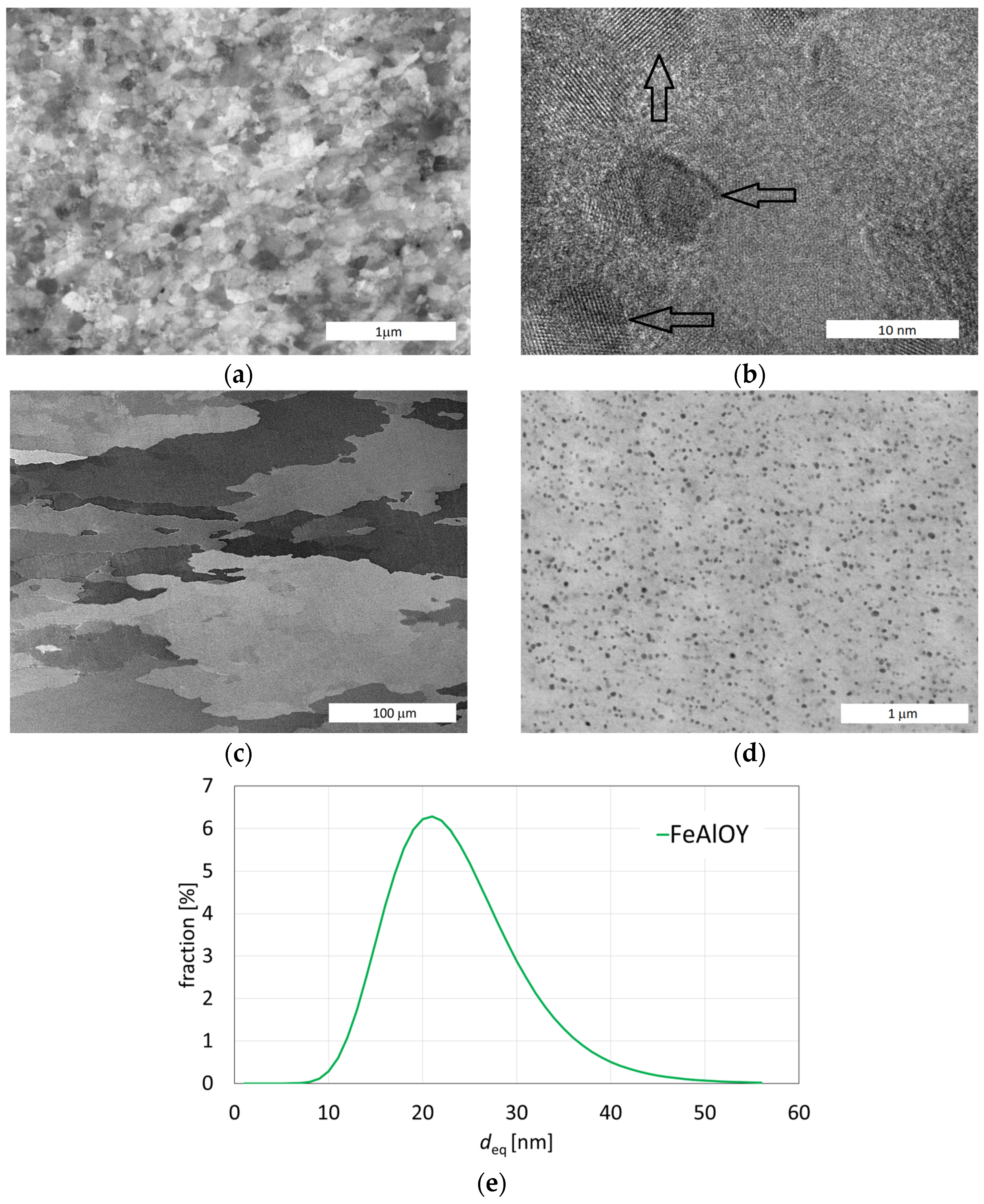
3.2. Powder Consolidation and Microstructure
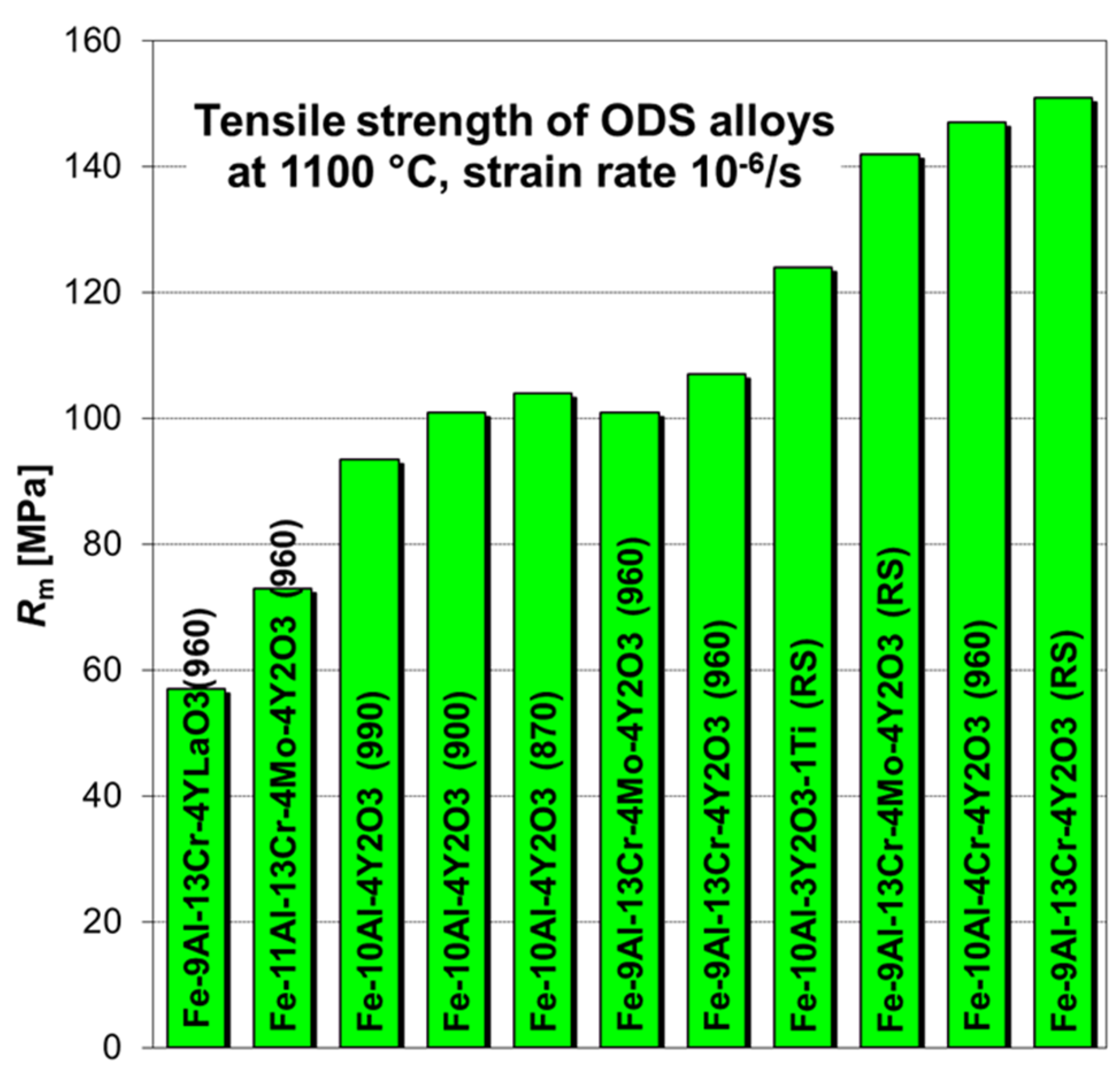
3.3. Low Strain Rate Tensile Testing to Find Optimum Alloy
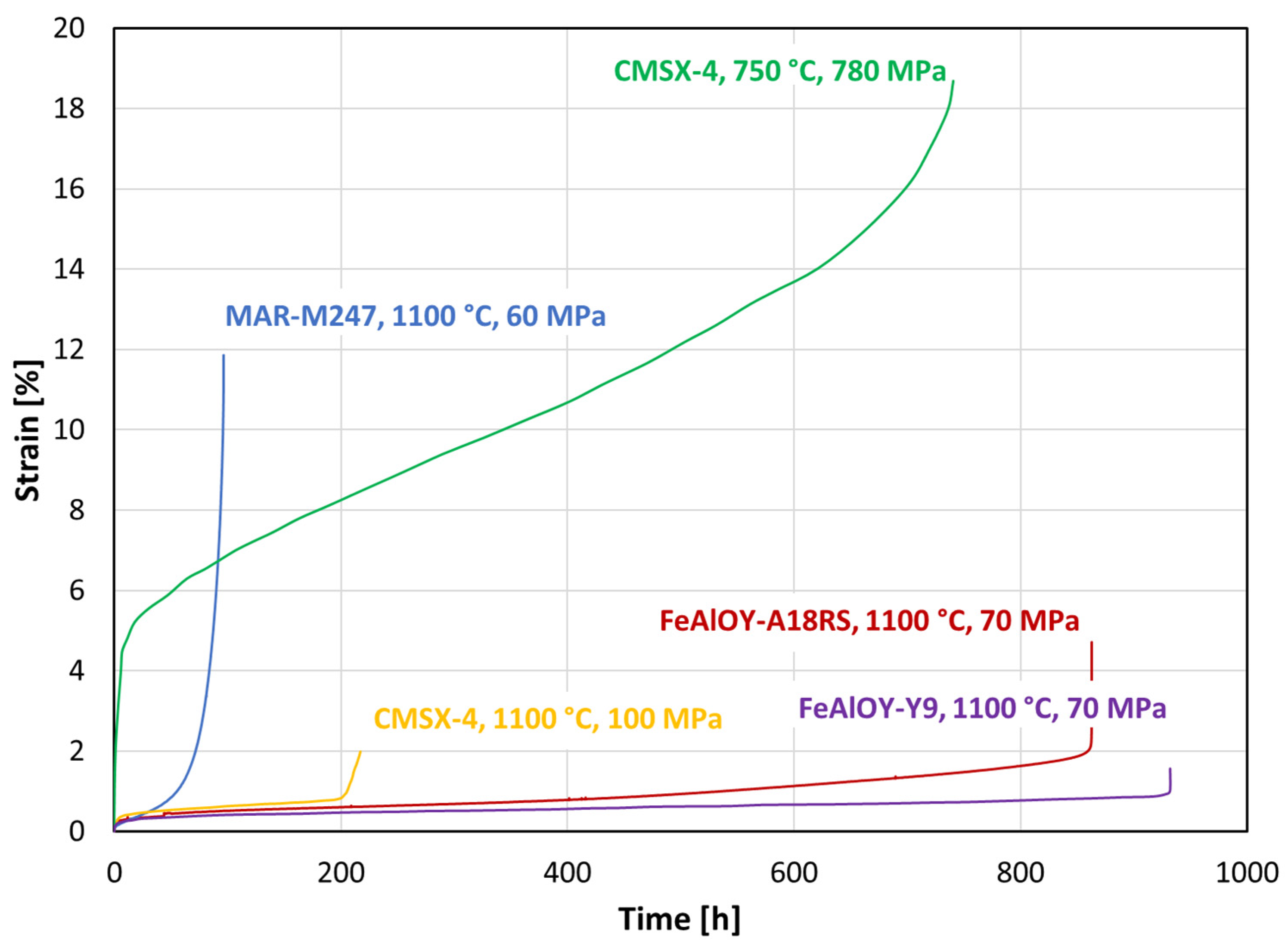
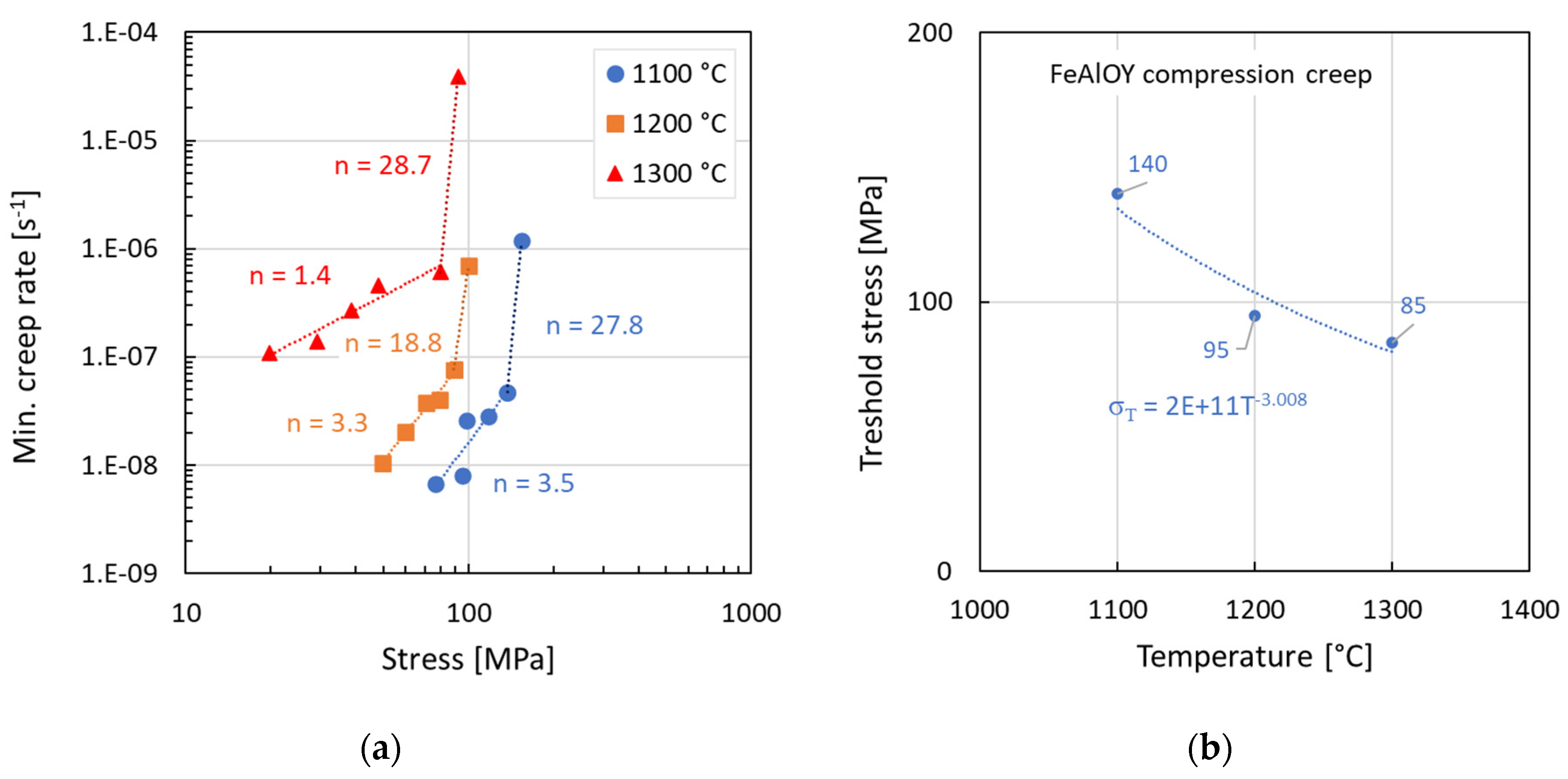
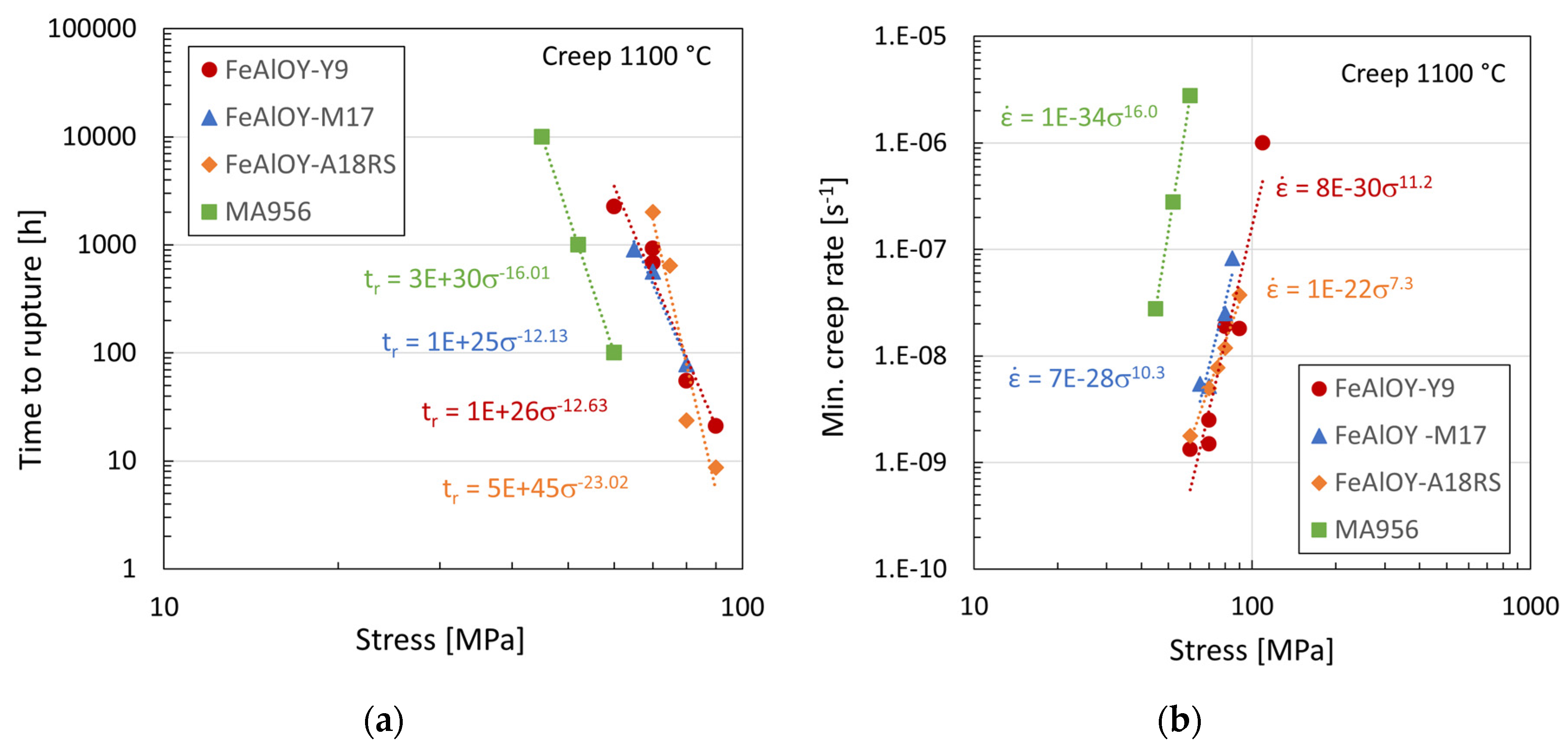
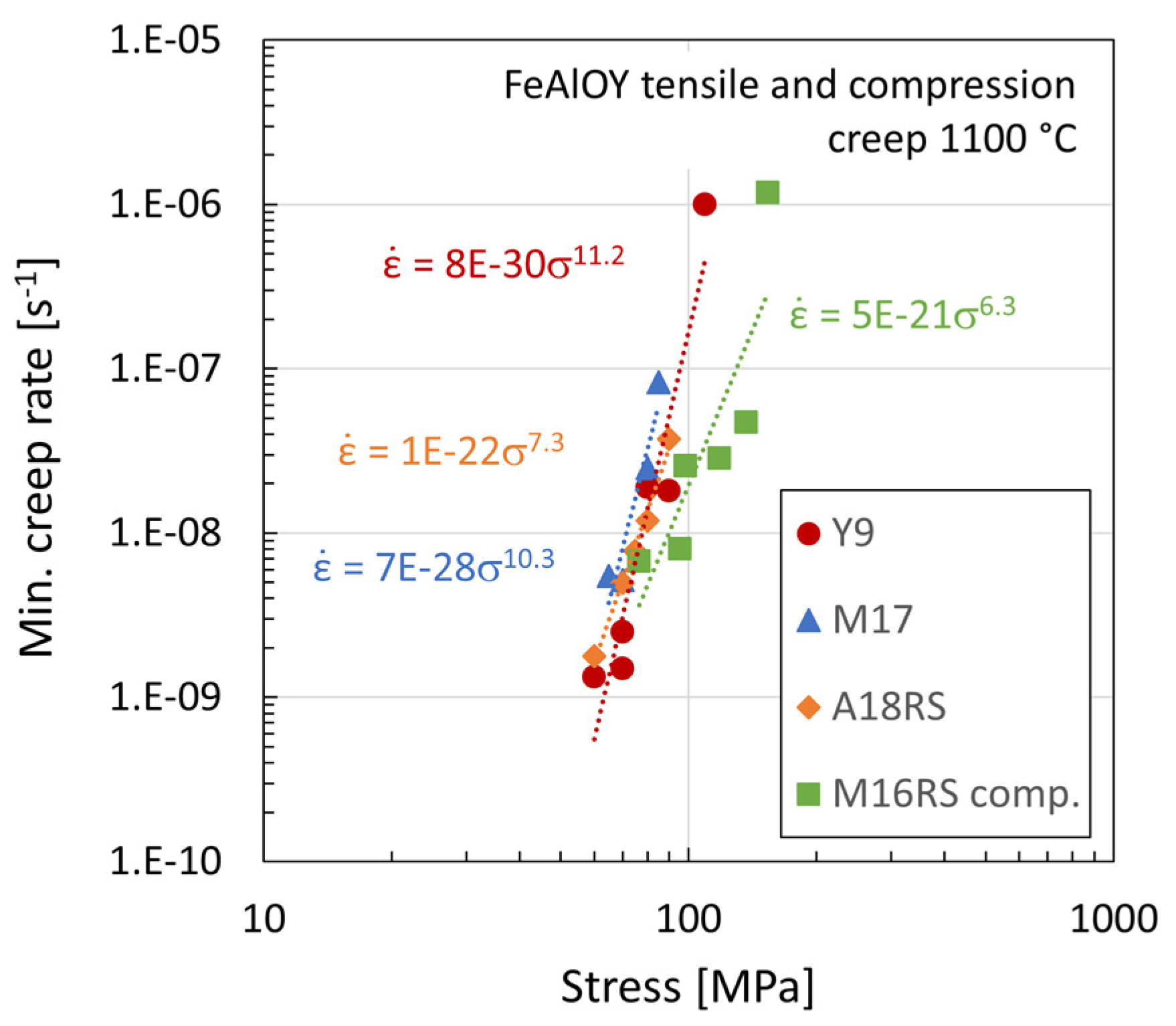
3.4. Creep Testing
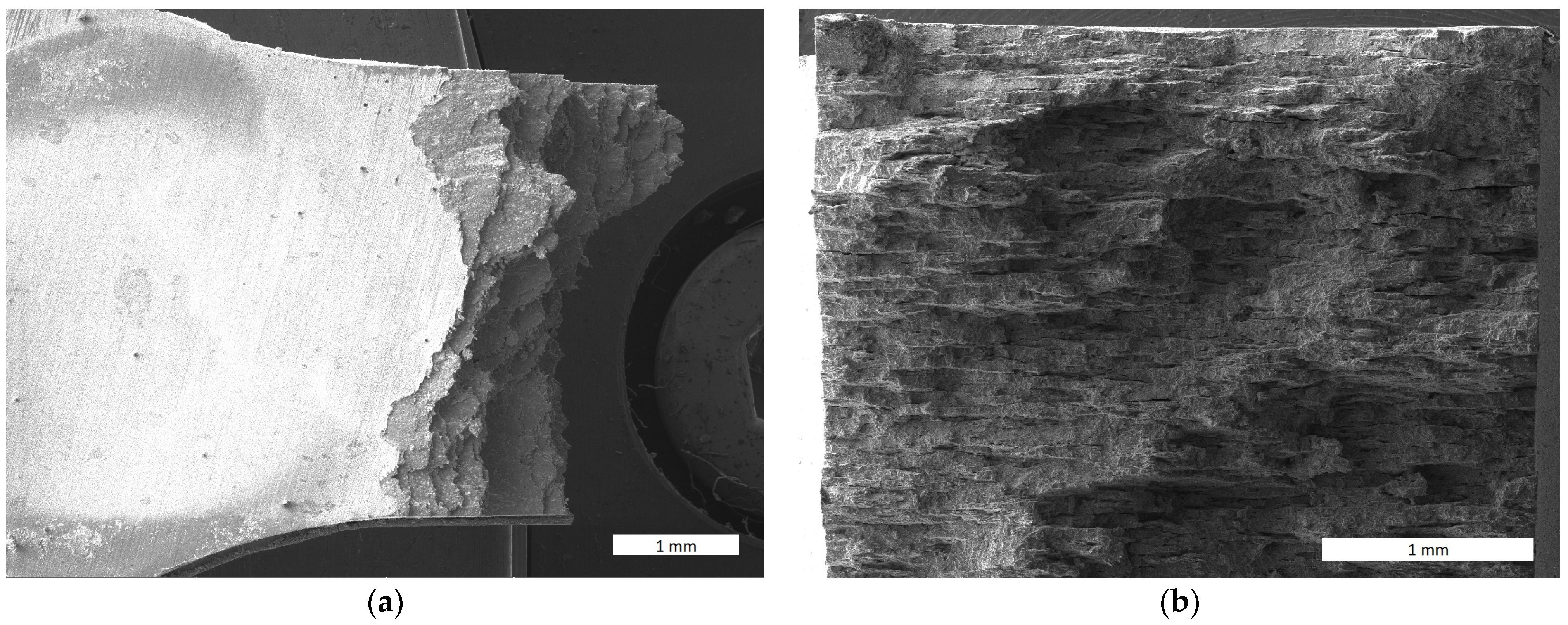
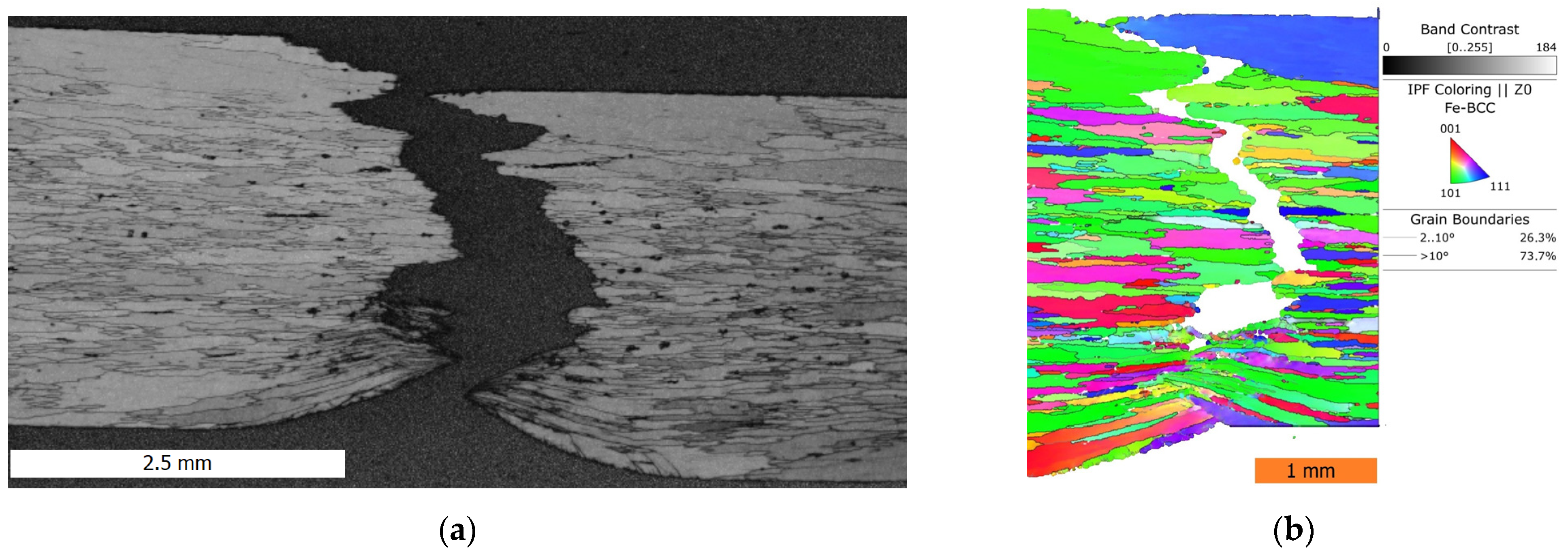
3.5. Fractographic and Microstructure Analyses of Ruptured Specimens
4. Discussion
5. Conclusions
- Long-term oxidation resistance up to 1300 °C.
- Long-term stability of strengthening nanodispersoid up to 1300 °C.
- High creep resistance at 1100–1300 °C exceeding the ODS alloy MA956.
- Over 100 MPa tensile and compression strength at 1100–1300 °C.
- Nearly no primary creep and low creep rate at 1100–1200 °C.
- Machinability in secondary recrystallized state comparable to carbon steels.
- Good hot formability in nanocrystalline state after hot consolidation.
- Production of bars up to 50 mm in diameter and sheets up to 100 mm in width by hot rolling is under development.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yano, Y.; Sekio, Y.; Tanno, T.; Kato, S.; Inoue, T.; Oka, H.; Ohtsuka, S.; Furukawa, T.; Uwaba, T.; Kaito, T.; et al. Ultra-High Temperature Creep Rupture and Transient Burst Strength of ODS Steel Claddings. J. Nucl. Mater. 2019, 516, 347–353. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X. Research Progress of ODS FeCrAl Alloys—A Review of Composition Design. Materials 2023, 16, 6280. [Google Scholar] [CrossRef] [PubMed]
- Gamanov, Š.; Luptáková, N.; Bořil, P.; Jarý, M.; Mašek, B.; Dymáček, P.; Svoboda, J. Mechanisms of Plastic Deformation and Fracture in Coarse Grained Fe–10Al–4Cr–4Y2O3 ODS Nanocomposite at 20–1300 °C. J. Mater. Res. Technol. 2023, 24, 4863–4874. [Google Scholar] [CrossRef]
- Luptáková, N.; Svoboda, J.; Bártková, D.; Weiser, A.; Dlouhý, A. The Irradiation Effects in Ferritic, Ferritic–Martensitic and Austenitic Oxide Dispersion Strengthened Alloys: A Review. Materials 2024, 17, 3409. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Y.; Wang, Y.; Han, L.; Zhang, Y.; Zhou, Z. Recent Progress on Creep Properties of ODS FeCrAl Alloys for Advanced Reactors. Materials 2023, 16, 3497. [Google Scholar] [CrossRef]
- Long, D.; Qiu, S.; Liu, W.; Sun, Y.; Luo, W.; Liu, H.; Zhang, R. Hot Deformation Behavior and Microstructure Features of FeCrAl–ODS Alloy. J. Iron Steel Res. Int. 2022, 29, 1455–1463. [Google Scholar] [CrossRef]
- Hadraba, H.; Husak, R.; Stratil, L.; Siska, F.; Chlup, Z.; Puchy, V.; Michalicka, J. Survey of Oxide Candidate for Advanced 9%, 14% and 17%Cr ODS Steels for Fusion Applications. Fusion Eng. Des. 2017, 124, 1028–1032. [Google Scholar] [CrossRef]
- Hojná, A.; Pazderová, M.; Rozumová, L.; Vít, J.; Hadraba, H.; Stratil, L.; Čížek, J. Performance of Sc-Y-ODS Variant of Eurofer Steel in Stagnant PbLi at 600 °C. J. Nucl. Mater. 2023, 575, 154227. [Google Scholar] [CrossRef]
- Miura, N.; Harada, N.; Kondo, Y.; Okabe, M.; Matsuo, T. Stress Exponent of Minimum Creep Rate and Activation Energy of Creep for Oxide Dispersion-Strengthened Nickel-Based Superalloy MA754. ISIJ Int. 2012, 52, 140–146. [Google Scholar] [CrossRef]
- Dobeš, F.; Hadraba, H.; Chlup, Z.; Matějíček, J. Different Types of Particle Effects in Creep Tests of CoCrFeNiMn High-Entropy Alloy. Materials 2022, 15, 7363. [Google Scholar] [CrossRef]
- Hadraba, H.; Chlup, Z.; Dlouhy, A.; Dobes, F.; Roupcova, P.; Vilemova, M.; Matejicek, J. Oxide Dispersion Strengthened CoCrFeNiMn High-Entropy Alloy. Mater. Sci. Eng. A 2017, 689, 252–256. [Google Scholar] [CrossRef]
- Sahragard-Monfared, G.; Zhang, M.; Smith, T.M.; Minor, A.M.; Gibeling, J.C. Superior Tensile Creep Behavior of a Novel Oxide Dispersion Strengthened CrCoNi Multi-Principal Element Alloy. Acta Mater. 2023, 255, 119032. [Google Scholar] [CrossRef]
- UNS S67956; Material Datasheet INCOLOY® Alloy MA956. Special Metals Corporation: Huntington, WV, USA, 2008.
- UNS S67957; Material Datasheet INCOLOY® Alloy MA957. Special Metals Corporation: Huntington, WV, USA, 2008.
- Korb, G.; Rühle, M.; Martinz, H.-P. New Iron-Based ODS-Superalloys for High Demanding Applications. In Proceedings of the Volume 5: Manufacturing Materials and Metallurgy; Ceramics; Structures and Dynamics; Controls, Diagnostics and Instrumentation; Education; IGTI Scholar Award; General; American Society of Mechanical Engineers, Orlando, FL, USA, 3–6 June 1991. [Google Scholar]
- Wasilkowska, A.; Bartsch, M.; Messerschmidt, U.; Herzog, R.; Czyrska-Filemonowicz, A. Creep Mechanisms of Ferritic Oxide Dispersion Strengthened Alloys. J. Mater. Process. Technol. 2003, 133, 218–224. [Google Scholar] [CrossRef]
- Kazimierzak, B.; Prignon, J.M.; Fromont, R.I. An ODS Material with Outstanding Creep and Oxidation Resistance above 1100 °C. Mater. Des. 1992, 13, 67–70. [Google Scholar] [CrossRef]
- Ukai, S.; Harada, M.; Okada, H.; Inoue, M.; Nomura, S.; Shikakura, S.; Nishida, T.; Fujiwara, M.; Asabe, K. Tube Manufacturing and Mechanical Properties of Oxide Dispersion Strengthened Ferritic Steel. J. Nucl. Mater. 1993, 204, 74–80. [Google Scholar] [CrossRef]
- Schaeublin, R.; Leguey, T.; Spätig, P.; Baluc, N.; Victoria, M. Microstructure and Mechanical Properties of Two ODS Ferritic/Martensitic Steels. J. Nucl. Mater. 2002, 307–311, 778–782. [Google Scholar] [CrossRef]
- Byun, T.S.; Yoon, J.H.; Wee, S.H.; Hoelzer, D.T.; Maloy, S.A. Fracture Behavior of 9Cr Nanostructured Ferritic Alloy with Improved Fracture Toughness. J. Nucl. Mater. 2014, 449, 39–48. [Google Scholar] [CrossRef]
- Klueh, R.L.; Shingledecker, J.P.; Swindeman, R.W.; Hoelzer, D.T. Oxide Dispersion-Strengthened Steels: A Comparison of Some Commercial and Experimental Alloys. J. Nucl. Mater. 2005, 341, 103–114. [Google Scholar] [CrossRef]
- Kim, J.H.; Byun, T.S.; Hoelzer, D.T.; Kim, S.-W.; Lee, B.H. Temperature Dependence of Strengthening Mechanisms in the Nanostructured Ferritic Alloy 14YWT: Part I—Mechanical and Microstructural Observations. Mater. Sci. Eng. A 2013, 559, 101–110. [Google Scholar] [CrossRef]
- Holzer, J.; Gamanov, Š.; Luptáková, N.; Dlouhý, A.; Svoboda, J. Coarsening Kinetics of Y2O3 Dispersoid in New Grade of Fe-Al-Cr-Based ODS Alloy. Metals 2022, 12, 210. [Google Scholar] [CrossRef]
- Svoboda, J.; Bořil, P.; Holzer, J.; Luptáková, N.; Jarý, M.; Mašek, B.; Dymáček, P. Substantial Improvement of High Temperature Strength of New-Generation Nano-Oxide-Strengthened Alloys by Addition of Metallic Yttrium. Materials 2022, 15, 504. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, J.; Luptáková, N.; Jarý, M.; Dymáček, P. Influence of Hot Consolidation Conditions and Cr-Alloying on Microstructure and Creep in New-Generation ODS Alloy at 1100 °C. Materials 2020, 13, 5070. [Google Scholar] [CrossRef] [PubMed]
- Gamanov, Š.; Holzer, J.; Roupcová, P.; Svoboda, J. High Temperature Oxidation Kinetics of Fe-10Al-4Cr-4Y2O3 ODS Alloy at 1200–1400 °C. Corros. Sci. 2022, 206, 110498. [Google Scholar] [CrossRef]
- Svoboda, J.; Kunčická, L.; Luptáková, N.; Weiser, A.; Dymáček, P. Fundamental Improvement of Creep Resistance of New-Generation Nano-Oxide Strengthened Alloys via Hot Rotary Swaging Consolidation. Materials 2020, 13, 5217. [Google Scholar] [CrossRef]
- Dymáček, P.; Kocich, R.; Kunčická, L.; Jarý, M.; Luptáková, N.; Holzer, J.; Mašek, B.; Svoboda, J. Processing of Top Creep and Oxidation Resistant Fe-Al Based ODS Alloys. Procedia Struct. Integr. 2022, 42, 1576–1583. [Google Scholar] [CrossRef]
- Bártková, D.; Šmíd, M.; Mašek, B.; Svoboda, J.; Šiška, F. Kinetic Study of Static Recrystallization in an Fe–Al–O Ultra-Fine-Grained Nanocomposite. Philos. Mag. Lett. 2017, 97, 379–385. [Google Scholar] [CrossRef]
- Bhadauria, A.; Singh, L.K.; Ballal, A.R.; Vijay, R. Effect of Yttria Dispersion on Creep Properties of Pure Iron. Trans. Indian Inst. Met. 2016, 69, 253–259. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dymáček, P.; Jarý, M.; Bártková, D.; Luptáková, N.; Gamanov, Š.; Bořil, P.; Georgiev, V.; Svoboda, J. High-Temperature Creep Resistance of FeAlOY ODS Ferritic Alloy. Materials 2024, 17, 4984. https://doi.org/10.3390/ma17204984
Dymáček P, Jarý M, Bártková D, Luptáková N, Gamanov Š, Bořil P, Georgiev V, Svoboda J. High-Temperature Creep Resistance of FeAlOY ODS Ferritic Alloy. Materials. 2024; 17(20):4984. https://doi.org/10.3390/ma17204984
Chicago/Turabian StyleDymáček, Petr, Milan Jarý, Denisa Bártková, Natália Luptáková, Štepán Gamanov, Petr Bořil, Vjaceslav Georgiev, and Jiří Svoboda. 2024. "High-Temperature Creep Resistance of FeAlOY ODS Ferritic Alloy" Materials 17, no. 20: 4984. https://doi.org/10.3390/ma17204984
APA StyleDymáček, P., Jarý, M., Bártková, D., Luptáková, N., Gamanov, Š., Bořil, P., Georgiev, V., & Svoboda, J. (2024). High-Temperature Creep Resistance of FeAlOY ODS Ferritic Alloy. Materials, 17(20), 4984. https://doi.org/10.3390/ma17204984







