The Role of Methods for Applying Cucurbit[6]uril to Hydroxyapatite for the Morphological Tuning of Its Surface in the Process of Obtaining Composite Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments for Interpreting Results
2.1.1. Infrared Spectroscopy
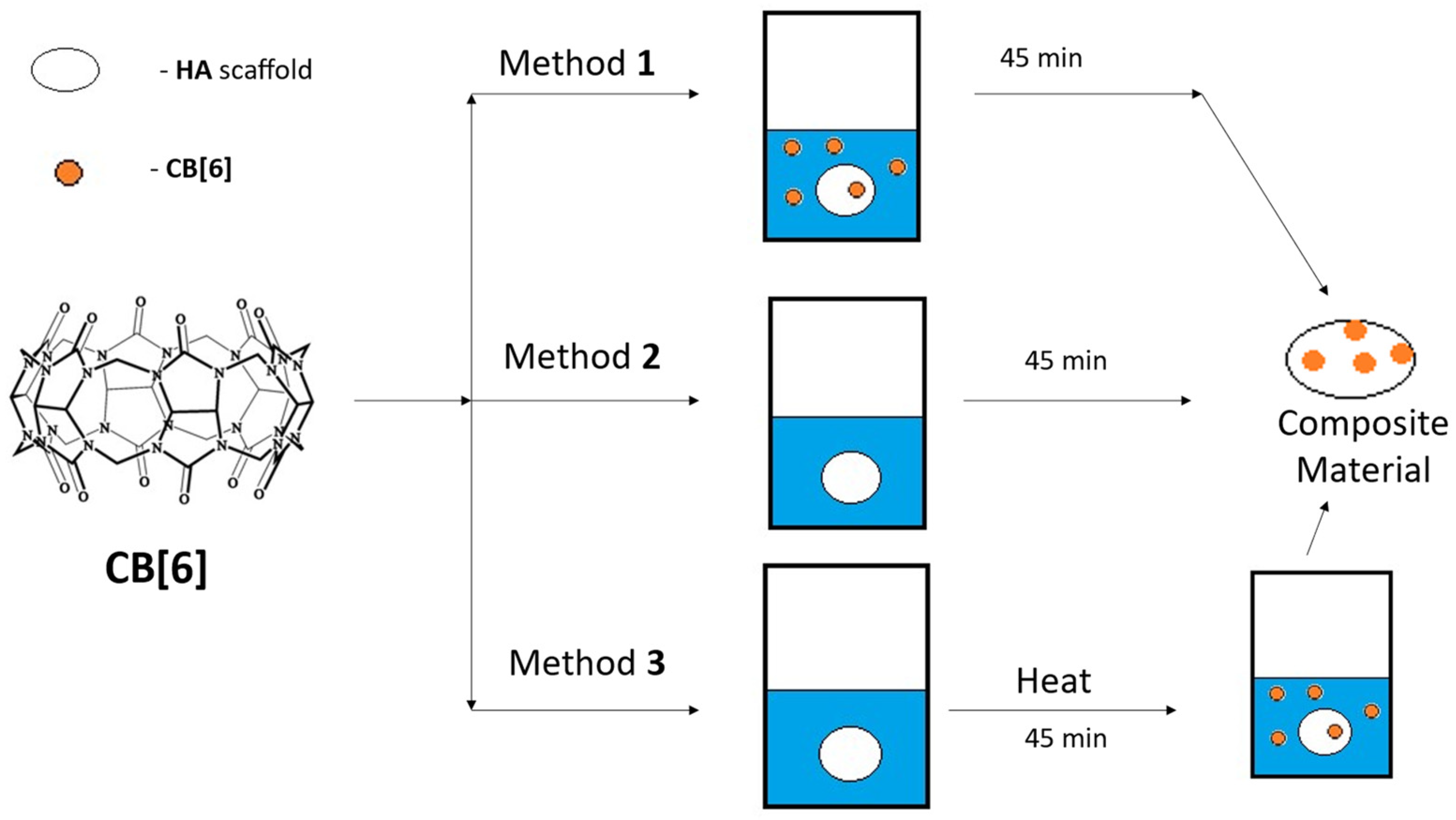
2.1.2. Scanning Electron Microscope (SEM) of CB[6] + HA Samples
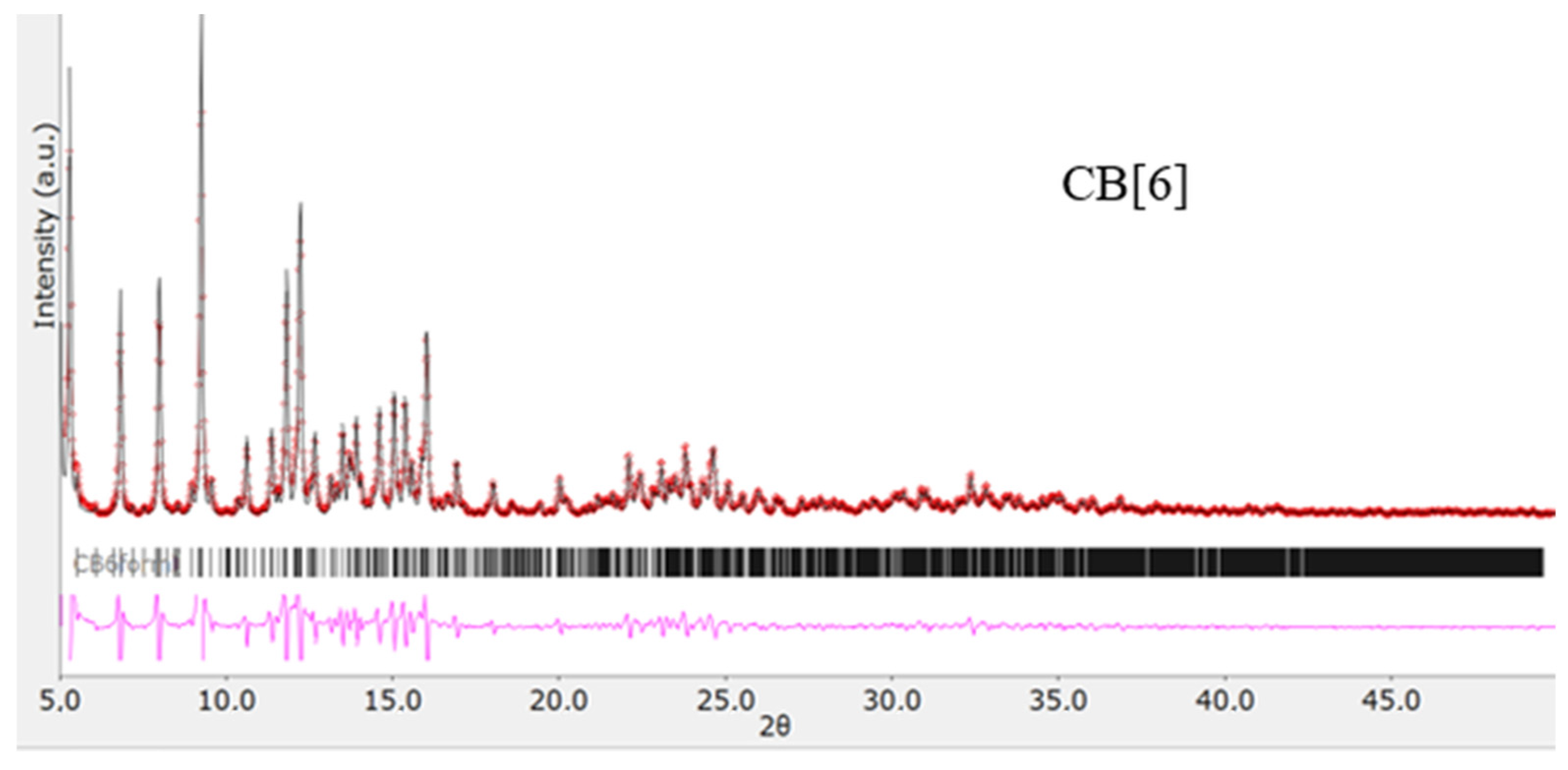
2.1.3. X-ray Diffraction Analysis
2.2. Preparation of Scaffolds from HA
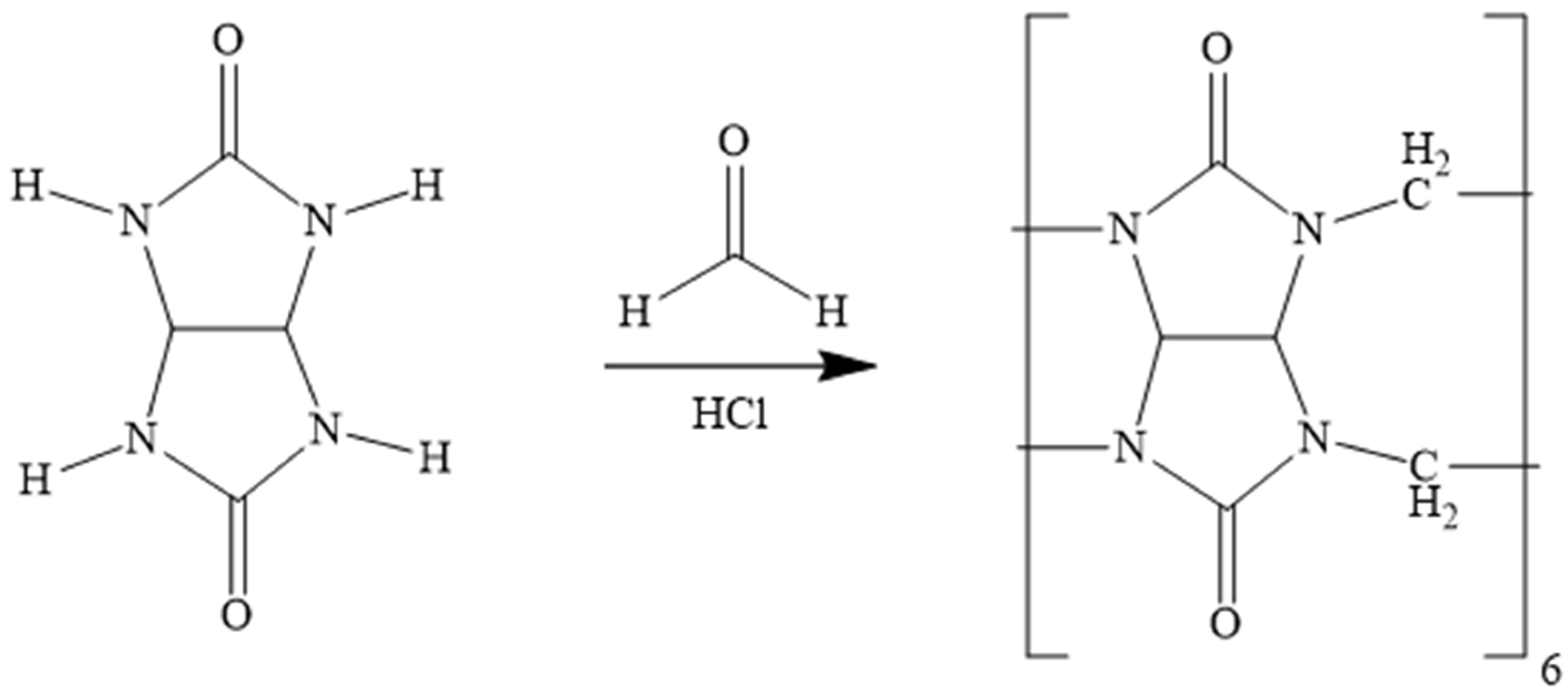
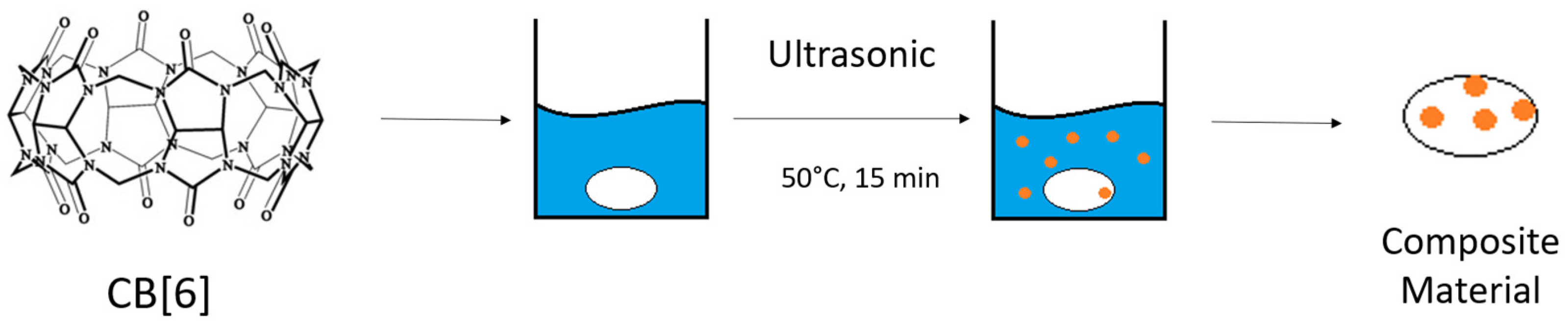
2.3. Synthesis and Purification of CB[6]
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vikhrov, S.P.; Kholomina, T.A.; Begun, P.I.; Afonin, P.N. Biomedical Materials Science; Hotline-Telecom: Moscow, Russia, 2006. [Google Scholar]
- Williams, D.F. Tissue, biomaterial interactions. J. Mater. Sci. 1987, 22, 3421–3445. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Elgendy, H. The biocompatibility and toxicity of degradable polymeric materials: Implication for drug delivery. Site Specif. Drug Deliv. 1994, 12, 27–46. [Google Scholar]
- Mikos, A.G.; McIntire, L.V.; Anderson, J.M.; Babensee, J.E. Host response to tissue engineered devices. Adv. Drug Deliv. Rev. 1998, 33, 111–139. [Google Scholar] [PubMed]
- Langer, R. Drug delivery. Drugs on target. Science 2001, 293, 58–59. [Google Scholar] [CrossRef]
- Santos, A.; Aw, M.S.; Bariana, M.; Kumeria, T.; Wang, Y.; Losic, D. Drug-releasing implants: Current progress, challenges and perspectives. J. Mater. Chem. B 2014, 2, 6157–6182. [Google Scholar] [CrossRef]
- Kulinets, I. Biomaterials and their applications in medicine. In Regulatory Affairs for Biomaterials and Medical Devices; Woodhead Publishing: Sawston, UK, 2015; pp. 1–10. [Google Scholar]
- Antoniac, I.V. Handbook of Bioceramics and Biocomposites; Springer: Berlin, Germany, 2016; ISBN 978-3-319-12461-2. [Google Scholar]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates in dentistry. J. Mater. Sci. Mater. Med. 2013, 24, 1335–1363. [Google Scholar] [CrossRef]
- Sun, H.; Su, F.Z.; Ni, J.; Cao, Y.; He, H.Y.; Fan, K.N. Gold supported on hydroxyapatite as a versatile multifunctional catalyst for the direct tandem synthesis of imines and oximes. Angew. Chem. Int. Ed. Engl. 2009, 48, 4390–4393. [Google Scholar] [CrossRef]
- Ong, J.L.; Chan, D. Hydroxyapatites and their use as coatings in dental implants: A review. Crit. Rev. Biomed. Eng. 1999, 28, 667–707. [Google Scholar] [CrossRef]
- Dey, A.; Bomans, P.; Müller, F.A.; Will, J.; Frederik, P.M.; With, G.; Sommerdijk, N. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. 2015, 55, 272–326. [Google Scholar]
- Yuan, Y.; Huang, P.; Peng, Q.; Zhang, C.; Weng, J. Osteogenesis of porous bioceramics scaffolds consisted of hydroxyapatite spherules after implanted in different non-osseous sites. Mater. Sci. Forum 2009, 610–613, 1335–1338. [Google Scholar] [CrossRef]
- Engin, N.O.; Tas, A.C. Manufacture of macroporous calcium hydroxyapatite bioceramics. J. Eur. Ceram. Soc. 1999, 19, 2569–2572. [Google Scholar] [CrossRef]
- Hou, Y.; Morrison, C.J.; Cramer, S.M. Classification of protein binding in hydroxyapatite chromatography: Synergistic interactions on the molecular scale. Anal. Chem. 2011, 83, 3709–3716. [Google Scholar] [CrossRef]
- Niimi, M.; Masuda, T.; Kaihatsu, K.; Kato, N.; Nakamura, S.; Nakaya, T.; Arai, F. Virus purification and enrichment by hydroxyapatite chromatography on a chip. Sens. Actuators 2014, 201, 185–190. [Google Scholar] [CrossRef]
- Feng, D.; Shi, J.; Wang, X.; Zhang, L.; Cao, S. Hollow hybrid hydroxyapatite microparticles with sustained and pH-responsive drug delivery properties. RSC Adv. 2013, 3, 24975–24982. [Google Scholar] [CrossRef]
- Kim, B.I.; Jeong, S.H.; Jang, S.O.; Kim, K.N.; Kwon, H.K.; Park, Y.D. Tooth whitening effect of toothpastes containing nano-hydroxyapatite. Key Eng. Mater. 2006, 309–311, 541–544. [Google Scholar]
- Shpinyak, S.P. Experimental study of antimicrobial activity of hydroxyappatite and metal nanoparticles in vitro. Mod. Probl. Sci. Educ. 2015, 6, 75–84. [Google Scholar]
- Kolesnichenko, I.V.; Anslyn, E.V. Practical applications of supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 2385–2390. [Google Scholar] [CrossRef]
- Xie, X.M.; Li, X.L.; Luo, H.H.; Lu, H.J.; Chen, F.F.; Li, W. The adsorption of reactive blue 19 dye onto cucurbit[8]uril and cucurbit[6]uril: An experimental and theoretical study. J. Phys. Chem. 2016, 120, 4131–4142. [Google Scholar] [CrossRef]
- Li, X.L.; Xie, X.M.; Luo, H.H.; Li, L.; Li, Z.; Xue, Z.Y.; Li, W. Adsorption of reactive yellow X-R G and reactive brilliant red X-3B onto cucurbit[8]uril and cucurbit[6]uril: Effect factors, adsorption behavior and mechanism study. J. Colloid Interface Sci. 2017, 498, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.H.; Huang, X.Y.; Luo, Y.H.; Li, Z.; Li, L.; Gao, C.; Xiong, J.Y.; Li, W. Adsorption behavior and mechanism of acidic blue 25 dye onto cucurbit[8]uril: A spectral and DFT study. Spectrochim. Acta Part A 2018, 193, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Burkhanbayeva, T.; Ukhov, A.; Fedorishin, D.; Gubankov, A.; Kurzina, I.; Bakibaev, A.; Yerkassov, R.; Mashan, T.; Suyundikova, F.; Nurmukhanbetova, N.; et al. Development of New Composite Materials by Modifying the Surface of Porous Hydroxyapatite Using Cucurbit[n]urils. Materials 2024, 17, 2041. [Google Scholar] [CrossRef]
- Marchenko, E.; Luchsheva, V.; Baigonakova, G.; Bakibaev, A.; Vorozhtsov, A. Functionalization of the Surface of Porous Nickel–Titanium Alloy with Macrocyclic Compounds. Materials 2023, 16, 66. [Google Scholar] [CrossRef]
- Zhumabayeva, G.; Turebayeva, P.; Ukhov, A.; Fedorishin, D.; Gubankov, A.; Luchsheva, V.; Kurzina, I.; Bakibaev, A.; Ryskaliyeva, R.; Abdullina, G.; et al. Development of novel composite biocompatible materials by surface modification of porous inorganic compounds using bambus[6]uril. Materials 2023, 16, 7257. [Google Scholar] [CrossRef]
- Rasskazova, L.A.; Korotchenko, N.M.; Zeer, G.M. Microwave Synthesis of Hydroxyapatite and Physicochemical Study of Its Properties. Phycicochemical Stud. Technol. 2013, 86, 691–695. [Google Scholar] [CrossRef]
- Bardelang, D.; Udachin, K.A.; Leek, D.M.; Margeson, J.C.; Chan, G.; Ratcliffe, C.I.; Ripmeester, J.A. Cucurbit[n]urils (n = 5–8): A Comprehensive Solid State Study. Cryst. Growth Des. 2011, 11, 5598–5614. [Google Scholar] [CrossRef]
- Gerasko, O.A.; Sokolov, M.N.; Fedin, V.P. Mono- and polynuclear aqua complexes and cucurbit[6]uril: Versatile building blocks for supramolecular chemistry. Pure Appl. Chem. 2004, 76, 1633–1646. [Google Scholar] [CrossRef]
- Ling, X.; Saretz, S.; Xiao, L.; Francescon, J.; Masson, E. Water vs. cucurbituril rim: A fierce competition for guest solvation. Chem. Sci 2016, 7, 3569. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Y.; Zhu, Q.; Xue, S.; Tao, Z. Host-guest complexes of a water soluble cucurbit[6]uril derivative with some dications of 1,ω-alkyldipyridines: 1H NMR and X-ray structures. Sci. China Ser. B-Chem. 2009, 52, 475–482. [Google Scholar] [CrossRef]
- Maslii, A.N.; Grishaeva, T.N.; Kuznetsov, A.M.; Bakovets, V.V. Quantum chemical study of the structuring of water in the cavity of cucurbit[6]uril. J. Struct. Chem. 2009, 50, 413–418. [Google Scholar] [CrossRef]
- Zhao, Y.; Buck, D.P.; Morris, D.L.; Pourgholami, M.H.; Day, A.I.; Collins, J.G. Solubilisation and cytotoxicity of albendazole encapsulated in cucurbit[n]uril. Org. Biomol. Chem 2008, 6, 4509–4515. [Google Scholar] [CrossRef] [PubMed]
- Blanch, R.J.; Sleeman, A.J.; White, T.J.; Arnold, A.P.; Day, A.I. Cucurbit[7]uril and o-carborane self-assemble to form a molecular ball bearing. Nano Lett. 2008, 2, 147–149. [Google Scholar] [CrossRef]
- Walker, S.; Kaur, R.; McInnes, F.J.; Wheate, N.J. Synthesis, processing and solid state excipient interactions of cucurbit[6]uril and its formulation into tablets for oral drug delivery. Mol. Pharm. 2002, 7, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
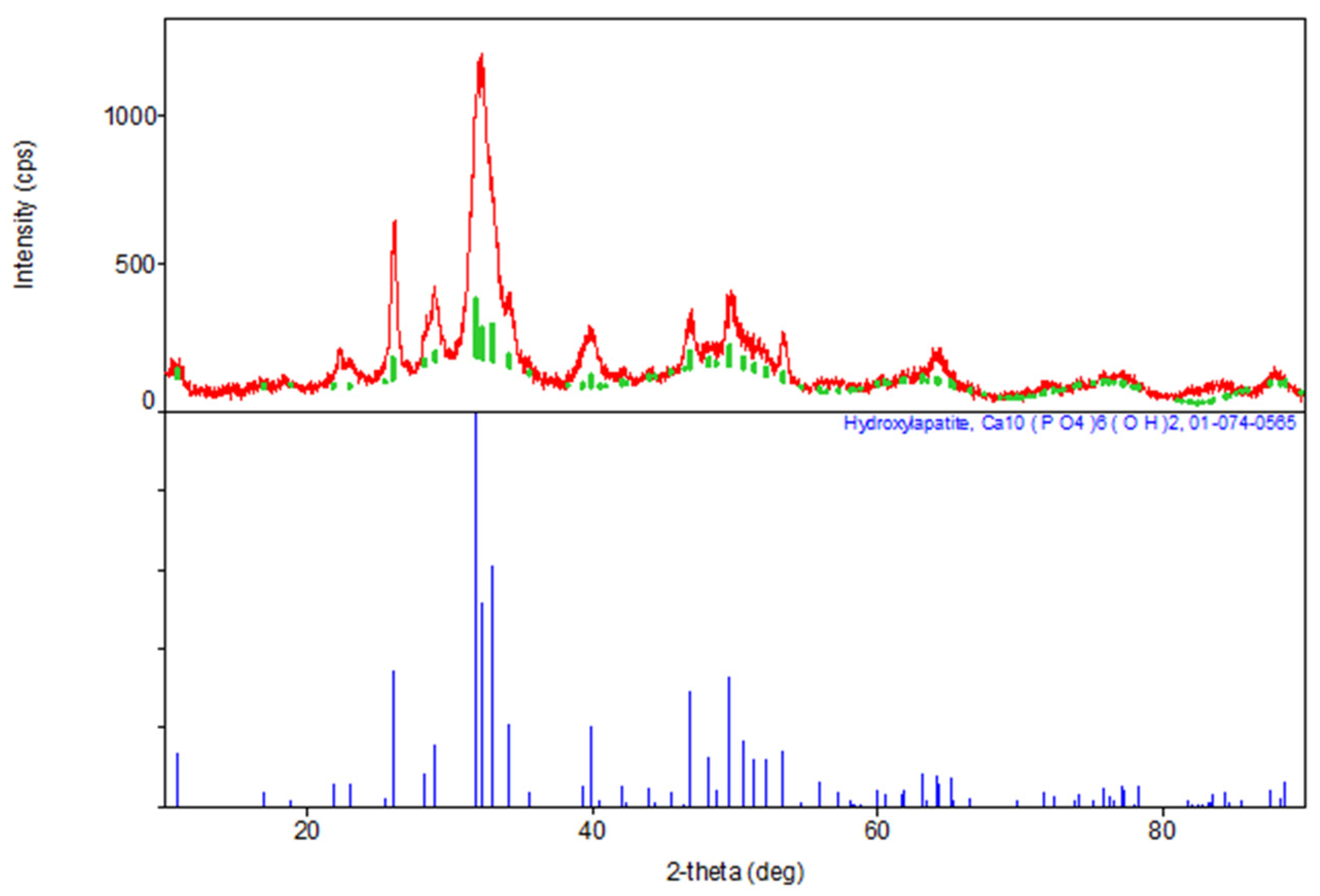
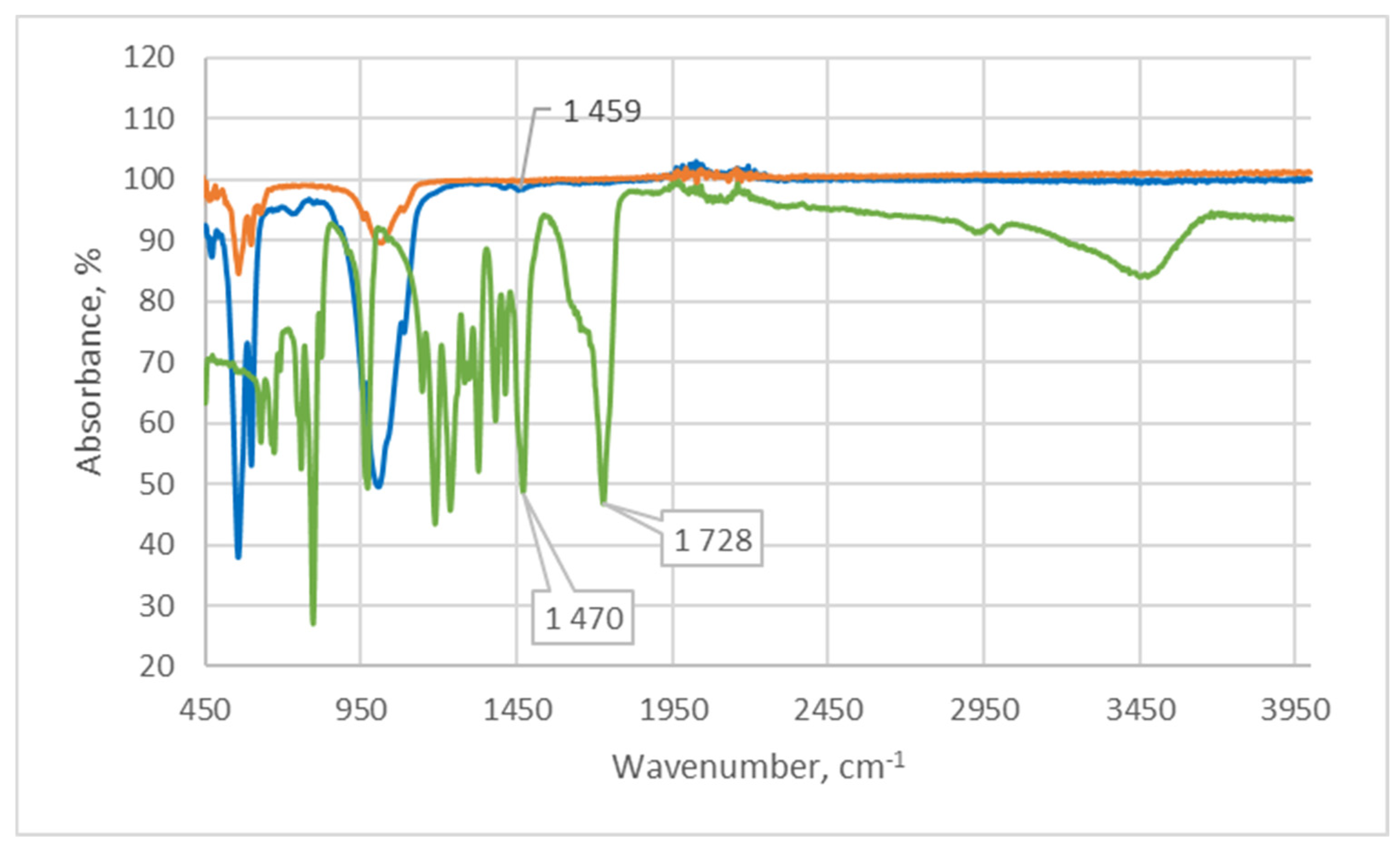
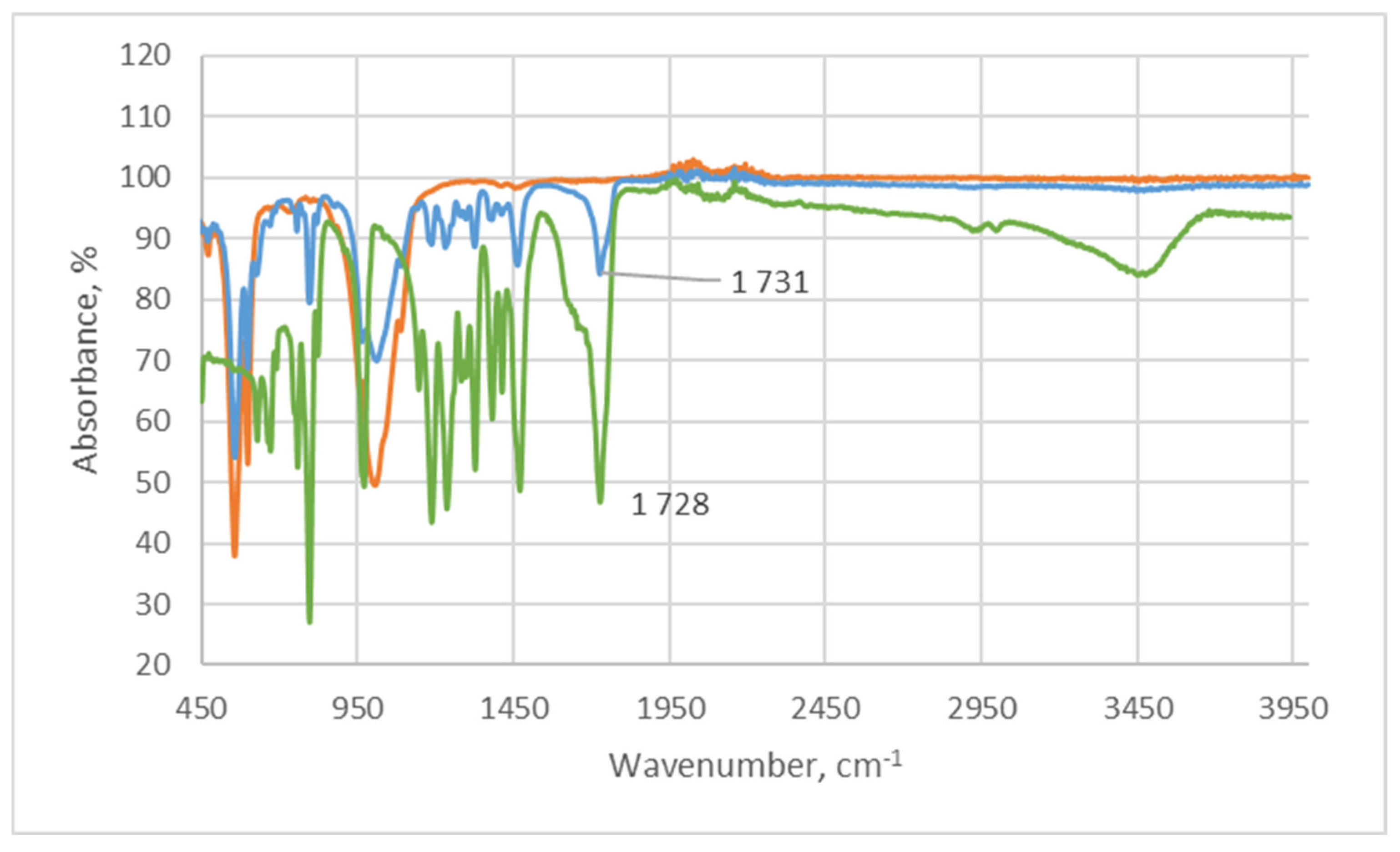
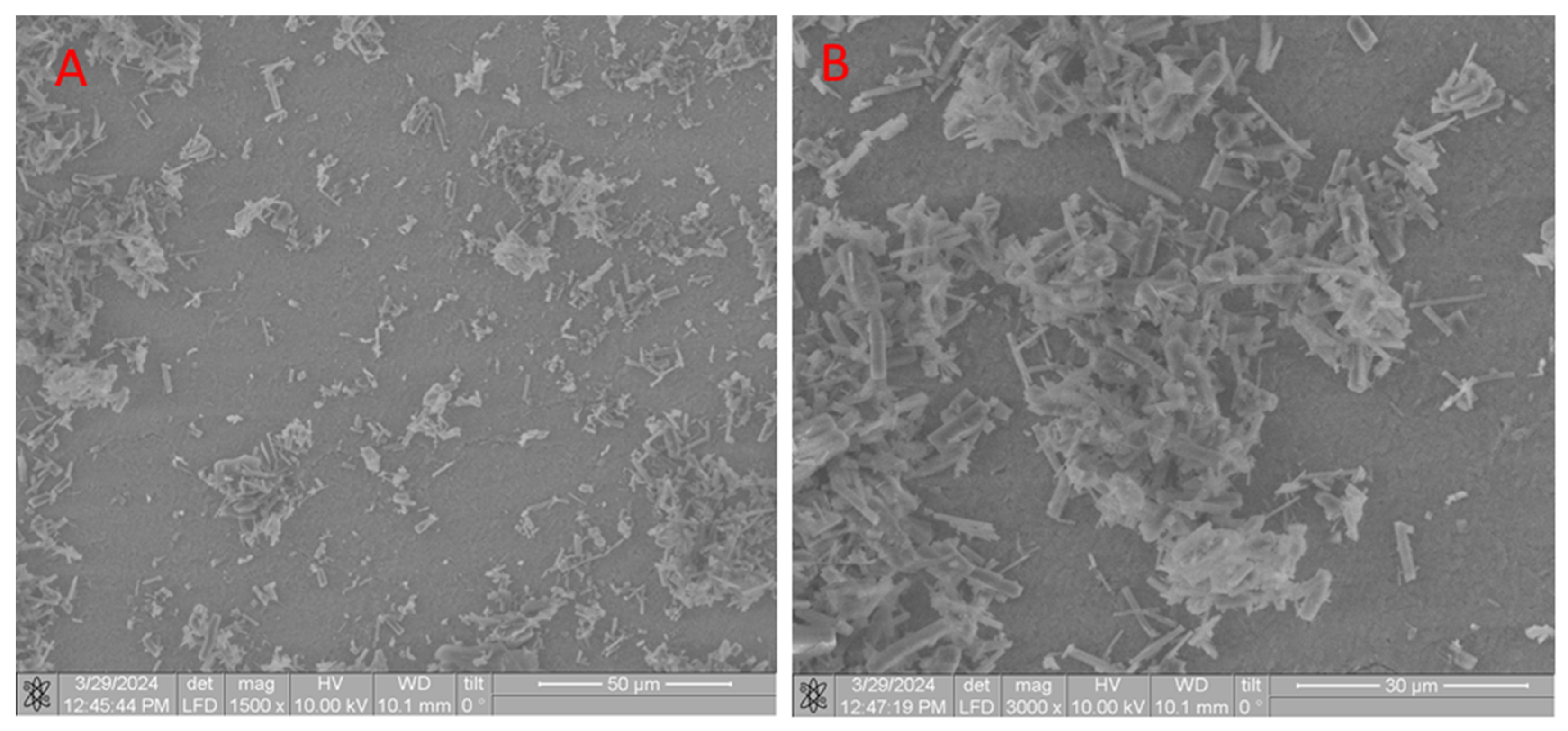

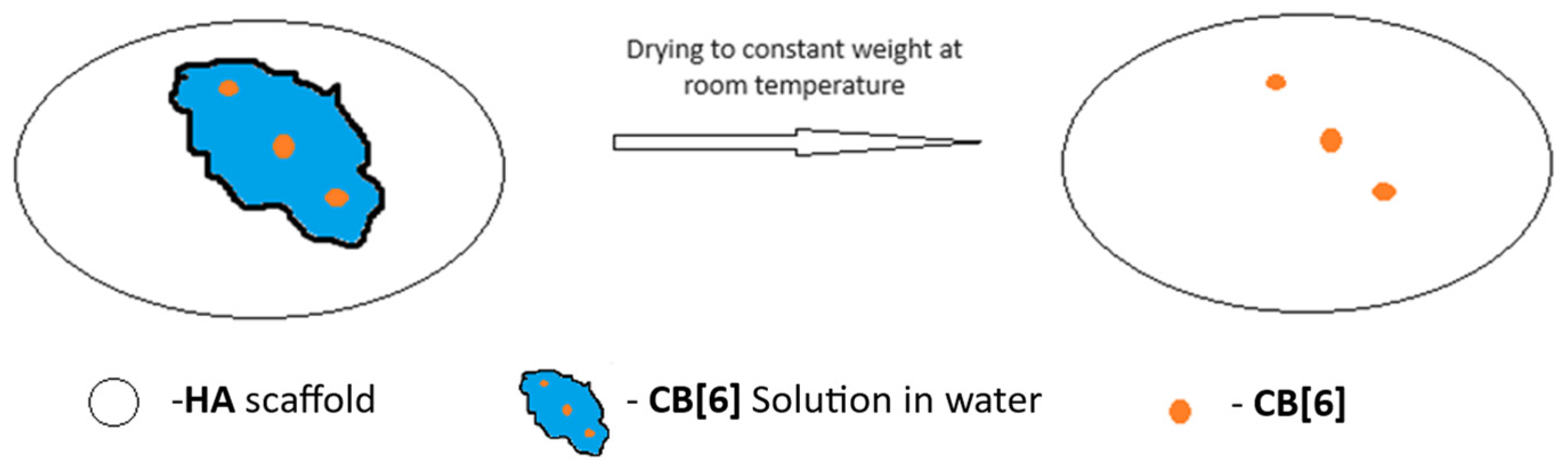
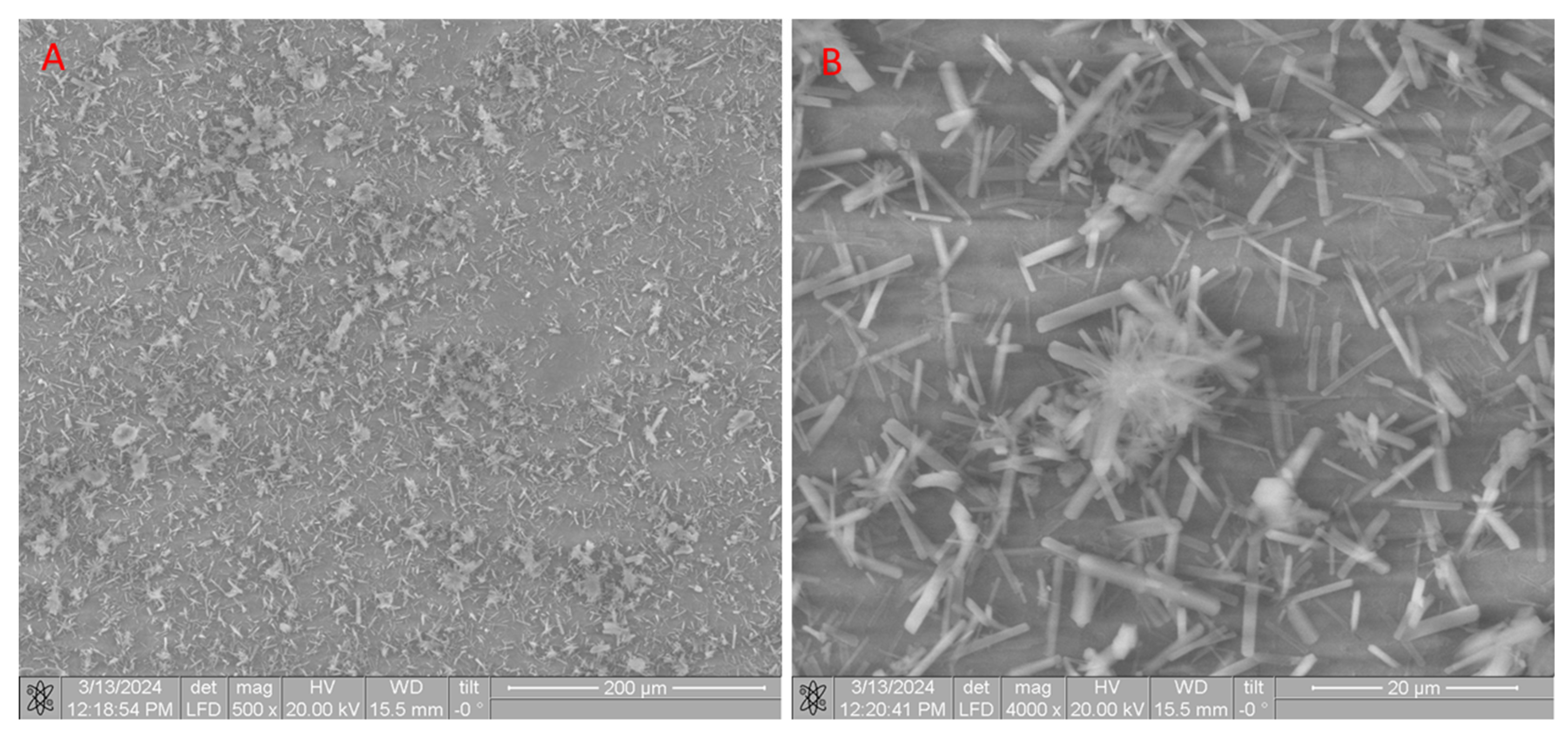

| Sample | The Inorganic Phase | Parameters of the Electronic Cell, Ǻ | |
|---|---|---|---|
| a | c | ||
| Synthesis Product (HA) | Ca10(PO4)6(OH)2 | 9411 | 6863 |
| JCPDS data, No.9-432 | Ca10(PO4)6(OH)2 | 9418 | 6884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkhanbayeva, T.; Ukhov, A.; Assylbekova, D.; Mussina, Z.; Kurzina, I.; Abilkasova, S.; Bakibaev, A.; Issabayeva, M.; Yerkassov, R.; Shaikhova, Z. The Role of Methods for Applying Cucurbit[6]uril to Hydroxyapatite for the Morphological Tuning of Its Surface in the Process of Obtaining Composite Materials. Materials 2024, 17, 4995. https://doi.org/10.3390/ma17204995
Burkhanbayeva T, Ukhov A, Assylbekova D, Mussina Z, Kurzina I, Abilkasova S, Bakibaev A, Issabayeva M, Yerkassov R, Shaikhova Z. The Role of Methods for Applying Cucurbit[6]uril to Hydroxyapatite for the Morphological Tuning of Its Surface in the Process of Obtaining Composite Materials. Materials. 2024; 17(20):4995. https://doi.org/10.3390/ma17204995
Chicago/Turabian StyleBurkhanbayeva, Tolkynay, Arthur Ukhov, Dina Assylbekova, Zukhra Mussina, Irina Kurzina, Sandugash Abilkasova, Abdigali Bakibaev, Manar Issabayeva, Rakhmetulla Yerkassov, and Zhanat Shaikhova. 2024. "The Role of Methods for Applying Cucurbit[6]uril to Hydroxyapatite for the Morphological Tuning of Its Surface in the Process of Obtaining Composite Materials" Materials 17, no. 20: 4995. https://doi.org/10.3390/ma17204995
APA StyleBurkhanbayeva, T., Ukhov, A., Assylbekova, D., Mussina, Z., Kurzina, I., Abilkasova, S., Bakibaev, A., Issabayeva, M., Yerkassov, R., & Shaikhova, Z. (2024). The Role of Methods for Applying Cucurbit[6]uril to Hydroxyapatite for the Morphological Tuning of Its Surface in the Process of Obtaining Composite Materials. Materials, 17(20), 4995. https://doi.org/10.3390/ma17204995








